Abstract
Aim
The aim was to assess study factors that impact the association of cognitive disorders in people with periodontal disease (PD).
Method
Medline, EMBASE and Cochrane databases were searched until February 2022 using keywords and MeSH: (periodon* OR tooth loss OR missing teeth) AND (dementia OR Alzheimer’s Disease OR cognitive*). Observational studies reporting prevalence or risk of cognitive decline, dementia or Alzheimer’s disease (AD) in people with PD compared with healthy controls were included. Meta-analysis quantified the prevalence and risk (relative risk[RR]) of cognitive decline, dementia/AD, respectively. Meta-regression/subgroup analysis explored the impact of study factors including PD severity and classification type, and gender.
Results
Overall, 39 studies were eligible for meta-analysis: 13 cross-sectional and 26 longitudinal studies. PD demonstrated increased risks of cognitive disorders (cognitive decline—RR = 1.33, 95% CI = 1.13–1.55; dementia/AD—RR = 1.22, 95% CI = 1.14–1.31). Risk of cognitive decline increased with PD severity (moderate—[RR] = 1.14, 95% confidence interval [CI] = 1.07–1.22; severe—RR = 1.25, 95% CI = 1.18–1.32). For every 10% population increase in females, the risk of cognitive decline increased by 34% (RR = 1.34, 95% CI = 1.16–1.55). Self-reported PD showed a lower risk of cognitive disorders compared with clinical classification (cognitive decline—RR = 0.77, 95% CI = 0.65–0.91; dementia/AD—RR = 0.86, 95% CI = 0.77–0.96).
Conclusion
The prevalence and risk estimates of cognitive disorders in association with PD can be influenced by gender, the disease classification of PD and its severity. Further homologous evidence taking these study factors into consideration is needed to form robust conclusions.
Keywords: Periodontitis, dementia, cognitive decline, cohort studies, systematic review, older people
Key Points
A systematic review that explores study factors impacting the association of cognitive disorders in periodontal disease (PD).
Study factors including PD severity, classification and sex influence prevalence and risk estimates of cognitive disorders.
Further homologous evidence from observational studies is needed to form robust conclusions.
Introduction
Periodontal disease (PD), a chronic inflammatory condition, is a major driver of tooth loss in older age and the sixth most prevalent non-communicable disease worldwide [1]. Dementia is the fifth leading cause of death globally and there are concerns that the disease prevalence could increase at an alarming rate as a result of the ageing population [2]. Observational evidence suggests that cognitive decline, as a pre-cursor to dementia, is associated with fewer teeth [3–6]. Recent evidence also suggests that there may be a reciprocal relationship between poor oral health and dementia [7]. Experimental studies have shown chronic systemic inflammation may be linked to onset of both dementia and PD [8, 9], and there is also evidence of increased levels of inflammatory markers associated with periodontal pathogens in people with Alzheimer’s disease (AD) [10]. Understanding the factors that could influence this association is imperative in view of tailoring public health initiatives promoting oral health towards dementia prevention.
Observational studies enable non-intrusive examination of exposures, outcomes and risk factors in the general population. Previous systematic reviews have sought to quantify the prevalence and risk of cognitive disorders in PD using observational studies; however, meta-analyses of effect sizes vary vastly and often conclude that further evidence is required to substantiate the findings [11–14]. One review suggested that combining cross-sectional and longitudinal studies in a meta-analysis caused around 16% of heterogeneity [12]. Given their real-world setting, observational studies can be subject to several biases including confounding and selection; it is therefore crucial to consider study factors when conducting systematic reviews and meta-analyses accordingly [15]. Furthermore, recent work has suggested there is risk of overestimating the link of PD and cognitive disorders from spurious associations identified in cross-sectional research [16].
We previously demonstrated the utility of meta-regression in revealing the effect of study factors such as sex, PD classification and study region on risk estimates of cardiovascular disease [17]. A recent systematic review used similar methods to explore effect of sample size, treatment during the follow-up and bias rating in studies estimating the risk of adverse pregnancy outcomes and diabetes in people with PD [18]. As yet, no study has examined the effects of study characteristics on prevalence and risk estimates of cognitive disorders, specifically cognitive decline/impairment and dementia, in people with PD. The aim of the current investigation was to assess the study factors that could impact the association of cognitive disorders in people with PD. In order to pool the results of individual studies, a meta-analysis will be used to quantify risk of dementia in PD populations and meta-regression will be used to evaluate the impact of key risk factors.
Methods
Study design—a systematic review of cross-sectional and longitudinal cohort studies that examine the prevalence and incidence of cognitive disorders in people with periodontitis.
Search strategy and selection criteria
The search string considered alternate terms incorporating several relevant key words and Medical Subject Headings (MeSH) headings. The final Boolean search string was: (periodon* OR tooth loss OR missing teeth) AND (dementia OR Alzheimer’s Disease OR cognitive*) (Supplementary Table 1). The search string was applied from database conception until 2 February 2022 to Medline, EMBASE and Cochrane databases to ensure retrieval of a broad scope of literature. Additional reference checking and ‘citation snowballing’ methods of key articles were also undertaken to maximise search sensitivity.
Study inclusion criteria were outlined as the following:
Cross-sectional or longitudinal retrospective/prospective cohort.
Clinically diagnosed or self-reported PD.
Clearly defined classification of dementia, AD and/or subtypes such as vascular dementia, or cognitive decline (including mild cognitive impairment). Diagnosis should be identified via appropriate disease classification codes such as ICD-10F00-F03, or clinical assessment using verified assessment tool such as MMSE or MoCA.
Provides estimates for prevalence (cross-sectional) or incidence (longitudinal) of dementia or cognitive decline, and/or when absent raw numbers are available for crude calculation.
Peer reviewed articles and published in English.
For full details of study selection, see Note S1 in the supplemental file.
Quality assessment
Quality assessment tools for observational studies can be contentious [19]; therefore, this review employed the Risk of Bias in Non-Randomised Studies of Interventions (ROBINS-I) recommended by Cochrane to determine the risk of bias in cohort and longitudinal observational studies [20]. Results from the risk of bias assessment were conferred with a second author and discrepancies discussed before finalising ROBINS-I assessment table.
The protocol for the present review was registered to PROSPERO before the study began (registration number: CRD42019154897).
Statistical analysis
Odds ratios (OR), hazard ratios (HR) and relative risks (RR) were used in different studies to quantify the risk of cognitive decline and dementia/AD. We examined cross-sectional and longitudinal studies separately. In cross-sectional studies that did not report the effect size, we used raw numbers of exposed/unexposed and case numbers to quantify a crude RR in pooling for meta-analysis. We converted ORs and HRs into RR in order to maximise the number of included studies for meta-analysis [21]. Where possible, adjusted RRs were used in the meta-analysis and adjustments of key confounders, such as smoking, gender and age, were screened for each study. For inclusion in meta-analysis, studies must have reported total population numbers for PD and non-PD cases, and RRs or converted RRs should also be available to be eligible for synthesis and pooling. For precision, studies should also have a minimum of 30 participants in the exposed PD/unexposed groups; studies that reported less than this were included as part of a sensitivity analysis.
Random effects meta-analysis was performed for prevalence or risk of cognitive decline or dementia according to the study type (cross-sectional or longitudinal). Subgroup analysis and meta-regression examined the impact of study and population factors such as age, smoking, PD classification (self-report or clinical), study region, sex and sample size. Study and population factors were selected according to previous literature and data availability. Average age (mean or median), smoking (population percentage), sex (female population percentage) were treated as continuous variables in meta-regression. Where age was reported in bands and the average was missing, the median value of the mode group was considered the average. PD classification, study region and sample size were treated as categorical variables. Sample size categories were determined according to the range in sample sizes within included studies and to maximise study numbers. Variables included in the meta-regression were dependent on data availability from included studies. I2 was used to measure the study heterogeneity. Publication bias was illustrated with funnel plots, which was quantified by Egger’s test. Forest plots were used to visualise the pooled results from meta-analysis.
Results
The search strategy retrieved 2,146 studies, with 1,726 studies eligible for title and abstract screening following duplicate removal. After title and abstract screening and hand searches, 232 studies were eligible for full text screening with 49 studies eligible for review. Most studies were excluded due to ineligible study design (n = 63). Of the 49 included studies, 21 were cross-sectional and 28 were of longitudinal design, including 11 and 11, and 20 and 9 studies examining dementia or cognitive decline, respectively. Two studies examined both dementia and cognitive decline as outcomes [22, 23]. One study was not eligible for meta-analysis due to missing raw data [24] and a further study was not eligible due to insufficient case numbers (number of cases in exposed = 0) [25]. One study was not eligible for meta-regression due to the across-region study population [22]. Seven studies were not eligible for meta-analysis as they reported below 30 participants in the exposed/unexposed groups [23, 26–31]. All included studies were published between 2007 and 2022 (Figure S1, Table 1).
Table 1.
Summary of included studies
| Study | Region | Age (average) | Females (%) | Sample size | PD classification | Total follow-up time (years) | ROBINS-I rating | |
|---|---|---|---|---|---|---|---|---|
| Cross-sectional studies | ||||||||
| Laugisch 2021*** | AD | Europe | 55 | 58.3 | 40 | Clinical | Serious | |
| Popovac 2021*** | AD | Europe | 62.6 | 76.06 | 179 | Clinical | Serious | |
| Tiisanoja 2019 | AD | Asia | 70 | 80.9 | 170 | Clinical | Moderate | |
| Tsuneishi 2021 | AD | Asia | 55.2 | 66.5 | 3,549,513 | Clinical | Serious | |
| Okamoto 2010 | Cognitive decline | Asia | 49.6 | 71 | 1964 | Clinical | Serious | |
| Winning 2022 | Cognitive decline | Europe | 55.3 | 65.5 | 2,258 | Clinical | Moderate | |
| Abdulhade Ganem 2019 | Cognitive decline | Asia | 100 | 48.2 | 79 | Clinical | Critical | |
| ALFotawi 2019 | Cognitive decline | Asia | 40 | 65.67 | 68 | Clinical | Serious | |
| Jockusch 2021*** | Cognitive decline | North America | 65.7 | 86 | 25 | Clinical | Serious | |
| Kim 2021 | Cognitive decline | Asia | 65.7 | 77.2 | 134 | Clinical | Serious | |
| Mizutani 2021 ** | Cognitive decline | Asia | 70 | 71 | 35 | Clinical | Serious | |
| Nilsson 2014 | Cognitive decline | Europe | 58.2 | 88.5 | 942 | Clinical | Serious | |
| Nilsson 2018 | Cognitive decline | Europe | 55 | 88.5 | 767 | Clinical | Serious | |
| Peres 2014 | Cognitive decline | South America | 62.5 | 62.5 | 1,122 | Self-report | Moderate | |
| Sharma 2021*** | Cognitive decline | Asia | 46.9 | 68.27 | 57 | Clinical | Serious | |
| Shin 2016 | Cognitive decline | Asia | 48.1 | 69.2 | 108 | Clinical | Serious | |
| Barbe 2019*** | Dementia | Europe | 73 | 82 | 40 | Clinical | Serious | |
| Chu 2015*** | Dementia | Asia | 79.7 | 80 | 97 | Clinical | Serious | |
| Gao 2020 | Dementia | Asia | 79 | 80.9 | 167 | Clinical | Serious | |
| Kato 2019 | Dementia | Asia | 51 | 78.1 | 210 | Clinical | Serious | |
| Saito 2021 | Dementia | Asia | 56.6 | 78 | 3,108 | Clinical | Moderate | |
| Longitudinal studies | ||||||||
| Adam 2022 | AD | North America | 58 | 76.5 | 162 | Clinical | 17 | Serious |
| Chen 2017 | AD | Asia | 47 | 54.2 | 27,963 | Clinical | 7 | Serious |
| Batty 2013 | Cognitive decline | Asia, Australasia, Europe and North America | 42.5 | 65.4 | 8,788 | Self-report | 5 | Serious |
| Hatta 2018 | Cognitive decline | Asia | 52.9 | 80 | 463 | Clinical | 3 | Serious |
| Nilsson 2018 | Cognitive decline | Europe | 56.4 | 67 | 566 | Clinical | 6 | Serious |
| Govindan 2021 | Cognitive decline | Asia | 69.3 | 72.5 | 120 | Clinical | 5 | Serious |
| Iwasaki 2019 | Cognitive decline | Asia | 52.4 | 80.1 | 179 | Clinical | 5 | Moderate |
| Okamoto 2015 | Cognitive decline | Asia | 49.4 | 71 | 2,155 | Clinical | 5 | Critical |
| Saito 2018 | Cognitive decline | Asia | 70.3 | 72.05 | 140 | Clinical | 11 | Serious |
| Xu 2021 | Cognitive decline | Asia | 49 | 81.4 | 6,721 | Self-report | 8 | Serious |
| Yang 2022 | Cognitive decline | Asia | 52.7 | 83 | 7,098 | Self-report | 16 | Serious |
| Arrive 2012 ** | Dementia | Europe | 54.6 | 70 | 405 | Clinical | 15 | Critical |
| Choi 2019* | Dementia | Asia | 49.3 | 60.4 | 262,349 | Clinical | 12 | Serious |
| Demmer 2020 | Dementia | North America | 54.4 | 63 | 3,258 | Clinical | 18.4 | Moderate |
| Holmer 2022 | Dementia | Europe | 43.8 | 61 | 37,174 | Clinical | 8 | Critical |
| Kiuchi 2021 | Dementia | Asia | 54 | 73.1 | 35,744 | Self-report | 6 | Serious |
| Lee 2017a* | Dementia | Asia | 50.5 | 54.5 | 117,476 | Clinical | 10 | Serious |
| Lee 2017b* | Dementia | Asia | 46 | 72.4 | 6,056 | Clinical | 12 | Serious |
| Lee 2020* | Dementia | Asia | 51.6 | 52 | 54,234 | Clinical | 13 | Serious |
| Malone 2021* | Dementia | North America | 38.4 | 67 | 439,760 | Clinical | 3 | Serious |
| Paganini-Hill 2012 | Dementia | North America | 69 | 81 | 1,169 | Self-report | 18 | Serious |
| Stein 2007*** | Dementia | North America | 100 | 84 | 101 | Clinical | 12 | Serious |
| Stewart 2015 | Dementia | Europe | 100 | 80 | 351 | Clinical | 38 | Serious |
| Takeuchi 2017 | Dementia | Asia | 55.7 | 75 | 1,241 | Clinical | 5 | Serious |
| Tzeng 2016* | Dementia | Asia | 61.4 | 44.5 | 8,828 | Clinical | 10 | Serious |
| Yamamoto 2012 | Dementia | Asia | 51.2 | 67 | 2,919 | Self-report | 4 | Serious |
| Yoo 2019* | Dementia | Asia | 66.5 | 64.5 | 209,806 | Clinical | 14 | Serious |
| Kim 2020* | Vascular dementia | Asia | 28.5 | 44.5 | 9,807 | Clinical | 14 | Serious |
Key: Alzheimer’s disease, AD; periodontal disease, PD. *Crude rate only, **received treatment during follow-up, ***not eligible for meta-analysis
Most study populations were from Asia (n = 18) and utilised a clinical diagnosis of PD to define the exposure (n = 22). Participants in eight dementia studies also received periodontal treatment during the follow-up as part of the study design. The median total study follow-up time for longitudinal studies was 10 years (interquartile range[IQR] = 5–14 years) (Table 1). Risk of bias assessment by ROBINS-I checklist demonstrated most included studies were of serious risk of bias due to risk of confounding or selection biases (n = 39; Table 2). Both cross-sectional and longitudinal cognitive decline studies had significant risk of publication bias, whereas this was not observed in dementia/AD studies (Figures S2 and S3).
Table 2.
Meta-regression of cross-sectional studies demonstrating change in the prevalence of cognitive disorders in people with periodontal disease by a unit change in study design factors
| Prevalence risk ratio (95% CI) | ||
|---|---|---|
| Cognitive decline | Dementia and AD | |
| PD severity | ||
| Moderate | Ref | Ref |
| Severe | 1.20 (0.78–1.85) | 2.27 (0.93–5.53) |
| Region | ||
| Europe | Ref | – |
| Asia | 0.75 (0.49–1.17) | – |
| Rate | ||
| Crude | Ref | Ref |
| Adjusted | 1.24 (0.81–1.89) | 1.83 (1.24–2.70)** |
| Sample size | ||
| < 1,000 | Ref | Ref |
| ≥ 1,000 | 0.92 (0.55–1.52) | 1.31 (0.65–2.65) |
| Bias rating | ||
| Moderate | Ref | Ref |
| Serious | 1.10 (0.72–1.70) | 0.67 (0.36–1.24) |
| Other factors | ||
| Females (for every 10% population increase) | 0.94 (0.86–1.03) | 1.03 (0.70–1.52) |
| Average age (for every 10-year increase) | 1.20 (1.11–1.29)*** | 0.95 (0.52–1.74) |
| Smoker (%)† | 1.00 (0.93–1.09) | 1.01 (0.92–1.10) |
Key: Alzheimer’s disease, AD; periodontal disease, PD. −Insufficient number of studies. †Separate model including only studies that reported smoking rate. Significant at *0.05, **0.01, ***0.001 levels
Cognitive decline
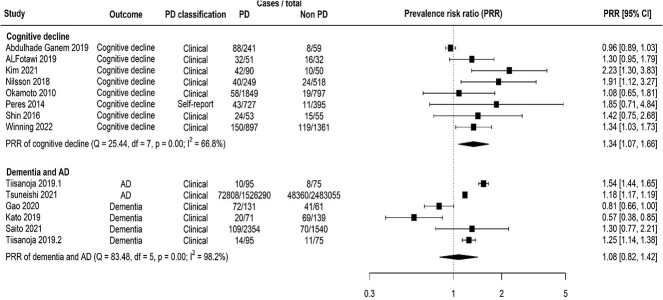
Random effects meta-analysis of cross-sectional studies showed that the prevalence of cognitive decline in people with PD was increased by 34% compared with those without PD (prevalence risk ratio [PRR] = 1.43, 95% confidence interval [CI] = 1.07–1.66; Figure 1). This outcome had moderate-high heterogeneity (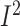 = 66.8%; Figure 1). The risk of developing cognitive decline in longitudinal studies was 33% higher in people with PD than those without (relative risk [RR] = 1.33, 95% CI = 1.13–1.55). The heterogeneity was high for longitudinal studies with this outcome (
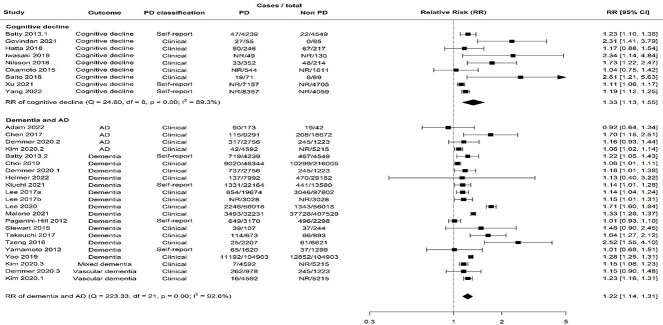
= 66.8%; Figure 1). The risk of developing cognitive decline in longitudinal studies was 33% higher in people with PD than those without (relative risk [RR] = 1.33, 95% CI = 1.13–1.55). The heterogeneity was high for longitudinal studies with this outcome (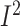 = 89.3%; Figure 2).
= 89.3%; Figure 2).
Figure 1.
Forest plot showing results from random effect meta-analysis for the prevalence of cognitive disorders. Key: Alzheimer’s disease, AD; degrees of freedom, df; periodontal disease, PD; prevalence risk ratio, PRR.
Figure 2.
Forest plot showing results from random effect meta-analysis for the incident risk of cognitive disorders. Key: Alzheimer’s disease, AD; degrees of freedom, df; case numbers not reported, NR; periodontal disease, PD; relative risk, RR.
Of note, a small study (n = 35) that was not eligible for meta-analysis identified no cases of cognitive decline in people without PD in non-smoking older Japanese outpatients [25].
Cognitive decline—study factors
Subgroup analysis of cross-sectional studies revealed an incremental increase in prevalence of cognitive decline by PD severity and a reduction in heterogeneity (moderate—PRR = 1.21, 95% CI = 0.85–1.72, 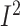 = 69.9%; severe—PRR = 1.35, 95% CI = 1.03–1.71,
= 69.9%; severe—PRR = 1.35, 95% CI = 1.03–1.71, 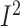 = 0%; Figure S4). The prevalence of cognitive decline was 20% higher in severe cases compared with moderate PD (PRR = 1.20, 95% CI = 0.78–1.85; Table 2). Prevalence estimates for cognitive decline were also impacted by study population (Figure S4) but meta-regression indicates this is not significant (Asia—PRR = 0.75, 95% CI = 0.49–1.17; Table 2). Older participants with PD had higher prevalence of cognitive decline compared with younger populations (younger—PRR = 1.17, 95% CI = 0.97–1.42; older—PRR = 2.06, 95% CI = 1.341–3.02; Figure S6). Meta-regression showed that for every 10 years increase in average age, there was a 20% increase in prevalence cognitive decline (PRR = 1.20, 95% CI = 1.11–1.29; Table 2).
= 0%; Figure S4). The prevalence of cognitive decline was 20% higher in severe cases compared with moderate PD (PRR = 1.20, 95% CI = 0.78–1.85; Table 2). Prevalence estimates for cognitive decline were also impacted by study population (Figure S4) but meta-regression indicates this is not significant (Asia—PRR = 0.75, 95% CI = 0.49–1.17; Table 2). Older participants with PD had higher prevalence of cognitive decline compared with younger populations (younger—PRR = 1.17, 95% CI = 0.97–1.42; older—PRR = 2.06, 95% CI = 1.341–3.02; Figure S6). Meta-regression showed that for every 10 years increase in average age, there was a 20% increase in prevalence cognitive decline (PRR = 1.20, 95% CI = 1.11–1.29; Table 2).
For longitudinal studies, an incremental increase in the risk of cognitive decline by PD severity was also observed (moderate—RR = 1.14, 95% CI = 1.07–1.22; severe—RR = 1.25, 95% CI = 1.18–1.32; Figure S7). In fact, the risk of cognitive decline was 8% higher for those with severe PD compared with moderate cases (RR = 1.08, 95% CI = 0.84–1.38; Table 3). Furthermore, for every 10% population increase in females in the study population, there was a 34% increased risk of cognitive decline (RR = 1.34, 95% CI = 1.16–1.55; Table 3). The risk of cognitive decline in studies stratified by age was similar (younger—RR = 1.40, 95% CI = 1.01–1.94; older—RR = 1.36, 95% CI = 1.08–1.71; Figure S8). Compared with studies of moderate risk of bias, those of serious and critical risk reported 57 and 66% lower risks, respectively (serious—RR = 0.53, 95% CI = 0.31–0.92; critical—RR = 0.44, 95% CI = 0.24–0.82; Table 3). Meta-regression also showed that studies that utilised self-reported PD diagnosis reported 23% lower risks compared with clinical diagnosis (RR = 0.77, 95% CI = 0.65–0.91) and those of bigger sample sizes reported lower risks compared with sample sizes of less than 1,000 participants (1,000–10,000—RR = 0.65, 95% CI = 0.54–0.79; 10,000–100,000—RR = 0.66, 95% CI = 0.53–0.82; Table 3).
Table 3.
Meta-regression of longitudinal studies demonstrating change in the incident risk of cognitive disorders in people with PD by a unit change in study design factors
| Relative risk (95% CI) | ||
|---|---|---|
| Cognitive decline | Dementia and AD | |
| PD severity | ||
| Mild | – | Ref |
| Moderate | Ref | 1.01 (0.77–1.34) |
| Severe | 1.08 (0.84–1.38) | 1.04 (0.79–1.38) |
| Sample size | ||
| < 1,000 | Ref | Ref |
| 1,000–10,000 | 0.65 (0.54–0.79)*** | 1.06 (0.83–1.36) |
| 10,000–100,000 | 0.66 (0.53–0.82)*** | 1.09 (0.82–1.46) |
| ≥ 100,000 | – | 1.17 (0.90–1.52) |
| PD classification | ||
| Clinical | Ref | Ref |
| Self-report | 0.77 (0.65–0.91)** | 0.86 (0.77–0.96)* |
| Region | ||
| Europe | – | Ref |
| Asia | – | 0.87 (0.60–1.26) |
| North America | – | 0.77 (0.53–1.12) |
| Bias rating | ||
| Moderate | – | Ref |
| Serious | 0.53 (0.31–0.92) * | 1.03 (0.89–1.20) |
| Critical | 0.44 (0.24–0.82) ** | – |
| PD treatment received | ||
| Unknown | – | Ref |
| Yes | – | 0.95 (0.85–1.05) |
| Other factors | ||
| Females (for every 10% population increase) | 1.34 (1.16–1.55)*** | 1.00 (0.96–1.04) |
| Average age (for every 10 year increase) | 0.87 (0.77–1.00) | 0.97 (0.93–1.01) |
| Smoker (%) | 1.00 (0.99–1.01) | 1.01 (1.00–1.01)** |
| Total follow-up (for every 5 years) | 0.94 (0.84–1.05) | 0.96 (0.92–1.01) |
Key: Alzheimer’s disease, AD; periodontal disease, PD. −Insufficient number of studies.
†Separate model including only studies that reported smoking rate. Significant at *0.05, **0.01, ***0.001 levels
Dementia and AD
Random effects meta-analysis of cross-sectional studies showed that the overall prevalence of dementia/AD was 8% higher in people with PD (PRR = 1.08, 95% CI = 0.82–1.42) with high heterogeneity (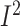 = 98.2%; Figure 1). The incident risk of dementia/AD was also increased in people with periodontal disease in longitudinal studies (RR = 1.22, 95% CI = 1.14–1.31,
= 98.2%; Figure 1). The incident risk of dementia/AD was also increased in people with periodontal disease in longitudinal studies (RR = 1.22, 95% CI = 1.14–1.31, 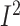 = 92.6%; Figure 2).
= 92.6%; Figure 2).
Dementia and AD—study factors
Incremental increase in prevalence of dementia/AD was observed in moderate PD (PRR = 0.98, 95% CI = 0.76–1.26) to severe cases (1.44, 95% CI = 0.89–2.32) when compared with those without PD (Figure S5). In fact, the prevalence of dementia and AD in severe PD was over 2-fold higher than those with moderate cases (PRR = 2.27, 95% CI = 0.93–5.53; Table 2).
PD severity did not appear to have impact on incident risk estimates of dementia and AD for longitudinal studies (Figure S7), with risks in moderate and severe PD similarly increased compared with mild cases (moderate—RR = 1.05, 95% CI = 0.81–1.37; severe—RR = 1.03, 95% CI = 0.78–1.35; Table 3). When compared with clinical PD diagnosis, self-reported PD showed reduced risks of dementia and AD (RR = 0.86, 95% CI = 0.77–0.96; Table 3). Risk of dementia and AD appeared highest in populations from Europe (RR = 1.41, 95% CI = 0.89–2.22; Figure S8). Meta-regression revealed lower risks of dementia and AD in studies from Asia and North America compared with those from Europe (Asia—RR = 0.87, 95% CI = 0.60–1.26; North America—RR = 0.77, 95% CI = 0.53–1.12; Table 3).
Sensitivity analysis
Meta-analysis of longitudinal studies that reported periodontal treatment during the follow-up (n = 8) revealed an increased risk of dementia in people with PD of a similar magnitude to the main analysis (RR = 1.30, 95% CI = 1.14–1.48; Figure S10). Meta-regression revealed a modest 6% increase in risk of dementia and AD compared with studies that did not report periodontal treatment (RR = 1.06, 95% CI = 0.95–1.17; Table 3). Furthermore, including studies with fewer than 30 participants within exposed/unexposed groups did not greatly impact results of the meta-analysis of prevalence and risk of cognitive disorders in cross-sectional or longitudinal studies (Figures S11–S12).
Discussion
In this systematic review, we examined 21 cross-sectional and 28 longitudinal studies reporting either prevalence or risk of cognitive decline, or dementia/AD. Overall, the prevalence and risk of cognitive decline was higher than dementia and AD in people with PD. Severe PD was associated with increased prevalence and risk of cognitive disorders. Meta-regression of study factors suggested that PD classification type, gender, age, study region and overall risk of bias may also attribute to variation observed in effect size estimates of observational studies.
The findings of this review align with previous systematic reviews that have found augmented risks for cognitive decline and dementia/AD in people with PD [11–14]. Contrariwise, a recent review concluded that the evidence regarding periodontal pathogens and AD onset is contentious and subject to bias, which may influence the robustness of previous findings [32]. Evidence shows that age is a risk factor for PD and both cognitive decline and dementia/AD, with cognitive decline typically developing prior to a formal diagnosis of dementia/AD [33, 34]. We found that the prevalence and risks for cognitive decline were higher than for dementia/AD. This supports the notion that signs of cognitive decline are the early markers for subsequent neuro-degeneration and eventual dementia-onset [35]; thus, cognitive decline is a more frequently diagnosed condition than dementia [36]. There may also be differences in the association of dementia with other disease subtypes. For example, the risk of vascular dementia increases 2-fold in people diagnosed with cardiovascular disease [37]. PD is linked to augmented risks cardiovascular disease development [16], which could implicate vascular dementia development further along the disease trajectory. Further primary work is required to dissect the association of PD with specific subtypes of dementia.
Meta-regression has shown merit in exploring the impact of study factors on estimates for the risk of systemic diseases. Meta-regression and subgroup analyses by key study factors in the present review demonstrated reductions to statistical heterogeneity. We previously demonstrated using meta-regression that PD severity and male gender may increase estimates for risk of cardiovascular disease [17]. The former finding aligns with the current systematic review as we revealed that PD severity is incrementally associated with the prevalence of cognitive decline. We also showed that a higher proportion of females was associated with increased risks of cognitive disorders, though this could be reflective of the higher proportion of females with dementia than males [38]. People with self-reported PD had reportedly lower risks of cognitive disorders than those with clinical classification. This contrasts previous work that suggests classification of PD has no effect on longitudinal risk of cardiovascular disease [16]. A possible explanation could be the differences in severity of self-reported responses. For example, previous oral health research in the UK Biobank has utilised responses of bleeding gums (mild periodontitis/gingivitis) to loose teeth (indicative of severe periodontitis) [39, 40]. It is possible that studies that utilise a self-reported classification such as bleeding gums, a noticeable sign of disease, may have a higher proportion with mild/moderate PD, which may have a lower risk of developing cognitive disorders. These studies are also at risk of reporting bias and therefore the results may not be precise; however, there is evidence that suggests self-reported tools for PD are accurate [41]. A recent systematic review with meta-regression also revealed the sample size and risk of bias can impact study estimates for risks of adverse pregnancy outcomes and diabetes [18]. We found that sample size had a variable effect on estimates for cognitive disorders, whereas studies at serious risk of bias also did not affect the association of PD on dementia/AD compared with those of moderate risk. Generally, studies rated at moderate risk of bias used methods such as inverse probability weighting to account for selection biases and stratified random sampling [42–44]. Most studies were rates at serious risk of bias due to failing to address confounding and selection biases, thereby meta-regression of this factor may be problematic; as such, there is a need for better quality primary research in the field and researchers should interpret findings of systematic reviews with caution.
This systematic review with meta-analysis is the first of its kind to assess using meta-regression, the impact of study factors on effect size estimates for dementia and AD in PD; as such, the study has notable strengths. Through including both cross-sectional and longitudinal studies, as well as two systemic disease outcomes—cognitive decline and dementia/AD, we were able to examine the associations with PD using a larger pool of included studies. The use of meta-regression enabled adjustments for several key factors of study design including gender, PD severity, study region, age, risk of bias and sample size. This ensured a thorough exploration of effect sizes in association studies of PD and cognitive disorders. Our review is further strengthened through adherence to the PRISMA guidelines [45].
Although the primary aim of this review was to explore the causes of methodological heterogeneity through meta-regression, a limitation was the risk of bias present in the included studies due to selection and unmeasured cofounding. Given that the studies included in this review were cross-sectional and longitudinal design, often using real-world datasets such as electronic health records, this leaves opportunity for residual bias and statistical heterogeneity that cannot be adjusted for post-hoc. Furthermore, the results of meta-regression are dependent on sufficient sample size and we were not able to explore the influence of some study factors due to the absence of information in certain studies. The impact of subgroups demonstrated reductions in statistical heterogeneity, thereby advocating future homologous studies with transparent reporting to account for between-study variation. Furthermore, although we strove to account for classification bias through stratifying PD classification into self-reported versus clinical, the classification guidelines of both PD and cognitive disorders can change over time. Thus, true identification of these conditions are therefore dependant on the classification system used and the time of the study. Another limitation of this review is that we were not able to extract adjusted estimates of prevalence from all cross-sectional studies. As a result, these studies were at serious risk of confounding. Evidence suggests that PD is associated with multimorbidity [40, 46]; multimorbidity is also linked to worse outcomes in older age, including dementia incidence [47]. We were not able to explore effect of co-morbidities, and future work should account for multimorbidity and seek to make necessary adjustments. Other confounding factors such as deprivation and socioeconomic status should also be explored further in future meta-regression studies as known drivers of adverse health outcomes that may influence effect sizes.
This study demonstrates the fragility of estimations of the association between PD and cognitive disorders, with study factors such as age, gender, study region and PD severity having strong influence on prevalence and risk estimates. The findings of this review contribute to understanding of PD prognosis and implicate the necessity for improved quality and reporting of observational studies in the field. The clinical implication of these findings is that dental and medical professionals should be made aware of the possible association and make appropriate treatment/prevention arrangements to care. Given the strain on dental appointments following the COVID-19 pandemic, self-managed oral hygiene should also be encouraged to prevent progression to severe PD.
Conclusion
The findings of this systematic review reveal that PD is more strongly associated with cognitive decline than dementia/AD. Meta-regression showed that some study factors may influence prevalence and risk estimates of cognitive disorders. More homologous observational evidence with clear adjustments for confounding and selection biases is required to determine the true direction of these associations. Specifically, future studies should utilise bias-reducing selection methods such as inverse probability weighting and random sampling of large and representative study populations with validated PD assessment tools to reduce the heterogeneity that is reflected in the current literature.
Supplementary Material
Contributor Information
Harriet Larvin, School of Dentistry, University of Leeds, Leeds, UK.
Chenyi Gao, School of Dentistry, University of Leeds, Leeds, UK.
Jing Kang, Oral Biology, School of Dentistry, University of Leeds, Leeds, UK.
Vishal R Aggarwal, School of Dentistry, University of Leeds, Leeds, UK.
Susan Pavitt, School of Dentistry, University of Leeds, Leeds, UK.
Jianhua Wu, School of Dentistry, University of Leeds, Leeds, UK; Centre for Primary Care, Wolfson Institute of Population Health, Queen Mary University of London, London, UK.
Data Availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declaration of Sources of Funding
H.L. was supported by the Frederick E. Hopper Scholarship at the University of Leeds. J.W. is supported by Barts Charity (MGU0504). The research is supported by the National Institute for Health Research (NIHR) infrastructure at Leeds.
Declaration of Conflicts of Interest
None.
References
- 1. Jin LJ, Lamster IB, Greenspan JS, Pitts NB, Scully C, Warnakulasuriya S. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis 2016; 22: 609–19. 10.1111/odi.12428. [DOI] [PubMed] [Google Scholar]
- 2. Nichols E, Szoeke CEI, Vollset SE et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li J, Xu H, Pan W, Wu B. Association between tooth loss and cognitive decline: a 13-year longitudinal study of Chinese older adults. PLoS One 2017; 12: e0171404–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okamoto N, Morikawa M, Tomioka K, Yanagi M, Amano N, Kurumatani N. Association between tooth loss and the development of mild memory impairment in the elderly: the Fujiwara-Kyo study. J Alzheimers Dis 2015; 44: 777–86. [DOI] [PubMed] [Google Scholar]
- 5. Kato H, Takahashi Y, Iseki C et al. Tooth loss-associated cognitive impairment in the elderly: a community-based study in Japan. Internal medicine (Tokyo, Japan) 2019; 58: 1411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kang J, Wu B, Bunce D, Ide M, Pavitt S, Wu J. Cognitive function and oral health among ageing adults. Community Dent Oral Epidemiol 2019; 47: 259–66. [DOI] [PubMed] [Google Scholar]
- 7. Kang J, Wu B, Bunce D et al. Bidirectional relations between cognitive function and oral health in ageing persons: a longitudinal cohort study. Age Ageing 2020; 49: 793–9. [DOI] [PubMed] [Google Scholar]
- 8. Delange N, Lindsay S, Lemus H, Finlayson TL, Kelley ST, Gottlieb RA. Periodontal disease and its connection to systemic biomarkers of cardiovascular disease in young American Indian/Alaskan natives. J Periodontol 2018; 89: 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sundelöf J, Kilander L, Helmersson J et al. Systemic inflammation and the risk of Alzheimer’s disease and dementia: a prospective population-based study. J Alzheimers Dis 2009; 18: 79–87. [DOI] [PubMed] [Google Scholar]
- 10. Noble JM, Scarmeas N, Celenti RS et al. Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PLoS One 2014; 9: e114959. 10.1371/journal.pone.0114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamer AR, Craig RG, Niederman R, Fortea J, de Leon MJ. Periodontal disease as a possible cause for Alzheimer’s disease. Periodontol 2000 2020; 83: 242–71. [DOI] [PubMed] [Google Scholar]
- 12. Fang WL, Jiang MJ, Gu BB et al. Tooth loss as a risk factor for dementia: systematic review and meta-analysis of 21 observational studies. BMC Psychiatry 2018; 18: 345. 10.1186/s12888-018-1927-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tonsekar PP, Jiang SS, Yue G. Periodontal disease, tooth loss and dementia: is there a link? A systematic review. Gerodontology 2017; 34: 151–63. [DOI] [PubMed] [Google Scholar]
- 14. Oh B, Han DH, Han KT et al. Association between residual teeth number in later life and incidence of dementia: a systematic review and meta-analysis. BMC Geriatr 2018; 18: 48. 10.1186/s12877-018-0729-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anglemyer A, Horvath HT, Bero L, Cochrane Methodology Review Group . Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev 2014; 2014: MR000034. 10.1002/14651858.MR000034.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomson WM, Barak Y. Tooth loss and dementia: a critical examination. J Dent Res 2021; 100: 226–31. [DOI] [PubMed] [Google Scholar]
- 17. Larvin H, Kang J, Aggarwal VR, Pavitt S, Wu J. Risk of incident cardiovascular disease in people with periodontal disease: a systematic review and meta-analysis. Clin Exp Dent Res 2021; 7: 109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oates TW, Guy V, Ni K et al. Meta-regression analysis of study heterogeneity for systemic outcomes after periodontal therapy. JDR Clin Trans Res 2023; 8: 6–15. [DOI] [PubMed] [Google Scholar]
- 19. Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 2007; 36: 666–76. [DOI] [PubMed] [Google Scholar]
- 20. Sterne JA, Hernan MA, Reeves BC et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shor E, Roelfs D, Vang ZM. The “Hispanic mortality paradox” revisited: meta-analysis and meta-regression of life-course differentials in Latin American and Caribbean immigrants' mortality. Soc Sci Med 2017; 186: 20–33. [DOI] [PubMed] [Google Scholar]
- 22. Batty GD, Li Q, Huxley R et al. Oral disease in relation to future risk of dementia and cognitive decline: prospective cohort study based on the action in diabetes and vascular disease: Preterax and Diamicron modified-release controlled evaluation (ADVANCE) trial. Eur Psychiatry 2013; 28: 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jockusch J, Hopfenmüller W, Nitschke I. Influence of cognitive impairment and dementia on oral health and the utilization of dental services: findings of the Oral Health, Bite Force and Dementia study (OrBiD). BMC Oral Health 2021; 21: 399. 10.1186/s12903-021-01753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arrive E, Letenneur L, Matharan F et al. Oral health condition of French elderly and risk of dementia: a longitudinal cohort study. Community Dent Oral Epidemiol 2012; 40: 230–8. [DOI] [PubMed] [Google Scholar]
- 25. Mizutani S, Egashira R, Yamaguchi M et al. Changes in oral and cognitive functions among older Japanese dental outpatients: a 2-year follow-up study. J Oral Rehabil 2021; 48: 1150–9. [DOI] [PubMed] [Google Scholar]
- 26. Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the Nun study. J Am Dent Assoc 2007; 138: 1314–22. [DOI] [PubMed] [Google Scholar]
- 27. Barbe AG, Küpeli LS, Hamacher S, Noack MJ. Impact of regular professional toothbrushing on oral health, related quality of life, and nutritional and cognitive status in nursing home residents. Int J Dent Hyg 2020; 18: 238–50. [DOI] [PubMed] [Google Scholar]
- 28. Laugisch O, Johnen A, Buergin W et al. Oral and periodontal health in patients with Alzheimer’s disease and other forms of dementia—a cross-sectional pilot study. Oral Health Prev Dent 2021; 19: 255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Popovac A, Čelebić A, Peršić S, Stefanova E, Milić Lemić A, Stančić I. Oral health status and nutritional habits as predictors for developing Alzheimer’s disease. Med Princ Pract 2021; 30: 448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chu CH, Ng A, Chau AM, Lo EC. Oral health status of elderly Chinese with dementia in Hong Kong. Oral Health Prev Dent 2015; 13: 51–7. [DOI] [PubMed] [Google Scholar]
- 31. Sharma S, Nayak SU, Uppoor A, Rao S, Pai K, Natarajan S. Evaluation of cognitive impairment in type 2 diabetic patients with chronic periodontitis: a cross-sectional study. J Int Soc Prev Community Dent 2021; 11: 50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elwishahy A, Antia K, Bhusari S, Ilechukwu NC, Horstick O, Winkler V. Porphyromonas Gingivalis as a risk factor to Alzheimer’s disease: a systematic review. J Alzheimers Dis Rep 2021; 5: 721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rheu G-B, Ji S, Ryu J-J et al. Risk assessment for clinical attachment loss of periodontal tissue in Korean adults. J Adv Prosthodont 2011; 3: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jansen WJ, Wilson RS, Visser PJ et al. Age and the association of dementia-related pathology with trajectories of cognitive decline. Neurobiol Aging 2018; 61: 138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Belleville S, Gauthier S, Lepage E, Kergoat MJ, Gilbert B. Predicting decline in mild cognitive impairment: a prospective cognitive study. Neuropsychology 2014; 28: 643–52. [DOI] [PubMed] [Google Scholar]
- 36. Plassman BL, Langa KM, Fisher GG et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med 2008; 148: 427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wolters FJ, Ikram MA. Epidemiology of vascular dementia. Arterioscler Thromb Vasc Biol 2019; 39: 1542–9. [DOI] [PubMed] [Google Scholar]
- 38. Wu YT, Beiser AS, Breteler MMB et al. The changing prevalence and incidence of dementia over time—current evidence. Nat Rev Neurol 2017; 13: 327–39. [DOI] [PubMed] [Google Scholar]
- 39. Kang J, Palmier-Claus J, Wu J et al. Periodontal disease in people with a history of psychosis: results from the UK biobank population-based study. Community Dent Oral Epidemiol 2022; 00: 1–12. [DOI] [PubMed] [Google Scholar]
- 40. Larvin H, Kang J, Aggarwal VR, Pavitt S, Wu J. Multimorbid disease trajectories for people with periodontitis. J Clin Periodontol 2021; 48: 1587–96. [DOI] [PubMed] [Google Scholar]
- 41. Abbood HM, Hinz J, Cherukara G, Macfarlane TV. Validity of self-reported periodontal disease: a systematic review and meta-analysis. J Periodontol 2016; 87: 1474–83. [DOI] [PubMed] [Google Scholar]
- 42. Winning L, Naseer A, De Looze C et al. Tooth loss and cognitive decline in community dwelling older Irish adults: a cross-sectional cohort study. J Dent 2022; 119: 104077. 10.1016/j.jdent.2022.104077. [DOI] [PubMed] [Google Scholar]
- 43. Demmer RT, Norby FL, Lakshminarayan K et al. Periodontal disease and incident dementia: the Atherosclerosis Risk in Communities study (ARIC). Neurology 2020; 95: e1660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iwasaki M, Kimura Y, Ogawa H et al. Periodontitis, periodontal inflammation, and mild cognitive impairment: a 5-year cohort study. J Periodontal Res 2019; 54: 233–40. [DOI] [PubMed] [Google Scholar]
- 45. Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Larvin H, Kang J, Aggarwal V, Pavitt S, Wu J. Systemic multimorbidity clusters in people with periodontitis. J Dent Res 2022; 101: 1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Seibert K, Stiefler S, Domhoff D, Wolf-Ostermann K, Peschke D. The influence of primary care quality on nursing home admissions in a multimorbid population with and without dementia in Germany: a retrospective cohort study using health insurance claims data. BMC Geriatr 2022; 22: 52–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].