Abstract
Introduction
Pulmonary veno-occlusive disease (PVOD) is a rare and severe subtype of pulmonary arterial hypertension (PAH). Although European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines advise assessing PAH severity at baseline and during follow-up, no existing risk assessment methods have been validated for PVOD. This study aimed to identify prognostic factors, examine the impact of treatment strategies and evaluate risk assessment methods for PVOD patients.
Methods
The study analysed all incident PVOD patients included in the French Pulmonary Hypertension Registry between 2006 and 2021. Survival was assessed based on initial treatment strategy and risk status and compared to a matched (age, sex, pulmonary vascular resistance) PAH group. Six risk assessment methods (number of four low-risk and three noninvasive low-risk variables, ESC/ERS guidelines three-strata and four-strata models, REVEAL 2.0 and Lite 2) were applied at baseline and early follow-up, and their accuracy was compared using Harrell's c-statistic.
Results
Among the 327 included PVOD patients, survival rates at 1, 3 and 5 years were 86%, 50% and 27%, respectively. Multivariate analysis showed that only 6-min walk distance was associated with survival, with no significant difference based on initial treatment strategy. All six risk assessment methods could discriminate mortality risk, and the ESC/ERS four-strata model was the most accurate at both baseline and follow-up (C-index 0.64 and 0.74). PVOD survival rates were consistently lower than PAH when comparing baseline risk status using the ESC/ERS four-strata model.
Conclusion
PVOD is associated with poor outcomes, and initial treatment strategies do not significantly affect survival. Risk assessment methods can be useful in predicting survival for PVOD patients.
Tweetable abstract
Initial treatment strategies do not significantly affect survival in pulmonary veno-occlusive disease (PVOD). Risk assessment methods are useful in predicting survival in PVOD. ESC/ERS 4-stratum model was the most accurate at both baseline and follow-up. https://bit.ly/49XFkkD
Introduction
Despite major improvements in the management of pulmonary arterial hypertension (PAH) in the last decades, the condition is still considered incurable [1, 2]. The current treatment goal is to enable patients to achieve a low mortality risk status, which is associated with improved outcomes [1, 2]. European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines suggest assessing the severity of PAH at both time of diagnosis and follow-up. Several risk stratification tools have been developed to assess mortality risk in patients with PAH and were validated in large registries including the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL), the Comparative Prospective Registry of Newly Initiated Therapies for PH (COMPERA) and the French Pulmonary Hypertension Network Registry [3–7]. These tools are based on the evaluation of invasive variables such as haemodynamic parameters measured by right heart catheterisation (RHC) and noninvasive variables including New York Heart Association (NYHA) functional class (FC), 6-min walk distance (6MWD) and plasma concentration of brain natriuretic peptide (BNP) or N-terminal proBNP (NT-proBNP). European Guidelines recommend to categorise newly diagnosed PAH patients into low risk (estimated 1-year mortality <5%), intermediate risk (5–20%) or high risk (>20%) [1, 2]. At follow-up, it is recommended to use a simplified four-strata multiparametric assessment model including only three noninvasive variables (NYHA FC, 6MWD and BNP or NT-proBNP) to classify PAH patients as low (treatment goal to achieve), intermediate–low, intermediate–high or high risk of mortality [1, 2, 8, 9].
PAH with features of venous and capillary involvement, more commonly known as pulmonary veno-occlusive disease (PVOD), is a rare form of pulmonary hypertension (PH) characterised by radiological abnormalities (interlobular septal lines, centrolobular ground-glass opacities, lymph node enlargement), low diffusing lung capacity for carbon monoxide (DLCO), severe hypoxaemia and a poor prognosis [10, 11] Response to approved PAH drugs in PVOD is uncommon and there is a risk of pulmonary oedema after initiating PAH therapy [12]. Therefore, no evidence-based therapy is recommended in this setting and lung transplantation is the preferred treatment option for eligible patients [1, 2]. None of the risk assessment strategies used in PAH have been evaluated in PVOD. The aims of this study were to identify prognostic factors in PVOD, evaluate the impact of different treatment strategies and compare different risk assessment methods.
Methods
Patient selection
We retrospectively reviewed all incident (i.e., newly diagnosed) patients aged ≥18 years with a diagnosis of PVOD enrolled in the French PH Registry between 1 January 2006 and 31 December 2021. Inclusion required a baseline RHC confirming precapillary PH as defined at the time of the study. We included PVOD patients with precapillary PH defined as a mean pulmonary arterial pressure (mPAP) ≥25 mmHg, pulmonary arterial wedge pressure (PAWP) ≤15 mmHg and pulmonary vascular resistance (PVR) >3 WU [13, 14]. Diagnosis of PVOD was based on a consensus among a multidisciplinary team of experts including experienced chest physicians and radiologists. Pulmonary hypertension with frequent venous and capillary involvement (PVOD-like), such as PH related to connective tissue disorders, sarcoidosis or histiocytosis, have not been included as they do not strictly belong to the PVOD subgroup in the ESC/ERS classification. Patients were excluded if they lacked a calculable risk status with right atrial pressure (RAP) and cardiac index (CI) measured by RHC, NYHA FC, 6MWD and BNP or NT-proBNP. We also excluded patients with significant chronic lung diseases based on computed tomography (CT) and/or pulmonary function tests (PFTs), i.e., patients with obstructive pattern defined as a forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) <0.7 with a FEV1 <60% of predicted value, or patients with restrictive pattern defined as a total lung capacity (TLC) <70% of predicted value. To compare survival of PVOD and PAH patients, we used an incident cohort of PAH patients enrolled in the French Registry between 1 January 2006 and 31 December 2021 [9].
This retrospective study complied with the declaration of Helsinki. French law does not require ethics committee or institutional review board approval or informed consent for retrospective data collection. All data were anonymised and compiled according to the requirements of the Commission National Informatique et Liberté, the organisation dedicated to privacy, information technology and civil rights in France. The committee approved the methods used to collect and analyse registry data on 24 May 2003 (approval number 842063).
Risk assessment
Patients were stratified at baseline and early follow-up (within the first year after diagnosis of PVOD) using different risk assessment methods:
Number of the four low-risk variables present or achieved (NYHA FC I–II, 6MWD >440 m, RAP <8 mmHg, CI ≥2.5 L·min−1·m−2) [7].
Number of the three noninvasive low-risk variables present or achieved (NYHA FC I–II, 6MWD >440 m, BNP <50 ng·L−1 or NT-proBNP <300 ng·L−1) [7].
ESC/ERS guidelines three-strata model (low-, intermediate-, high-risk) based on NYHA FC, 6MWD, RAP, CI, mixed venous oxygen saturation (SvO2), and BNP or NT-proBNP [1, 2, 5, 6].
ESC/ERS guidelines four-strata model (low, intermediate–low, intermediate–high, high risk) based on the three noninvasive variables (NYHA FC, 6MWD and BNP or NT-proBNP) [1, 2, 8, 9].
REVEAL 2.0 risk calculator score that includes both demographics variables, aetiology of PAH and invasive and noninvasive variables assessing the severity of PAH [4].
REVEAL Lite-2 risk calculator score that includes NYHA FC, 6MWD, BNP or NT-proBNP, heart rate, systolic blood pressure and estimated glomerular filtration rate [4].
Statistical analysis
Data were collected from the web-based French Registry (PAHTool®; Inovultus, Santa Maria da Feira, Portugal) and were stored in a personal computer-based data spreadsheet. Continuous data are presented as mean±SD or as median (interquartile range (IQR), 25–75%) and categorical data as number and percentage. The dataset as of 31 May 2022 was analysed. The primary outcome was all-cause mortality. Survival time was calculated from the date of diagnostic RHC until death or last recorded clinical contact. Patients who underwent lung transplantation were censored at the date of transplantation and those who were lost to follow-up at the date of the last contact. No imputations were made for missing data.
Univariable Cox proportional hazards regression models were performed to assess the risk of death according to baseline characteristics. The Harrell's c-statistic was used to compare accuracy and discrimination of the different risk stratification methods. Survival analyses were performed using the Kaplan–Meier method, and log-rank test was used to compare the different treatment strategies or risk status. To compare survival of PVOD and PAH, two additional survival analyses that used propensity score matching of age, sex and PVR were performed: overall survival and transplant-free survival. Analyses were performed using SPSS Statistics (version 26; SPSS, Chicago, IL, USA) and R version 4.0.0.
Results
Patients
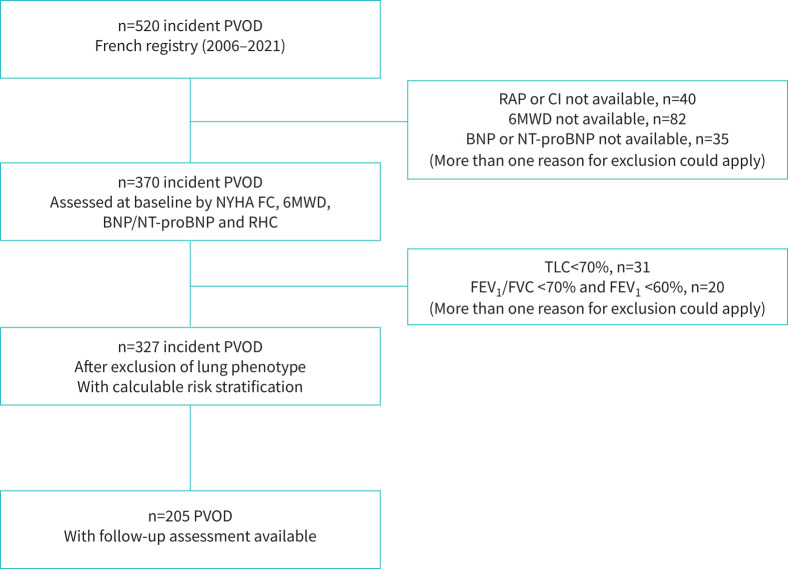
Between 1 January 2006, and 31 December 2021, 520 incident patients with a suspicion of PVOD were enrolled in the French PH Registry. After exclusion of patients who did not fulfil inclusion criteria, we identified 327 PVOD patients who had all variables available to calculate their risk status at baseline using the six previously described tools (figure 1). Baseline characteristics are shown in table 1. 69% of them were male, and the mean age was 65±14 years. 26 patients (8%) had heritable PVOD with identified biallelic EIF2AK4 pathogenic variants. Most patients (85%) were in NYHA FC III or IV at diagnosis with moderate to severe haemodynamic impairment. As expected, PFTs revealed low DLCO with a mean of 35±13% of predicted value and severe hypoxaemia with partial pressure of oxygen in arterial blood (PaO2) on room air of 57±13 mmHg. 205 patients (63%) were reassessed after a median follow-up of 4.4 (IQR 3.5–5.6) months with NYHA FC, 6MWD, BNP or NT-proBNP and RHC and had therefore a calculable risk stratification.
FIGURE 1.
Patient selection flow chart. BNP: brain natriuretic peptide; NYHA FC: New York Heart Association functional class; CI: cardiac index; NT-proBNP: N-terminal pro-brain natriuretic peptide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; PVOD: pulmonary veno-occlusive disease; RAP: right atrial pressure; 6 MWD: 6-min walk distance; TLC: total lung capacity.
TABLE 1.
Baseline characteristics of the study population (n=327)
| Characteristics | |
| Age years | 65±14 |
| Female/male | 100 (31)/227 (69) |
| Body mass index kg·m−2 | 26.9±5.2 |
| Biallelic EIF2AK4 pathogenic variants | 26 (8) |
| NYHA FC | |
| Class II | 49 (15) |
| Class III | 205 (63) |
| Class IV | 73 (22) |
| 6MWD m | 242±160 |
| Haemodynamics | |
| RAP mmHg | 8±5 |
| mPAP mmHg | 45±11 |
| PAWP mmHg | 9±4 |
| Cardiac output L·min−1 | 4.4±1.3 |
| Cardiac index L·min−1·m−2 | 2.4±0.7 |
| PVR WU | 9±4 |
| SvO2 % | 60±9 |
| Heart rate bpm | 78±16 |
| Stroke volume index mL | 33±11 |
| BNP ng·L−1 (n=175) | 273 (78–531) |
| NT-proBNP ng·L−1 (n=152) | 2040 (413–4491) |
| Increased BNP or NT-proBNP | 260 (80) |
| Pulmonary function tests | |
| FEV1 % pred | 88±20 |
| FVC % pred | 95±22 |
| TLC % pred | 93±17 |
| DLCO % pred | 35±13 |
| DLCO/AV % pred | 42±16 |
| Arterial blood gases on ambient room air | |
| PaO2 mmHg | 57±13 |
| PaCO2 mmHg | 32±5 |
| Initial PAH treatment strategy | |
| Monotherapy | 221 (68) |
| Combination therapy | 57 (17) |
| No therapy | 49 (15) |
| Therapy stopped | |
| Endothelin receptor antagonist | 40 (12) |
| Phosphodiesterase 5 inhibitor | 13 (4) |
| Prostacyclin | 3 (1) |
| Last therapy received | |
| Monotherapy | 136 (42) |
| Combination therapy | 134 (41) |
| No therapy | 57 (17) |
Data are presented as mean±sd, median (IQR) or n (%). EIF2AK4: Eukaryotic translation initiation factor 2-α kinase 4; NYHA FC: New York Heart Association functional class; 6MWD: 6-min walk distance; RAP: right atrial pressure; mPAP: mean pulmonary artery pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; SvO2: mixed venous oxygen saturation; BNP: brain natriuretic peptide; NT-proBNP: N-terminal pro-brain natriuretic peptide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLC: total lung capacity; DLCO: diffusing capacity for carbon monoxide; DLCO/AV: diffusing capacity for carbon monoxide to alveolar volume ratio; PaO2: partial pressure of oxygen; PaCO2: partial pressure of carbon dioxide.
Survival and prognostic factors
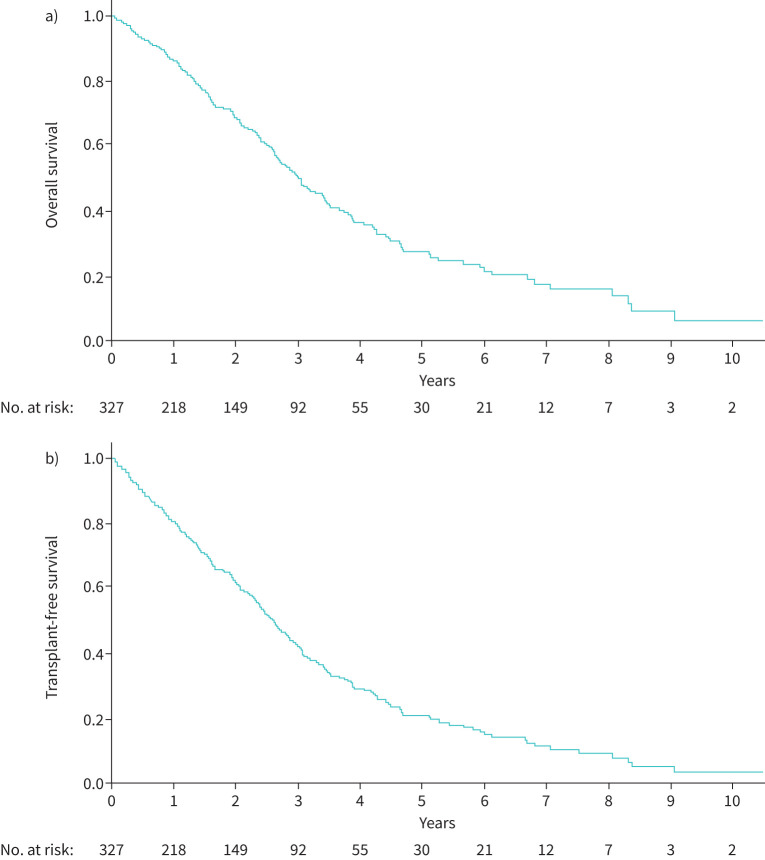
Overall survival and transplant-free survival are shown in figure 2a and b. After a median follow-up of 21 (IQR 8–38) months, 167 patients (51%) died and 41 (13%) underwent lung transplantation (figure 2). The median survival time was 36 (IQR 31–41) months. The 1-, 2-, 3- and 5-year overall-survival rates were respectively 86%, 68%, 50% and 27%.
FIGURE 2.
a) Overall survival and b) transplant-free survival of pulmonary veno-occlusive disease patients.
In univariable Cox regression analysis, age, heritable form of PVOD, NYHA FC, 6MWD, NT-proBNP level, cardiac output, CI and SvO2 were associated with survival whereas sex, body mass index, other haemodynamic variables and initial treatment strategy were not (table 2). In addition PaO2 on room air and DLCO were associated with survival. In the three multivariate models with NYHA FC, 6MWD, NT-proBNP (model A) and PaO2 (model B) or DLCO (model C), only 6MWD was associated with survival (supplementary table S1).
TABLE 2.
Univariable Cox analyses
| Hazard ratio | 95% CI | p-value | |
| Sex (male) | 1.330 | 0.939–1.882 | 0.108 |
| Age, per year | 1.032 | 1.018–1.046 | <0.001 |
| Body mass index, per kg·m−2 | 1.007 | 0.978–1.036 | 0.646 |
| Biallelic EIF2AK4 mutations | 3.176 | 1.177–8.570 | 0.023 |
| NYHA FC, per class | 1.697 | 1.303–2.209 | <0.001 |
| 6MWD, per m | 0.997 | 0.996–0.998 | <0.001 |
| BNP, per ng·L−1 | 1.000 | 1.000–1.000 | 0.053 |
| NT-proBNP, per ng·L−1 | 1.000 | 1.000–1.000 | 0.019 |
| RAP, per mmHg | 1.026 | 0.991–1.062 | 0.146 |
| mPAP, per mmHg | 1.001 | 0.987–1.015 | 0.874 |
| PAWP, per mmHg | 0.957 | 0.914–1.001 | 0.058 |
| Cardiac output, per L·min−1 | 0.856 | 0.753–0.973 | 0.017 |
| Cardiac index, per L·min−1·m−2 | 0.673 | 0.524–0.864 | 0.002 |
| PVR, per WU | 1.018 | 0.985–1.053 | 0.290 |
| S vO2 , per % | 0.970 | 0.948–0.992 | 0.007 |
| P aO2 , per mmHg | 0.979 | 0.966–0.993 | 0.003 |
| P aCO2 , per mmHg | 1.012 | 0.980–1.044 | 0.472 |
| TLC, per % | 0.997 | 0.988–1.007 | 0.599 |
| FEV1, per % | 0.999 | 0.991–1.007 | 0.781 |
| D LCO , per % | 0.980 | 0.967–0.993 | 0.002 |
| D LCO /AV, per % | 0.990 | 0.980–1.000 | 0.054 |
| Number of PAH therapies | 0.793 | 0.597–1.053 | 0.108 |
EIF2AK4: Eukaryotic translation initiation factor 2-α kinase 4; NYHA FC: New York Heart Association functional class; 6MWD: 6-min walk distance; BNP: brain natriuretic peptide; NT-proBNP: N-terminal pro-brain natriuretic peptide; RAP: right atrial pressure; mPAP: mean pulmonary artery pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; SvO2: mixed venous oxygen saturation; PaO2: partial pressure of oxygen; PaCO2: partial pressure of carbon dioxide; TLC: total lung capacity; FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity for carbon monoxide; DLCO/AV: diffusing capacity for carbon monoxide to alveolar volume ratio; PAH: pulmonary arterial hypertension.
Impact of PAH-approved drugs on PVOD survival
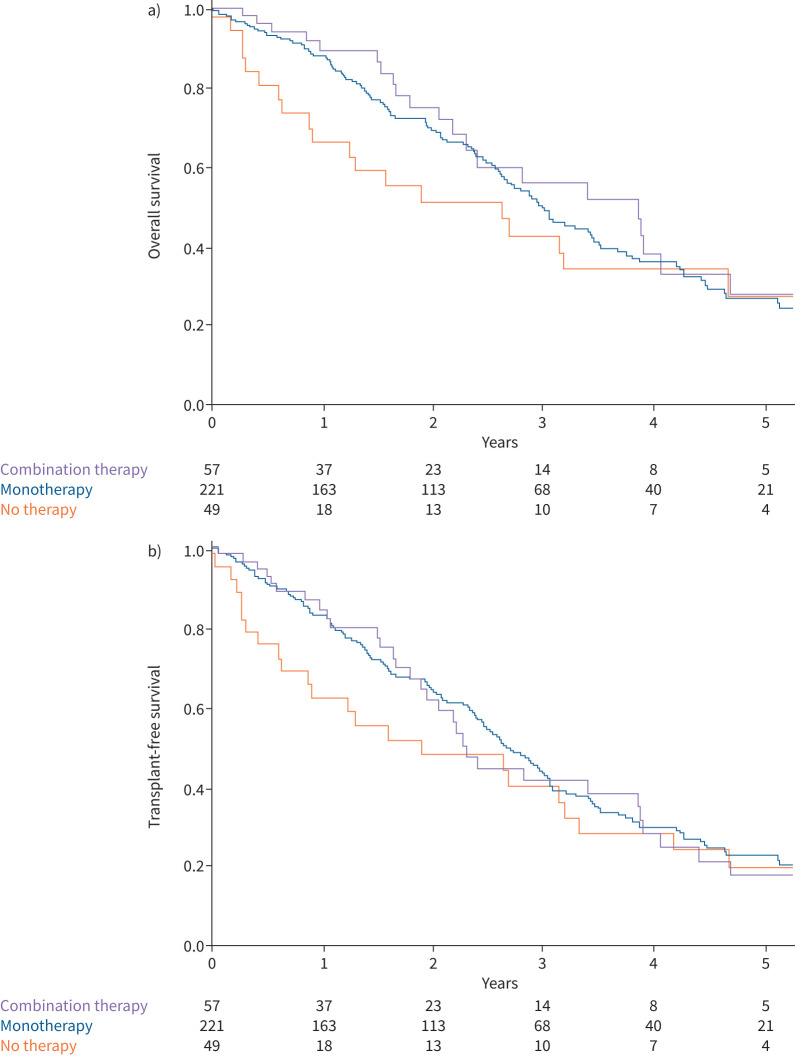
68% of PVOD (n=221) were initiated on monotherapy (141 on endothelin receptor antagonist (ERA), 73 on phosphodiesterase 5 inhibitors (PDE5i) and five on prostacyclin). 17% (n=57) received initial dual combination therapy (48 with ERA and PDE5i, and nine with prostacyclin and oral drugs). Finally, 15% (n=49) patients did not receive PAH-targeted therapy within the first 6 months of diagnosis. There was no significant difference either in overall survival or in transplant-free survival according to initial treatment strategy (no PAH-targeted therapy within the first 6 months, initial monotherapy or initial dual combination therapy) (figure 3a and b). In addition, there was no significant difference in survival (neither overall nor transplant-free survival) when comparing treatment to no treatment.
FIGURE 3.
a) Overall survival and b) transplant-free survival of pulmonary veno-occlusive disease patients according to initial treatment strategy. a) log-rank comparisons tests, p=0.402; b) log-rank comparisons tests, p=0.572.
Risk assessment at the time of diagnosis and at first follow-up assessment
At the time of PVOD diagnosis, 46 patients (14%) were classified at low-risk according to the ESC/ERS guidelines three-strata model, and 19 (6%) according to the four-strata risk stratification model (supplementary table S2). Only 12 patients (3.5%) had three noninvasive low-risk criteria present at the time of diagnosis. According to REVEAL 2.0 and REVEAL Lite 2 risk score calculators, 59 (18%) and 66 (20%) patients were considered at low risk, respectively. The proportion of patients classified as high risk varied from17% to 67% according to the method utilised to evaluate the risk status: 17% (n=57) and 20% (n=67) with the ESC/ERS guidelines three-strata and four-strata models, respectively; 67% (n=218) and 61% (n=199) with REVEAL 2.0 and REVEAL Lite 2 risk calculators, respectively.
At first follow-up visit (4.4 (IQR 3.5–5.6) months after diagnosis), the proportion of patients achieving a low-risk status varied from 14% to 36.5%: 14% (n=29) when the ESC/ERS guidelines four-strata model was used, 24% (n=49) using the ESC/ERS guidelines three-strata model, 31.5% (n=65) and 36.5% (n=75) using REVEAL 2.0 and REVEAL Lite 2.0 risk calculators, respectively. Finally, only seven patients (3%) achieved a low-risk status defined by the presence of three noninvasive low-risk criteria.
Determination of the most accurate risk assessment tool in PVOD
All risk scores were accurate to identify high-risk patients (c-statistic between 0.59 and 0.64 at baseline and 0.64 and 0.74 at follow-up). Discrimination between the different risk stratification methods, as measured by the c-statistic, was slightly greater for the ESC/ERS risk stratification in four strata than for other risk assessment strategies, at both baseline (c-statistic 0.64) and follow-up (c-statistic 0.74) (table 3). A sensitivity analysis has been performed in 81 patients under 60 years of age. Comparison of the discrimination of risk scores calculated at baseline and at first follow-up using Harrell's c-statistics in this subset of patients is presented in supplementary table S3.
TABLE 3.
Comparison of the discrimination of risk scores calculated at baseline and at first follow-up using Harrell's c-statistic
|
Baseline
C-index (95% CI) |
Follow-up
C-index (95% CI) |
|
| REVEAL 2.0 | 0.619 (0.584–0.654) | 0.720 (0.683–0.757) |
| REVEAL Lite 2 | 0.610 (0.571–0.649) | 0.730 (0.691–0.769) |
| ESC/ERS risk score in three strata | 0.605 (0.566–0.644) | 0.682 (0.633–0.731) |
| ESC/ERS risk score in four strata | 0.643 (0.602–0.684) | 0.743 (0.696–0.790) |
| Number of low-risk criteria (out of four) | 0.617 (0.574–0.660) | 0.635 (0.574–0.696) |
| Number of noninvasive low-risk criteria (out of three) | 0.589 (0.552–0.626) | 0.710 (0.669–0.751) |
ESC/ERS: European Society of Cardiology/European Respiratory Society.
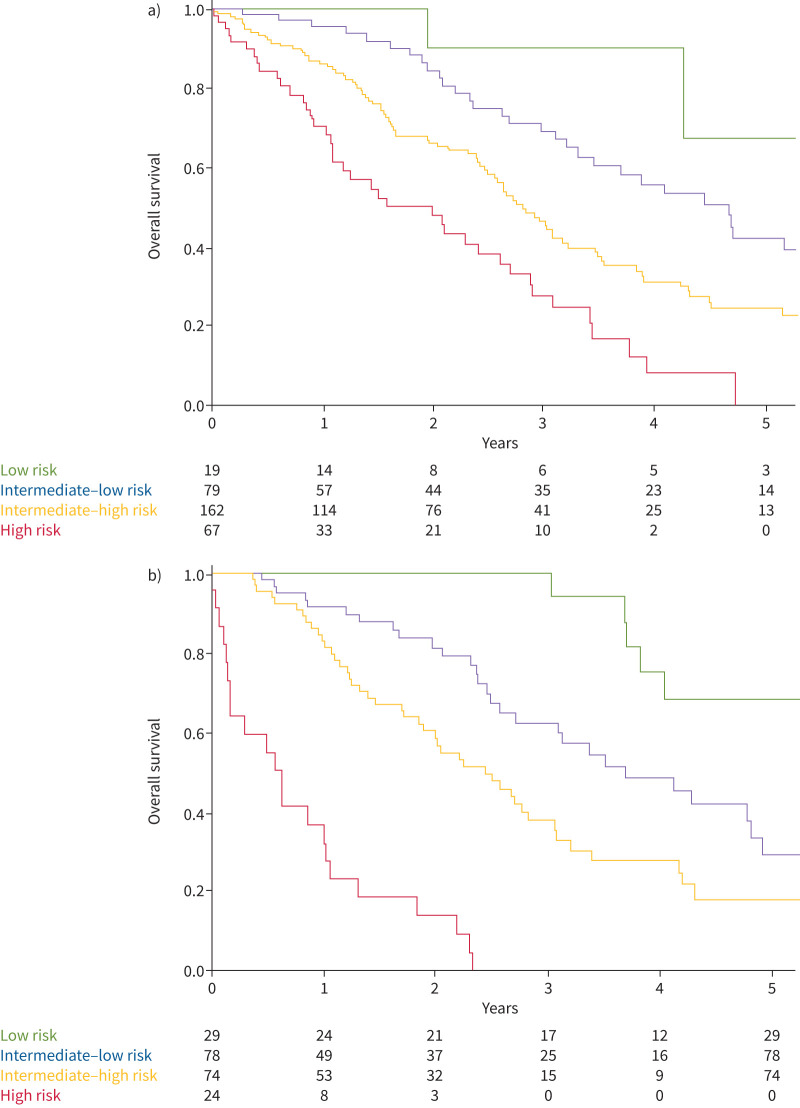
The Kaplan–Meier survival curves according to the ESC/ERS guidelines four-strata model at baseline and follow-up are presented in figure 4a and b. The 1-year survival rates were 100%, 96%, 86% and 71% for patients at baseline low, intermediate–low, intermediate–high and high risk, respectively (p<0.001). The granularity between the four-risk status was greater at follow-up, with a 1-year survival rate after assessment of respectively 100%, 92%, 82% and 32% for low-, intermediate–low, intermediate–high and high-risk status (p<0.001).
FIGURE 4.
a) Overall survival according to European Society of Cardiology/European Respiratory Society guidelines four-strata model at baseline and b) first follow-up reassessment. a) log-rank comparisons tests, p<0.001; b) log-rank comparisons tests, p<0.001.
According to the ESC/ERS guidelines four-strata risk stratification model, 35% of patients improved their risk status from baseline to follow-up, while 22% worsened it. In addition, 29 patients, mainly at intermediate–high or high risk at baseline, died or underwent lung transplantation within a year before any reassessment.
Comparisons of survival between PVOD and PAH patients
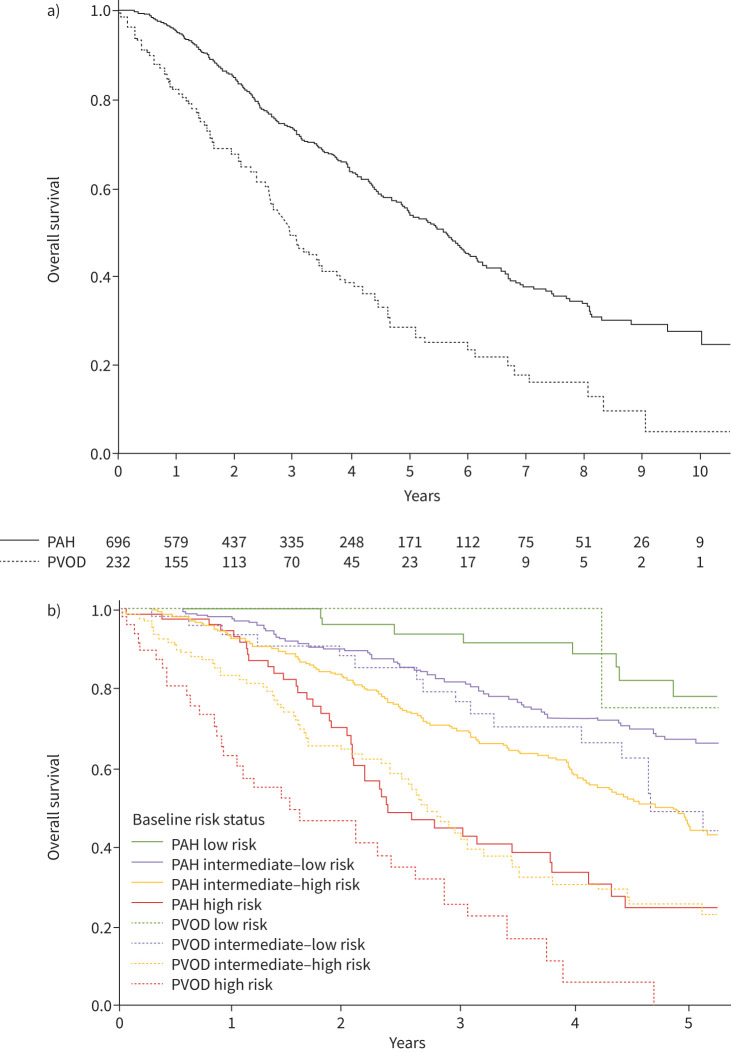
We compared survival of PVOD patients and PAH patients diagnosed in the same period [9] after matching on age, sex and PVR. Baseline characteristics of matched PAH patients are presented in the supplementary table S4. The overall survival of PVOD patients was poorer than that of PAH patients (figure 5a). When comparing baseline risk status in four strata in PVOD and PAH, the survival of intermediate–high and high status in PVOD was poorer than in PAH, while there was no significant difference in survival between low-risk status in PAH and PVOD, nor between low–intermediate-risk status in PVOD and that in PAH (figure 5b).
FIGURE 5.
Kaplan–Meier survival curves in PAH and in PVOD after matching on age, sex and pulmonary vascular resistance: a) whole cohorts and b) according to baseline risk status in four strata. a) log-rank comparisons tests, p<0.001. b) log-rank comparisons tests: PAH low risk versus PVOD low risk, p=0.523; PAH intermediate–low risk versus PVOD intermediate–low risk, p=0.070; PAH intermediate–high risk versus PVOD intermediate–high risk, p<0.001; PAH high risk versus PVOD high risk, p<0.001. PAH: pulmonary arterial hypertension; PVOD: pulmonary veno-occlusive disease.
Discussion
Current ESC/ERS pulmonary hypertension guidelines recommend using multiparametric instruments to assess the severity of PAH at the time of diagnosis and during follow-up in order to guide treatment strategy for PAH patients without comorbidities [1, 2]. However, there is no recommendation regarding risk assessment and treatment strategy in PVOD patients [1, 2]. Our large cohort study confirmed that PVOD is associated with a worse prognosis than PAH. Unlike PAH, we found no evidence of an impact of initial treatment strategy on PVOD outcomes. Therefore, we identified by univariable analysis several variables associated with survival, including NYHA FC, 6MWD and NT-proBNP. Although all six different risk assessment methods were useful to discriminate risk of mortality in PVOD patients, the ESC/ERS risk stratification in four strata was the best model for predicting survival at both baseline and follow-up, which is consistent with previous studies performed in PAH [8, 9]. However, only a small number of patients with PVOD were categorised as low risk at baseline, and their prognosis appears to be similar to that of PAH patients. Conversely, patients at intermediate- or high-risk status had a poorer prognosis than PAH patients with equivalent risk. Specifically, PVOD patients classified as intermediate–high or high risk had a survival rate lower than 50% after 3 years, which warrants consideration for transplantation eligibility, especially as the effects of treatments appears limited.
In a prior large cohort study of 2879 PAH patients, we demonstrated that the discrimination for overall mortality was slightly higher with the four-strata risk assessment (Harrell's c-index 0.64) than the three-strata model (Harrell's c-index 0.61) at baseline, and at follow-up (respectively 0.67 and 0.63) [9]. In our PVOD population, the c-index values were even higher than those found in PAH (PVOD c-index 0.64 at baseline and 0.74 at follow-up) [9].
More than half of the patients reassessed within a year achieved a low (14%) or intermediate–low-risk status (38%) according to the ESC/ERS four-strata method. Nevertheless, in contrast to PAH, in PVOD, the prognosis of low- and intermediate–low-risk status remained poor. Indeed, the 5-year survival rates of low-risk and intermediate–low-risk status were only 68% and 42% at baseline, and 68% and 29% at follow-up, respectively. Despite similar haemodynamic severity at baseline between the PAH and PVOD cohorts, PVOD patients had worse NYHA FC, lower exercise capacity and higher BNP/NT-proBNP levels than PAH patients. Consequently, only 6% and 24% of PVOD patients were at low- and intermediate–low-risk status at baseline compared to respectively 12% and 33% in the PAH cohort. These differences likely explain the distinct outcomes in the two cohorts, as the three noninvasive variables were associated with the overall survival in PVOD using univariable analysis at baseline, whereas haemodynamic variables such as RAP or PVR were not.
In addition, NYHA FC, 6MWD and BNP/NT-proBNP are part of risk assessment models, especially the ESC/ERS risk score in four strata, which was the most accurate in our study. As combination therapy is not recommended in PVOD, risk assessment in PVOD is unlikely to be helpful in determining the optimal initial treatment strategy or the need for treatment escalation at follow-up. Indeed, we have previously reported that patients with a heritable form of PVOD had better outcomes than sporadic PVOD patients, due to an increased likelihood of access to lung transplantation [12]. Thus, risk assessment may be useful to discriminate the most severe patients requiring lung transplantation, either through conventional listing or high-emergency organ allocation.
In our study, we found that higher DLCO and PaO2 at baseline were associated with better overall survival in PVOD in univariable analysis. A decrease in DLCO and consequent hypoxaemia is an indirect indicator of the importance of capillary remodelling and the severity of the disease. This observation is in line with previous studies in PAH, which have shown that patients with a lower DLCO have a worse prognosis than those with higher levels [15, 16]. Interestingly, in multivariable analyses including NYHA FC, 6MWD and BNP/NT-proBNP, these variables did not remain significant. These results highlight that the clinical impact of exercise capacity limitation and of right heart failure are potent prognostic markers in PVOD and PAH, despite different pathophysiology.
The major strengths of our study were the large cohort of PVOD patients and the availability of complete data including RHC and noninvasive variables at diagnosis and during the first year following PVOD diagnosis, allowing us to calculate risk stratification at both baseline and follow-up according to the six methods assessed. However, our study had limitations. First, PVOD most often remains a probable diagnosis without definite confirmation, apart from heritable PVOD patients carrying EIF2AK4 pathogenic variants (n=26) or in case of lung transplantation (n=41). Indeed, to avoid the cofounding effect of associated respiratory diseases and thus minimise bias related to overlap with the lung phenotype subgroup, we excluded patients with CT scans showing underling chronic respiratory disease (COPD, emphysema, interstitial lung disease) and patients with significant obstructive (defined by a FEV1/FVC <0.7 and a FEV1 <60% of theorical value) and restrictive (TLC <70% of theorical value) patterns. Furthermore, some patients included in our study underwent lung transplantation and all of them had significant venular and capillary involvement on histological analysis, arguing in favour of PVOD. This underlines the reliability of the PVOD diagnosis in our cohort.
Secondly, patients were included according to the definition of precapillary PH in force at the time of the inclusion in the PH registry [13, 14]. Thus, this study does not include PVOD patients with mPAP between 20 and 25 mmHg or PVR between 2 and 3 WU [1, 2]. We recognise some limitations given the retrospective nature of the study including the incomplete or missing data for some patients at baseline or the lack of follow-up assessment, which prevented the calculation of risk scores. The exclusion from the analysis of patients with missing data could lead to bias. Another limitation was the lack of echocardiographic data, although all risk assessment methods remained calculable without these data. Despite the retrospective design of the study, we included all patients with PVOD enrolled in the French PH Registry over a 15-year period from multiple centres across France, thus reporting the largest PVOD cohort.
In conclusion, our study supports the use of a risk assessment approach in PVOD. It is worth noting that several risk assessment methods could be used. Notably, The ESC/ERS risk stratification in four strata demonstrated promising accuracy in predicting survival outcomes both at baseline and during follow-up in the PVOD patient cohort. We also confirmed that PVOD is associated with a worse prognosis than PAH, and that even PVOD patients with low- or intermediate–low-risk status have poor overall survival. Unlike in PAH, we found no evidence of an impact of the initial treatment strategy on outcomes in PVOD. Risk assessment may be useful for identifying the most severe patients and determining which patients are the most suitable candidates for lung transplantation.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00612-2023.SUPPLEMENT (350.4KB, pdf)
Acknowledgements
We thank the patients, their families and healthcare providers from the French Pulmonary Hypertension Network for agreeing to collaborate.
Provenance: Submitted article, peer reviewed.
Conflict of interest: A. Boucly reports grants or contracts from Acceleron, Janssen and MSD, outside the submitted work; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Janssen, Merck, AOP Orphan and Ferrer, outside the submitted work; support for attending meetings and/or travel from Janssen and MSD, outside the submitted work.
Conflict of interest: X. Jaïs reports grants or contracts from Acceleron, Janssen, MSD and Bayer HealthCare, outside the submitted work; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Janssen, and MSD, outside the submitted work.
Conflict of interest: M. Jevnikar reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Janssen, outside the submitted work.
Conflict of interest: A. Bourdin reports grants or contracts from AstraZeneca and Boehringer Ingelheim, outside the submitted work; consulting fees from AstraZeneca, GSK, Novartis, Sanofi Regeneron, Boehringer Ingelheim and Chiesi, outside the submitted work; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, GSK, Novartis, Sanofi Regeneron, Boeringher Ingelheim and Chiesi, outside the submitted work; support for attending meetings and/or travel from AstraZeneca, GSK, Novartis, Sanofi Regeneron, Boehringer Ingelheim and Chiesi, outside the submitted work; and participation on a data safety monitoring or advisory board for AB Science, outside the submitted work.
Conflict of interest: A. Chaouat reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from GSK, MSD and Chiesi, outside the submitted work; and support for attending meetings and/or travel from Janssen and AstraZeneca, outside the submitted work.
Conflict of interest: V. Cottin reports consulting fees from Ferrer/United Therapeutics, outside the submitted work; payment for lectures and consulting from Ferrer/United Therapeutics, outside the submitted work; participation on data and safety monitoring board for Galapagos, Galecto and GSK, outside the submitted work; and adjudication committee for Fibrogen, outside the submitted work.
Conflict of interest: L. Bertoletti reports grant to the institution for research studies from MSD and Bayer, outside the submitted work; consulting fees from MSD, outside the submitted work; payments for honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from MSD, BMS/Pfizer, LEO-Pharma and Viatris, outside the submitted work; support for attending meetings and/or travel from Johnson and Johnson, BMS/Pfizer, and LEO-Pharma, outside the submitted work; participation on a data safety monitoring or advisory board for Bayer, outside the submitted work; receipt of medical writing services from BMS/Pfizer, outside the submitted work.
Conflict of interest: L. Savale reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Janssen, outside the submitted work; support for attending meetings and/or travel from Merck, outside the submitted work; and participation on a data safety monitoring or advisory board for Janssen, outside the submitted work.
Conflict of interest: M. Humbert reports grants or contracts from Acceleron, AOP Orphan, Janssen, Merck and Shou Ti, outside the submitted work; consulting fees from Acceleron, Aerovate, Altavant, AOP Orphan, Bayer, Chiesi, Ferrer, Janssen, Merck, MorphogenIX, Shou Ti, Tiakis and United Therapeutics, outside the submitted work; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Janssen and Merck, outside the submitted work; and participation on a data safety monitoring or advisory board for Acceleron, Altavant, Janssen, Merck, and United Therapeutics, outside the submitted work.
Conflict of interest: O. Sitbon reports grants or contracts from Acceleron (now MSD), AOP Orphan, Janssen (formerly Actelion) and MSD, outside the submitted work; consulting fees from Acceleron (now MSD), Altavant (now Enzyvant), AOP Orphan, Ferrer, Gossamer Bio, Janssen (formerly Actelion) and MSD, outside the submitted work; honoraria for speaking at conferences from AOP Orphan, Janssen (formerly Actelion), Ferrer and MSD, outside the submitted work; and honoraria received for Trial Steering Committee membership (topic: pulmonary hypertension) from Altavant (now Enzyvant), Gossamer Bio and Janssen (formerly Actelion), outside the submitted work.
Conflict of interest: D. Montani reports grants or contracts from Acceleron, Janssen and Merck MSD, outside the submitted work; consulting fees from Acceleron, Janssen, Merck MSD and Ferrer, outside the submitted work; and payment or honoraria for speakers’ bureaus from Bayer, Janssen, Boehringer, Chiesi, GSK, Ferrer and Merck MSD, outside the submitted work.
References
- 1.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 3.Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. doi: 10.1016/j.chest.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 4.Benza RL, Kanwar MK, Raina A, et al. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL Lite 2, for use in patients with pulmonary arterial hypertension. Chest 2021; 159: 337–346. doi: 10.1016/j.chest.2020.08.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kylhammar D, Kjellström B, Hjalmarsson C, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2018; 39: 4175–4181. doi: 10.1093/eurheartj/ehx257 [DOI] [PubMed] [Google Scholar]
- 6.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. doi: 10.1183/13993003.00740-2017 [DOI] [PubMed] [Google Scholar]
- 7.Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. doi: 10.1183/13993003.00889-2017 [DOI] [PubMed] [Google Scholar]
- 8.Hoeper MM, Pausch C, Olsson KM, et al. COMPERA 2.0: a refined 4-strata risk assessment model for pulmonary arterial hypertension. Eur Respir J 2022; 60: 2102311. doi: 10.1183/13993003.02311-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucly A, Weatherald J, Savale L, et al. External validation of a refined 4-strata risk assessment score from the French pulmonary hypertension Registry. Eur Respir J 2022; 59: 2102419. doi: 10.1183/13993003.02419-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montani D, Lau EM, Dorfmüller P, et al. Pulmonary veno-occlusive disease. Eur Respir J . 2016; 47: 1518–1534. doi: 10.1183/13993003.00026-2016 [DOI] [PubMed] [Google Scholar]
- 11.Nossent EJ, Antigny F, Montani D, et al. Pulmonary vascular remodeling patterns and expression of general control nonderepressible 2 (GCN2) in pulmonary veno-occlusive disease. J Heart Lung Transplant 2018; 37: 647–655. doi: 10.1016/j.healun.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 12.Montani D, Girerd B, Jaïs X, et al. Clinical phenotypes and outcomes of heritable and sporadic pulmonary veno-occlusive disease: a population-based study. Lancet Respir Med 2017; 5: 125–134. doi: 10.1016/S2213-2600(16)30438-6 [DOI] [PubMed] [Google Scholar]
- 13.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 14.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 15.Hoeper MM, Pausch C, Grünig E, et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant 2020; 39: 1435–1444. doi: 10.1016/j.healun.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 16.Lewis RA, Thompson AAR, Billings CG, et al. Mild parenchymal lung disease and/or low diffusion capacity impacts survival and treatment response in patients diagnosed with idiopathic pulmonary arterial hypertension. Eur Respir J 2020; 55: 2000041. doi: 10.1183/13993003.00041-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00612-2023.SUPPLEMENT (350.4KB, pdf)