Abstract
Objective
The aim of this study was to create a prognostic instrument for COPD with a multidimensional perspective that includes physical activity (PA). The score also included health status, dyspnoea and forced expiratory volume in 1 s (HADO.2 score).
Methods
A prospective, observational, non-intervention study was carried out. Patients were recruited from the six outpatient clinics of the respiratory service of a single university hospital. The component variables of the HADO.2 score and BODE index were studied, and PA was measured using an accelerometer. The outcomes for the HADO.2 score were mortality and hospitalisations during follow-up and an exploration of the correlation with health-related quality of life at the moment of inclusion in the study.
Results
401 patients were included in the study and followed up for three years. The HADO.2 score showed good predictive capacity for mortality: C-index 0.79 (0.72–0.85). The C-index for hospitalisations was 0.72 (0.66–0.77) and the predictive ability for quality of life, as measured by R2, was 0.63 and 0.53 respectively for the Saint George's Respiratory Questionnaire and COPD Assessment Test.
Conclusions
There was no statistically significant difference between the mortality predictive capacity of the HADO.2 score and the BODE index. Adding PA to the original BODE index significantly improved the predictive capacity of the index. The HADO.2 score, which includes PA as a key variable, showed good predictive capacity for mortality and hospitalisations. There were no differences in the predictive capacity of the HADO.2 score and the BODE index.
Tweetable abstract
Physical activity is a key variable in the evaluation of COPD patients and should be included in the multidimensional prognosis scores of COPD. https://bit.ly/491hOm2
Introduction
COPD is a heterogeneous lung condition in which patients suffer diverse symptoms due to structural changes in the airways and lung parenchyma [1]. Moreover, the consequences of COPD for homeostasis and the function of other organs and systems extend beyond the respiratory system, making this disease a multifaceted condition [2].
This “systemic scenario” impacts the evaluation and treatment of the different subtypes of COPD patient, resulting in a more precise medicine. In this approach, it is essential to establish an accurate prognosis.
In recent years, the use of multidimensional tools in the evaluation and prognosis of COPD patients has become increasingly widespread. The BODE index (body mass index, airflow obstruction, dyspnoea, exercise capacity) has marked a turning point in prediction of the prognosis (mortality) of COPD patients [3] and is now the most widely used tool for this task. Taking the BODE index as a reference, several other indexes have been created that seek to improve on its prognostic capacity and usability [4–6].
On the presumption that there is no one gold-standard predictive instrument, we should all, ideally, “speak the same language” (for example, BODE). However, given that there are multiple facets to the disease that are being overlooked in the predictive indexes currently being used, it is essential to improve the predictive capacity of these tools until such time as new forms of evaluation can be implemented, based on new technologies.
Physical activity (PA) plays a key role in health and disease. In COPD its importance has been established in epidemiological and observational studies and through measurement with questionnaires and accelerometers [7–9]. Indeed, PA has been indicated to be the main predictor of mortality in COPD patients [10]. The HADO (health, activity, dyspnoea, obstruction) score was created to be a predictive instrument of mortality at least as good as BODE [11] that uses self-reported PA as a key element for scoring (C-statistic 0.682) [12]. Surprisingly, given that PA is a key element in COPD prognosis, no other index has incorporated this measurement from the original HADO.
The aims of this study were to emphasise the importance of PA in COPD; to improve the usability of the original HADO score in daily clinical practice and its predictive capacity by including objectively-measured PA (HADO.2 score); and to compare its predictive capacity to the reference tool, the BODE index.
Methods
Participants and data collection
This was a prospective, observational, non-intervention study. Patients were recruited after being treated for COPD in one of six outpatient respiratory clinics run by the Respiratory Service of Galdakao University Hospital. Functional inclusion criteria were forced expiratory volume in 1 s (FEV1) <80% of the predicted value and a FEV1/forced vital capacity ratio <70%. Patients were enrolled consecutively in the study if they had been diagnosed with COPD for at least 6 months and had been stable for at least 6 weeks. Patients were not eligible for the study if they had been diagnosed with asthma, any other major respiratory diseases, or psychiatric or neurological problems that might hinder effective collaboration. The protocol was approved by the hospital's ethics and research committees (16/2014). All candidate patients were given detailed information about the study, and all those included provided written informed consent.
Study protocol
Sociodemographic variables were recorded. The level of dyspnoea was established using the modified Medical Research Council (mMRC) dyspnoea scale [13]. Comorbidities were identified by reviewing the patients’ entire electronic medical record and summarised using the Charlson comorbidity index [14]. Health-related quality of life (HRQoL) was assessed using the validated Spanish versions of the Saint George's Respiratory Questionnaire (SGRQ) [15] and the COPD assessment Test (CAT) [16].
Complete pulmonary function tests were carried out. These tests were performed in accordance with the standards of the Spanish Society of Respiratory Medicine and Thoracic Surgery (SEPAR) [17]. For theoretical values, we considered the values of the European Community for Steel and Coal [18].
Two 6-min walk tests (6MWT) were performed as per American Thoracic Society guidelines [19]. PA was measured using an accelerometer (SenseWear Pro 3; Body Media Inc, Pittsburgh, PA, USA). Patients wore the armband for 7 consecutive days, at all times except during their daily personal hygiene.
Self-reported general health was assessed with the question “How is your health status in general?” with a 5-option response “excellent, very good, good, fair, or poor” [20].
Follow-up
Patients were followed up for 3 years. The interview and assessments were then repeated yearly amongst survivors. No interventions were performed related to this study, and the research team did not take part in patients’ routine care or the treatment of any exacerbations.
Patient medical records and the hospital database on hospitalisations were reviewed at each assessment during the 3-year follow-up period. Vital status was established by reviewing medical records, the hospital database and public death registries.
HADO and variations of HADO and BODE
To construct the new HADO (HADO.2 score) we used the 12-point score of the original HADO and, importantly, with a higher score indicating better clinical condition [11]. In the HADO.2 score the patient-reported PA of the original tool was substituted for PA as measured by an accelerometer. The four categories of PA ranged from <3500 steps to >12 000 steps. These were established as <3500 (“sedentary”=3 points), 3500–6499 (“low active”=2 points), 6500–11 999 (“somewhat active”=1 point) and ≥12 000 (“active”=0 points). These cut-off points have been selected using descriptive analysis techniques such as quartiles.
These four categories of PA were kept in the three other different variations which were analysed in the study (adding 6MWT in the HADO; adding PA in the BODE and replacing 6MWT for PA in the BODE). We also used the categories of the BODE index original [3].
Statistical analysis
Descriptive statistics of all the variables were performed, using frequencies and percentages for categorical variables and mean and standard deviation or median and 1st and 2nd quartiles for continuous variables. The characteristics of live and dead participants after 3 years of follow-up were compared using the Chi-square test for categorical variables and non-parametric Wilcoxon test for continuous variables.
We categorised the scores into four different levels of risk. The optimal thresholds in the continuous risk score were determined with the CatPredi function of the R package CatPredi [21].
Cox proportional hazard regression analyses were performed to assess the capacity of the different scores to predict mortality at 3 years. Hazard ratio, 95% CI and p-value were calculated for the categories of each score. We also calculated the C-statistic to study the predictive ability of the score, where the null value for the C-statistic is 0.5 with a maximum value of 1.0. Internal validation was carried out using the bootstrap method. Significant differences between C-statistics of each score were also analysed using the bootstrap method.
Survival and linear models for the prediction of mortality and HRQoL (CAT and SGRQ) at 3 years have been created adjusted for baseline characteristics. The goodness-of-fit of the linear models was calculated using the R2 statistic. Differences between the number of hospitalisations and HADO were analysed using the Chi-square test.
Kaplan–Meier curves were performed for mortality after 3 years of follow-up for the different scores. All statistical analyses were performed using SAS for Windows, version 9.4 (SAS Institute, Carey, NC, USA) and R, version 4.1.1.
Results
We included 401 consecutive patients in our study. Women represented 26.4% of the cohort. The cohort had a mean±sd age of 64.2±8.4. Current smokers represented 29.7% of the cohort. Other clinical and functional characteristics of the cohort were body mass index (BMI) 27.5±5.4 kg·m−2, FEV1 56.9±17.6% predicted and 6MWT 476.7±108.3 m; the median PA was 6330 (3573–9586) steps·day−1, and 43.9% considered their health status to be “good” and 41.4% “fair”. The median HADO.2 score of the cohort was 6 (4–8) and the BODE index 2 (1–3). Mortality at 3 years was 11%.
The bivariate analysis showed statistical differences between those that remained alive and those who died during the 3-year follow-up period (table 1).
TABLE 1.
Baseline characteristics of study participants and characteristics by vital status after 3 years follow-up
| All | Patients | p-value | ||
| Alive | Dead | |||
| Total | 401 (100.00) | 357 (89.03) | 44 (10.97) | |
| Age years | 64.19±8.41 | 63.59±8.36 | 69.00±7.26 | <0.0001 |
| Male | 295 (73.57) | 255 (71.43) | 40 (90.91) | 0.0057 |
| BMI kg·m−2 | 27.50±5.44 | 28.25±12.27 | 26.18±5.69 | 0.1304 |
| BMI >21 | 358 (89.95) | 326 (91.32) | 32 (78.05) | 0.0074 |
| Hospital admission (2 previous years) | 132 (32.92) | 102 (28.57) | 29 (65.91) | <0.0001 |
| Smoking habit pack-years | 44 (31–65) | 43 (30–60) | 62 (39–77.50) | 0.0038 |
| FEV1 L | 1.47±0.51 | 1.52±0.50 | 1.05±0.44 | <0.0001 |
| FEV1 % pred | 56.95±17.61 | 58.63±16.89 | 43.51±17.64 | <0.0001 |
| FEV1/FVC | 51.20±10.11) | 51.92±9.87 | 45.40±10.28 | <0.0001 |
| 6-min walk distance m | 476.73±108.34 | 493.03±95.49 | 340.98±114.66 | <0.0001 |
| Dyspnoea (mMRC) scale | 1 (1–2) | 1 (1–2) | 2 (2–3) | <0.0001 |
| Physical activity steps·day−1 | 6330 (3573–9586) | 6687 (4188–9782) | 2618 (997–4000) | <0.0001 |
| Health status | 0.0075 | |||
| Bad | 42 (10.47) | 32 (8.96) | 10 (22.73) | |
| Fair | 166 (41.40) | 145 (40.62) | 21 (47.73) | |
| Good | 176 (43.89) | 163 (45.66) | 13 (29.55) | |
| Very good/excellent | 17 (4.24) | 17 (4.76) | 0 (0.00) | |
| Charlson Comorbidity Index | 1 (1–2) | 1 (1–2) | 2 (1.50–3) | <0.0001 |
| St George's Respiratory Questionnaire total | 41.18±17.57 | 39.54±17.27 | 52.79±15.45 | 0.0012 |
| COPD Assessment Test | 12.74±7.66 | 12.14±7.40 | 17.59±8.07 | <0.0001 |
| Hospital Anxiety and Depression Scale | ||||
| Anxiety | 5 (2–8) | 5 (1–8) | 7 (3–10.50) | 0.0124 |
| Depression | 3 (1–6) | 3 (1–5) | 5 (2–8.50) | 0.0005 |
| HADO.2 score (steps·day−1) | 6 (4–8) | 7 (5–8) | 3 (2–4.5) | <0.0001 |
| BODE index | 2 (1–3) | 1 (1–2) | 4 (2–6) | <0.0001 |
Data are presented as n (%), mean±sd or median (interquartile range). BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; mMRC: modified Medical Research Council dyspnoea scale; HADO: health, activity, dyspnoea, obstruction; BODE: body mass index, obstruction, dyspnoea, exercise.
Table 2 shows the predictive ability of all the variables making up the HADO.2 score and the BODE index for 3-year survival. Dyspnoea and PA (steps·day−1) were the variables with the highest predictive capacity (C-statistics 0.757 and 0.753 respectively). By contrast, the lowest predictive capacity was for BMI (C-statistic 0.562).
TABLE 2.
3-year death survival univariable analysis for the variables of the HADO and BODE scores
| Variables | n (%) | HR (95% CI) | p-value | C-statistic |
| HADO variables | ||||
| FEV1 % | 0.722 | |||
| ≤35 | 17 (40.48) | Ref. | Ref. | |
| 36–49 | 13 (14.13) | 0.311 (0.151–0.642) | 0.0017 | |
| 50–64 | 6 (4.41) | 0.091 (0.036–0.231) | <0.0001 | |
| ≥65 | 8 (6.11) | 0.128 (0.055–0.296) | <0.0001 | |
| Dyspnoea (mMRC) scale | 0.757 | |||
| 3–4 | 21 (45.65) | Ref. | Ref. | |
| 2 | 14 (10.53) | 0.181 (0.092–0.357) | <0.0001 | |
| 1 | 7 (4.55) | 0.076 (0.032–0.178) | <0.0001 | |
| 0 | 2 (2.94) | 0.049 (0.011–0.209) | <0.0001 | |
| Physical activity | 0.753 | |||
| 0 (<3500 steps·day−1) | 28 (29.47) | Ref. | Ref. | |
| 1 (3500–6499 steps·day−1) | 10 (9.01) | 0.274 (0.133–0.563) | 0.0004 | |
| 2 (6500–11 999 steps·day−1) | 4 (2.84) | 0.083 (0.029–0.236) | <0.0001 | |
| 3 (≥12 000 steps·day−1) | 2 (3.70) | 0.108 (0.026–0.455) | 0.0024 | |
| Health status | 0.631 | |||
| Bad | 10 (23.81) | Ref. | Ref. | |
| Fair | 21 (12.65) | 0.497 (0.234–1.056) | 0.0689 | |
| Good | 13 (7.39) | 0.279 (0.122–0.637) | 0.0024 | |
| Very good/excellent | 0 (0.00) | - | 0.9963 | |
| 6MWT m | 0.706 | |||
| ≤149 | 2 (100.00) | Ref. | Ref. | |
| 150–249 | 8 (44.44) | 0.378 (0.080–1.790) | 0.2200 | |
| 250–349 | 13 (38.24) | 0.288 (0.064–1.287) | 0.1030 | |
| ≥350 | 21 (6.04) | 0.040 (0.009–0.171) | <0.0001 | |
| BODE variables | ||||
| FEV1 % | 0.727 | |||
| ≥65 | 8 (6.11) | Ref. | Ref. | |
| 50–64 | 6 (4.41) | 0.712 (0.247–2.053) | 0.5300 | |
| 36–49 | 13 (14.13) | 2.442 (1.012–5.891) | 0.0470 | |
| ≤35 | 17 (40.48) | 7.843 (3.381–18.193) | <0.0001 | |
| Dyspnoea (mMRC) scale | 0.753 | |||
| 0–1 | 9 (20.46) | Ref. | Ref. | |
| 2 | 14 (31.82) | 2.687 (1.163–6.209) | 0.0207 | |
| 3 | 13 (29.55) | 13.460 (5.748–31.521) | <0.0001 | |
| 4 | 8 (18.18) | 17.733 (6.823–46.092) | <0.0001 | |
| 6MWT m | 0.706 | |||
| ≥350 | 21 (6.04) | Ref. | Ref. | |
| 250–349 | 13 (38.24) | 7.293 (3.649–14.580) | <0.0001 | |
| 150–249 | 8 (44.44) | 9.558 (4.226–21.620) | <0.0001 | |
| ≤149 | 2 (100.00) | 25.316 (5.865–109.28) | <0.0001 | |
| BMI kg·m−2 | 0.562 | |||
| ≤21 | 9 (22.50) | 2.696 (1.287–5.649) | 0.0086 | |
| Physical activity | 0.753 | |||
| 0 (≥12 000 steps·day−1) | 2 (3.70) | Ref. | Ref. | |
| 1 (6500–11 999 steps·day−1) | 4 (2.84) | 0.765 (0.140–4.174) | 0.7565 | |
| 2 (3500–6499 steps·day−1) | 10 (9.01) | 2.524 (0.553–11.519) | 0.2320 | |
| 3 (<3500 steps·day−1) | 28 (29.47) | 9.227 (2.198–38.743) | 0.0024 |
HADO: health, activity, dyspnoea, obstruction; BODE: body mass index, obstruction, dyspnoea, exercise; HR: hazard ratio; FEV1: forced expiratory volume in 1 s; mMRC: modified Medical Research Council dyspnoea scale; 6MWT: 6-min walk test; BMI: body mass index.
A 1-point decrease in the continuous HADO.2 score increased mortality by almost 60% (HR: 1.598), similar to the BODE index (HR: 1.602) (table 3). The C-statistic for the categorised HADO.2 score was 0.79 (0.725–0.855) and for the BODE index 0.767 (0.692–0.842). There were no significant differences between the c-index of the different models except between the BODE index and BODE (+PA) index. Figure 1 shows the survival curve of the HADO.2 score categories.
TABLE 3.
3-year death survival analysis for the different scores
| Variables | n (%) | HR (95% CI) | p-value | C-statistic (95% CI) |
| HADO.2 score (cont.) # | 3 (2–4.5) | 1.598 (1.409–1.812) | <0.0001 | 0.800 (0.735–0.864) |
| HADO.2 score | 0.790 (0.725–0.855) | |||
| 7–12 | 3 (2.03) | Ref. | Ref. | |
| 0–1 | 11 (7.14) | 32.850 (9.749–110.700) | <0.0001 | |
| 2–4 | 10 (17.24) | 9.206 (2.533–33.450) | 0.0007 | |
| 5–6 | 20 (48.78) | 3.595 (1.003–12.890) | 0.0495 | |
| Bootstrap (cat.) | 0.791 (0.720–0.848) | |||
| HADO.2 score (+6MWT) (cont.) # | 4 (2–6) | 1.463 (1.327–1.614) | <0.0001 | 0.796 (0.727–0.866) |
| HADO.2 score (+6MWT) | 0.773 (0.703–0.843) | |||
| 10–15 | 7 (3.41) | Ref. | Ref. | |
| 7–9 | 6 (6.52) | 19.383 (7.888–47.627) | <0.0001 | |
| 4–6 | 16 (21.62) | 7.053 (2.901–17.148) | <0.0001 | |
| 0–3 | 15 (50.00) | 1.948 (0.655–5.796) | 0.2310 | |
| Bootstrap (cat.) | 0.775 (0.698–0.842) | |||
| BODE index (cont.) # | 4 (2–6) | 1.583 (1.421–1.763) | <0.0001 | 0.792 (0.719–0.865) |
| BODE index | 0.767 (0.692–0.842) | |||
| 0–2 | 11 (3.90) | Ref. | Ref. | |
| 3–4 | 10 (13.33) | 3.593 (1.526–8.460) | 0.0034 | |
| 5–6 | 13 (46.43) | 15.233 (6.815–34.050) | <0.0001 | |
| 7–10 | 7 (53.85) | 18.395 (7.103–47.640) | <0.0001 | |
| Bootstrap (cat.) | 0.769 (0.693–0.845) | |||
| BODE index (−6MWT+PA) (cont.) # | 7 (5–7) | 1.687 (1.479–1.924) | <0.0001 | 0.805 (0.734–0.877) |
| BODE index (−6MWT+PA) | 0.773 (0.698–0.848) | |||
| 0–3 | 5 (3.31) | Ref. | Ref. | |
| 4–5 | 10 (5.59) | 1.718 (0.587–5.027) | 0.3230 | |
| 6–7 | 16 (31.37) | 11.222 (4.109–30.645) | <0.0001 | |
| 8–10 | 10 (58.82) | 25.433 (8.661–74.683) | <0.0001 | |
| Bootstrap (cat.) | 0.777 (0.704–0.846) | |||
| BODE index (+PA) (cont.) # | 7 (5–9) | 1.488 (1.356–1.634) | <0.0001 | 0.807 (0.735–0.879) |
| BODE index (+PA) | 0.804 (0.735–0.873) | |||
| 0–3 | 6 (2.69) | Ref. | Ref. | |
| 4–5 | 7 (7.14) | 2.737 (0.920–8.143) | 0.0704 | |
| 6–7 | 9 (21.95) | 9.064 (3.226–25.469) | <0.0001 | |
| 8–13 | 19 (52.78) | 26.629 (10.609–66.841) | <0.0001 | |
| Bootstrap (cat.) | 0.806 (0.728–0.875) |
Significant differences only found between BODE index and BODE (+PA) index and HADO (+6MWT) score and BODE (+PA) index, where the confidence interval of the difference is (−0.077 to −0.001) and (−0.042 to −0.006) respectively. HR: hazard ratio; HADO: health, activity, dyspnoea, obstruction; CI: confidence interval; 6MWT: 6 min walk test; BODE: body mass index, obstruction, dyspnoea, exercise; Cont: continuous score; Bootstrap (cat.): refers to the C-statistic results of the internal validation of each score by bootstrap. #: the probability of death is calculated for each unit the score decreases in the HADO.2, while in the BODE the probability of death is calculated for each unit the score increases.
FIGURE 1.
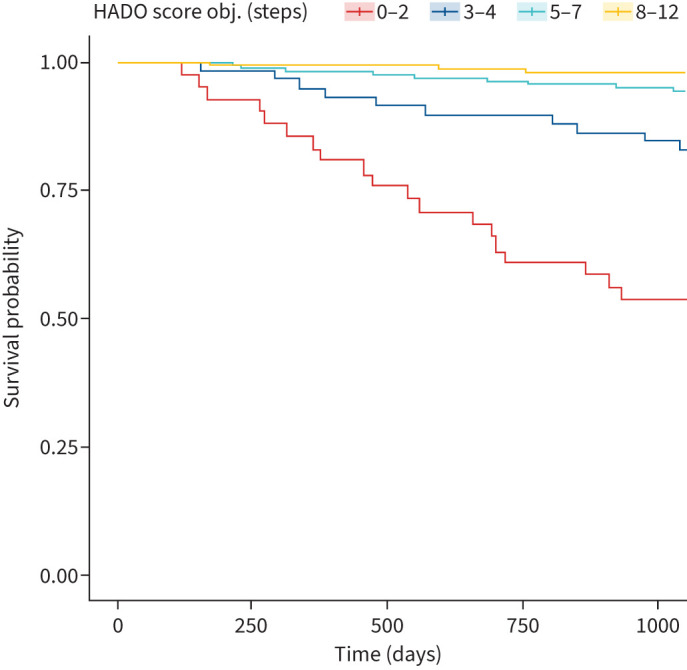
3-year survival curve of the HADO.2 score categories. obj: objective.
Indexes variations
Given that each index includes one of two variables related to PA accepted as being very important for COPD management (PA and 6MWT), we performed the exercise of exchanging the variable between the tools. When 6MWT was added to the continuous HADO.2 score, the C-statistic was 0.796 (0.727–0.866). Exchanging 6MWT for PA, the C-statistic for the continuous BODE index was 0.805 (0.734–0.877), while adding PA it was 0.807 (0.735–0.879). Among all the scores analysed, the only statistical differences found were between the HADO with the 6MWT score versus the BODE with PA index and between the BODE index versus BODE with PA index (table 3). Internal validation was performed by bootstrap; the C-index achieved through this internal validation was 0.791 for the HADO.2 score and 0.769 for the BODE index (table 3).
HADO.2 score properties
Adjusted by age and comorbidities (Charlson index), the HADO.2-score showed a good predictive capacity for mortality (C-index 0.85) with an increasing HR risk of death by HADO.2 severity categories. Moreover, the HADO.2 score categories were also associated with an increasing HR of hospitalisation during the 3-year follow-up (table 4). We also looked at the relationship of the HADO.2 with two HRQoL measures, the CAT and SGRQ, where the adjustment by the respective baseline HRQoL score in each case was also added to each model, with increasingly worse scores in each HADO.2 increasing the severity category in each HRQoL tool (SGRQ and CAT) and with good predictive ability, as measured by R2 of 0.63 and 0.53 respectively.
TABLE 4.
3-year death survival model, hospitalisation logistic regression and linear models for the CAT and SGRQ scores changes from baseline to 3 years
| Variables | n (%) | 3-year mortality | Hospitalisations | CAT changes | SGRQ changes | ||||||||
| n | HR (95% CI) | p-value | n | OR (95% CI) | p-value | n | β (95% CI) | p-value | n | β (95% CI) | p-value | ||
| HADO-score | |||||||||||||
| 8–12 | 148 (36.91) | 3 | Ref. | Ref. | 16 | Ref. | Ref. | 145 | Ref. | Ref. | 143 | Ref. | Ref. |
| 5–7 | 154 (38.40) | 11 | 2.97 (0.82–10.70) | 0.0963 | 43 | 3.24 (1.71–6.12) | 0.0003 | 143 | 1.62 (0.40–2.83) | 0.0095 | 140 | 3.38 (0.44–6.32) | 0.0424 |
| 3–4 | 58 (14.46) | 10 | 8.00 (2.20–29.18) | 0.0016 | 20 | 4.28 (2.00–9.18) | 0.0002 | 50 | 4.74 (3.00–6.49) | <0.0001 | 50 | 9.85 (5.60–14.11) | <0.0001 |
| 0–2 | 41 (10.22) | 20 | 29.32 (8.67–99.16) | <0.0001 | 21 | 9.47 (4.13–21.73) | <0.0001 | 22 | 3.57 (1.14–6.00) | 0.0041 | 22 | 6.31 (0.24–12.38) | 0.0249 |
| C-index (95% CI) | 0.85 (0.80–0.89) | 0.72 (0.66–0.77) | |||||||||||
| R2 | 0.53 | 0.63 | |||||||||||
All models have been adjusted by age, Charlson comorbidity index and baseline health-related quality of life respective scores. CAT: COPD Assessment Test; SGRQ: Saint George's Respiratory Questionnaire; HR: hazard ratio; HADO: health, activity, dyspnoea, obstruction.
Discussion
In our cohort of COPD patients: 1) the HADO.2 score – which uses PA measured by an accelerometer, as opposed to a questionnaire as in the original version of the HADO – showed better predictive capacity for mortality at 3 years; 2) at the same time, the HADO.2 score showed a similar predictive capacity to the BODE index; and 3) adding our proposal of measuring PA by accelerometer to the BODE (BODE+PA) also improved the predictive capacity of the original BODE.
The HADO.2 score was made up of several variables which had already been shown to be important in the prognosis of COPD patients. Variables such as FEV1% and dyspnoea are well-known predictive factors of mortality in COPD patients [22, 23]. Indeed, both variables, especially FEV1, are frequently included in the prognosis scores [24].
It has been shown that HRQoL, which globally reflects patients’ general and subjective respiratory clinical condition, was associated with short-term prognosis [25]. However, we sought to incorporate this aspect by using a simpler tool, in this case in the form of a single question. We based this choice on a number of other studies, summarised in a meta-analysis of all-cause mortality prediction based on 14 studies comparing individuals reporting their health status as “fair” and “poor” versus “excellent”, giving a result of OR 1.44 (95% CI 1.21–1.72) and 1.92 (95% CI 1.64–2.25) [20]. Considering that this subjective measurement is correlated with other objective measurements [26], it should be noted that in our cohort, self-reported health status did not have the highest predictive capacity (table 2), but was better than BMI, which had the lowest predictive capacity. This aspect of BMI has been noted in other studies; Soler-Cataluña et al. [4] found that the only variable not significantly associated with mortality in their cohort was BMI.
What deserves more attention in this study is PA and its relationship with exercise capacity (6MWT). Previous evidence has determined several key aspects in exercise capacity and PA. Exercise capacity measured by 6MWT has proved to be an important prognostic tool in different scenarios. Indeed, 6MWT was an independent predictor of mortality in a cohort of severe COPD patients, with a risk-of-death rate of 0.82 per 50-m increase in 6MWT [27]. 6MWT was a predictor of mortality in a retrospective study that included observational studies and clinical trials, with an area under the curve from the receiver operating characteristic curve of 0.725 and 0.684 at 6 and 12 months respectively [28]. In women with COPD a threshold of 350 m in the 6MWT was a valid differentiator of survival [29].
PA also has an important role in COPD. Watz et al. [8] showed that PA is reduced in the mild level of COPD and continues to deteriorate increasingly over time [30]. Furthermore, PA was distinguished as an important predictor of several outcomes in COPD. Waschki et al. [10], using PA level or steps per day, identified it as the strongest predictor of all causes of mortality. Moreover, PA has been linked to mortality in studies based on different methodologies and designs [7, 8], and even a relationship to dose response has been demonstrated [9].
Starting from this scenario, it is clear that PA and exercise capacity settle in different constructs. In COPD patients, the relationship between PA versus exercise capacity measured with an incremental cycle ergometer test and incremental and endurance shuttle walking test was established from moderate to weak [31]. In another cross-sectional study that included mild–moderate patients from primary care, there was no significant correlation between several variables of PA and 6MWT [32]. Furthermore, exercise training was associated with a small effect on PA, as demonstrated in a systematic review and meta-analysis [33]. Furthermore, after a rehabilitation programme, an immediate improvement in exercise capacity (6MWT and incremental shuttle walking test) ensued, with no change in PA performance [34].
Summarising, PA and exercise capacity contribute to a better perspective of the clinical situation of the patient and thus to better management of COPD patients [35]. These two perspectives should be considered together, not only with regard to the aspects discussed above, but also in the prognosis evaluation, as demonstrated in our study. Strikingly, a recent study found that the higher the level of 6MWT, the lower the risk of mortality in the 6-year follow-up [36]. In this study, PA seems to play a minor role in the predictive role of mortality.
In our study it is worth noting that the C-statistics of the HADO.2 score, which can be qualified as good, are 0.80 when the score is considered as continuous and 0.79 when categorised, without losing predictive ability. In our original HADO we did not get a particularly good predictive capacity (C-statistic 0.682) by using a questionnaire for PA. But what is also remarkable is the improvement in the score of BODE+PA as opposed to BODE. This could be interpreted as the complementarity of PA and exercise capacity in COPD evaluation and reinforces the role of PA as a mortality predictor, contributing to the debate on its importance and providing another perspective on the results of Vaes et al. [10, 35, 36]. Indeed, there was a statistical difference in the C-statistics between the BODE+PA versus the BODE (table 3). That finding reinforces the importance of PA.
Differences between HADO.2 score and BODE cohorts should be mentioned. The BODE cohorts come from hospitals [3], but the HADO.2 cohort was built from patients from a monographic COPD surgery (hospital patients), and patients from other surgeries in our service (which involves patients neither in hospital nor in primary care but in outpatient respiratory surgeries). Our patients are therefore milder in severity than those from the BODE cohort, but both the HADO.2 score and the BODE index work well in our cohort in the mid-term.
Our study has some limitations: 1) the HADO.2 score was cross-sectional and other clinical and therapeutic circumstances that occurred during the follow-up in this study were not taken into consideration; 2) there was a low number of deaths during the study, which may have conditioned the results; 3) an external validation was not carried out, although an internal validation by bootstrap was performed; 4) the long-term predictive capacity in the HADO.2 score was not established, although this was not the aim of the study and the prognosis of each patient should probably be evaluated bearing in mind the constant change in factors having some influence on these patients’ life expectancy; 5) other variables related to PA obtained from accelerometers could have been used in order to improve the predictive capacity of the model, but the objective was to use easily obtainable parameters to provide the easiest tool for use in clinical practice; 6) in the study an analysis of subgroups like bronchitis and emphysema were not carried out, due to a lack of a precise diagnosis of both entities and their overlap in some patients; and 7) the measurement of PA by accelerometers imply a limitation in its implementation; however measurement of PA is day by day becoming more accessible for everyone, so this kind of measurement (mobile phone) just needs a validation and PA will become an easy-to-use tool with very important clinical implications for the patients.
Among the strengths of this study, apart from the quality of the records, we have shown that the HADO.2 score has a good association with the most important outcomes in COPD, namely mortality, hospitalisations during the follow-up and change in quality of life measured by two well-known specific tools for COPD patients, which supports the suitability of the tool (table 4).
We need to be aware that the task of establishing a precise prognosis has not been concluded, given that several facets of COPD must still be explored. Patients’ evaluation should be based mainly on objective measurements (metabolic, hormonal, inflammatory, etc.), but humans are unable to capture the entire complex relationship between the mix of variables that play a role in COPD (genetics, exposome, microbiome, metabolomics, etc.). We therefore need to recruit other agents (i.e., artificial intelligence) for the task. Until such time, we propose incorporating PA in the prognosis scores of the COPD patients, since it improves the efficacy of these prognosis scores and will probably make health professionals (at all levels of care) and patients more aware of the importance of keeping moving.
Footnotes
Provenance: Submitted article, peer reviewed.
Availability of data and material: Data available on request from the authors.
Conflict of interest: No financial, consultative, institutional and other relationships that might lead to bias or a conflict of interest exist for any of the authors of this study.
Support statement: This work was supported in part by a grant from the Instituto de Salud Carlos III (PI13/02352). The project has received an unrestricted grant from Laboratorios Menarini. Funding information for this article has been deposited with the Crossref Funder Registry.
Ethics statement: All patients were required to provide written informed consent to participate in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol was approved by the Ethics Committee of the Hospital University Galdakao (reference PI2016/14).
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . 2023 Global Strategy for the Diagnosis, Management and Prevention of COPD. https://goldcopd.org/2023-gold-report-2/
- 2.Reid LV, Spalluto CM, Watson A, et al. . The role of extracellular vesicles as a shared disease mechanism contributing to multimorbidity in patients with COPD. Front Immunol 2021; 12: 754004. doi: 10.3389/fimmu.2021.754004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celli BR, Cote CG, Marin JM, et al. . The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005–1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 4.Soler-Cataluña JJ, Martínez-García MA, Sánchez LS, et al. . Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med 2009; 103: 692–699. doi: 10.1016/j.rmed.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 5.Golpe R, Esteban C, Figueira-GonÇalves JM, et al. . Development and validation of a prognostic index (BODEXS90) for mortality in stable chronic obstructive pulmonary disease. Pulmonology 2023; 29: 276–283. [DOI] [PubMed] [Google Scholar]
- 6.Cote CG, Pinto-Plata VM, Marin JM, et al. . The modified BODE index: validation with mortality in COPD. Eur Respir J 2008; 32: 1269–1274. doi: 10.1183/09031936.00138507 [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Aymerich J, Lange P, Benet M, et al. . Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006; 61: 772–778. doi: 10.1136/thx.2006.060145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watz H, Waschki B, Meyer T, et al. . Physical activity in patients with COPD. Eur Respir J 2009; 33: 262–272. doi: 10.1183/09031936.00024608 [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Rio F, Rojo B, Casitas R, et al. . Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest 2012; 142: 338–346. doi: 10.1378/chest.11-2014 [DOI] [PubMed] [Google Scholar]
- 10.Waschki B, Kirsten A, Holz O, et al. . Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011; 140: 331–342. doi: 10.1378/chest.10-2521 [DOI] [PubMed] [Google Scholar]
- 11.Esteban C, Quintana JM, Moraza J, et al. . BODE-Index vs HADO-score in chronic obstructive pulmonary disease: which one to use in general practice? BMC Med 2010; 8: 28. doi: 10.1186/1741-7015-8-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esteban C, Quintana JM, Aburto M, et al. . A simple score for assessing stable chronic obstructive pulmonary disease. QJM 2006; 99: 751–759. doi: 10.1093/qjmed/hcl110 [DOI] [PubMed] [Google Scholar]
- 13.Bestall JC, Paul EA, Garrod R, et al. . Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54: 581–586. doi: 10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 15.Ferrer M, Alonso J, Prieto L, et al. . Validity and reliability of the St George's Respiratory Questionnaire after adaptation to a different language and culture: the Spanish example. Eur Respir J 1996; 9: 1160–1166. doi: 10.1183/09031936.96.09061160 [DOI] [PubMed] [Google Scholar]
- 16.Agustí A, Soler JJ, Molina J, et al. . Is the CAT questionnaire sensitive to changes in health status in patients with severe COPD exacerbations? COPD 2012; 9: 492–498. doi: 10.3109/15412555.2012.692409 [DOI] [PubMed] [Google Scholar]
- 17.García-Río F, Calle M, Burgos F, et al. . Spirometry. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Arch Bronconeumol 2013; 49: 388–401. doi: 10.1016/j.arbres.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 18.Quanjer PH, Tammeling GJ, Cotes JE, et al. . Lung volumes and forced ventilatory flows. Report working party standardization of lung function test, European Community for Steel and Coal. Official statement of the European Respiratory Society. Eur Respir J 1993; 16: 5–40. doi: 10.1183/09041950.005s1693 [DOI] [PubMed] [Google Scholar]
- 19.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 20.DeSalvo KB, Bloser N, Reynolds K, et al. . Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med 2006; 21: 267–275. doi: 10.1111/j.1525-1497.2005.00291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrio I, Arostegui I, Rodríguez-Álvarez MX, et al. . A new approach to categorising continuous variables in prediction models: proposal and validation. Stat Methods Med Res 2017; 26: 2586–2602. doi: 10.1177/0962280215601873 [DOI] [PubMed] [Google Scholar]
- 22.Anthonisen NR, Wright EC, Hodgkin JE. IPPB Trial Group. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 133: 14–20. doi: 10.1164/arrd.1986.133.1.14 [DOI] [PubMed] [Google Scholar]
- 23.Nishimura K, Takateru I, Tsukino M, et al. . Dyspnea is a better predictor of 5 year survival than airway obstruction in patients with COPD. Chest 2002; 121: 1434–1440. doi: 10.1378/chest.121.5.1434 [DOI] [PubMed] [Google Scholar]
- 24.Bellou V, Belbasis L, Konstantinidis AK, et al. . Prognostic models for outcome prediction in patients with chronic obstructive pulmonary disease: systematic review and critical appraisal. BMJ 2019; 367: l5358. doi: 10.1136/bmj.l5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esteban C, Arostegui I, Aramburu A, et al. . Changes in health-related quality of life as a marker in the prognosis in COPD patients. ERJ Open Res 2022; 8: 00181-2021. doi: 10.1183/23120541.00181-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorem G, Cook S, Leon DA, et al. . Self-reported health as a predictor of mortality: a cohort study of its relation to other health measurements and observation time. Sci Rep 2020; 10: 4886.. doi: 10.1038/s41598-020-61603-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto-Plata VM, Cote C, Cabral H, et al. . The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J 2004; 23: 28–33. doi: 10.1183/09031936.03.00034603 [DOI] [PubMed] [Google Scholar]
- 28.Celli B, Tetzlaff K, Criner G, et al. . The 6–minute-walk distance test as a chronic obstructive pulmonary disease stratification tool. Insights from the COPD Biomarker Qualification Consortium. Am J Respir Crit Care Med 2016; 194: 1483–1493. doi: 10.1164/rccm.201508-1653OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Torres JP, Casanova C, Cote CG, et al. . Six-minute walking distance in women with COPD. COPD 2011; 8: 300–305. doi: 10.3109/15412555.2011.589870 [DOI] [PubMed] [Google Scholar]
- 30.Waschki B, Kirsten AM, Holz O, et al. . Disease progression and changes in physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 295–306. doi: 10.1164/rccm.201501-0081OC [DOI] [PubMed] [Google Scholar]
- 31.Zwerink M, van der Palen J, van der Valk P, et al. . Relationship between daily physical activity and exercise capacity in patients with COPD. Respir Med 2013; 107: 242–248. doi: 10.1016/j.rmed.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 32.Fastenau A, van Schayck OC, Gosselink R, et al. . Discrepancy between functional exercise capacity and daily physical activity: a cross-sectional study in patients with mild to moderate COPD. Prim Care Respir J 2013; 22: 425–430. doi: 10.4104/pcrj.2013.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cindy Ng LW, Mackney J, Jenkins S, et al. . Does exercise training change physical activity in people with COPD? A systematic review and meta-analysis. Chron Respir Dis 2012; 9: 17–26. doi: 10.1177/1479972311430335 [DOI] [PubMed] [Google Scholar]
- 34.Egan C, Deering BM, Blake C, et al. . Short term and long-term effects of pulmonary rehabilitation on physical activity in COPD. Respir Med 2012; 106: 1671–1679. doi: 10.1016/j.rmed.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 35.Koolen EH, van Hees HW, van Lummel RC, et al. . “Can do” versus “do do”: a novel concept to better understand physical functioning in patients with chronic obstructive pulmonary disease. J Clin Med 2019; 8: 340. doi: 10.3390/jcm8030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaes AW, Spruit MA, Koolen EH, et al. . “Can do, do do” quadrants and 6-year all-cause mortality in patients with COPD. Chest 2022; 161: 1494–1504. doi: 10.1016/j.chest.2021.12.657 [DOI] [PubMed] [Google Scholar]


