Abstract
Objective
Developing and evaluating new treatment guidelines for rheumatoid arthritis (RA) based on observational data requires a quantitative understanding of patterns in current treatment practice with biologic and targeted synthetic disease‐modifying antirheumatic drugs (b/tsDMARDs).
Methods
We used data from the CorEvitas RA registry to study patients starting their first b/tsDMARD therapy, defined as the first line of therapy, between 2012 and the end of 2021. We identified treatment patterns as unique sequences of therapy changes following and including the first‐line therapy. Therapy cycling was defined as switching back to a treatment from a previously used therapeutic class.
Results
A total of 6015 b/tsDMARD‐naïve patients (77% female) were included in the analysis. Their median age was 58 years, and their median disease duration was 3 years. In 2012–2014, 80% of the patients started a tumor necrosis factor inhibitor (TNFi) as their first b/tsDMARD. However, the use of TNFi decreased in favor of Janus kinase inhibitors since 2015. Although the number of treatment patterns was large, therapy cycling was relatively common. For example, 601 patterns were observed among 1133 patients who changed therapy at least four times, of whom 85.3% experienced therapy cycling. Furthermore, the duration of each of the first three lines of therapy decreased over the past decade. For example, the median duration of the first‐line therapy was 153 days in 2018–2021 compared to 208 days in 2015–2017 (P < 0.001).
Conclusion
First‐line therapy was almost always TNFi, but diversity in treatment choice was high after that. This practice variation allows for proposing and evaluating new guidelines for sequential treatment of RA. It also presents statistical challenges to compare patients with different treatment sequences.
INTRODUCTION
The treatment of patients with rheumatoid arthritis (RA) with disease‐modifying antirheumatic drugs (DMARDs) is often sequential and requires trial and error. Although prescribing conventional synthetic DMARDs (csDMARDs) is the recommended first treatment strategy (1, 2, 3), there is no consensus on how to choose from biologic and targeted synthetic DMARDs (b/tsDMARDs) when the initial csDMARD therapy fails. Tumor necrosis factor inhibitors (TNFi), a group of bDMARDs developed in the late 1990s, are routinely used after initial csDMARD failure (4, 5); however, neither the American College of Rheumatology guideline nor the European Alliance of Associations for Rheumatology (formerly the European League Against Rheumatism) recommendations express any preference of bDMARDs over tsDMARDs in this situation (1, 2). With a growing number of medications available, practice variation in RA treatment continues to increase.
SIGNIFICANCE & INNOVATIONS.
Understanding current practice in the sequential treatment of patients with rheumatoid arthritis starting a biologic or targeted synthetic disease‐modifying antirheumatic drug is an important step to finding optimal treatment strategies. Previous work has mostly focused on individual lines of therapy or transitions between them, not entire sequences of therapies.
Tumor necrosis factor inhibitors dominate as the first line of therapy, although the use of Janus kinase inhibitors increased in recent years. Interestingly, the duration of each of the first three lines of therapy decreased over the past decade.
Substantial variety in later‐line therapy selections leads to many distinct treatment patterns. Therapy cycling is one of few recurring patterns. This practice variation presents statistical challenges but allows for evaluation of new treatment strategies using observational data.
Implementing active control trials of sequential treatment strategies is difficult but would help with an evidence basis for stronger treatment guidelines. As an alternative, observational data on treatment decisions and outcomes might provide opportunities to evaluate new strategies without requiring the expenses and/or prolonged duration of randomized controlled trials (6). For such an evaluation to be most useful, it is necessary that alternative treatment strategies of interest are regularly observed in routine data and thus can be assessed using real‐world evidence (7). Characterizing common treatment sequences is therefore an important step in finding optimal strategies for the sequential treatment of RA.
Much attention has been given to patterns in the treatment of patients with RA who experience an insufficient response to their first TNFi after already having tried a csDMARD (8, 9). These patients may be treated with either a second TNFi or a medication with a new mechanism of action (10, 11, 12). However, these studies are mostly limited to the choice of second‐line b/tsDMARD and do not give a complete picture of current practice. Other studies have extended the duration of follow‐up and examined sequential therapies using observational designs (13, 14). Most often, these studies demonstrate transitions between therapies in a flow diagram, typically in the form of a Sankey diagram, and not the whole sequences of treatments. For example, a transition from a TNFi to a non‐TNFi b/tsDMARD appears the same in a Sankey diagram regardless of previous treatments. A different set of studies have focused on pathways to a particular type of therapy, for example, tocilizumab monotherapy (15) or therapies with baricitinib (16), but they do not summarize the entire treatment strategy across the disease course.
In the current set of analyses, we aimed to provide a more complete description of common patterns in the sequential treatment of RA. Using data from the CorEvitas RA registry (17) (previously the Corrona RA registry), we defined the first b/tsDMARD therapy as the first line of therapy. We then described first‐line therapy selection and the most common patterns of sequential therapies. Most patients received an initial csDMARD therapy before starting their first b/tsDMARD therapy. Given the increase in the number of available DMARDs over the past decade, we also studied changes in these patterns over time. Insights from these analyses give directions for using observational data in evaluating sequential therapies for RA.
MATERIAL AND METHODS
Study design and population
We used data from 42,068 patients enrolled in the CorEvitas RA registry (17), an ongoing longitudinal clinical registry in the US, between January 2012 and December 2021. A total of 1186 patients were excluded because of missing data, such as patient age and dates of therapy changes, resulting in 40,882 patients in a cleaned data set. As of December 31, 2021, data on 57,543 patients with RA were collected in the registry and include 452,967 patient visits and 218,228 patient‐years of follow‐up observation time. The mean duration of patient follow‐up is 4.8 years (median 3.4). In addition to treatment changes and treatment history, the data include, for example, patient demographics, clinical disease characteristics, comorbidities, infections, and adverse events.
The aim of this study was to describe patterns in treatment sequences starting with the first b/tsDMARD therapy. We therefore selected a cohort of b/tsDMARD‐naïve patients who initiated a b/tsDMARD treatment at or after enrollment in the registry. The visit for the first reported b/tsDMARD initiation was considered the baseline visit, and subsequent visits were defined as the follow‐up period. No restrictions, for example, in terms of regularity, were placed on the follow‐up visits, although the registry protocol recommends visits every 6 months per clinical practice. In the selected cohort, 29% of the patients had at least one registry visit every 6‐month period starting with the baseline visit and 75% had at least one registry visit every 12‐month period. Furthermore, each patient was observed until their last recorded visit or the data cut date of December 31, 2021 (whichever occurred first), resulting in the number of registry visits and duration of follow‐up varying across patients.
Classes of drugs and therapies
More than 20 individual drugs were at some point prescribed in the selected data. To limit the number of treatment patterns (see Treatment patterns section), we studied the following classes of drugs rather than individual drugs: csDMARDs (hydroxychloroquine, leflunomide, methotrexate, sulfasalazine, cyclosporine, azathioprine, and minocycline hydrochloride), TNFi (adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, and biosimilar TNFi), interleukin‐6 receptor inhibitors (IL‐6Ri) (sarilumab and tocilizumab), T cell inhibitors (abatacept), B cell inhibitors (rituximab), and Janus kinase inhibitors (JAKi) (baricitinib, tofacitinib, and upadacitinib). Interleukin‐1 receptor inhibitors were excluded because of the small sample size. For the sake of simplicity, we refer to abatacept and rituximab instead of T cell inhibitors and B cell inhibitors hereafter.
Based on these drug classes, we labeled prescribed therapies for each patient in the data. We included both monotherapies and combination therapies with potentially multiple csDMARDs in combination with one b/tsDMARD. We did not distinguish between csDMARD monotherapies and csDMARD‐only combination therapies. For example, both a methotrexate monotherapy and a methotrexate plus leflunomide combination therapy were labeled as csDMARD therapy. Furthermore, we always highlighted the use of a b/tsDMARD. For example, a therapy with csDMARDs in combination with TNFi was considered a TNFi combination therapy, regardless of which drug was added last and which csDMARDs were used. A therapy without any DMARDs was labeled “no DMARD.” We did not categorize combinations of b/tsDMARDs (eg, TNFi in combination with JAKi) because they are not recommended clinically. Such therapies were rare (less than 1% of all prescribed therapies) and were classified as “other.”
In summary, we studied the following classes of therapies: csDMARD therapy, TNFi monotherapy, TNFi combination therapy, IL‐6Ri monotherapy, IL‐6Ri combination therapy, abatacept monotherapy, abatacept combination therapy, rituximab monotherapy, rituximab combination therapy, JAKi monotherapy, JAKi combination therapy, and no DMARD therapy. The initial b/tsDMARD therapy was defined as the first line of therapy.
Treatment patterns
We defined a treatment pattern of length m as a unique sequence consisting of a first‐line therapy and the m − 1 therapy changes following the first‐line therapy. For example, if a patient is given an initial TNFi combination therapy, replacing the TNFi with a JAKi means a change of therapy to a JAKi combination therapy, and stopping all csDMARDs results in a TNFi monotherapy. While a sequence may refer to m arbitrarily consecutive therapies, a pattern is a particular sequence, for example, for m = 3, TNFi combination therapy to TNFi monotherapy to no DMARD. Cycling between drugs within the same drug class, for example, replacing etanercept with adalimumab in a TNFi monotherapy, is not considered a therapy change with our definition of therapy classes. This choice was made for statistical reasons to limit the number of possible treatment patterns. We defined therapy cycling as returning to a previously used therapy class.
For a fixed sequence length m (ie, for a fixed number of m − 1 therapy switches), we collected all patients in the selected cohort who changed therapy at least m − 1 times from baseline and onward. We counted the number of occurrences of each unique therapy sequence (each pattern) and created visualizations of the most common ones. For m = 1 (ie, at baseline), we summarized the most common treatments using bar plots. For a given m ≥ 3, we presented the most frequent patterns in a single figure. To make this visualization as informative as possible, we grouped similar patterns together, and we set the length of each therapy segment to the median duration of that therapy in the data.
We included all types of changes between the therapies listed above. That is, we did not distinguish between actual therapy changes and washout periods. For example, patients switching from a TNFi combination therapy to a JAKi combination therapy via a period of csDMARD‐only therapy were considered to follow the sequence TNFi combination therapy to csDMARD therapy to JAKi combination therapy. In some cases, the period of csDMARD‐only therapy may represent a washout period from the previous TNFi.
Statistical analysis
Patient characteristics at baseline were summarized with descriptive statistics. Categorical variables were summarized using frequency counts and percentages; continuous variables were summarized by median, first quartile, and third quartile.
Selected results were studied from a time perspective. Specifically, we divided the data into three distinct groups based on the date of the baseline visit: 2012–2014, 2015–2017, and 2018–2021. The number of groups was limited to three to ensure that all groups contained a sufficient number of patients, and the intervals were chosen to be almost evenly distributed. Follow‐up visits were allowed to occur beyond the distinct calendar year groupings. When studying the duration of the mth therapy across time periods, we included only patients who were treated with at least m + 1 therapies to avoid censoring. We recognize that this requirement introduced bias because patients who remained on the mth therapy were excluded. However, requiring a minimum follow‐up duration for patients in the cohort would not resolve this issue because patients change therapies at different frequencies. We compared distribution medians using Kruskal–Wallis H tests with a significance level of α = 0.001.
We used Python version 3.10 to conduct the data analyses. The data were processed using Pandas (18) and NumPy (19), figures were created using Matplotlib (20) and Seaborn (21), and statistical tests were performed using SciPy (22).
Data are available from CorEvitas, LLC through a commercial subscription agreement and are not publicly available. No additional data are available from the authors.
Ethics
All participating investigators were required to obtain full board approval for conducting research involving human participants. Sponsor approval and continuing review was obtained through a central institutional review board (IRB) (New England Independent Review Board, NEIRB No. 120160610). For academic investigative sites that did not receive a waiver to use the central IRB, approval was obtained from the respective governing IRBs and documentation of approval was submitted to the sponsor prior to initiating any study procedures. All registry participants were required to provide written informed consent prior to participating.
RESULTS
Baseline characteristics
We identified 6015 unique patients (77% female) who met the study criteria. Of the 40,882 patients in the cleaned data set, 21,507 (53%) had a history of b/tsDMARD usage at the first visit registered in the data, and 13,360 (33%) never started a b/tsDMARD treatment. At baseline, the median age of the selected patients was 58 years, and the median disease duration was 3 years. Additional baseline characteristics are given in Table 1.
Table 1.
Baseline characteristics of patients in the selected cohort
| Characteristic | N | Statistics |
|---|---|---|
| Age, median (IQR), years | 5784 | 58 (49–67) |
| Female, n (%) | 5784 | 4450 (76.9) |
| Race (self‐reported), n (%) | 5719 | |
| White | 4539 (79.4) | |
| Hispanic | 525 (9.2) | |
| Black | 448 (7.8) | |
| Asian | 115 (2.0) | |
| Other | 92 (1.6) | |
| Final education, n (%) | 5577 | |
| Primary school | 149 (2.7) | |
| High school | 2190 (39.3) | |
| College | 3195 (57.3) | |
| Health insurance, n (%) | 5784 | |
| Private | 4089 (70.7) | |
| Medicare | 1874 (32.4) | |
| Medicaid | 382 (6.6) | |
| None | 115 (2.0) | |
| BMI, median (IQR) | 5653 | 29.2 (25.0–34.7) |
| Smoker, n (%) | 4993 | 859 (17.2) |
| Work status (self‐reported), n (%) | 5656 | |
| Full‐time | 2362 (41.8) | |
| Part‐time | 472 (8.3) | |
| Work at home | 491 (8.7) | |
| Student | 74 (1.3) | |
| Disabled | 611 (10.8) | |
| Retired | 1646 (29.1) | |
| Disease duration, median (IQR), years | 5707 | 3 (1–8) |
| RF positive, n (%) | 1427 | 858 (60.1) |
| CCP positive, n (%) | 1372 | 775 (56.5) |
| Comorbidities, n (%) | ||
| History of cardiovascular disease a | 5784 | 378 (6.5) |
| History of cancer b | 5784 | 376 (6.5) |
| Hypertension | 5784 | 832 (14.4) |
| Hyperlipidemia | 5782 | 427 (7.4) |
| Diabetes | 5782 | 316 (5.5) |
| Anxiety | 5772 | 1128 (19.5) |
| Depression | 5322 | 430 (8.1) |
| Serious infections | 5772 | 79 (1.4) |
| Disease activity, median (IQR) | ||
| Tender joint count (0–28) | 5713 | 4 (1–10) |
| Swollen joint count (0–28) | 5714 | 3 (0–8) |
| CDAI | 5676 | 17.0 (8.9–27.2) |
| DAS28 | 3303 | 4.2 (3.0–5.2) |
| Patient self‐assessment | ||
| Pain, median (IQR) | 5760 | 50 (20–70) |
| Fatigue, median (IQR) | 5730 | 50 (20–75) |
| Morning stiffness, n (%) | 5706 | 4845 (85.0) |
| Glucocorticoids | ||
| Prednisone prescribed, n (%) | 5784 | 1787 (30.9) |
| Prednisone dose, median (IQR) | 1740 | 5 (5–10) |
| Prednisone prescribed at first follow‐up visit, n (%) | 4760 | 1140 (23.9) |
| Prednisone dose at first follow‐up visit, median (IQR) | 1137 | 5 (5–10) |
Note: N represents the number of patients with nonmissing data; n represents the number of patients with baseline demographic or clinical characteristic.
Abbreviations: BMI, body mass index; CCP, cyclic citrullinated peptide antibody; CDAI, Clinical Disease Activity Index; DAS28, Disease Activity Score in 28 joints; IQR, interquartile range; RF, rheumatoid factor.
Coronary heart disease, stroke, transient ischemic attack, carotid artery disease, peripheral arterial disease, deep vein thrombosis, pulmonary embolism, heart attack.
Breast cancer, lung cancer, lymphoma, melanoma skin cancer, and any other type of cancer. Nonmelanoma skin cancer is not included.
First‐line therapy selection over the past decade
Figure 1 shows the distribution of the first b/tsDMARD therapy for different time periods in the last decade. Therapies with TNFi dominated, up to 80% of the patients received either a TNFi monotherapy or TNFi in combination with csDMARDs), but the use of JAKi steadily increased. In the most recent interval, almost 20% of the patients were treated with JAKi therapies as their first b/tsDMARD therapy. This change appears concurrent with a reduction of TNFi prescriptions; the use of other therapies remained relatively constant.
Figure 1.
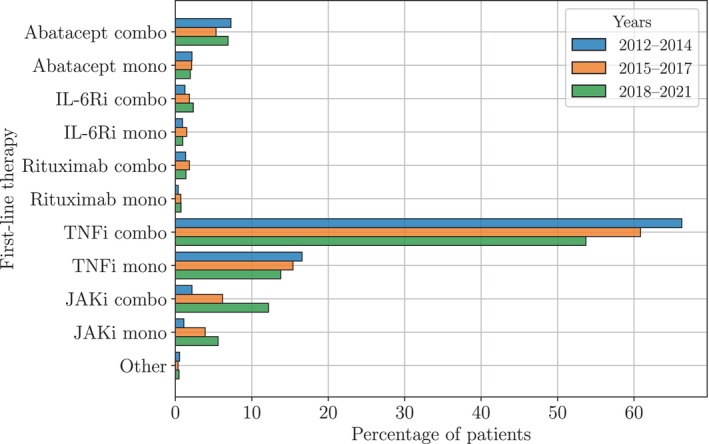
Distribution of first‐line therapies for different time periods in the last decade. Up to 80% of the patients started a TNFi as their first b/tsDMARD either as monotherapy or in combination with csDMARDs. However, in recent years, the use of TNFi decreased, whereas the use of JAKi increased. The use of the other drugs remained relatively constant. Abbreviations: b/tsDMARD, biologic or targeted synthetic disease‐modifying antirheumatic drug; combo, combination therapy; csDMARD, conventional synthetic disease‐modifying antirheumatic drug; IL‐6Ri, interleukin‐6 receptor inhibitor; JAKi, Janus kinase inhibitor; mono, monotherapy; TNFi, tumor necrosis factor inhibitor.
From consensus to heterogeneity
With the aim of describing patterns in current treatment practice, we studied the ratio between the number of patients and the number of observed patterns. Figure 2 shows how this ratio varies with the number of therapies in sequence. For example, all 6015 patients in the selected cohort were treated with at least one therapy, but only one‐sixth of the patients were treated with five therapies or more. The number of observed patterns increases from 11 to 601, approaching the maximum number of patterns. In other words, there were fewer recurring patterns for longer sequences.
Figure 2.
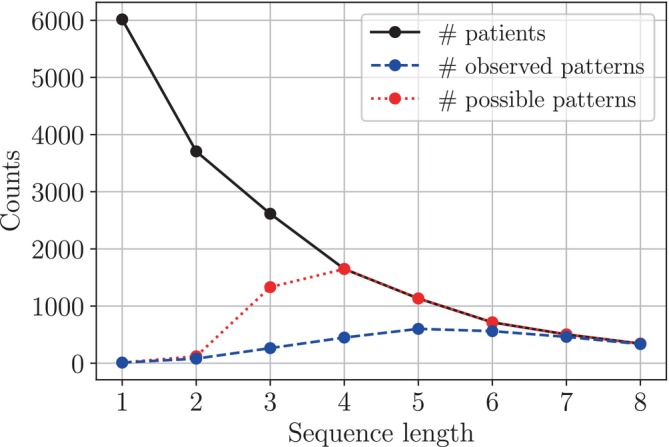
Number of patients and patterns against number of consecutive therapies. The number of patients treated with at least m − 1 therapies following the baseline therapy (solid black line) and the number of observed patterns (dashed blue line) as a function of m, the number of therapies in sequence. The number of possible patterns (dotted red line) is included as a reference. For m = 1, 2, …, 8, it is calculated as the minimum of 11 m and the number of patients, in which 11 is the number of available therapies. Recall that a pattern is defined as a unique therapy sequence.
Patterns in the first three to five lines of therapy
For sequences of six therapies or more, almost all observed sequences were unique (Figure 2). We therefore focused on sequences of lengths three to five, for which there were some recurrent patterns. Figure 3 shows the most common patterns of length three; the most common patterns of lengths four and five are provided in Supplementary Figures 1 and 2. In total, 2615 patients (43% of the patients in the selected cohort) were treated with at least three therapies. In the two main groups of patients starting a TNFi combination therapy and a TNFi monotherapy, TNFi removal was the most common first intervention, leading to a csDMARD‐only and a no DMARD therapy. In the next step, most patients restarted a TNFi, but some switched to another b/tsDMARD, mainly JAKi or abatacept. Specifically, 508 patients (19%) followed the patterns TNFi combination therapy to csDMARD therapy to TNFi combination therapy and TNFi monotherapy to no DMARD to TNFi monotherapy, whereas 361 patients (14%) started in the same way but instead used a non‐TNFi b/tsDMARD therapy in the third line. The main primary reasons for changing DMARDs in the most common patterns are presented in Supplementary Table 1.
Figure 3.
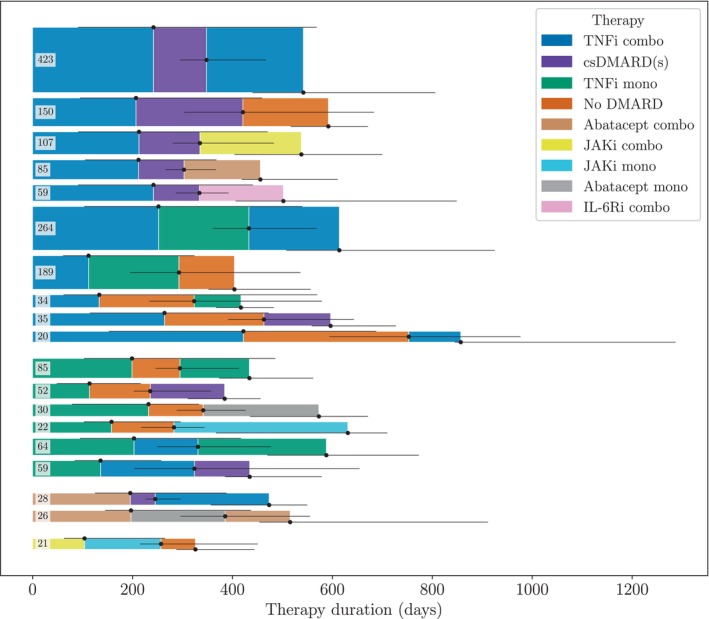
The most common treatment patterns of length three. Some of the patients may have been treated with additional therapies after the third therapy. The numbers indicate how many patients were treated according to each pattern. Only patterns that occurred at least 20 times in the data are shown. The height of the sequences corresponds to the number of observations of each pattern, and the length of the segments corresponds to the median therapy duration in the data. The horizontal black lines show the interquartile range of the therapy durations. Two sequences involving therapies classified as “other” were excluded to enhance readability. Abbreviations: combo, combination therapy; csDMARD, conventional synthetic disease‐modifying antirheumatic drug; csDMARD(s), csDMARD therapy; IL‐6Ri, interleukin‐6 receptor inhibitor; JAKi, Janus kinase inhibitor; mono, monotherapy; TNFi, tumor necrosis factor inhibitor.
The strategy of restarting a therapy from a previously used therapy class was defined as therapy cycling. We observe this pattern not only in Figure 3 but also for longer sequences, that is, for more therapy changes (see Supplementary Figures 1 and 2). Table 2 shows the percentage distribution of patients by the number of restarted therapies and the number of therapies in sequence. For the first three therapies, most patients (59.5%) tried a new medication in each line of therapy. However, for sequences of four and five therapies, the majority of the patients returned to at least one previous treatment during the course of medication. Two‐therapy cyclers were a specific group of returners who switched between only two distinct therapies. We see that 40.5% of the patients restarted their first b/tsDMARD therapy in the third line of therapy. For longer sequences, the percentage of two‐therapy cyclers drops below 20% and 10%, respectively.
Table 2.
Percentage distribution of patients by number of restarted therapies and number of therapies in sequence
| Number of therapies in sequence | Patient distribution over the number of restarted therapies, % | |||
|---|---|---|---|---|
| 0 restarts | 1 restart | 2 restarts | 3 restarts | |
| 3 | 59.5 | 40.5 | ‐ | ‐ |
| 4 | 32.6 | 49.5 | 17.9 | ‐ |
| 5 | 14.7 | 41.7 | 35.4 | 8.2 |
Note: By our definition, the second therapy must be different from the first therapy, so for a sequence of the first three therapies, only one restart is possible. The values in the rightmost nonempty cells indicate the percentage of two‐therapy cyclers.
Therapy duration over time
Figure 1 shows that the distribution of first‐line therapies shifted over time. In Table 3, we present the duration, given in days, of the first three lines of therapy for different time periods in the last decade. We report median therapy duration and interquartile range. As we can see, the median duration of each of the first three therapies decreased during the study period. We also compared the therapy duration distributions within each line of therapy. We found that the difference between the medians of all pairwise distributions for 2015–2017 and 2018–2021 was nonzero with statistical significance (P < 0.001). The test statistics were 49.0, 36.0, and 14.7 for the first, second, and third lines of therapy, respectively.
Table 3.
Duration of the first three lines of therapy for different periods in the last decade
| Line of therapy | Median (interquartile range) therapy duration over time, days | ||
|---|---|---|---|
| 2012–2014 | 2015–2017 | 2018–2021 | |
| 1 | 215 (98–518) | 208 (92–486) | 153 (75–309) |
| 2 | 149 (64–341) | 150 (64–333) | 108 (61–196) |
| 3 | 172 (79–385) | 151 (71–334) | 117 (61–242) |
Note: Median duration and the interquartile range are reported. The duration of each line of therapy decreased during the study period.
DISCUSSION
The goal of this work was to provide an overview of common patterns in the sequential treatment of RA starting with the first b/tsDMARD. Most patients began with a TNFi therapy, although we observed a recent shift toward JAKi therapies over the decade‐long study period (2012–2021). Although the choice of first‐line therapy was near deterministic as a TNFi, there was substantial variation in subsequent treatment selections, leading to many distinct treatment patterns. We identified the most common sequences of up to four therapy changes and found that therapy cycling (restarting a therapy from a previously used therapy class) was a frequent pattern. We also found that the average duration of the first three therapies decreased over the study period. We did not provide data on patient characteristics, effectiveness, disease activity, or adverse events for the observed patterns. The most common patterns are not necessarily the recommended best practices. Nevertheless, identifying frequent patterns in current treatment of RA is an important step toward developing and evaluating new treatment strategies.
Real‐world observational data provides a unique view of patients’ responses to treatments and could be used to identify the effectiveness of different sequences of therapies. However, conducting such a study requires a quantitative understanding of current practice. For example, which patterns do we see often enough to evaluate retrospectively? First, in the current set of analyses, we found that sequences that did not start with an initial TNFi therapy were rare. Evaluating such patterns retrospectively would require very large data sets of patients to arrive at statistically sound results. Second, we found large practice variation in longer therapy sequences, which may be exploited to identify successful strategies that deviate from current guidelines. By providing an overview of current practice, this study takes a first step in advancing RA treatment. The next steps include identifying strategies for sequential treatment with sufficient support in observed data, estimating the historical propensity for following these strategies, and adjusting for selection bias to compare their value over current guidelines.
Patients with difficult‐to‐treat RA (23) often undergo multiple therapies in search of a working medication. Acquiring a better knowledge of successful therapy sequences would be particularly beneficial for this group of patients. Recent work has indicated that many nonresponders eventually benefit from a fourth‐line therapy (14), but there is little evidence of which of these “extended” sequences may work better than others. We suspect that these patients account for many of the observed patterns in this work. However, most of these patterns are not shown in our sequence visualizations in Figure 3 because they are too rare. To draw conclusions about patients with difficult‐to‐treat RA and evaluate different treatment strategies for these patients, one would need to make additional assumptions and group similar sequences together. For example, patients who have tried the same set of therapies, with similar responses, may be comparable even if the order in which they tried the therapies differs.
A finding of our work is that the use of JAKi as first‐line therapy increased in recent years. This trend can partially be explained by the increasing availability of these drugs. However, the use of JAKi after 2021 may have been affected by the results from the ORAL Surveillance trial (24), which have caused the US Food and Drug Administration to update its recommendations for JAKi use (25).
Another finding is that the duration of the three initial therapies decreased in the last decade. A possible explanation for this result is that the number of available treatment options greatly increased during this time period. An alternative explanation could be that within‐class cycling (ie, switching between drugs with the same mechanism of action) was more common in the past. However, when studying the number of within‐class switches for patients who started with a TNFi combination therapy, we found no clear support for that theory. Finally, the number of outliers (eg, patients who stayed on their first therapy for several years before they suddenly changed therapy) is naturally higher in the first interval and skews those distributions.
The trend of decreasing therapy duration was observed also in recent work by Mease et al (26), although they used data of patients enrolled in the CorEvitas RA registry between 2004 and 2015 and studied a smaller set of therapies. There exist some studies that have tried to describe sequential therapies using Sankey diagrams (13, 14). Our sequence visualizations contain more information in the sense that they show entire therapy trajectories and not only transitions between consecutive therapies. Still, it is worth noting that Zhao et al (14) identified the transition between TNFi and rituximab as the second most common transition between the first two lines of treatment. In contrast, there are no sequences starting in this way in Figure 3. This may reflect differences in typical care by country (UK vs US). Another notable difference between the US and other countries lies in the use of IL‐6Ri. In Europe and Japan, it has been reported that 11% and 22% of the patients, respectively, use tocilizumab as their first b/tsDMARD therapy (5). We found that only 3% of the patients used IL‐6Ri as their first‐line therapy (see Figure 1), indicating that these agents may be underused in the US.
The primary strengths of this study are the focus on sequential therapies and the use of a large real‐world data set from the CorEvitas RA registry. There are also limitations, for example, that we did not place any restrictions on the regularity of the registry visits and that we did not take special account of washout periods. As well, the median duration of follow‐up in the CorEvitas RA registry is 3.4 years, which limits the amount of data for patients treated with many sequential therapies. We expect the variation in patterns to be even greater with increased duration of follow‐up. Because the CorEvitas RA registry only includes patients from the US, it should be noted that the findings of this work reflect RA management in the US and not necessarily other countries. Further, biologic data were not included in the current analyses, limiting the ability to understand the pathobiology of difficult‐to‐treat RA. We also did not study the sequential use of glucocorticoids because they are used intermittently at variable doses and are often not accurately reported.
The fact that we did not distinguish between individual drugs is another limitation of our work. We chose this approach to reduce the number of therapy permutations and reinforce patterns of b/tsDMARD treatment strategies, our main focus. For the same reason, we did not consider switching between drugs from the same class as a change of therapy, preventing such transitions from appearing in our patterns. Finally, we included only subsequences starting with the first b/tsDMARD prescription in our analysis. Most patients were treated with an initial csDMARD therapy before starting their first b/tsDMARD, and not distinguishing patients based on this information may be considered a limitation. Understanding the effects of early exploration on the therapy decisions that follow is an important challenge for future work.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Matsson, Solomon, Crabtree, Harrison, Litman, Johansson.
Analysis and interpretation of data
Matsson, Solomon, Crabtree, Harrison, Litman, Johansson.
ROLE OF THE STUDY SPONSOR
This study was sponsored by CorEvitas, LLC. CorEvitas has been supported through contracted subscriptions in the past 2 years by AbbVie, Amgen, Inc., Arena, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai, Eli Lilly and Company, Genentech, Gilead Sciences, Inc., GlaxoSmithKline, Janssen Pharmaceuticals, Inc., LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Inc., Regeneron Pharmaceuticals, Inc., Sanofi, Sun Pharmaceutical Industries Ltd., and UCB S.A. CorEvitas biostatisticians contributed to the study design, analysis and interpretation of the data, and in the review and approval of the publication.
ADDITIONAL DISCLOSURES
Authors Crabtree and Litman are employees of CorEvitas, LLC. Author Harrison is currently an employee of Biogen Inc., but was employed by CorEvitas, LLC during the time the study was conducted. The Wallenberg AI, Autonomous Systems and Software Program acts as external funder of the research lab at Chalmers University of Technology led by author Johansson, of which author Matsson is a member.
Supporting information
Disclosure form
Appendix: Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank all the participating providers and patients in the CorEvitas RA registry who contributed data for this study.
Supported by CorEvitas, LLC and the NIH (grant P30‐AR‐072577 to Dr. Solomon). CorEvitas has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, Inc., Arena, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai, Eli Lilly and Company, Genentech, Gilead Sciences, Inc., GlaxoSmithKline, Janssen Pharmaceuticals, Inc., LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Inc., Regeneron Pharmaceuticals, Inc., Sanofi, Sun Pharmaceutical Industries Ltd., and UCB S.A. Partially supported by the Wallenberg AI, Autonomous Systems and Software Program, funded by the Knut and Alice Wallenberg Foundation.
Additional supplementary information cited in this article can be found online in the Supporting Information section (https://onlinelibrary.wiley.com/doi/10.1002/acr2.11621).
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11621.
REFERENCES
- 1. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2021;73:924–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smolen JS, Landewé RB, Bijlsma JW, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 3. Mian A, Ibrahim F, Scott DL. A systematic review of guidelines for managing rheumatoid arthritis. BMC Rheumatol 2019;3:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sullivan E, Kershaw J, Blackburn S, et al. Biologic disease‐modifying antirheumatic drug prescription patterns for rheumatoid arthritis among United States physicians. Rheumatology and Therapy. 2020;7:383–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sullivan E, Kershaw J, Blackburn S, et al. Biologic disease‐modifying antirheumatic drug prescription patterns among rheumatologists in Europe and Japan. Rheumatol Ther 2020;7:517–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenbaum PR. Design of observational studies. New York: Springer New York; 2010. [Google Scholar]
- 7. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 8. Salliot C, Finckh A, Katchamart W, et al. Indirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease‐modifying antirheumatic drugs or to an anti‐tumour necrosis factor agent: a meta‐analysis. Ann Rheum Dis 2011;70:266–71. [DOI] [PubMed] [Google Scholar]
- 9. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis 2009;68:216–21. [DOI] [PubMed] [Google Scholar]
- 10. Bonafede MM, Curtis JR, McMorrow D, et al. Treatment effectiveness and treatment patterns among rheumatoid arthritis patients after switching from a tumor necrosis factor inhibitor to another medication. Clinicoecon Outcomes Res 2016;8:707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park DJ, Choi SE, Kang JH, et al. Comparison of the efficacy and risk of discontinuation between non‐TNF‐targeted treatment and a second TNF inhibitor in patients with rheumatoid arthritis after first TNF inhibitor failure. Ther Adv Musculoskelet Dis 2022;14:1759720X221091450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei W, Knapp K, Wang L, et al. Treatment persistence and clinical outcomes of tumor necrosis factor inhibitor cycling or switching to a new mechanism of action therapy: real‐world observational study of rheumatoid arthritis patients in the United States with prior tumor necrosis factor inhibitor therapy. Adv Ther 2017;34:1936–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fletcher A, Lassere M, March L, et al. Patterns of biologic and targeted‐synthetic disease‐modifying antirheumatic drug use in rheumatoid arthritis in Australia. Rheumatology (Oxford) 2022;61:3939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao SS, Kearsley‐Fleet L, Bosworth A, et al. Effectiveness of sequential biologic and targeted disease modifying anti‐rheumatic drugs for rheumatoid arthritis. Rheumatology (Oxford) 2022;61:4678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Solomon DH, Xu C, Collins J, et al. The sequence of disease‐modifying anti‐rheumatic drugs: pathways to and predictors of tocilizumab monotherapy. Arthritis Res Ther 2021;23:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perrone V, Losi S, Rogai V, et al. Real‐world analysis of therapeutic patterns in patients affected by rheumatoid arthritis in Italy: a focus on baricitinib. Rheumatol Ther 2020;7:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kremer J. The CORRONA database. Ann Rheum Dis 2005;64 Suppl 4:iv37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McKinney W. Data structures for statistical computing in Python. In: van der Walt S, Millman J, editors. Proceedings of the 9th Python in Science Conference; 2010 Jun 28–Jul 3; Austin, TX. 2010. p. 56–61. [Google Scholar]
- 19. Harris CR, Millman KJ, van der Walt SJ, et al. Array programming with NumPy. Nature 2020;585:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunter JD. Matplotlib: a 2D graphics environment. Comput Sci Eng 2007;9:90–5. [Google Scholar]
- 21. Waskom ML. seaborn: statistical data visualization. J Open Source Softw 2021;6:3021. [Google Scholar]
- 22. Virtanen P, Gommers R, Oliphant TE, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nature Methods 2020;17:261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagy G, Roodenrijs NM, Welsing PM, et al. EULAR definition of difficult‐to‐treat rheumatoid arthritis. Ann Rheum Dis 2021;80:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. [DOI] [PubMed] [Google Scholar]
- 25. Winthrop KL, Cohen SB. Oral surveillance and JAK inhibitor safety: the theory of relativity. Nat Rev Rheumatol 2022;18:301–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mease PJ, Stryker S, Liu M, et al. Treatment patterns in rheumatoid arthritis patients newly initiated on biologic and conventional synthetic disease‐modifying antirheumatic drug therapy and enrolled in a North American clinical registry. Arthritis Res Ther 2021;23:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure form
Appendix: Supplementary Material


