Abstract
Objective:
Numerous studies have consistently found that reduced SMN protein expression does not severely affect cognitive function in SMA patients. However, the average intelligence quotient of SMA patients has ranged above to below average in different studies. The cognitive development of SMA patients identified through newborn screening remains largely unknown.
Methods:
40 of 47 eligible SMA patients (23 females/17 males) from 39 families identified through newborn screening between January 2018 and December 2020 underwent developmental testing using Bayley III (BSID) after the 2 years of age. The mean age was 29.25 months (23–42 months). 17 patients had 2, 11 patients had 3 and 12 patients had ≥4 copies of SMN2.
Results:
cognitive scale: mean 94.55 (SD 24.01); language scale: mean 86.09 (SD 26.41); motor scale: 81.28 (SD 28.07). Overall, the cognitive scales show that 14 children were below average, 20 children were average and 6 children were above average. 10/14 children with below average scores had 2 SMN2 copies. The post-hoc pairwise comparisons showed that the cognition main scale was significantly more sensitive to the number of SMN2 copies than the motor main scale of the BSID (MΔ= 10.27, p = 0.014). There is also evidence that cognition scored higher than the language main scale (MΔ= 7.11, p = 0.090).
Conclusion:
The impaired cognitive development of SMA children with 2 SMN2 copies, despite early initiation of therapy, underscores the critical role of the SMN protein in the early stages of brain development.
Keywords: Cognitive development, SMN protein, newborn screening, spinal muscular atrophy, BSID-III
INTRODUCTION
5q-Spinal muscular atrophy (SMA) is the most common neurodegenerative disease in childhood and the second most common autosomal recessive disorder with an incidence rate of 1:6,000 to 1:11,000. Homozygous deletion of the “survival motor neuron” (SMN) gene, SMN1, is the main cause [36]. Motor neurons are fundamentally dependent on the SMN1 gene product, the SMN protein. SMN is differentially expressed during development, found in all cell types and is critical for survival [5]. SMN is also thought to play a developmental role in different regions of the forebrain [4]. Neuropathological changes in brainstem nuclei, pigmented nuclei, thalami, basal ganglia, hippocampi and cerebellum have been reported in SMA type 1 patients, while different abnormalities are reported in neuropathological studies in SMA type 2 patients [2, 12, 17] and mouse model studies [37]. This suggests that SMN plays an important role in non-motor regions of the nervous system [7]. There is a wide phenotypic spectrum of the disease, ranging from early-onset forms that are fatal in infancy if untreated, to later-onset forms that may not present until adulthood. SMN2, a paralogous gene that is almost identical to SMN1 but encodes a less stable form of the SMN protein, is the most important disease modifier. Milder phenotypes are associated with higher SMN2 copy numbers [35]. Historically, SMA was classified into three pediatric forms (SMA types I-III) based on the age of onset of symptoms and best motor function. Newly available therapies, specifically SMN2 splicing modifiers [10, 20] and gene replacement therapy, have dramatically changed survival and overall disease progression [19]. With an increasing number of long-term survivors of SMA, it has become obvious that treated children exhibit new phenotypes that include changes in motor function and other areas of functionality, including cognition and language development.
Newborn screening (NBS) programs aim to achieve a pre-symptomatic diagnosis of treatable disorders allowing for early initiation of treatment, and thus prevention or reduction of morbidity and mortality. Pilot projects on NBS for SMA starting in January 2018 in Germany showed better motor outcomes in early-treated patients with expected severe forms of the disease [32]. Consequently, NBS for SMA was implemented in the nationwide German NBS program in October 2021 [21].
Existing data show that a reduction of SMN-protein does not have a severe effect on the development of cognitive function in adults with SMA. Nevertheless, the mean intelligence quotient (IQ) in SMA patients has been contradictorily described as above [33] or below average compared to healthy subjects [15, 17, 28].
Cognitive development in SMA patients detected by NBS remains widely unexplored. In particular, the cognitive development of SMA type 1 patients, who rarely survived if untreated, is unknown. It is unclear whether a lack of SMN protein during pregnancy has an impact on the cognitive functions of the children. To investigate this, cognitive testing was done in a cohort of patients diagnosed by SMA-NBS, in which SMN was restored shortly after birth.
MATERIALS AND METHODS
Study protocol
SMA patients that were diagnosed by NBS in the German pilot projects and that were born between January 15, 2018 and December 31, 2020 were included. Homozygous SMN1 deletion and number of SMN2 copies was confirmed in all patients by a second genetic test [22, 32].
Treatment recommendations during the first two years of the pilot project were equal to the recommendations of the 2018 “American SMA-NBS Multidisciplinary Working Group” [11]: Immediate treatment with Nusinersen was recommended for children with two or three SMN2 copies and a “watchful waiting” strategy for children with≥four copies. Treatment by that time was initiated solely with Nusinersen, as Onasemnogene Abeparvovec was not available in Germany until 2021.
The neurological development was assessed by the Bayley Scales of Infant and Toddler Development-III (BSID) scale. BSID was started 24 months after NBS at the earliest by trained colleagues in each of the participating centers.
Study group
A total of 47 SMA patients from 46 families identified through newborn screening between January 2018 and December 2020 were contacted for developmental testing at participating centers beginning on the child’s second birthday. Seven families refused developmental testing and/or an additional appointment to the clinic to be screened. An apparent reason was the Covid pandemic and parents’ concern about exposing themselves or their child to an additional risk of infection.
The Bayley Scales of Infant and Toddler Development–Third Edition (BSID-III)
Bayley III is a revision of the frequently used and well-known Bayley Scales of Infant Development–Second Edition (BSID-II; Bayley, 1993) including Cognitive, Language, Motor, Social-Emotional, and Adaptive Behavior scales [1]. The following five scales are assessed with the German Bayley-III: Cognition, receptive language, expressive language, motor skills fine, motor skills coarse. In the Bayley III, the tasks, whose difficulty increases continuously, are given to the child in a predetermined order for each of the five subtests. Scores below 85 are classified as below average, between 85 and 115 as average, and greater than or equal 116 as above average.
Statistical analysis
A chi-square test was performed to analyse the distribution of females and males of this sub-sample among the groups of patients that have two, three or four copies. Gender effects were evaluated by a two-sided t-test for independent groups for the variables that are used in the following analyses. ANCOVA was performed to measure a general effect of the copy number on the main scale scores.
RESULTS
Characteristics of infantile SMA patients
Forty patients could be included with a mean age at the time of examination of 29.25 months (SD = 4.86), ranging from a minimum age of 23 to a maximum age of 42 months. In our cohort 42.5% (17 patients) had two SMN2 copies, 27.5% (11 patients) had three SMN2 copies, and 30% (12 patients) had≥four SMN2 copies. Of all subjects, 57.5% (23) were female and 42.5% (17) were male. Thirty-six patients were treated exclusively with nusinersen or switched to onasemnogene abeparvovec at the time of testing. The reasons for switching therapy varied. Four children, two with four SMN2 and two with five SMN2 copies were without medication at the time of testing, in agreement with the parents.
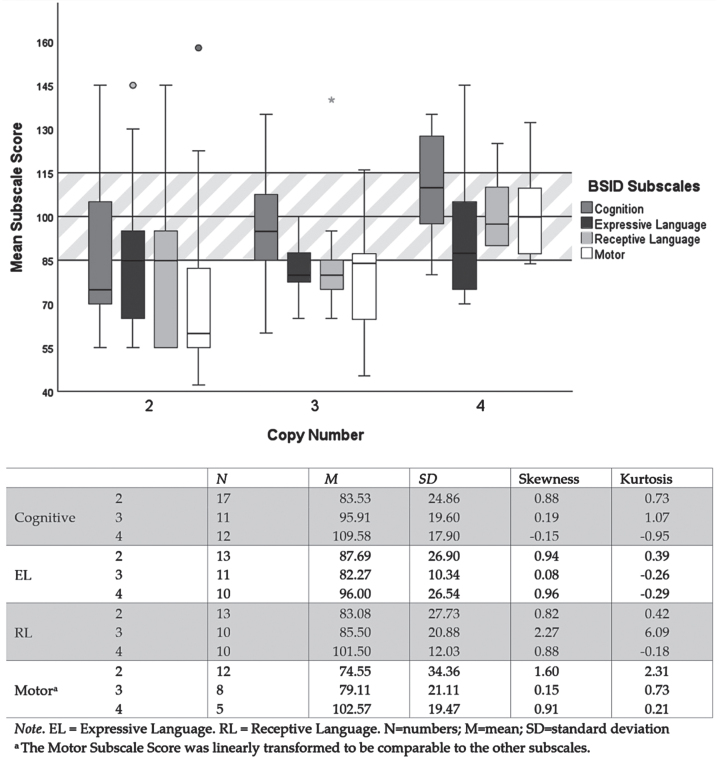
Fig. 1.
Descriptive analysis regarding the scale scores by scale type and copy number. The mean values of the three main scales of the BSID are indicated on the y-axis by the different shades of gray. These mean values are normally distributed (M = 100, SD = 15). On the x-axis, the groups of patients with 2 to > 4 copies are indicated.
Thirty-four of 40 children were asymptomatic at the start of treatment and four with 2 SMN2 copies of 40 showed signs of disease onset at start of the treatment during the first weeks of life. Treatment at 11 months of age was initiated in two (twins) with three SMN2 copies of 40 patients at the explicit request of the parents after the children had developed clear symptoms of the disease. At the time of testing, 34/40 patients could walk independently and were able to climb some stairs. Four out of 40 could sit independently, one out of 40 reached the milestone of head control and can sit with support, and one out of 40 did not reach a motor milestone but can also sit with support. One patient required additional feeding via a PEG tube. Two patients had intermittent nocturnal non-invasive ventilation.
Bayley scales
At the age of 24 months, not all children are able to complete the Bayley test in one session. As we did not want to overburden the parents, we focused on the cognitive scales at the appointment and then performed all the other scales as far as the children were able to do them well, so that a reliable evaluation is possible. There was no negative influence of motor deficits on the test results.
Cognition Scale: A score below average was found in 14 of 40 children tested, 20 of 40 showed an average score and six of 40 showed an above average score. Ten of 14 with a below average score have two SMN2 copies.
Motor Scale: Fourteen of 25 of children showed a below average score in the motor scale, seven of 25 showed an average score and four of 25 showed an above average score. Nine of 14 with a below average score have two SMN2 copies.
Language scale: Twelve of 33 children showed a below average score in the receptive language subscale, 16 of 33 showed an average score and five of 33 showed an above average score. SMN2 copy numbers are evenly distributed in all domains. Eleven of 34 of the tested children showed a below average score in the expressive language subscale, 13 of 34 showed an average score and four of 34 showed an above average score. SMN2 copy numbers are evenly distributed in all domains.
In Table 2, the Pearson Correlation Analysis shows substantial inter-correlations between the scales of the BSID, as expected based on the basic structure of the BSID. The correlations between the copy number and the scales of the BSID only showed a substantial correlation with the cognitive scale of the BSID.
Table 2.
Pearson Correlation Analysis between the main scale scores of the BSID, including the copy number
| BSID Main Scale Scores | ||||
| CN | Cognitive | Language | Motor | |
| CN | – | |||
| Cognitive | 0.48** (0.002) | – | ||
| Language | 0.23 (0.202) | 0.73*** | – | |
| Motor | 0.30 (0.143) | 0.86*** | 0.83*** | – |
Note. CN = Copy Number. P-values above 0.001 are shown in brackets below. Pairwise correlations are listed below the diagonal. aSignificant after adjusting the p-values by the sample size. **p < 0.01 ***p < 0.001.
Table 1.
Gender and BSID subscales. Analysis for Gender Differences among the Main Scale and Subscale Scores of the BSID: In each main scale and subscale of the BSID, females showed a significantly better performance than males (Table1), particularly pronounced in the motor scales.
| t | df | p | d | Mfemale | Mmale | ||
| Cognitive | Main Scale | 2.09 | 38 | 0.044* | 0.67 | 101.09 | 85.71 |
| Subscale | 2.20 | 38 | 0.034* | 0.70 | 101.50 | 85.60 | |
| Language | Main Scale | 2.80 | 31 | 0.009** | 0.98 | 96.11 | 72.50 |
| ES | 2.72 | 32 | 0.011* | 0.95 | 96.50 | 76.80 | |
| RS | 2.85 | 31 | 0.008** | 1.00 | 98.15 | 77.50 | |
| Motor | Main Scale | 3.74 | 23 | 0.001** | 1.50 | 97.54 | 63.67 |
| Subscalea | 3.17 | 23 | 0.004** | 1.27 | 96.80 | 65.20 |
RS = Receptive Subscale. ES = Expressive Subscale; aThe Motor Subscale Score of the BSID was linearly transformed to be comparable with the other subscale scores. *p < 0.05 **p < 0.01.
To test whether the gender of the participants had a larger effect on cognitive development, we performed an ANCOVA. The result showed that the main effect of SMN2 copy number remained. An in-depth statistical analysis of the results of the three main scales in relation to the SMN2 copies showed that the cognition main scale scored significantly higher than the motor main scale of the BSID (MΔ= 10.27, p = 0.014). Additionally, there is an indication that cognition scored higher than the language main scale (MΔ= 7.11, p = 0.090). The effect of SMN2 copy number on the language main scale score of the BSID is not statistically significant.
DISCUSSION
The results show that cognitive development of children with SMA detected by newborn screening depends on SMN2 copy number. Fourteen of 40 (35%) participants showed a below average score in the cognitive scale, ten of 14 (71%) have two SMN2 copies. The lower the SMN2 copy number, the lower the cognitive development score determined by BSID. This effect on cognitive development is present even though all children with two SMN2 copies were treated in the first weeks of life. In total, 34 of 40 children were treated in the first weeks of life, in which the need for SMN protein is particularly high in the developing nervous system [3, 13]. Cognitive impairment in presymptomatic SMA patients with a copy number≤two may be the result of brain hypoperfusion [38] or low expression of SMN protein in cortical neurons [12], which cannot be fully corrected by current disease-modifying treatments started postnatally. This is supported by the fact that during the third trimester of pregnancy, the brain undergoes important maturational development [18]. SMN protein plays a major role during early development. Levels in humans have been shown to decrease during development in spinal cord and brain tissue, with a substantial decline between fetal and postnatal stages, reaching a nadir after three months of age [26]. All children with less than three SMN2 copies were initially treated with Nusinersen intrathecally according to a standardized procedure. The difference between the groups (two vs three or more SMN2 copies) may either indicate that the amount of SMN-protein available in the CNS is lower in patients with a lower SMN2-copy number, even when treated with Nusinersen, or that the prenatal SMN-protein deficiency is responsible. A significant difference in the physical interaction with their surroundings, which is important for normal cognitive development [29], is unlikely in our patients, as they generally have good motor performance and can therefore interact normally with their environment.
A recent study of 22 adult patients with SMA type III showed that greater motor difficulties were associated with poorer performance in attention and working memory and better performance in language, visuospatial skills and memory. The authors concluded that the cognitive changes in SMA type III may reflect the presence of intrinsic brain pathology and the capacity of cognitive adaptive mechanisms in the presence of physical dysfunction. In men, but not women, performance on cognitive tests was associated with motor function [16]. This shows that despite normal intelligence in patients with SMA type III, a detailed analysis of the subtests reveals an impairment that can be compensated through good motor functions. With regard to our patient group, it must be noted that the BSID is a pure developmental test, therefore statements on the specific cognitive subscales for our patients can only be made at a later stage after an intelligence test has been performed. In addition, it was shown that in severe SMA mouse models, changes in the developmental processes of the brain are associated with low SMN levels [37].
These findings and our results of cognitive development in children with two SMN2 copies, despite early initiation of therapy, highlight the crucial role of SMN protein in the early stages of brain development.
There is conflicting information in the literature regarding cognition in SMA patients. In 1967, Dubowitz was the first to postulate normal intelligence in children with SMA [8]. Another study in 1987 found no difference in cognitive abilities between children with SMA and children with DMD [34]. Since then, several studies about cognitive development have been published. In symptomatic patients with SMA type 1 treated with disease-modifying therapies, global developmental delay and defects in gross motor functions have been found in the majority, whereas scores obtained on scales to assess learning and language skills suggest a positive trend in the development of general neurocognitive skills [31]. Furthermore, in a study of 18 children with SMA, four children were identified with BSID III score below average on cognitive scales [23].
All of these findings are consistent with the results of our study, which was conducted in a larger group of older children (≥ two years-of-age) who were predominantly treated pre-symptomatically, and thus, had more time to benefit from treatment. Furthermore, these findings are similar to phenomena already known from other neuromuscular diseases. Although there is no common pathomechanism, from a clinical point of view there is similarity in presentation. The severe courses with congenital onset are often multisystem disorders as in Pompe disease, MTM1 or myotonic dystrophy type 1 and then also associated with cognitive impairment. While the adult and adolescent patients usually have a mild phenotype with a more purely neuromuscular phenotype [9].
Our study shows that female patients performed better than male patients. This is an unexpected finding. The influence of gender on the phenotype of SMA has been debated. Some studies have reported that the symptoms of female siblings in affected SMA families were milder than those of male siblings due to plastin 3 as a modifier [24, 30]. Studies have also reported that female patients with SMA type III predominated [6]. In other studies, a predominance of male patients was observed with milder forms of SMA [14]. Results from a larger group will be necessary to confirm these findings.
The evaluation of the BSID showed that the children predominantly show a normal development in the main scales of BSID with outliers upwards and downwards. The mean scores demonstrate a clear dependence of cognitive development on the SMN2 copies, whereas the other scales do not show this effect so clearly.
When assessing the motor scales, it should be kept in mind that 36 of 40 children were treated with different disease-modifying therapies at the time of testing. Another important point when looking at the results of the children’s motor performance is that we only tested 25 children with the motor Bayley Scale. The children who were already able to master the Hammersmith functional motor Scales extended (HFMSE) at the age of two years of life were tested with the HFMSE. These results have already been published [32]. Unfortunately, the two tests are not comparable, so we did not conduct a combined evaluation.
The analysis of the children’s language development in our study showed no dependence on SMN2 copy number and an overall normal development. The literature suggests that symptomatic children with spinal muscular atrophy (SMA) show precocious grammar development and spatial cognition, suggesting that they devote their resources to developing their language skills and observing their environment because their motor impairments limit their ability to explore the world [25]. In our patients, who mostly have a normal motor development, a similar shift towards grammar development was not observed.
In summary, our findings indicate important aspects of cognitive development still unknown in SMA patients. This includes the impact of new pharmacological treatments on cognitive and language functions and the role of regained motor skills on brain development. In the future, these aspects may become increasingly important regarding the choice between different drugs and in the planning of personalized rehabilitation programs, especially in SMA children with neuromuscular dysfunction already in the first weeks of life despite drug treatment and who also have a developmental disorder. Fetal treatment has been assessed in mice and should be considered in humans to prevent the precocious loss of the SMN protein [27].
Our findings support the underlying hypothesis and emphasize the need for (1) early initiation of medical treatment, possibly even intrauterine, especially when less than two SMN2 copies are present, (2) performance of standardized longitudinal brain function assessments, especially in children expected to develop SMA type 1, and (3) initiation of early intervention programmes for children identified by newborn screening.
Limitations of the study
The study group of 40 children is very small, to perform a reliable statistical analysis between all subgroups. Furthermore, other effects, such as drug regimen, start of treatment, and other supportive measures like family environment support, could not be taken into account. The BISC is a developmental test in which all three scales are strongly dependent on each other, so the results can still indicate changes especially in the first years of life. From an age of >24 months, the cognitive scale approaches a real intelligence test, and is, therefore, generally used as a predictor for preschool testing in the follow-up of premature and at-risk babies. However, these children should be retested at the age of five years to provide a valid statement about their cognitive development.
AUTHOR CONTRIBUTIONS
Heike Kölbel, Wolfgang Müller-Felber and Oliver Schwartz conceptualized and designed the study, collected questionnaires and clinical data, drafted the initial manuscript, and reviewed and revised the manuscript. Katharina Vill conceptualized and designed the study, collected questionnaires and clinical data and reviewed the manuscript. Laura Modler and Marius Kopka conducted the analysis and reviewed the manuscript. Astrid Blaschek and Ulrike Schara-Schmidt collected clinical data and reviewed the manuscript. All authors have approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
FUNDING
The Cystinosis Foundation funded the SMA NBS pilot project between January 2018 and May 2019. We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
INSTITUTIONAL REVIEW BOARD STATEMENT
The local ethics committee of the participating universities (project no. 18-269) has approved the study (University Duisburg-Essen: 19-8698-BO). Written informed consent for all diagnostic steps as well as the permission to publish clinical data were obtained from both parents.
INFORMED CONSENT STATEMENT
Written informed consent for all diagnostic steps as well as the permission to publish clinical data were obtained from both parents.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
ACKNOWLEDGMENTS
The Cystinosis Foundation initiated, designed and conducted the pilot project for genetic newborn screening for SMA and cystinosis in Germany in 2017. Within this pilot project (January 2018 to May 2019) 200.901 newborns were tested and a total of 29 newborns with a homozygous deletion in the SMN1 gene were identified [12]. The Cystinosis Foundation had no impact on the interpretation and publication of the clinical data. The authors thank all the parents of the infants who participated in this study; they also thank the study nurse Britta Holtkamp and the Cystinosis Foundation for their initial support.
All of the authors of this publication are members of the European Reference Network for Neuromuscular Diseases - Project ID N° 870177.
CONFLICTS OF INTEREST
HK is serving on a scientific advisory board for Avexis and received travel expenses and speaker honoraria from Biogen, Pfizer, Roche, and Sanofi-Aventis. KV received travel expenses and speaker honoraria from Biogen and Santhera. LM and MK has nothing to disclose. AB received speaker honoraria from Roche, Novartis Gene Therapies (formerly Avexis), Novartis, and Sanofi Genzyme. USS is serving on a scientific advisory board or data safety monitoring board for Biogen, Avexis, and Novartis and received speaker honoraria from Biogen, Avexis, PTC, and Sanofi-Aventis. OS is serving on a scientific advisory board for Avexis and received travel expenses and speaker honoraria from Biogen.WMF is serving on a scientific advisory board for Biogen, Avexis, PTC, Sanofi-Aventis, Roche, and Cytokinetics and received travel expenses and speaker honoraria from Biogen, Avexis, PTC, and Sanofi-Aventis.
REFERENCES
- [1]. Albers CA, Grieve AJ 2007;Test Review: Bayley, N. (2006). Bayley Scales of Infant and Toddler Development–Third Edition. San Antonio, TX: Harcourt Assessment. Journal of Psychoeducational Assessment 25:180–90. DOI: 10.1177/0734282906297199. [DOI] [Google Scholar]
- [2]. Araki S, Hayashi M, Tamagawa K, Saito M, Kato S, Komori T, Sakakihara Y, Mizutani T, Oda M. Neuropathological analysis in spinal muscular atrophy type II. Acta Neuropathol 2003;106:441–8. DOI: 10.1007/s00401-003-0743-9. [DOI] [PubMed] [Google Scholar]
- [3]. Bowerman M, Becker CG, Yanez-Munoz RJ, Ning K, Wood MJA, Gillingwater TH, Talbot K. Therapeutic strategies for spinal muscular atrophy: SMN and beyond. Disease Models & Mechanisms 2017;10: 943–54. DOI: 10.1242/dmm.030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Briese M, Richter DU, Sattelle DB, Ulfig N. SMN, the product of the spinal muscular atrophy-determining gene, is expressed widely but selectively in the developing human forebrain. J Comp Neurol 2006;497, 808–16. DOI: 10.1002/cne.21010. [DOI] [PubMed] [Google Scholar]
- [5]. Burlet P, Huber C, Bertrandy S, Ludosky MA, Zwaenepoel I, Clermont O, Roume J, Delezoide AL, Cartaud J, Munnich A, et al. The distribution of SMN protein complex in human fetal tissues and its alteration in spinal muscular atrophy. Human Molecular Genetics 1998;7:1927–33. DOI: 10.1093/hmg/7.12.1927. [DOI] [PubMed] [Google Scholar]
- [6]. Chung BH, Wong VC, Ip P. Spinal muscular atrophy: Survival pattern and functional status. Pediatrics 2004;114:e548–53. DOI: 10.1542/peds.2004-0668. [DOI] [PubMed] [Google Scholar]
- [7]. Detering NT, Zambon A, Hensel N, Kothary R, Swoboda K, Gillingwater TH, Baranello G. 264th ENMC International Workshop: Multi-system involvement in spinal muscular atrophy Hoofddorp, the Netherlands, November 19th - 21st 2021. Neuromuscular Disorders: NMD 2022;32:697–705. DOI: 10.1016/j.nmd.2022.06.005. [DOI] [PubMed] [Google Scholar]
- [8]. Dubowitz V Infantile muscular atrophy–a broad spectrum. Clin Proc Child Hosp Dist Columbia 1967;23:223–39. [PubMed] [Google Scholar]
- [9]. Ebbink BJ, Aarsen FK, van Gelder CM, van den Hout JM, Weisglas-Kuperus N, Jaeken J, Lequin MH, Arts WF, van der Ploeg AT. Cognitive outcome of patients with classic infantile Pompe disease receiving enzyme therapy. Neurology 2012;78:1512–8. DOI: 10.1212/WNL.0b013e3182553c11. [DOI] [PubMed] [Google Scholar]
- [10]. Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, Chiriboga CA, Saito K, Servais L, Tizzano E, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. The New England Journal of Medicine 2017;377:1723–32. DOI: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- [11]. Glascock J, Sampson J, Haidet-Phillips A, Connolly A, Darras B, Day J, Finkel R, Howell RR, Klinger K, Kuntz N, et al. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. Journal of Neuromuscular Diseases 2018;5:145–58. DOI: 10.3233/jnd-180304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Harding BN, Kariya S, Monani UR, Chung WK, Benton M, Yum SW, Tennekoon G, Finkel RS. Spectrum of neuropathophysiology in spinal muscular atrophy type I. J Neuropathol Exp Neurol 2015;74:15–24. DOI: 10.1097/nen.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Iwatani S, Harahap NIF, Nurputra DK, Tairaku S, Shono A, Kurokawa D, Yamana K, Thwin KKM, Yoshida M, Mizobuchi M, et al. Gestational age-dependent increase of survival motor neuron protein in umbilical cord-derived mesenchymal stem cells. Front Pediatr 2017;5:194. DOI: 10.3389/fped.2017.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Jedrzejowska M, Milewski M, Zimowski J, Borkowska J, Kostera-Pruszczyk A, Sielska D, Jurek M, Hausmanowa-Petrusewicz I. Phenotype modifiers of spinal muscular atrophy: The number of SMN2 gene copies, deletion in the NAIP gene and probably gender influence the course of the disease. Acta Biochim Pol 2009;56:103–8. [PubMed] [Google Scholar]
- [15]. Kizina K, Akkaya Y, Jokisch D, Stolte B, Totzeck A, Munoz-Rosales J, Thimm A, Bolz S, Brakemeier S, Pul R, et al. Cognitive impairment in adult patients with 5q-associated spinal muscular atrophy. Brain Sci 2021;11. DOI: 10.3390/brainsci11091184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Lenzoni S, Semenza C, Calligaro D, Turcano P, Caumo L, Pegoraro E, Wennberg A. Cognitive profiles and clinical factors in type III spinal muscular atrophy: A preliminary study. Neuromuscular Disorders: NMD 2022;32:672–7. DOI: 10.1016/j.nmd.2022.05.005. [DOI] [PubMed] [Google Scholar]
- [17]. Masson R, Brusa C, Scoto M, Baranello G. Brain, cognition, and language development in spinal muscular atrophy type A scoping review. Dev Med Child Neurol 2021;63:527–36. DOI: 10.1111/dmcn.14798. [DOI] [PubMed] [Google Scholar]
- [18]. McGowan JE, Alderdice FA, Holmes VA, Johnston L. Early childhood development of late-preterm infants: A systematic review. Pediatrics 2011;127:1111–24. DOI: 10.1542/peds.2010-2257. [DOI] [PubMed] [Google Scholar]
- [19]. Mendell JR, Al-Zaidy SA, Lehman KJ, McColly M, Lowes LP, Alfano LN, Reash NF, Iammarino MA, Church KR, Kleyn A, et al. Five-year extension results of the phase 1 START trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurology 2021;78:834–41. DOI: 10.1001/jamaneurol.2021.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, Iannaccone ST, Kirschner J, Kuntz NL, Saito K, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. The New England Journal of Medicine 2018;378:625–35. DOI: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- [21]. Müller-Felber W, Blaschek A, Schwartz O, Gläser D, Nennstiel U, Brockow I, Wirth B, Burggraf S, Röschinger W, Becker M, et al. Newbornscreening SMA - from pilot project to nationwide screening in germany. Journal of Neuromuscular Diseases 2023;10:55–65. DOI: 10.3233/jnd-221577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Muller-Felber W, Vill K, Schwartz O, Glaser D, Nennstiel U, Wirth B, Burggraf S, Roschinger W, Becker M, Durner J, et al. Infants diagnosed with spinal muscular atrophy and 4 SMN2 copies through newborn screening - opportunity or burden? J Neuromuscul Dis 2020;7:109–17. DOI: 10.3233/JND-200475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Ngawa M, Dal Farra F, Marinescu AD, Servais L. Longitudinal developmental profile of newborns and toddlers treated for spinal muscular atrophy. Therapeutic Advances in Neurological Disorders 2023;16:17562864231154335. DOI: 10.1177/17562864231154335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Oprea GE, Kröber S, McWhorter ML, Rossoll W, Müller S, Krawczak M, Bassell GJ, Beattie CE, Wirth B. Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science (New York, NY) 2008;320:524–7. DOI: 10.1126/science.1155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Oudgenoeg-Paz O, Leseman PP, Volman MC. Exploration as a mediator of the relation between the attainment of motor milestones and the development of spatial cognition and spatial language. Dev Psychol 2015;51:1241–53. DOI: 10.1037/a0039572. [DOI] [PubMed] [Google Scholar]
- [26]. Ramos DM, d’Ydewalle C, Gabbeta V, Dakka A, Klein SK, Norris DA, Matson J, Taylor SJ, Zaworski PG, Prior TW, et al. Age-dependent SMN expression in disease-relevant tissue and implications for SMA treatment. The Journal of Clinical Investigation 2019;129:4817–31. DOI: 10.1172/JCI124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Rashnonejad A, Amini Chermahini G, Gündüz C, Onay H, Aykut A, Durmaz B, Baka M, Su Q, Gao G, Özkınay F. Fetal gene therapy using a single injection of recombinant AAV9 rescued SMA phenotype in mice. Molecular Therapy: The Journal of the American Society of Gene Therapy 2019;27:2123–33. DOI: 10.1016/j.ymthe.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Riviére J, Lècuyer R. Spatial cognition in young children with spinal muscular atrophy. Developmental Neuropsychology 2002;21:273–83. DOI: 10.1207/S15326942DN2103_4. [DOI] [PubMed] [Google Scholar]
- [29]. Smith L, Gasser M. The development of embodied cognition: Six lessons from babies. Artif Life 2005;11:13–29. DOI: 10.1162/1064546053278973. [DOI] [PubMed] [Google Scholar]
- [30]. Stratigopoulos G, Lanzano P, Deng L, Guo J, Kaufmann P, Darras B, Finkel R, Tawil R, McDermott MP, Martens W, et al. Association of plastin 3 expression with disease severity in spinal muscular atrophy only in postpubertal females. Archives of Neurology 2010;67:1252–6. DOI: 10.1001/archneurol.2010.239. [DOI] [PubMed] [Google Scholar]
- [31]. Tosi M, Cumbo F, Catteruccia M, Carlesi A, Mizzoni I, De Luca G, Cherchi C, Cutrera R, Bertini E, D’Amico A. Neurocognitive profile of a cohort of SMA type 1 pediatric patients and emotional aspects, resilience and coping strategies of their caregivers. European Journal of Paediatric Neurology: EJPN: Official Journal of the European Paediatric Neurology Society 2023;43:36–43. DOI: 10.1016/j.ejpn.2023.02.004. [DOI] [PubMed] [Google Scholar]
- [32]. Vill K, Schwartz O, Blaschek A, Gläser D, Nennstiel U, Wirth B, Burggraf S, Röschinger W, Becker M, Czibere L, et al. Newborn screening for spinal muscular atrophy in Germany: Clinical results after 2 years. Orphanet Journal of Rare Diseases 2021;16:153. DOI: 10.1186/s13023-021-01783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. von Gontard A, Zerres K, Backes M, Laufersweiler-Plass C, Wendland C, Melchers P, Lehmkuhl G, Rudnik-Schöneborn S. Intelligence and cognitive function in children and adolescents with spinal muscular atrophy. Neuromuscular Disorders: NMD 2002;12:130–6. DOI: 10.1016/s0960-8966(01)00274-7. [DOI] [PubMed] [Google Scholar]
- [34]. Whelan TB. Neuropsychological performance of children with Duchenne muscular dystrophy and spinal muscle atrophy. Dev Med Child Neurol 1987;29:212–20. DOI: 10.1111/j.1469-8749.1987.tb02138.x. [DOI] [PubMed] [Google Scholar]
- [35]. Wirth B, Brichta L, Schrank B, Lochmüller H, Blick S, Baasner A, Heller R. Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum Genet 2006;119:422–8. DOI: 10.1007/s00439-006-0156-7. [DOI] [PubMed] [Google Scholar]
- [36]. Wirth B, Karakaya M, Kye MJ, Mendoza-Ferreira N. Twenty-five years of spinal muscular atrophy research: From phenotype to genotype to therapy, and what comes next. Annu Rev Genomics Hum Genet 2020;21:231–61 DOI: 10.1146/annurev-genom-102319-103602. [DOI] [PubMed] [Google Scholar]
- [37]. Wishart TM, Huang JP, Murray LM, Lamont DJ, Mutsaers CA, Ross J, Geldsetzer P, Ansorge O, Talbot K, Parson SH, et al. SMN deficiency disrupts brain development in a mouse model of severe spinal muscular atrophy. Human Molecular Genetics 2010;19:4216–28. DOI: 10.1093/hmg/ddq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Yohannan M, Patel P, Kolawole T, Malabarey T, Mahdi A. Brain atrophy in Werdnig-Hoffmann disease. Acta Neurologica Scandinavica 1991;84,426–8. DOI: 10.1111/j.1600-0404.1991.tb04982.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.