Abstract
Background:
Spinal muscular atrophy (SMA) is a genetic neurodegenerative disorder with onset predominantly in infants and children. In recent years, newborn screening and three treatments, including gene replacement therapy (Onasemnogene abeparvovec-xioi), have become available in the United States, aiding in the diagnosis and treatment of children with SMA.
Objective:
To evaluate parents’ experiences with newborn screening and gene replacement therapy and to explore best practices for positive newborn screen disclosure and counseling of families.
Methods:
We conducted semi-structured interviews (n = 32) and online surveys (n = 79) of parents whose children were diagnosed with SMA (on newborn screening or symptomatically) and treated with gene replacement therapy.
Results:
Gene replacement therapy was most parents’ first treatment choice, although concerns regarding long term efficacy (65%) and safety (51%) were common. Information provided during the newborn screening disclosure was quite variable. Only 34% of parents reported the information provided was sufficient and expressed need for more information about treatment. Although many parents experienced denial of the diagnosis at initial disclosure, 94% were in favor of inclusion of SMA on newborn screening. Parents were almost universally anxious following diagnosis and over half remained anxious at the time of study participation with uncertainty of the future being a key concern. Many parents had difficulty processing information provided during their first clinic appointment due to its complexity and their emotional state at the time.
Conclusions:
Utilizing this data, we provide a recommendation for the information provided in newborn screening disclosure, propose adjustments to education and counseling during the first clinic visit, and bring awareness of parents’ mental health difficulties.
Keywords: Spinal muscular atrophy, neonatal screening, genetic therapy, Onasemnogene abeparvovec-xioi
INTRODUCTION
Spinal muscular atrophy (SMA) is an autosomal recessive neurodegenerative disease that affects approximately 1 in 5,000 to 1 in 11,000 individuals. The carrier frequency for the common exon 7 deletion is 1–3%, based on ancestry [1–7]. Prior to the availability of treatment, SMA was the most common genetic cause of death in children under the age of two. Due to the high disease incidence and a common deletion accounting for approximately 95% of cases, SMA was suggested as an addition to the recommended uniform screening panel (RUSP) in 2008. However, this was denied due to the lack of pilot data showing feasibility to screen for SMA utilizing available technology and treatment options. From January 2016 to January 2017, a pilot study of over 3,800 newborns in New York City demonstrated feasibility of utilizing multiplex real time PCR on dried blood spot cards to detect homozygous deletions of SMN1 as a means of newborn screening (NBS) for SMA. The study also showed general population acceptance of the screening with 93% of parents opting in [2, 8]. In December 2016, Nusinersen became the first disease modifying treatment approved by the Food and Drug Administration (FDA). Based on these two changes, SMA was added to the RUSP in July 2018. A positive NBS for SMA is highly specific for disease status; however, confirmatory testing, including SMN2 copy number, is required prior to treatment [2, 9–12]. Newborn screening for SMA is available in 48 of the United States (accounting for 98% of infants born) as of January 2023 [13]. Since the addition of SMA to the RUSP, two additional therapies have been FDA approved –Onasemnogene abeparvovec-xioi in May 2019 and Risdiplam in August 2020.
Outside the United States, NBS for SMA is also available in regions in Taiwan, Germany, Belgium, Australia, Italy, Russia, Canada, and Japan. This accounts for approximately two percent of all newborns worldwide [14]. As of January 2023, Onasemnogene abeparvovec-xioi has been approved for use in 45 countries [15].
Previous studies show individuals and families with SMA, the general population, and expectant parents with no history of SMA all supported NBS for SMA even prior to approval of disease modifying therapies [16–20]. However, because both NBS and gene replacement therapy are recent advances, limited research exists assessing parental experiences surrounding these interventions. Experiences with Onasemnogene abeparvovec-xioi, rather than other treatment options, were of interest given it is the first FDA approved systemic gene replacement therapy and the first long acting treatment option for SMA. The goal of this study was to describe parents’ experiences with NBS and gene replacement therapy and to explore best practices for positive newborn screen disclosure and counseling of families.
MATERIALS AND METHODS
Participants
Parents of children diagnosed with SMA who received Onasemnogene abeparvovec-xioi were recruited between July 2021 and August 2022. Children could be diagnosed symptomatically or pre-symptomatically by NBS. Recruitment occurred through the Nationwide Children’s Hospital (NCH) Neuromuscular Clinic and/or CureSMA outreach efforts.
Study design
This was a two-part study, with a semi-structured interview and an online Redcap survey. Interviews and surveys were only offered in English. Information collected in both parts included parents’ experiences and reactions to the NBS process (if applicable), first neuromuscular clinic appointment, treatment decisions and administration experience, and outlook on their child’s diagnosis post-treatment. IRB approval for the study was obtained from Nationwide Children’s IRB. Consent was obtained verbally for interviews and online for surveys.
Interviews were offered to parents of patients cared for in the neuromuscular clinic at NCH. They were performed via phone or during clinic appointments. Separate interview guides were developed for parents of children diagnosed on NBS and those diagnosed symptomatically, with input from all senior authors.
Surveys were completed by families identified at NCH and through CureSMA. Responses utilized 5-point Likert scales, multiple choice and open ended questions. Eight questions were added to the survey after study initiation to solicit additional details of newborn screen disclosure and genetic counseling involvement in patient care. A minimum of 42 responses were analyzed for each question.
Data analysis
Thematic coding of verified interview transcripts was conducted by four study team members. Conventional content analysis using a constant comparative approach was used with subsets of interviews within each group. Sequential coding team discussions were used to determine consensus of themes and exemplar quotes. Coding was conducted separately for parents of children diagnosed symptomatically (Symptomatic group) and by NBS (NBS group). Survey results were analyzed via comparative statistics. Chi square analyses were utilized to investigate factors influencing parents’ responses to NBS disclosure and anxiety related to diagnosis at the time of survey completion.
RESULTS
Survey results
The survey was sent to 338 individuals and 79 complete responses were received; a response rate of 23%. Parents were mostly mothers (82%), white (84%), had a bachelor’s or master’s degree (51%), and considered themselves at least somewhat religious (72%). Twenty-nine (36.7%) had children diagnosed symptomatically and 50 had children diagnosed on NBS (63.3%). Six parents (7.6%) had multiple children with SMA, seventeen (22%) were aware of their carrier status of SMA prenatally, and eight obtained a prenatal diagnosis of SMA (10%). The full demographic information of the survey participants is provided in Table 1.
Table 1.
Demographics of survey participants
| Symptomatic (n = 29) | Newborn Screening (n = 50) | |
| Mean Parent Age (years) | 29.7 | 30.8 |
| Relationship to Child | ||
| Mother | 79% (23) | 84% (42) |
| Father | 21% (6) | 16% (8) |
| Parent Education Level | ||
| High school or less | 17% (5) | 20% (10) |
| Some College | 31% (9) | 8% (4) |
| Associate/Bachelor’s Degree | 41% (26) | 52% (26) |
| Master’s degree | 10% (3) | 20% (10) |
| Parent Ethnicity | ||
| White | 79% (23) | 86% (43) |
| African-American/Black | 7% (2) | 10% (5) |
| Hispanic | 3% (1) | 2% (1) |
| Asian | 3% (1) | 0 |
| Native American | 3% (1) | 0 |
| Multi-Ethnic | 3% (1) | 2% (1) |
| Parent Religious Status | ||
| Yes | 38% (11) | 46% (23) |
| No | 34% (10) | 24% (12) |
| Somewhat | 28% (8) | 30% (15) |
| Prior Experience with SMA | ||
| Affected Sibling | 7% (2) | 8% (4) |
| Carrier Screening | 21% (6) | 22% (11) |
| Mean Age of Child (months) | 35.5 | 17.3 |
| Mean Time since Diagnosis (months) | 30 | 17.3 |
| Mean Child age at Treatment (months) | 9.8 | 2.5 |
| Treatment Before GRT* | ||
| Spinraza | 11 | 12 |
| Risdiplam | 3 | 1 |
| None | 15 | 38 |
| Ambulatory Status | ||
| Wheelchair Full Time | 76% (22) | 4% (2) |
| Wheelchair Part Time | 10% (3) | 2% (1) |
| Ambulatory Full Time | 4% (1) | 50% (25) |
| Too Young To Tell | 10% (3) | 44% (22) |
| Other Medical Needs* | ||
| G-tube | 69% (20) | 12% (6) |
| Ventilator | 17% (5) | 4% (2) |
| Bi-Pap | 48% (14) | 8% (4) |
*Values may be larger than number of patients in the cohort due to use of multiple treatments in some patients.
Newborn screening
A total of fifty (63%) parents had children diagnosed on NBS. Thirty-eight (76%) reported being aware that their child was receiving NBS. Nine (18%) were aware that SMA was included on this screening, all of whom were either known carriers (n = 8) or had an older child with SMA (n = 1). Pediatricians most commonly disclosed the positive NBS result (71%, n = 30) followed by neurologists (12%, n = 5), genetics providers (12%, n = 5) or OBGYNs (5%, n = 2). Information provided in the disclosures included the name of the diagnosis for 95% (n = 40), symptoms of SMA for 60% (n = 25), existence of treatments for 57% (n = 24), and details of treatment options for 40% (n = 17).
Only 42% (n = 21) of parents felt that the NBS disclosure allowed them to understand the diagnosis. Twenty-eight (56%) felt the disclosure helped them understand the necessary next steps in their child’s healthcare.
Parents who were well informed about symptoms of SMA, treatment availability, and details of treatment options reported better understanding their child’s NBS result, diagnosis, and next steps required for their child’s medical care. These comparisons are summarized in Table 2. In addition, parents who received information in more of these categories reported better understanding of the NBS results (p = .004), diagnosis (p = .003), and next steps in care (p = .003). Disclosing provider (pediatrician vs non-pediatrician) did not impact the inclusion of diagnosis name (p = .49), symptoms of SMA (p = .55), existence of treatment (p = .92) or details of treatments (p = .43) in the NBS result call. Provider type did not impact parents’ understanding of the results (p = .09), diagnosis (p = .31) or next steps (p = .49). The majority of parents (88%) were anxious following the disclosure, regardless of amount of information provided (p = .16) or disclosing provider (p = .30). Despite this, parents were generally glad that their child had NBS for SMA (94%, n = 47) and felt that it positively impacted their child’s health (94%, n = 47).
Table 2.
Factors influencing parents’ response to NBS disclosure
| Information disclosed in first phone call after NBS | ||||||||||
| Symptoms of SMA | Treatment exists | Treatment details | ||||||||
| Parents’ responses | Y | N | P-value | Y | N | P-value | Y | N | P-value | |
| The disclosure allowed me to understand my child’s diagnosis | Disagree | 6 | 11 | .03 | 5 | 12 | .007 | 1 | 16 | .001 |
| Neutral | 6 | 2 | 5 | 3 | 4 | 4 | ||||
| Agree | 13 | 4 | 14 | 3 | 12 | 5 | ||||
| I felt well informed about my child’s NBS result after the disclosure | Disagree | 5 | 12 | .004 | 7 | 10 | .03 | 0 | 17 | <.001 |
| Neutral | 8 | 3 | 5 | 6 | 6 | 5 | ||||
| Agree | 12 | 2 | 12 | 2 | 11 | 3 | ||||
| The disclosure allowed me to understand the next steps for my child’s healthcare | Disagree | 5 | 11 | .01 | 5 | 11 | .03 | 0 | 16 | <.001 |
| Neutral | 3 | 1 | 3 | 1 | 2 | 2 | ||||
| Agree | 17 | 5 | 16 | 6 | 15 | 7 | ||||
| Anxiety post disclosure* | Disagree/Neutral | 2 | 3 | .34 | 1 | 4 | .07 | 0 | 5 | .05 |
| Agree | 23 | 14 | 23 | 14 | 17 | 20 | ||||
*Due to low incidence of responses conveying a lack of anxiety at disclosure, ‘disagree’ and ‘neutral’ responses were combined into a single category.
Treatment with gene replacement therapy
The majority of parents in both groups (Symptomatic, N = 29 and NBS, N = 50) felt hopeful following discussion about available treatment options (80% Symptomatic; 94% NBS). Most felt that gene replacement therapy would improve their child’s symptoms (84% Symptomatic; 94% NBS), but understood that it is not a cure for the condition (72% Symptomatic; 62% NBS). Parents often had safety concerns related to gene replacement therapy (58% Symptomatic; 46% NBS). Although almost all felt grateful that their child received gene replacement therapy (97% Symptomatic; 98% NBS), the majority did worry about the possibility of its effectiveness “wearing off” (58% Symptomatic; 68% NBS).
Impact of diagnosis on parent and outlook on future
Parents from both groups (Symptomatic, N = 29 and NBS, N = 50) endorsed feeling scared (93% Symptomatic; 92% NBS) and anxious (100% Symptomatic; 96% NBS) following their child’s diagnosis of SMA. Although fewer reported these feelings at the time of study, the majority still expressed these emotions (62% Symptomatic, 50% NBS scared; 86% Symptomatic, 52% NBS anxious).
Parent age (p = .87), sex (p = .52), and education level (p = .44) did not impact current anxiety. Parents with children who were unable to sit (p = .002) or walk independently (p = .001) or who required use of a wheelchair (p = .002), feeding support (p = .03) or respiratory support (p = .03) were more likely to report anxiety at the time of survey. Parents in the NBS group were more likely to report anxiety if their child was younger (p = .04). These findings are summarized in Table 3.
Table 3.
Factors about child influencing parental anxiety at time of study
| Parent anxious about child’s diagnosis | |||||
| at time of study | |||||
| Child characteristics | Disagree | Neutral | Agree | P-value | |
| n | n | n | |||
| Can sit independently | Y | 11 | 15 | 28 | .002 |
| N | 1 | 1 | 23 | ||
| Can walk independently | Y | 8 | 5 | 7 | .001 |
| N | 4 | 11 | 44 | ||
| Uses wheelchair | Y | 0 | 4 | 24 | .002 |
| N | 9 | 7 | 10 | ||
| Uses feeding support | Y | 1 | 3 | 22 | .03 |
| N | 11 | 13 | 29 | ||
| Uses respiratory support | Y | 0 | 3 | 18 | .03 |
| N | 12 | 13 | 33 | ||
| M (SD) | M (SD) | M (SD) | |||
| Child age (both groups) | Months | 25.0 (14.6) | 23.4 (18.3) | 24.0 (19.6) | .97 |
| Child age (NBS group) | Months | 25.0 (14.6) | 17.8 (13.8) | 13.6 (10.5) | .04 |
A small proportion of parents felt that the diagnosis of SMA made it difficult to bond with their child (13% Symptomatic; 14% NBS) and/or felt disconnected from their child (10% Symptomatic; 14% NBS). Twenty-one (42%) in the NBS group felt that ‘receiving the diagnosis so early’ prevented them from enjoying the newborn period with their child.Most agreed that the diagnosis of SMA was serious (65% Symptomatic; 66% NBS), but did not think that their child’s current health status was serious (24% Symptomatic; 14% NBS). When asked about their child’s future, more parents in the NBS group thought that their child would have a “normal life” (74%) compared to the Symptomatic group (38%), but similar proportions felt that their child would have a “meaningful life” (93% Symptomatic; 90% NBS) and felt hopeful for their child’s future (93% Symptomatic; 92% NBS).
Care and counseling related to SMA
Parents were happy with the clinical care their child receives for SMA (100% Symptomatic; 96% NBS) and were trusting of their child’s care team (100% Symptomatic; 96% NBS). Both groups understood the need for continued medical care related to their child’s SMA diagnosis following treatment (100% Symptomatic; 94% NBS).Regarding counseling following diagnosis, parents generally felt the amount of information provided for disease (79% Symptomatic; 84% NBS), testing (69% Symptomatic; 78% NBS), genetics (69% Symptomatic; 88% NBS), and treatment (83% Symptomatic; 92% NBS) was appropriate. Timing of information was mostly thought to be appropriate for disease, genetics, and treatment information (83–86% Symptomatic; 82–90% NBS) or later than desired (14–17% Symptomatic; 10–18% NBS). Regarding information about testing for SMA, 28% of parents in the Symptomatic group felt this came too late. Verbal communication was felt to be most useful for all topics (79-86% Symptomatic; 76-88% NBS), but a large proportion also agreed that written (55–69% Symptomatic; 66–76%) or visual (52–62% Symptomatic; 40–58% NBS) formats would be helpful. More parents in the symptomatic group (97%, n = 28) utilized the internet to learn about SMA than in the NBS group (78%, n = 39) and more often found it to be useful across all topics (62–73% Symptomatic; 44–56% NBS).Genetic counseling was more commonly received by parents in the NBS group (72%, n = 36) than the Symptomatic group (59%, n = 17). This usually occurred at an appointment immediately following diagnosis (58%, n = 21) or at a separate appointment prior to gene replacement therapy (28%, n = 10). Most parents felt that meeting with a genetic counselor immediately following diagnosis would be the most beneficial time (77%, n = 44). Genetic counseling was seen as most useful in assisting in the understanding of diagnosis (82% Symptomatic; 83% NBS), and risk for themselves (76% Symptomatic; 92% NBS) or family members (88% Symptomatic; 83% NBS) to be carriers of SMA. To a lesser extent, parents felt it was helpful in understanding treatments (59% Symptomatic; 64% NBS).
Thematic analysis of qualitative interviews
Thirty-two parents (of twenty-six children) completed the interview process. Twenty-five (78.1%) were mothers and seven (21.9%) were fathers. Of these, eighteen (56.3%) were parents of children diagnosed on NBS and thirteen (40.6%) were parents of children diagnosed symptomatically. Nineteen had children (59.4%) with 2 copies of SMN2, ten (31.3%) with 3 copies, and three (9.4%) with four copies. One parent had two children with SMA, one in the NBS group and one in the symptomatic group, and both experiences were discussed during the interview. Two parents in the NBS group had a previous child who had died of SMA, but were interviewed with respect to their living child. One couple in the Symptomatic group were identified as carriers for SMA prenatally. Full demographic information for the cohort studied is provided in Table 4.
Table 4.
Demographics of interview participants
| Symptomatic (n = 14; 10 families) | Newborn Screening (n = 18; 16 families) | |
| Relationship to Child | ||
| Mother | 64% (9) | 89% (16) |
| Father | 36% (5) | 11% (2) |
| Prior Experience with SMA | ||
| Affected Sibling | 0 | 11% (2) |
| Carrier Screening | 15% (2) | 11% (2) |
| Other Family hx of SMA | 8% (1) | 6% (1) |
| Mean Age of Child (months) | 46.2 | 17.7 |
| Mean Time Since Diagnosis (months) | 40 | 17.7 |
| Child SMN 2# | ||
| 2 | 79% (11) | 44% (8) |
| 3 | 21% (3) | 39% (7) |
| 4 | 0 | 17% (3) |
| Mean Age at Treatment (months) | 10.3 | 1.9 |
| Treatment Mode | ||
| Clinical Trial | 29% (4) | 11% (2) |
| EAP/MAP | 21% (3) | 0 |
| Commercial | 50% (7) | 89% (16) |
| Treatment Before GRT* | ||
| Spinraza | 7 | 1 |
| Risdiplam | 0 | 1 |
| None | 7 | 17 |
| Treatment After GRT* | ||
| Spinraza | 3 | 0 |
| Risdiplam | 13 | 2 |
| None | 3 | 16 |
| Ambulatory Status | ||
| Wheelchair Full Time | 10 (100%) | 0 |
| Wheelchair Part Time | 0 | 0 |
| Ambulatory Full Time | 0 | 9 (56%) |
| Too Young To Tell | 0 | 7 (44%) |
| Other Medical Needs* | ||
| G-tube | 6 (43%) | 0 |
| Ventilator | 2 (14%) | 0 |
| Bi-Pap | 3 (33%) | 0 |
*Values may be larger than number of patients in the cohort due to use of multiple treatments in some patients.
Receiving symptomatic diagnosis (symptomatic group)
Parents experience significant delays in diagnosis despite persistent advocacy
Parents of symptomatic children described a lengthy diagnostic process and had to fight for appropriate referrals and testing. A variety of factors led to delays in diagnosis including lack of provider familiarity with SMA, wait times for specialty appointments, and receiving incorrect diagnoses prior to their SMA diagnosis.
And I was like, well, can we test for SMA?...I feel like he’s got all the symptoms . . . And he said, ‘oh, I don’t think that’s necessary . . . kids with SMA, they are they’re way sicker than him. They struggle to breathe. They can’t hold their head up’ . . . He goes, ‘I would be very surprised if he has SMA.’ And he was, I mean, very adamant that it wasn’t that. So I was like okay. I guess that’s a sigh of relief, it’s not that. (Symptomatic, Mother)
Receiving newborn screening diagnosis (NBS group)
Parents have limited knowledge of NBS prior to child’s diagnosis
Excluding one, all parents were aware that their child had NBS completed and recall the sample being collected. None understood the types of diseases included on the screening. Only parents with prior children diagnosed with SMA were aware that SMA was included on the screening.
We just knew that there was a general . . . newborn screening that they did at the hospital. But we didn’t know that they were screening for anything like that. We had no idea. (NBS, Mother)
NBS disclosure leaves parents wanting more information, highlights need for up-to-date information
Parents received the positive NBS result from a variety of providers, most commonly pediatricians. The amount and type of information disclosed was quite variable. Parents generally wished more information had been provided in the initial phone call, with many specifically desiring more discussion about treatment.
She said that we got some results back that may be concerning, and that they wanted me to take her immediately the next day to get tested again to be sure that it was positive or not, but they didn’t tell me what it was because they didn’t want me to worry. But that made me worry more because I had no idea what was going on with her. They just said it was really urgent and life threatening, so that scared me. (NBS, Mother)
And I think you got to just be able to just say, hey, you know, here are some options that are available . . . Here’s what our next steps will be. Here’s how you can start working towards what we’re hopeful for. You know, there was none of that . . . I’m sure if they were to reflect on that, they would have said they would agree. But, you know, certainly offer hope, offer solutions, not just like a reign of terror kind of thing. (NBS, Father)In some cases, the information provided to families was outdated or inaccurate, often leaving parents with a bleak impression of their child’s future.I do remember her saying . . . that children who normally are born with this, either they can’t walk, they can’t crawl, they can’t eat. Most of them are wheelchair bound. And she said some of them are like a vegetable. And I remember her saying that . . . I broke down...you can’t tell a parent that. (NBS, Mother)Diagnosis in a “healthy” child leads to disbelief and denial in parentsReceiving the diagnosis of SMA on NBS was difficult for parents to accept because their child appeared healthy. Many pointed out the struggle of being told their child had a disease while in the pre-symptomatic stage and the shock of receiving an unsolicited diagnosis so shortly after birth.I remember thinking that, you know, my son looks perfect. And how could he look so perfect and have something so wrong? (NBS, Mother)But, you know, we’re looking at our son and . . . he’s doing great . . . you would have never known that anything was going on without that newborn screening . . . we’re looking at our son and I’m like, I don’t, I don’t know . . . it’s hard for me to believe what you’re telling me right now. (NBS, Father)Parents hold on to hope that NBS is a false positive while awaiting diagnostic resultsInaccuracies in information provided in the NBS disclosure and parents’ disbelief in the diagnosis due to lack of symptoms led parents to hope that the NBS results would be a false positive.She said he came up positive on the screening for something called SMA . . . and then she said that . . . he could just be a carrier. Which I guess was not the case, but we, at the time, thought that that was true . . . I guess, we got a little bit of false hope . . . from that call. (NBS, Mother)Oh, you know, sometimes . . . its trial and error when it comes to that kind of stuff. Sometimes you get a false positive reading and it’s really false...You know, so I was kind of hoping that’s what it was. But, you know, come to find out that’s not what it was. (NBS, Father)
Parents’ opinions on NBS for SMA (Both groups)
Parents supportive of NBS for SMA, recognizing need for early diagnosisParents in both groups overwhelmingly supported the use of NBS for pre-symptomatic diagnosis of SMA, emphasizing the importance of early diagnosis on disease outcomes. Despite this, many in the NBS group also expressed the difficulty of receiving the diagnosis when their child was so young.Yes, I am ever so grateful that they, you know, included it on the infant screening because, in all likelihood, the fact that he got treatment as quickly as he did is why he’s doing as well as he is . . . And, you know, getting the diagnosis was hard, but it was crucial to making sure that my son gets to live the life that he should. (NBS, Mother)I have no regrets about finding out when we did . . . yes, innocence, it was robbed. But at the same time, I, I’m very thankful for that because of the, just the urgency and the action that we’re able to take and the time that we did...It changed our lives. (NBS, Father) Parents of children diagnosed symptomatically were left wondering how their children’s lives may have been different with NBSParents in the Symptomatic group had firsthand experience with the importance of NBS and early diagnosis. Many parents expressed how NBS would have shortened the diagnostic process. They wished that their children had the opportunity of pre-symptomatic diagnosis and treatment, wondering how this may have changed their outcome.Like, there was a kid who got diagnosed on newborn screen lives right down the road from us. And he wears little ankle braces, but he has hit every milestone and walks just fine. So, I, it’s hard . . . I just wonder what she would be like. It’s just four more months, like if I had, if I had just waited to get pregnant four more months . . . yeah, you think she’d be so typical if she was on the newborn screen. (Symptomatic, Mother)
Counseling for parents following SMA diagnosis (Both groups)
First appointment is overwhelming for newly diagnosed familiesParents in both groups commented on how overwhelming the first neuromuscular clinic appointment was, specifically calling out the amount of people and information included and the difficulty to process the information while still in a state of shock.We just had this perfect little tiny baby and she’s saying that it wasn’t. It was the worst. And I just remember asking a lot of questions and being in such a haze and not understanding like what she was saying... I just remember asking the same things over and over and over but I like could not grasp what she was saying. (Symptomatic, Mother)I remember meeting with so many doctors, I feel like I saw like 8 or 10 people that day. And I remember thinking, ‘it’s just taking forever, and it’s just it’s so much information, and I can’t process all of this information.’ (NBS, Mother)Provider compassion is key for parents at the initial clinic appointmentAlthough the initial appointment was overwhelming, parents did remember the compassion and support they received from providers during this time.I think the people we met with were all very gentle in talking to us, and that was good. It was like they were sensitive to how shaken up we all were. (NBS, Mother)They let me sit there and cry. It wasn’t like ‘here, let’s finish up and here’s some tissues and let’s move on to the next subject’, you know, because I couldn’t help it. (NBS, Mother)I was so nervous but felt comfortable being there with everybody just because they all seemed so confident and like this is what’s going to happen. This is how things are going to go. We’ve got you, we’ve got your back. Don’t worry. (Symptomatic, Mother)
Treatment with gene replacement therapy (both groups)
Gene replacement therapy was parents’ first choice for treatmentParents in both groups overwhelmingly preferred and pursued gene replacement therapy for their children. Factors repeatedly mentioned as influencing this decision included administration route and one-time dosing.So when we sat and listened to the explanation of those treatments, we said that we wanted to go home and talk about it...But when we got home, we both immediately said, you know, the gene replacement therapy is what we want. This is a one-time thing. It’s less invasive. This is what we want to do. (NBS, Mother)My initial reaction or thought was quality of life for my daughter. I wanted her to potentially just not really always have to go to a clinic or hospital every three months...I wanted her quality of life to be outside of regular hospital visits. (Symptomatic, Father)Some parents used personal experiences or experiences of other families to aid in their decision between treatment options.Well, I guess from what we saw with our friends that had that used the gene therapy versus the Spinraza, we could see a big difference. It just seemed that children were doing a whole lot better . . . so, of course, naturally, that’s the path we wanted to take too. We are parents that wanted to give our child everything we could to have a normal life. (NBS, Mother)My first daughter, she was taking, Spinraza. And we saw like there were, uh, there was improvement . . . So we discussed more about gene therapy and the other drug the child would take, take throughout his life. So we decided to go with the gene therapy since it’s the one time treatment the child can take and the child will be ok. (NBS, Mother)Parents seeking gene replacement therapy for symptomatic children race against disease progressionParents from the Symptomatic group expressed desperation in seeking out gene replacement therapy for their child, as their condition worsened.I mean, at that point, we really didn’t have much of an option. It was either do nothing or try something. (Symptomatic, Father)It was like this . . . golden, magical thing that everybody was trying to figure out a way to get their hands on it. And everybody was talking about it . . . there was so many people who wanted to ask us . . . ’and how did you get it and why did you get it?’...and really we just got really lucky. (Symptomatic, Mother)Parents with children diagnosed on NBS anxiously await signs that gene replacement therapy workedParents from the NBS group struggled with the lack of evidence that therapy was working. For them, the absence of deterioration in their child was the indication of successful treatment which meant waiting for the appearance of normal milestones. Sometimes this led to increased vigilance over their child and skepticism in whether the treatment worked.But you also have a little worry of like, well, this is what we’re putting our trust in. I hope it’s, you know, doing what we need it to do. But you just don’t know . . . I don’t think you ever get rid of that feeling, really. You know, hey, did it work?...I watch my son every day just to make sure that he’s, you know, not regressing or showing signs and, you know, that’s the only thing we can do. That’s the only thing I can control. (NBS, Father)Parents with symptomatic children were able to see improvements more quicklyAlthough parents from the Symptomatic group still worried about how well the gene replacement therapy was working, they saw improvements in their child’s symptoms that pointed towards its success.Oh, I cried...I was so excited . . . he hasn’t lifted his leg up in two months . . . To see him be able to lift his leg, it was just it was amazing. And the first like two days after the therapy, like we couldn’t sleep because we were just staring at him. We were like, what’s next?...We didn’t want to miss anything. (Symptomatic, Mother)
Impact of diagnosis on parent and outlook on the future (both groups)
Parental anxiety improves with time, but does not disappear while uncertainty about the future remainsWhile most parents in both groups reported ongoing anxiety regarding their child’s diagnosis and the uncertainty of their future health, they did also note this lessened over time. Several parents reported their mental health improving after their child had treatment or after seeing their child achieve or regain motor milestones.I was like at three months I know he has to reach his milestones . . . my eyes are on him, like how he will sit, how he will crawl, how he will stand and do everything . . . But he [sat], he crawled . . . he started walking too . . . we are happy with everything. We don’t think about SMA anymore. (NBS, Mother)I would just say that, Zolgensma is not only a treatment, but it’s a life saver. Like it really saved all of us, not just [child]. It gave us all of our life back, not just him. (Symptomatic, Mother)Parents from both groups struggled with the uncertainty associated with their child receiving a novel therapy, stemming from the lack of knowledge of long term outcomes and whether the therapeutic effect would eventually fade.The long term: does it really work forever? Because they don’t know, it is predicted, “it is predicted”: the famous phrase of Zolgensma . . . they don’t know the long term. That’s scary . . . (NBS, Mother)Like you’re incredibly grateful for these treatments, but yet you still don’t even know when it’s happening. You don’t know what you’re doing. You don’t know what’s going to be happening from that treatment, and you don’t know what your child’s life is going to be like. (Symptomatic, Mother)Parents remain cautiously optimistic about their child’s futureParents in both groups were hopeful for their child’s future, but remained cautious given the unknowns of the treatment longevity. More parents in the Symptomatic group expressed viewpoints of acceptance or new outlook on the physical limitations that may still exist for their children.So at the beginning of this, I thought my son’s future was not anything that I had imagined. All of our dreams and our hopes for him had kind of gone away . . . and now I see my son running around like his cousins. And [chokes up] sorry. I see a really bright future for him . . . .those hopes and dreams may have changed a little bit in our mind from when he was first born . . . .I do believe that he is going to live a very close if not normal lifestyle. (NBS, Mother)But he’s, this kid has this personality that he’s just like he’s not going to let it stop him, you know what I mean? Like, he, I mean, is he going to play football? No . . . But I can see him like being on the sidelines . . . I still see him being involved in things, just in a different capacity than a normal child his age. (Symptomatic, Mother)
DISCUSSION
In this cohort, gene replacement therapy was parents’ first choice for treatment, similar to the findings of Deng et al. performed in the United States [21]. Parents voiced similar rationale for this choice: one time dosing, less invasive administration route, and perceived better outcomes compared to the other treatment options [21]. Despite this, about half of survey respondents reported safety concerns with the therapy, including its novelty, side effects, young age at treatment, and concerns about the procedure.
After receiving gene replacement therapy, parents in the NBS group experienced anxiety while awaiting signs that the therapy was working, leading to increased watchfulness of their child. This lessened with the emergence of normal motor milestones. Parents in the Symptomatic group were more quickly reassured by improvements seen in their children’s motor function following treatment.
A theme of cautious hope emerged regarding parents’ views of their child’s diagnosis and future following gene replacement therapy. Lack of long term outcome data and uncertainty in their child’s future health were the most common concerns voiced by parents. This finding was similar to the U.S. study and a similar study conducted in Australia, where the majority of parents (65%) had concerns related to the treatment longevity [21, 22].
Newborn screening for SMA was highly supported in our cohort, with 94% of parents glad that their child was screened. Many parents cited the importance of early diagnosis and treatment in best outcomes for their child. These findings were consistent with both the Australian and U.S. studies, where 100% and 94.4% of the parents were satisfied with the program, respectively [21, 22]. Additionally, a major theme in the Symptomatic group was the significant delays experienced in obtaining their child’s SMA diagnosis, which has also been shown in prior studies, highlighting the need for NBS for efficient diagnosis and treatment [23, 24].
In both our cohort and the Australian cohort, only 76% of parents were aware that their child had NBS. Additionally, most were unaware of the types of conditions that were screened [22]. Lack of parental knowledge and education about NBS is an issue that has been highlighted in other studies and has been associated with increased shock and confusion for parents receiving a positive screen [25–27].
Only 34% of parents felt the information provided in the NBS disclosure was sufficient, a smaller proportion compared to the Australian study, where 59% felt the information provided was acceptable [22]. Insufficient depth of information in disclosure and perception that providers are uninformed about the diagnosis they are disclosing have been described previously for other NBS conditions [26–30]. Parents participating in interviews specifically wanted more information about treatments during the disclosure; an important finding given only 40% were given details of treatment options. In our study, parents receiving more information in the NBS disclosure reported better understanding of the NBS result, diagnosis, and next steps in care.
Parents in the NBS group commonly reported denial and disbelief in their child’s diagnosis due to lack of visible symptoms. This, coupled with inaccuracies in information provided during initial disclosure, led to inflated hope that the NBS was a false positive or that their child may be an unaffected carrier. The hope for false positive in our study was also demonstrated in the Australian study, where 48% of parents hoped for a false positive and reported disbelief that their healthy appearing child could have SMA [22]. Shock and disbelief have also been commonly reported in parents receiving a positive NBS for other diagnoses [26, 29–31].
Following NBS disclosure, most parents felt anxious and scared, similar to the findings in the Australian study where 83% reported anxiety [22]. Anxiety immediately following NBS disclosure was not relieved by information provided in the call or type of provider giving the information. Disclosing providers should anticipate that anxiety in this time period is universal. Until this phone call, parents see their child as healthy. This call shatters that perception. Our goal should not be to alleviate these emotions immediately, but rather to support families as they grapple with this transition.
While many parents reported anxiety reduced with time, over half were still anxious at the time of taking the survey. We found that parents with more symptomatic children (with lower motor function or need for respiratory or feeding support) were more likely to report ongoing anxiety. Additionally, parents with children diagnosed on NBS were more likely to report anxiety if their child was younger, likely due to them still adjusting to the diagnosis and awaiting normal milestones to develop.
Regarding clinical follow up and counseling after positive NBS for SMA, two main themes emerged. First, parents felt overwhelmed at the first clinic appointment. They struggled to process the amount of information provided while still in a state of shock. These sentiments were shared by 41% of parents in the Australian cohort who noted emotional response to the diagnosis and complexity of the information being presented as barriers to comprehension [22]. Parents have reported similar feeling regarding initial clinic appointments following positive NBS for metabolic disorders [26]. Second, parents took notice of providers’ compassion at this first appointment. They appreciated providers taking time to thoroughly explain the aspects of their child’s diagnosis and allowing them space for their feelings during the appointment. These findings were similar to a study focused on NBS results disclosure for cystic fibrosis and congenital hypothyroidism [27]. While the majority of new SMA diagnoses in the United States will be on NBS, these themes were consistent in the Symptomatic group. This is relevant for counseling families of children with variants not detectable by NBS and in areas where NBS is not available.
Genetic counseling was more commonly provided in the NBS group (75%). Those who received genetic counseling reported it was useful in understanding the diagnosis, carrier risks for themselves and family members, and treatments. Most (77%) felt that meeting with a genetic counselor immediately following diagnosis would be most beneficial.
Parents felt receiving information verbally was most useful for understanding of disease, testing, genetics, and treatment, but the majority felt that written or visual information would also be helpful. Parents in the prior U.S. study also felt additional formats of information related to treatment options were helpful [21]. Additionally, parental interest in written summary materials has been described in reference to other conditions on the NBS [27].
Limitations of this study include that interviews and surveys were only offered to parents of children who received gene replacement therapy. Although in our experience at NCH, every family with a child under the age of two years chose to pursue gene replacement (only one of which could not receive it due to elevated AAV9 antibodies), responses to questions regarding treatment choice are limited by the participant inclusion criteria. Additionally, in the survey, responses to questions are dependent on participant interpretation. This is especially true in the more open-ended questions, including those related to education preferences following diagnosis. Finally, interviews and surveys were only offered in English so we are unable to comment on whether parents who do not read and/or speak English would have differing experiences with these processes.
CONCLUSIONS AND RECOMMENDATIONS
This study identified three key aspects of the parental experience to target for improved communication.
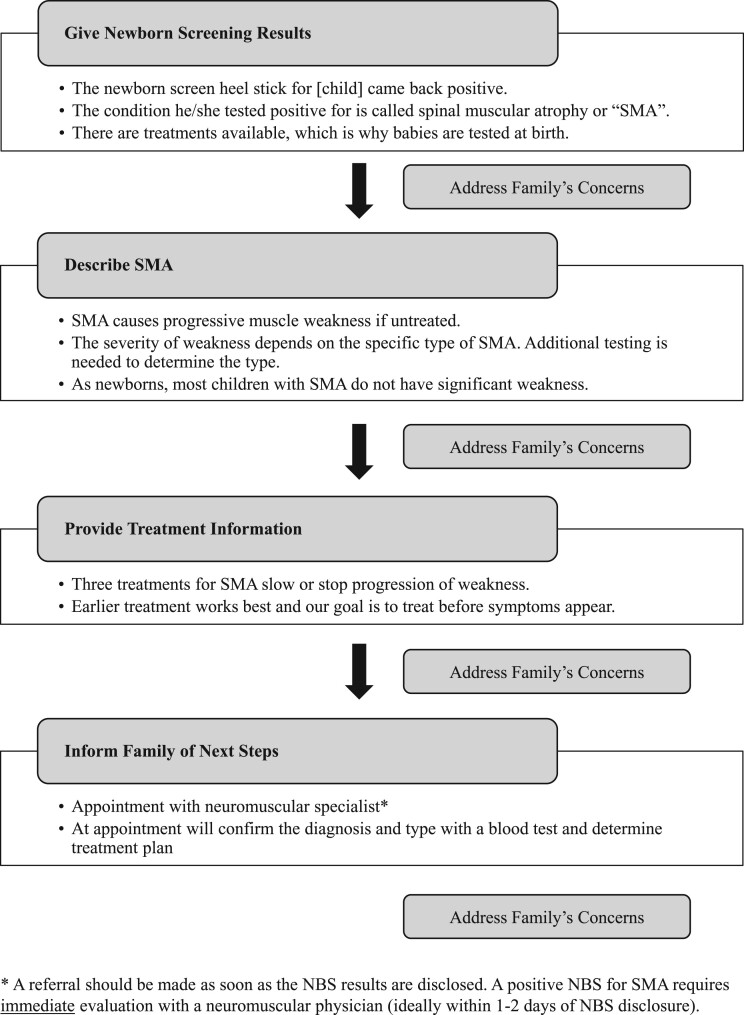
1. NBS disclosure call: The amount, type, and accuracy of information parents received was inconsistent, suggesting a need for standardization. Additionally, our results highlight topics that parents identified as desirable including availability of treatments and establishing a clear follow up plan. Our specific recommendations for the disclosure call are provided in Fig. 1.
Fig. 1.
Suggested content for disclosure of a positive NBS for SMA.
2. First clinic appointment: Families in this study as well as another cohort expressed difficulty in comprehending the information provided during this first encounter. Paradoxically, parents also reported the amount and timing of information provided to them as appropriate. We recommend: 1) limiting the number of providers involved to those most pertinent to the initial visit, 2) providing written and visual summary information for families to take home, and 3) providing the recommendation for parents to bring a support person to this first appointment to help with processing information and asking appropriate questions.
3. Parents’ Mental Health: Feelings of anxiety and fear were not only expressed following diagnosis, but persisted months and years, suggesting that time alone will not lead to resolution for everyone. This could be partially due to the theme of uncertainty that parents expressed regarding their child’s future. Given these findings, we identify the need for mental health screening and care for parents. How best to address this would be an area of future research, but we recommend checking on newly diagnosed families in the appointments following diagnosis as well as on parents at higher risk of persistent anxiety and offering mental health resources as needed. This is often done by embedded clinic social workers, but should be done by another staff member if a social worker is unavailable.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank CureSMA for their assistance in distributing study recruitment information to their members and all of the parents who took the time to share their experiences.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-230082.
FUNDING
This work was supported by Award Number UL1TR002733 from the National Center for Advancing Translational Sciences and the National Society of Genetic Counselors Pediatric and Clinical Special Interest Group. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Contributed to study conception and design: A.M., M.W., A.C., K.V., N.H.
Conducted participant interviews: A.M.
Analyzed and interpreted data: A.M., M.W., A.C., K.V., N.H., A.H., A.D., P.P, A.W.
Drafted and reviewed the final publication: A.M., M.W., A.C., K.V., N.H., A.H., A.D., P.P, A.W.
CONFLICTS OF INTEREST
Dr. Waldrop has received clinical trial support from Sarepta therapeutics and Novartis Gene Therapies and has received consulting fees from Sarepta therapeutics. Dr. Connolly has served on advisory boards for Biohaven, Scholar Rock, Sarepta Therapeutics, Edgewise, Avexis, and Genentech-Roche. She receives clinical trial support from Novartis Gene Therapies, Biohaven and Scholar Rock. The remaining authors have no relevant disclosures.
DATA AVAILABILITY
The data supporting the findings of this study are available within the article and/or its supplementary material.
REFERENCES
- [1]. Dejsuphong D, Taweewongsounton A, Khemthong P, et al. Carrier frequency of spinal muscular atrophy in Thailand. Neurol Sci. 2019;40:1729–1732. [DOI] [PubMed] [Google Scholar]
- [2]. Kraszewski JN, Kay DM, Stevens CF, et al. Pilot study of population-based newborn screening for spinal muscular atrophy in New York state. Genet Med. 2018;20:608–613. [DOI] [PubMed] [Google Scholar]
- [3]. Nilay M, Moirangthem A, Saxena D, Mandal K, Phadke SR. Carrier frequency of SMN1-related spinal muscular atrophy in north Indian population: The need for population based screening program. Am J Med Genet A. 2021;185:274–277. [DOI] [PubMed] [Google Scholar]
- [4]. Solov’ev AA, Grishchenko NV, Livshits LA. [Spinal muscular atrophy carrier frequency in Ukraine]. Genetika. 2013;49:1126–8. [PubMed] [Google Scholar]
- [5]. Verhaart IEC, Robertson A, Wilson IJ, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature review. Orphanet J Rare Dis. 2017;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Zhang J, Wang Y, Ma D, et al. Carrier Screening and Prenatal Diagnosis for Spinal Muscular Atrophy in 13,069 Chinese Pregnant Women. J Mol Diagn. 2020;22:817–822. [DOI] [PubMed] [Google Scholar]
- [7]. Hendrickson BC, Donohoe C, Akmaev VR, et al. Differences in SMN1 allele frequencies among ethnic groups within North America. J Med Genet. 2009;46:641–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Taylor JL, Lee FK, Yazdanpanah GK, et al. Newborn blood spot screening test using multiplexed real-time PCR to simultaneously screen for spinal muscular atrophy and severe combined immunodeficiency. Clin Chem. 2015;61:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Hale JE, Darras BT, Swoboda KJ, et al. Massachusetts’ Findings from Statewide Newborn Screening for Spinal Muscular Atrophy. Int J Neonatal Screen. 2021;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Baker MW, Mochal ST, Dawe SJ, et al. Newborn screening for spinal muscular atrophy: The Wisconsin first year experience. Neuromuscul Disord. 2022;32:135–141. [DOI] [PubMed] [Google Scholar]
- [11]. Abiusi E, Vaisfeld A, Fiori S, et al. Experience of a 2-year spinal muscular atrophy NBS pilot study in Italy: Towards specific guidelines and standard operating procedures for the molecular diagnosis. J Med Genet. 2022. [DOI] [PubMed]
- [12]. Kariyawasam DST, Russell JS, Wiley V, Alexander IE, Farrar MA. The implementation of newborn screening for spinal muscular atrophy: The Australian experience. Genet Med. 2020;22:557–565. [DOI] [PubMed] [Google Scholar]
- [13]. Newborn Screening State Program Map.. 2022 [cited. 2023 1/19/2023]; Available from: https://www.curesma.org/newborn-screening-for-sma/.
- [14]. Dangouloff T, Vrscaj E, Servais L, Osredkar D, Group SNWS. Newborn screening programs for spinal muscular atrophy worldwide: Where we stand and where to go. Neuromuscul Disord. 2021;31:574–582. [DOI] [PubMed] [Google Scholar]
- [15]. Zolgensma Global Managed Access Program in. 2023.. 2023 [cited. 2023; Available from: https://www.novartis.com/news/zolgensma-global-managed-access-program-2023..
- [16]. Boardman FK, Young PJ, Griffiths FE. Newborn screening for spinal muscular atrophy: The views of affected families and adults. Am J Med Genet A. 2017;173:1546–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Boardman FK, Young PJ, Warren O, Griffiths FE. The role of experiential knowledge within attitudes towards genetic carrier screening: A comparison of people with and without experience of spinal muscular atrophy. Health Expect. 2018;21:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Boardman FK, Young PJ, Griffiths FE. Population screening for spinal muscular atrophy: A mixed methods study of the views of affected families. Am J Med Genet A. 2017;173:421–434. [DOI] [PubMed] [Google Scholar]
- [19]. Wood MF, Hughes SC, Hache LP, et al. Parental attitudes toward newborn screening for Duchenne/Becker muscular dystrophy and spinal muscular atrophy. Muscle Nerve. 2014;49:822–8. [DOI] [PubMed] [Google Scholar]
- [20]. Qian Y, McGraw S, Henne J, Jarecki J, Hobby K, Yeh WS. Understanding the experiences and needs of individuals with Spinal Muscular Atrophy and their parents: A qualitative study. BMC Neurol. 2015;15:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Deng S, Lee BH, Ciafaloni E. Parent Perceptions in Choosing Treatment for Infants With Spinal Muscular Atrophy Diagnosed Through Newborn Screening. J Child Neurol. 2022;37:43–49. [DOI] [PubMed] [Google Scholar]
- [22]. Kariyawasam DST, D’Silva AM, Vetsch J, Wakefield CE, Wiley V, Farrar MA. “We needed this”: Perspectives of parents and healthcare professionals involved in a pilot newborn screening program for spinal muscular atrophy. EClinicalMedicine. 2021;33:100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Lin CW, Kalb SJ, Yeh WS. Delay in Diagnosis of Spinal Muscular Atrophy: A Systematic Literature Review. Pediatr Neurol. 2015;53:293–300. [DOI] [PubMed] [Google Scholar]
- [24]. Cao Y, Cheng M, Qu Y, et al. Factors associated with delayed diagnosis of spinal muscular atrophy in China and changes in diagnostic delay. Neuromuscul Disord. 2021;31:519–527. [DOI] [PubMed] [Google Scholar]
- [25]. Tluczek A, Orland KM, Nick SW, Brown RL. Newborn screening: An appeal for improved parent education. J Perinat Neonatal Nurs. 2009;23:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. DeLuca JM, Kearney MH, Norton SA, Arnold GL. Parents’ experiences of expanded newborn screening evaluations. Pediatrics. 2011;128:53–61. [DOI] [PubMed] [Google Scholar]
- [27]. Salm N, Yetter E, Tluczek A. Informing parents about positive newborn screen results: Parents’ recommendations. J Child Health Care. 2012;16:367–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Conway M, Vuong TT, Hart K, Rohrwasser A, Eilbeck K. Pain points in parents’ interactions with newborn screening systems: A qualitative study. BMC Pediatr. 2022;22:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Chudleigh J, Buckingham S, Dignan J, et al. Parents’ Experiences of Receiving the Initial Positive Newborn Screening (NBS) Result for Cystic Fibrosis and Sickle Cell Disease. J Genet Couns. 2016;25:1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Buchbinder M, Timmermans S. Newborn screening for metabolic disorders: Parental perceptions of the initial communication of results. Clin Pediatr (Phila). 2012;51:739–44. [DOI] [PubMed] [Google Scholar]
- [31]. Grob R. Is my sick child healthy? Is my healthy child sick?: Changing parental experiences of cystic fibrosis in the age of expanded newborn screening. Soc Sci Med. 2008;67:1056–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and/or its supplementary material.