Abstract
Docetaxel (DTX) and cabazitaxel (CBZ) are guideline-chemotherapy treatments for metastatic castration-resistant prostate cancer (mCRPC), which comprises the majority of prostate cancer (PCa) deaths. TNF-related apoptosis inducing ligand (TRAIL) is an anti-cancer agent that is selectively cytotoxic to cancer cells; however, many human cancers are resistant to TRAIL. In this study, we sensitized androgen-independent and TRAIL-resistant PCa cells to TRAIL-mediated apoptosis via taxane therapy and examined the mechanism of sensitization. DU145 and PC3 cells displayed no significant reduction in cell viability when treated with soluble TRAIL, DTX, or CBZ alone indicating that both cell lines are resistant to TRAIL and taxanes individually. Taxane and TRAIL combination synergistically amplified apoptosis strongly suggesting that taxanes sensitize prostate cancer cells to TRAIL. A Jun N-terminal kinases (JNK) inhibitor inhibited apoptosis in treated cells and significantly reduced death receptor expression indicating JNK activation by ER stress sensitizes PCa cells to TRAIL-induced apoptosis by upregulating DR4/DR5 expression. In addition, suppression of C/EBP homologous protein (CHOP) reduced TRAIL sensitization in both cell lines indicating that ER stress-related apoptosis is mediated, in part by CHOP. Cytochrome c knockdown showed a significant decrease in sensitivity in PC3 cells, but not in Bax-deficient DU145 cells. A computational model was used to simulate apoptosis for cells treated with taxane and TRAIL therapy as demonstrated in in vitro experiments. Pretreatment with taxanes sensitized cells to apoptosis induced by TRAIL-mediated apoptosis, demonstrating that combining TRAIL with ER stress inducers is a promising therapy to reverse TRAIL resistance to treat mCRPC.
Keywords: Prostate cancer, taxanes, TRAIL, ER stress, synergy
Introduction
Prostate cancer is the second leading cause of death in U.S. men.(1, 2) Once PCa has reached an advanced stage, that no longer responds to hormone treatment or chemotherapy, a term called metastatic castration-resistant prostate cancer (mCRPC), treatment options are limited.(3) FDA-approved chemotherapies for mCRPC are taxanes, docetaxel (DTX) and cabazitaxel (CBZ). DTX is the first line of treatment given to mCRPC patients who stop responding to conventional therapies.(4–6) Unfortunately, most patients develop resistance to DTX. Cabazitaxel, the dimethoxy derivative of DTX, becomes the second line of treatment demonstrating a survival benefit for DTX-refractory mCRPC patients.(6, 7) Effective treatment options for mCRPC patients still remain a significant clinical problem since resistance to chemotherapy treatment and metastatic progression still occur. In the last decade, there has been more attention and focus on cancer stem cells (CSCs) and their role in tumor recurrence and poorer prognosis in mCRPC patients. Like stem cells, cancer stem cells constitute a small subpopulation in tumors that have clonogenic and self-renewal abilities that can help explain their tumorigenicity, metastatic potential, and chemotherapy/radiotherapy resistance.(8–11)
A promising anticancer therapeutic agent is TNF-related apoptosis-inducing ligand (TRAIL), a type II transmembrane protein that selectively kills cancer cells sparing normal cells. Apoptosis is induced when TRAIL binds to death receptors DR4 and DR5 on the surface of a cell.(12, 13) Previous studies in our lab have used TRAIL liposomes to effectively target and kill circulating tumor cells (CTCs) while reducing metastatic tumor burden in various animal models.(14–16) TRAIL does have a limitation due to the variety of mechanisms cancer cells undergo to develop TRAIL resistance.(17, 19)
The endoplasmic reticulum (ER) is highly dynamic organelle that is responsible for protein and lipid synthesis in eukaryotic cells.(20, 21) Protein production, folding, transport, and maintaining protein homeostasis is one of the main functions of the ER and is important in the viability of cells. When the normal functions of the ER are disturbed, a cellular stress response is initiated called the unfolded protein response (UPR), that attempts to reestablish normal ER function and restore homeostasis.(22–24) If ER stress is chronic or prolonged and irreversible cell damage occurs, there is a switch from pro-survival to pro-apoptotic signaling pathways involving distinct ER stress sensors.(25, 26)
Previous studies have shown the sensitization of prostate cancer cells to TRAIL-induced apoptosis when treated with different small molecules.(27–36) Although there have been numerous studies that have examined TRAIL-sensitization in PCa cells with clinically-relevant chemotherapies, these studies do not fully explore the role of ER stress in the mechanism behind TRAIL-sensitization using these taxanes.(37–41) In this study, we investigated the role of ER stress in the mechanism of sensitization of PCa cells to TRAIL-induced apoptosis via taxane pretreatment, and we adopted a computational model to predict this synergistic effect of apoptosis in each cell line. We confirmed that pretreatment with taxanes significantly enhanced the susceptibility of DU145 and PC3 cells to TRAIL-induced apoptosis compared to taxane or TRAIL alone. This synergistic effect was mediated by CHOP and JNK, proapoptotic factors associated with chronic ER stress. Taxane treatment resulted in a marked increase in DR5 expression. This study provides new insight into the mechanism of TRAIL sensitization by ER stress and mitochondrial pathways in vitro and presents a useful computational model of these processes.
Materials and Methods
Cell Culture
Prostate cancer cell lines DU145 (ATCC #HTB-81) and PC3 (ATCC #CRL-1435) were obtained from American Type Culture Collection (Manassas, VA, USA). DU145 cells were maintained in Eagle’s Minimum Essential Medium (EMEM) cell culture media (Corning, Corning, NY, USA). PC3 cells were cultured in F12 cell culture media (Gibco, ThermoFisher, Waltham, MA, USA). Media was supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) PenStrep (both purchased from Gibco) under humidified conditions at 37˚C and 5% CO2. All cell lines were obtained in Spring 2018 and placed in liquid nitrogen until required for use. Cells were tested twice using a Mycoplasma Detection Kit (ATCC 30–1012K) following manufacturer’s protocol. Testing occurred once after thawing at passage 3 and then near the end of experiments at passage 15. Test results were negative. Cells were last tested 04/01/2020.
Chemicals/Reagents
Cabazitaxel (ADV465749196) and docetaxel (01885) were purchased from Sigma Aldrich (St. Louis, MO, USA). Both taxanes were dissolved in a 1:10 solution of DMSO:PBS (pH 7.4) to a final concentration of 100 μM. Dimethylsulfoxide (DMSO) was purchased from ATCC. Soluble histidine-tagged TNF-related apoptosis-inducing ligand (TRAIL) (BML-SE721–0100) was purchased from Enzo Life Sciences (Farmingdale, NY, USA). Azoramide (5796), SB 202190 p38 MAPK inhibitor (1264), and SP 600125 JNK inhibitor (1496) were all purchased from Tocris (Minneapolis, MN, USA).
Small Interfering RNA (siRNA) Transfection
Two siRNA oligonucleotide sequences were used to target Smac (siDiablo/Smac; Assay ID: 290077 and 182519), Cytochrome C (siCYCS; Assay ID: 132742 and 132743), and CHOP (siDDIT3; Assay ID: s3995 and s3996). A negative control siRNA (Scr; AM4611) was used to control for siRNA delivery effects. All were purchased from Thermo Fisher Scientific (Grand Island, NY, USA). Cells were plated in 12-well plates and transfected using the siRNAs, Lipofectamine RNAIMAX reagent (Invitrogen), and Opti-MEM (Gibco) according to the manufacturer’s instructions. Cell media was replaced after 48 hours and then cells were treated with taxanes alone (24 hr), sTRAIL (24hr) alone or in combination (24 + 24 = 48hr). The final concentration for each siRNA was 30 nM.
Annexin V/PI Apoptosis Assay
Human prostate cancer cell lines DU145 and PC3 (5×104 cells) were seeded in 24-well plates overnight and then exposed to TRAIL (100 ng/mL), taxane drug (0.25μM DTX or CBZ), inhibitor (20μM Azoramide, SB202190, or SP600125), or a combination for 24 hr. For pretreatment studies, cells were treated with inhibitor for 24 hr, then taxane for 24hr, then TRAIL for 24hr. For post-treatment studies, cells were treated with a taxane for 24 hr, then inhibitor for 24hr, then TRAIL for 24hr. The sequential treatment plan was chosen to determine the ability and capacity of sensitization that occurs with taxane alone without TRAIL treatment and to allow adequate time for protein translation. Cells were harvested using Accutase™ (STEMCELL Technologies, Cambridge, MA, USA) and analyzed by an Annexin V/PI flow cytometry assay to assess cell viability. FITC Annexin V (556419) and propidium iodide (556463) staining solutions were purchased from BD Biosciences (San Diego, CA, USA). Staining of untreated and treated cells was carried out according to the manufacturer instructions. Cells were incubated for 15 min with reagents at RT in the dark and immediately analyzed using a Guava EasyCyte 12HT benchtop flow cytometer (MilliporeSigma). Viable cells were classified as AV-/PI-, early apoptotic cells as AV+/PI-, late-stage apoptotic cells as AV+/PI+, and necrotic cells as AV-/PI+. Flow cytometry plots were analyzed using FlowJo software (FlowJo, Ashland, OR, USA). The following control samples were used to calibrate the instrument: unlabeled cell samples to evaluate the level of autofluorescence and adjust the instrument accordingly, and cell samples labeled individually with Annexin V and PI to define the boundaries of each cell population.
Synergistic Evaluation by Jin’s Formula and Chou-Talalay Method
The synergistic effect of combined taxane and TRAIL was analyzed by Jin’s formula (36, 42) and Chou-Talalay method.(43, 44) For Jin’s formula, , where , and are the average inhibitory effects of the combination treatment, taxane only and TRAIL only, respectively. In this method, Q < 0.85 indicates antagonism, 0.85 < Q < 1.15 indicates additive effects and Q > 1.15 indicates synergism. The , and values were obtained from the apoptosis assay. These values were analyzed further using CompuSyn software (45) to obtain combination index (CI) values for additive effect (CI = 1), synergism (CI < 1), and antagonism (CI >1) in drug combinations.
Cancer Stem Cell Identification in Surviving Fraction
Cancer stem cells (CSCs) were characterized as CD44+/CD24- subpopulations. Following the apoptosis assay protocol described previously, cells from each treatment group were also labeled using anti-CD44-APC (338805) and anti-CD24-Brilliant Violet 421™ (311121) monoclonal antibodies (BioLegend San Diego, CA, USA). Mouse IgG1 APC (400119) and mouse IgG2a Brilliant Violet 421™ (400259) constituted isotype controls (BioLegend). The following control samples were used to calibrate the instrument: unlabeled, Annexin-V only, PI only, AV/PI, mouse IgG1 APC only, mouse IgG2a Brilliant Violet 421™ only, anti-CD44-APC only, and anti-CD24-Brilant Violet 421™. Flow cytometry plots were analyzed using FlowJo software (FlowJo, Ashland, OR, USA).
Endoplasmic Reticulum Stress Assay
PC3 and DU145 cells were seeded on 6-well plates at a density of 400,000 cells per well. The cells were incubated at 37°C and allowed to adhere overnight. The next day the cancer cells were treated with 0.25¼M of DTX or CBZ, or left untreated for 24 hr. After treatment, the cells were washed with HBSS without calcium or magnesium (Thermo Fisher; 14170112) and lifted with trypsin (Thermo Fisher; 25200072). The cells were collected and fixed with 4% paraformaldehyde (Electron Microscopy Sciences; 15714-S). After fixation, the cells were permeabilized using Triton X-100 (Sigma-Aldrich; T8787). The cells were then blocked using 5% bovine serum albumin (Sigma-Aldrich; A4161), and subsequently stained with a PE conjugated antibody against XBP1 (Biolegend; 647503) and an Alexa-Fluor 488 conjugated antibody against GRP78 (Thermo Fisher; 53–9768-80). Fluorescence was measured using a flow cytometer and median fluorescence intensity was used to determine expression of the ER stress markers XBP1 and GRP78.
MTT Assay
PC3 and DU145 cells were seeded on a 96 well plate at a density of 10,000 cells per well. The cells were incubated at 37°C and allowed to adhere overnight. The next day the cancer cells were either left untreated or treated with 0.25 ¼M of DTX or CBZ for 24 hr. The cells were then washed with HBSS without calcium or magnesium. After washing, the cells were treated with or without 25 ¼M Z-VAD-FMK (Tocris; 2163) for 30 min before treating cells with or without 100 ng/mL TRAIL. The cells were incubated with combinations of Z-VAD-FMK and TRAIL for 24 hr. After the treatment, cell viability and proliferation were measured using the MTT assay (Abcam; ab211091) according to the manufacturer’s instructions. Cells were incubated with 50 ¼L of serum free media and 50¼L of MTT reagent for 3 hours. The media was then aspirated and the cells were incubated with the MTT solvent for 15 min. Finally, the absorbance was measured at OD 570 nm using a microplate reader.
Death Receptor Expression
TRAIL receptor surface expression was analyzed using Human TruStain blocking solution (422302), PE Mouse IgG1 isotype control (400112), PE anti-human DR4 (307206), and PE anti-human DR5 (307406). Reagents were purchased from BioLegend (San Diego, CA, USA). Cells were plated in 24-well plates and treated with CBZ and DTX for 24 hr. Cell were then lifted, fixed with 4% paraformaldehyde, blocked with TruStain in bovine serum albumin (BSA), and incubated with respective antibodies before performing flow cytometry. The median fluorescence intensity was measured and analyzed to determine relative expression.
Antibodies and Western Blot Analysis
DU145 and PC3 cells were transfected with siRNA oligonucleotides or incubated overnight and subsequently treated with different treatments for the indicated times. Afterwards, cells were rinsed with sterile PBS and lysed with 4x Laemmli sample buffer (Bio-Rad; 1610747) and then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) [7% (w/v) for DR4 and DR5, 15% (w/v) for Diablo/SMAC, cytochrome c, and CHOP] and transferred to PVDF membranes. After transfer, membranes were blocked with 5% milk (Boston BioProducts, Ashland, MA, USA) in tris-buffered saline supplied with 0.1% Tween (Thermo Fisher Scientific). Primary antibodies were prepared at 1:500 dilution in 5% milk in the case of DR5 (Abcam; ab199357) and DDIT3 (Abcam; ab1419). Smac (Cell Signaling 15108) primary antibody was prepared at 1:1000 dilution in 5% milk. In the case of DR4 (Abcam; ab8414), cytochrome c (Abcam; ab133504), GAPDH (Millipore; MAB374), primary antibodies were prepared at 1:5000 dilution in 5% milk. Anti-rabbit or anti-mouse secondary antibodies conjugated to horseradish peroxidase (Rockland, Pottstown, PA, USA) were prepared at 1:2000 dilution in 5% milk. Membranes were imaged with West Pico (Thermo Fisher Scientific) per their respective protocols, using an ImageQuant LAS-4000 system (GE Healthcare, Chicago, IL, USA).
Computational Model
The computational simulation was performed in MATLAB using the systems-ODE solving function ‘ode15s’. Figures utilizing the simulation were created in MATLAB. The systems of ODEs used in the simulation were derived from the Hope-King and Albeck-Sorger models.(46, 47) The biochemical reactions were modeled using the mass-action kinetic equations shown in Hope et. al. A cell was considered apoptotic when the concentration of cPARP was half the initial condition of PARP. Mitochondrial permeability was determined by measuring the concentration of cytochrome c present in the cytosol. To create an ER-stressed or taxane-treated condition, the initial conditions of Bcl-2, cIAP1/2, and DR4/5 were altered as shown in Supplementary Table 1. To account for the different apoptotic responses of DU145 and PC3, cells different initial conditions were used for Bax and x-linked inhibitor of apoptosis protein (XIAP) as shown in Supplementary Table 2.
Statistical Analysis
GraphPad Prism 8 (San Diego, CA, USA) software was used to plot and analyze data sets. Student’s t-test was used for comparisons between two groups, with p < 0.05 considered significant. ANOVA was used for comparing multiple groups with p < 0.05 considered significant. Data are presented as mean ± SD with at least three independent replicates used for each experiment..
Results
Pretreatment with CBZ and DTX sensitize DU145 and PC3 cells to TRAIL-induced apoptosis synergistically
To determine whether metastatic, hormone-independent DU145 and PC3 cells were resistant to TRAIL, cells were treated with 100 ng/mL of soluble TRAIL for 24 hr and analyzed by flow cytometry (Supplementary Figure S1A). PC3 cells displayed no significant reduction in cell viability compared to the untreated control cells, confirming their resistance to TRAIL-induced apoptosis. DU145 cells displayed a more significant reduction in cell viability compared to untreated control cells, indicating an increased susceptibility to apoptosis although cell viability remained high at ~80% (Supplementary Figure S1B).
Since taxanes can inhibit cell growth through G2-M arrest, cell proliferation was explored and measured using an MTT assay to capture growth inhibition as well as cell death. Additionally, a caspase inhibitor, Z-VAD- FMK, was used to illustrate growth inhibition through the inhibition of apoptosis (Supplementary Figure S4). For both cell lines, MTT absorbance decreased when treated with CBZ only, DTX only, or CBZ/DTX in combination with TRAIL demonstrating the growth inhibitory effects of taxanes alone and of the combination. For DU145 cells, Z-VAD-FMK had no effect on any treatment including the combination. For PC3 cells, increased MTT absorbance was seen in Z-VAD-FMK cells illustrating that growth inhibition is impeded by the apoptosis inhibition (Supplementary Figures S4A and S4B).
To determine cell viability when exposed to taxanes, DU145 and PC3 cells were treated for 24 hr with CBZ and DCX at concentrations ranging from 0.01 μM to 1μM. At these dosages, cell viability for both cell lines was >80% when treated with taxanes alone (Supplementary Figure S1C). Cells were also treated with each taxane over a 24–96 hr period to assess apoptosis over prolonged exposure times (Supplementary Figure S1D). The cell lines exhibited a time-dependent response to each taxane, demonstrating decreasing cell viability over time, with DU145 cells showing increased cell death compared to PC3 cells. When treated with either docetaxel or cabazitaxel, DU145 and PC3 cells showed rounder morphologies and were moderately detached from the plates but still viable after 24 hr.
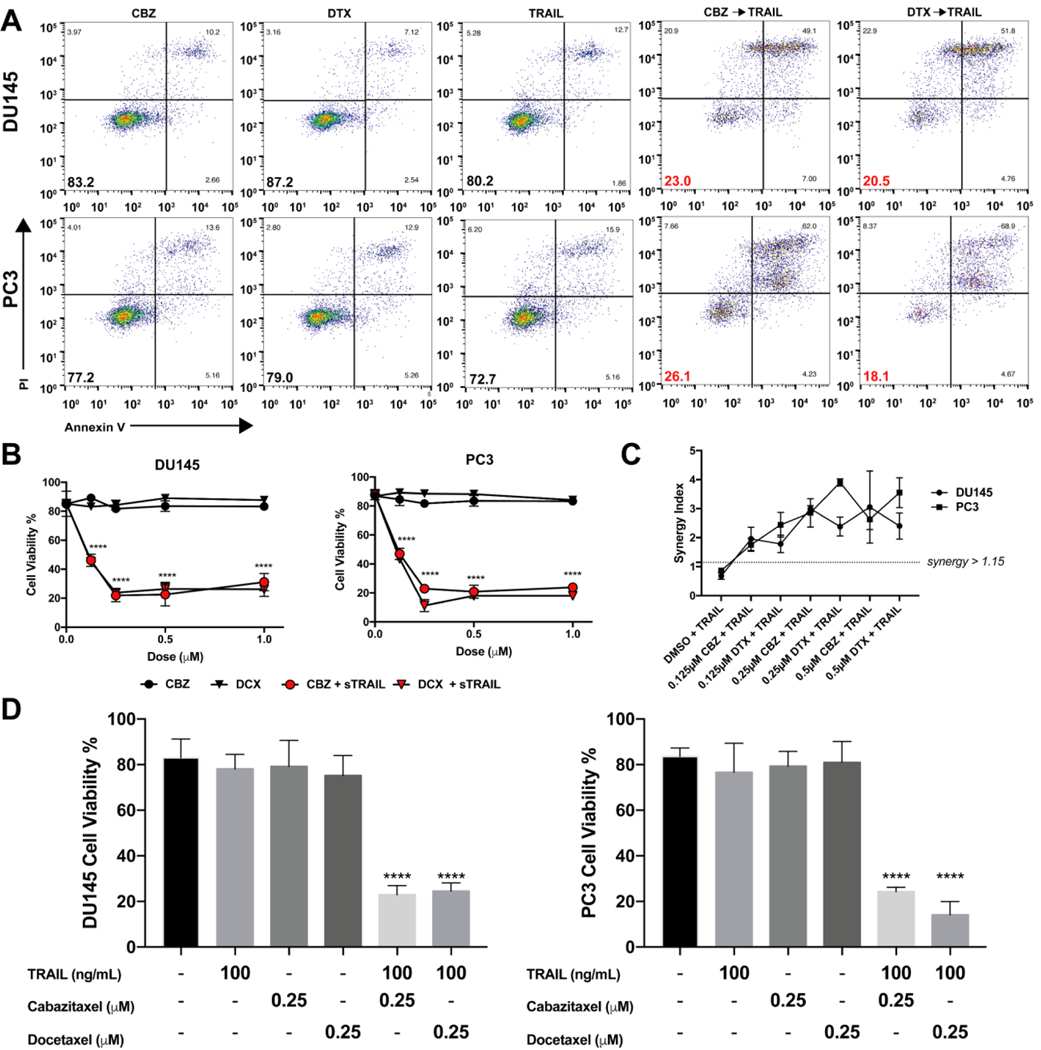
In both cells, pretreatment with various concentrations (0–1μM) of each taxane for 24 hr and then treatment with 100 ng/mL of TRAIL was efficient in inducing apoptosis in DU145 and PC3 cells (Figure 1A). Cell viability decreased two-fold when exposed to the lowest concentration of taxane at 0.125 μM in combination with TRAIL. At 0.25 μM, cell viability decreased almost four-fold in DU145 and remained at this level with increasing concentration of taxane (Figure 1B). We determined the most effective concentration to be 0.25 μM to produce a significant response of TRAIL-induced apoptosis. Further increasing the concentration did not have an additional effect on cell viability for the sequential therapy. Pretreating cells with taxanes for 24 hr followed by TRAIL significantly decreased cell viability from ~80% down to ~20% (Figure 1D). Furthermore, taxane and TRAIL exerted a synergistic inhibitory effect (Q > 1.15, CI < 1) in both cell lines. Such synergy was observed when TRAIL was combined with either taxane (Figure 1C and Supplementary Table S3). These data confirm that TRAIL-induced apoptosis is promoted in DU145 and PC3 cells pretreated with either CBZ or DTX, and demonstrates a significant synergistic effect.
Figure 1.
Synergistic effect of taxane plus TRAIL treatment in TRAIL-mediated apoptosis of DU145 and PC3 cells. A. Representative flow cytometry plots of DU145 and PC3 cells after treatment with taxane alone, TRAIL alone, or taxane then TRAIL. B. Cell viability of DU145 and PC3 cells when treated with varying concentrations of taxane alone or in combination with TRAIL C. Synergistic anti-tumor effect of combined taxane and TRAIL in DU145 and PC3 cells by Jin’s formula. D. Cell viability of DU145 and PC3 cells when treated with 0.25 μM taxane alone or in combination with TRAIL. The values represent the mean ± SD (n=6). **** p ≤ 0.0001 compared to control.
Cancer stem cells comprise the surviving fraction in DU145 and PC3 cells
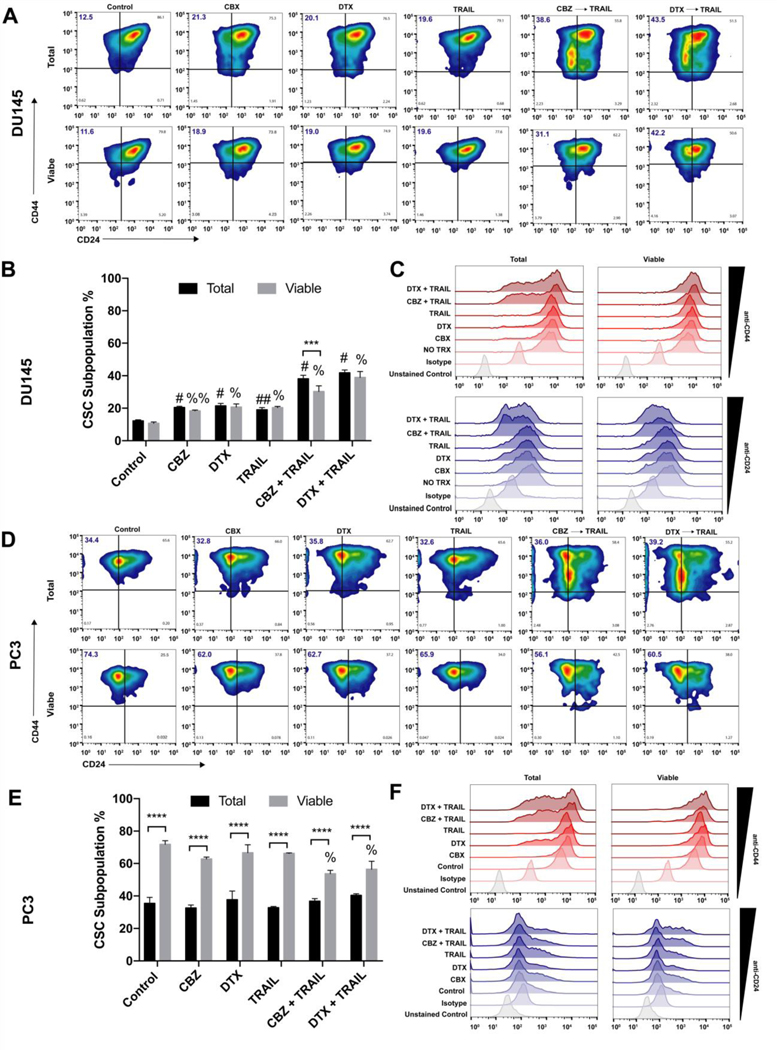
We used flow cytometry to help characterize the surviving fraction of stem-like tumor cells in the taxane plus TRAIL treated groups. We identified the cancer cells with stem-like characteristics as the CD44+/CD24- subpopulation (Figure 2A, Figure 2D). In the total cancer cell population of DU145 cells, the CSCs subpopulation is relatively low at ~12% in the control group and linearly increases to ~40% in both combination treatment groups. The CSC subpopulation in the viable cell population follows the same pattern of ~11% in the control group and ~40% in both combination treatment groups (Figure 2B). There was a significant difference when compared to the control of each population and their respective treatment groups; however, no significant difference was observed between total population vs viable subpopulation in each treatment group suggesting that CSCs in this cell line may slowly differentiate and proliferate into more CSCs in response to each progressive therapy (Figure 2C).
Figure 2.
Analysis of CD44+/CD22- stem cell population in viable percentage of DU145 and PC3 cells. A, D. Representative CD44/CD24 flow cytometry plots of DU145 and PC3 cells after each treatment displying total percentage of CD44+/CD22- stem cells in first quadrant. B,E. Mean fluorescence intensity of CD44+ and CD24+ cell populations in DU145 and PC3 comparing total and viable populations.
C,F. Stacked histograms displaying cell CD44+ and CD24+ cell population shifts in DU145 and PC3 cells across treatment groups. The values represent the mean ± SD (n=6). *** p < 0.001, **** p ≤ 0.0001, significantly different from total vs viable. ## p ≤ 0.001, # p ≤ 0.0001, significantly different from total population control. %% p ≤ 0.001, % p ≤ 0.0001, significantly different from viable supopulation control.
In the total cancer cell population of PC3 cells, the CSCs subpopulation is moderately higher at ~35% in the control group and remains at this percentage even in both combination treatment groups. Surprisingly, the CSC subpopulation in the viable cell subpopulation was relatively high at ~65% in all groups except the combination groups in which there was a 10% decrease (Figure 2E). A significant difference was observed between the total population and the viable subpopulation in each group, but none was observed when comparing each population to their respective controls except for in the viable combination group. Despite the difference in CSCs percentage compared to DU145 cells, PC3 cells displayed the same pattern in distribution among populations and treatment groups (Figure 2F).
Mitochondrial and ER stress pathways of apoptosis affect sensitization
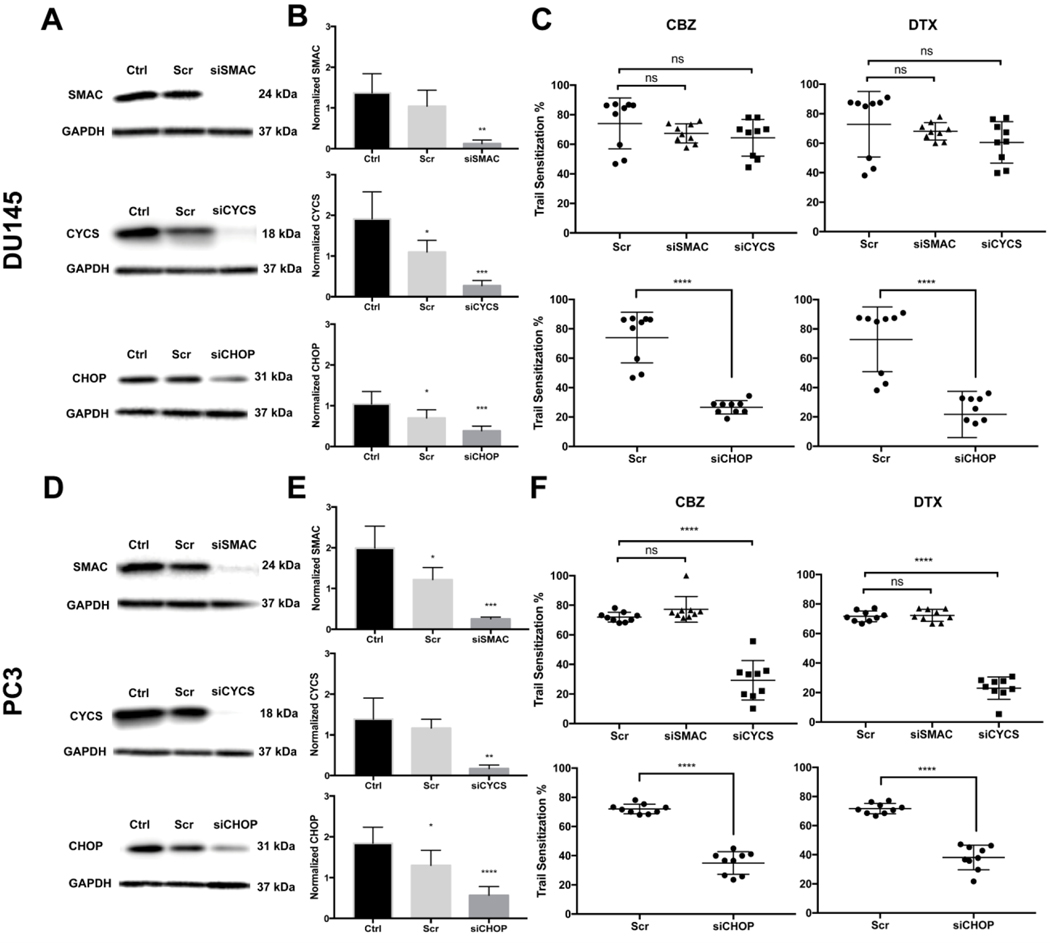
DU145 and PC3 cells were transfected with the siRNA targeting SMAC and CYCS and then treated with TRAIL alone or TRAIL with taxane to calculate the degree of TRAIL sensitization. The efficiency of each siRNA knockdown in each cell line was confirmed via Western blot (Figure 3A and 3D). The relative expression of SMAC and CYCS showed a significant decrease compare to control indicating the knockdown was effective (Figure 3B and 3E). Inhibition of SMAC showed no significant difference in cell death when compared to controls of both cell lines (Figure 3C and 3F). In DU145 cells, cytochrome c inhibition resulted in no significant sensitization effect; however, PC3 cells showed a significant decrease in taxane-mediated TRAIL sensitization (Figure 3C and 3F). Sensitization of these cells was reduced to 29.3% and 23.04% for CBZ- or DTX-treated cells with knocked down cytochrome c compared to 72% and 71.3% in scrambled-siRNA CBZ- or DTX-treated cells, respectively. DU145 cells are deficient in Bax, indicating that mechanisms other than mitochondrial apoptosis induction are activated.(48)
Figure 3.
Cytochrome c knockdown reduces TRAIL-induced apoptosis in PC3 cells, but not in Bax-deficient DU145 cells. CHOP knockdown reduces TRAIL-induced apoptosis in DU145 and PC3 cells. A,D. Western blot of SMAC, cytochrome c, and CHOP expression in DU145 and PC3 cells after siRNA knockdown. B,E. Relative expression of SMAC, cytochrome c, and CHOP after siRNA knockdown compared to control. C,F. TRAIL sensitization of DU145 and PC3 cells when treated with respective taxane and TRAIL after scrambled siRNA, SMAC/Diablo, and cytochrome c knockdown. The values represent the mean ± SD (n=6 or 9). * p < 0.05, ** p < 0.005, *** p < 0.001, **** p ≤ 0.0001.
In order to demonstrate the activation of ER stress, the modulation of the expression of GRP78 (a biomarker for ER stress) and XBP1 (downstream transcription factor of JNK) were analyzed (Supplementary Figures S4C and S4D). In DU145 cells, GRP78 expression significantly increased upon treatment with CBZ and DTX and XBP1 expression was more pronounced. In PC3 cells, only DTX treated cells showed an increased in expression compared to untreated control and XBP1 expression did not significantly change. These results indicate that ER stress is occurring for both cells lines when treated with DTX. PC3 cells treated with CBZ showed no significant increase in the ER stress surface markers (Supplementary Figures S4C and S4D).
Chronic ER stress can initiate apoptotic mechanisms, like CHOP, that lead to cell death.(49) DU145 and PC3 cells were transfected with CHOP siRNA, and then treated with TRAIL and/or taxane to calculate sensitization. The efficiency of each siRNA knockdown in each cell line was confirmed via Western blot (Figure 3A and 3D). The relative expression of CHOP displayed a significant decrease compare to control indicating the knockdown was effective (Figure 3B and 3E). In both cell lines, CHOP inhibition showed a significant effect on TRAIL sensitization and effectively attenuated taxane/TRAIL-induced death. Knockdown of CHOP in DU145 cells reduced sensitization from 74% and 72.8% for scrambled-siRNA CBZ or DTX treated cells, compared to 26.6% and 21.7% in siCHOP CBZ or DTX treated cells, respectively (Figure 3C). Knockdown of CHOP in PC3 cells reduced sensitization from 72% and 71.7% for scrambled-siRNA CBZ or DTX treated cells compared to 35% and 38.1% in siCHOP CBZ- or DTX-treated cells, respectively (Figure 3F).
Increased DR5 expression in PCa cells after treatment with CBZ and DCX
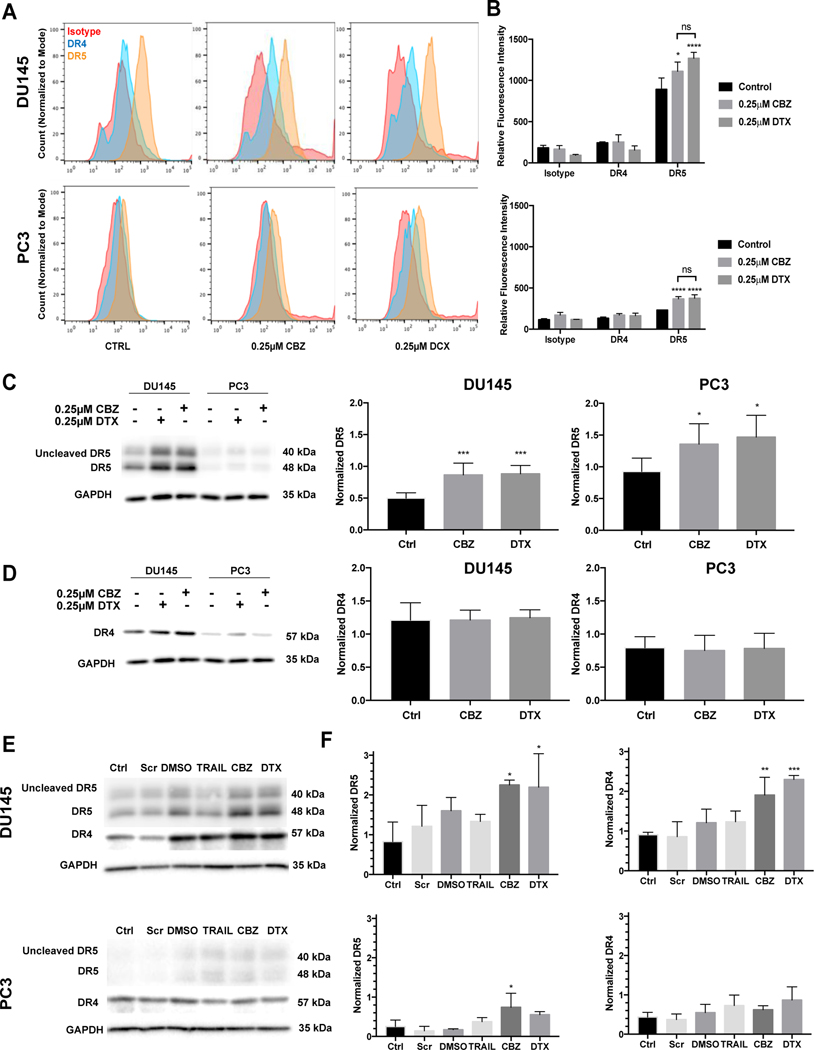
Treatment with taxanes alone caused a marked increase in cell surface expression of DR5 compared to no treatment for both DU145 and PC3 cells, but not DR4 (Figure 4A). There is a higher baseline expression of DR5 in DU145 when compared to PC3 innately; however, both cell lines express higher levels of DR5 when treated with taxanes (Figure 4B). The relative fluorescence intensity for DR5 in DU145 cells exhibited a 24.5% increase when treated with CBZ and a 42.3% increase when treated with DCX compared to control. The relative fluorescence intensity for DR5 in PC3 cells exhibited a 58.7% increase when treated with CBZ and a 61.8% increase when treated with DCX compared to control. When measuring the protein expression using Western blot, we observed a significant increase in DR5 expression upon exposure to 0.25 μM of each taxane in both cell lines (Figure 4C). DR4 expression did not significantly change after exposure to each taxane (Figure 4D).
Figure 4.
Taxane pre-treatment increases death receptor expression. A. Representative histograms of death receptor expression using flow cytometry. B. Mean fluorescent intensity of DR4 and DR5 compared to isotype control. C. Western blot and relative expression of DR5 in DU145 and PC3 cells treated with 0.25μM CBZ or DTX. D. Representative Western blot and relative expression of DR4 in DU145 and PC3 cells treated with 0.25μM CBZ or DTX. E. Western blot of death receptor expression in siCHOP-knockdown DU145 and PC3 cells treated with DMSO, TRAIL, CBZ or DTX. F. Relative death receptor expression in siCHOP-knockdown DU145 and PC3 cells treated with DMSO, TRAIL, CBZ or DTX. The values represent the mean ± SD (n=6). * p < 0.05, ** p < 0.01, *** p ≤ 0.005, **** p< 0.0001.
There have been conflicting reports about DR5 regulation by CHOP in relation to ER stress and TRAIL sensitization.(50, 51) An increase in DR5 expression was observed in both cell types in response to taxane exposure with negligible effects on DR4 upregulation (Figure 4E and 4F). The inhibition of CHOP expression did not attenuate DR5 up-regulation, suggesting that taxane-induced DR5 expression is independent of CHOP. This further implies that CHOP is not the only or even a major regulator of DR5 induction in DU145 and PC3 cells; however, CHOP does have a role in the initiation of apoptosis in this taxane-induced ER stress-sensitization to TRAIL.
Death receptor regulation and synergistic apoptosis is mediated by JNK activation
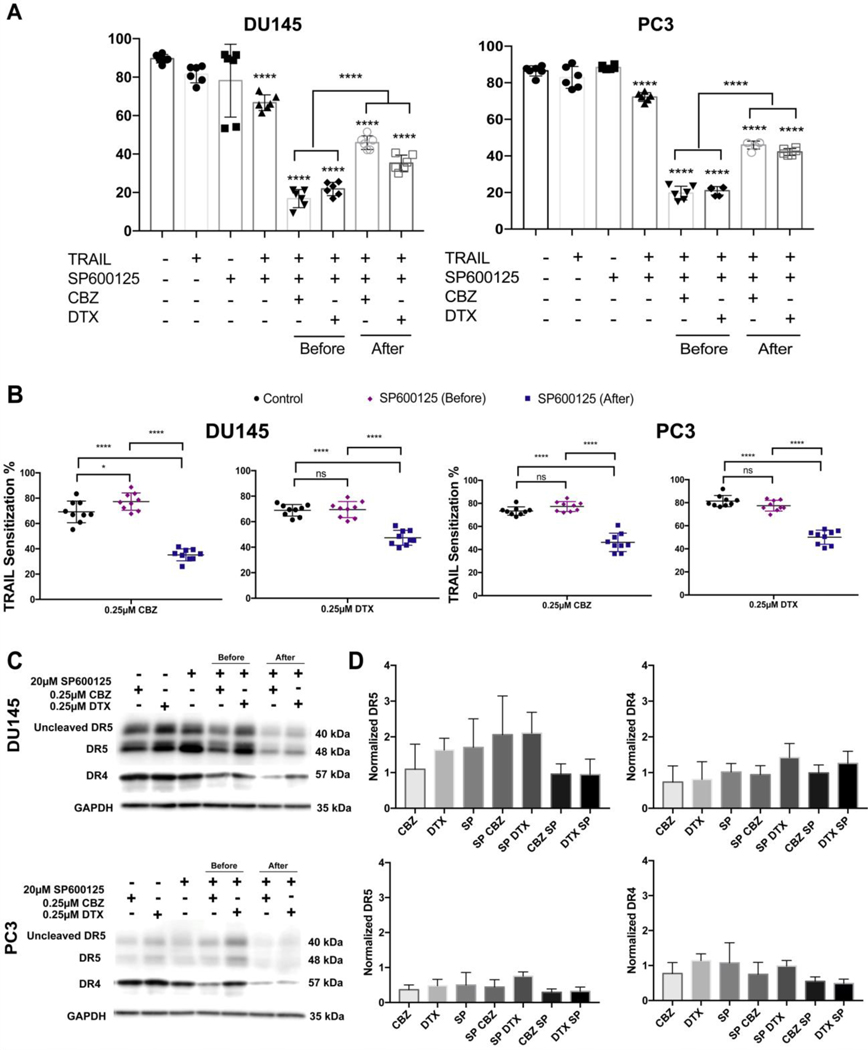
The post-transcriptional regulation of CHOP is mediated by the p38 MAP kinase family coinciding with JNK activation. In this study we inhibited p38 MAP kinase and JNK with SB202190 and SP600125 inhibitors. We also used a small molecule modulator of UPR, azoramide.(52) Azoramide pre- and post-treatment displayed a significant decrease in cell viability in both cell lines similar to taxane plus TRAIL alone (Supplementary Figure S2A and S2B). These data illustrate that azoramide does not improve ER-stressed cells that are committed to apoptosis. Pre- and post-treatment with SB202190 displayed a significant decrease in cell viability indicating that the p38 MAP kinase pathway does not contribute to the sensitization of DU145 and PC3 cells (Supplementary Figure S2A and S2B). Post-treatment and not pre-treatment with JNK inhibitor SP600125 was sufficient to abrogate the effects of TRAIL-mediated apoptosis after taxane sensitization (Figure 5A and 5B). These data demonstrate that the JNK signaling pathway is involved and necessary for the apoptotic response to ER stress.
Figure 5.
JNK activation is significant in ER-stress related apoptosis and death receptor regulation. A. Cell viability of DU145 and PC3 cells after pre- and post-treatement with 20 μM SP600125 (JNK inhibitor). B. TRAIL sensitization of DU145 and PC3 cells cells treated with JNK inhibitor before or after taxane (0.25 μM) therapy followed by TRAIL treatment. C. Western blot of death receptor expression in DU145 and PC3 cells treated with either CBZ, DTX, and JNK inhibitor alone or in combination. Cells were also treated with the JNK inhibitor before or after taxane exposure. D. Relative death receptor expression in DU145 and PC3 cells treated with either CBZ, DTX, and JNK inhibitor alone or in combination. Cells were also treated with the JNK inhibitor before or after taxane exposure. The values represent the mean ± SD (n=6 or 9). * p < 0.05, **** p< 0.0001.
To determine whether JNK is responsible for the up-regulation of DR5, DU145 and PC3 cells were treated with SP600125 and their lysates were analyzed for DR4 and DR5 expression via Western blot (Figure 5C). We observed a decrease in DR4 and DR5 expression in DU145 and PC3 cells treated with the JNK inhibitor after taxane exposure (Figure 5D). These data illustrate that CBZ and DTX induce death receptor expression via JNK activation with JNK acting as a regulator of DR4 and DR5 expression. It is proposed that post-treatment with the JNK inhibitor attenuated JNK activation and inhibited DR4 and DR5 upregulation.
Computational model of taxane and TRAIL synergy
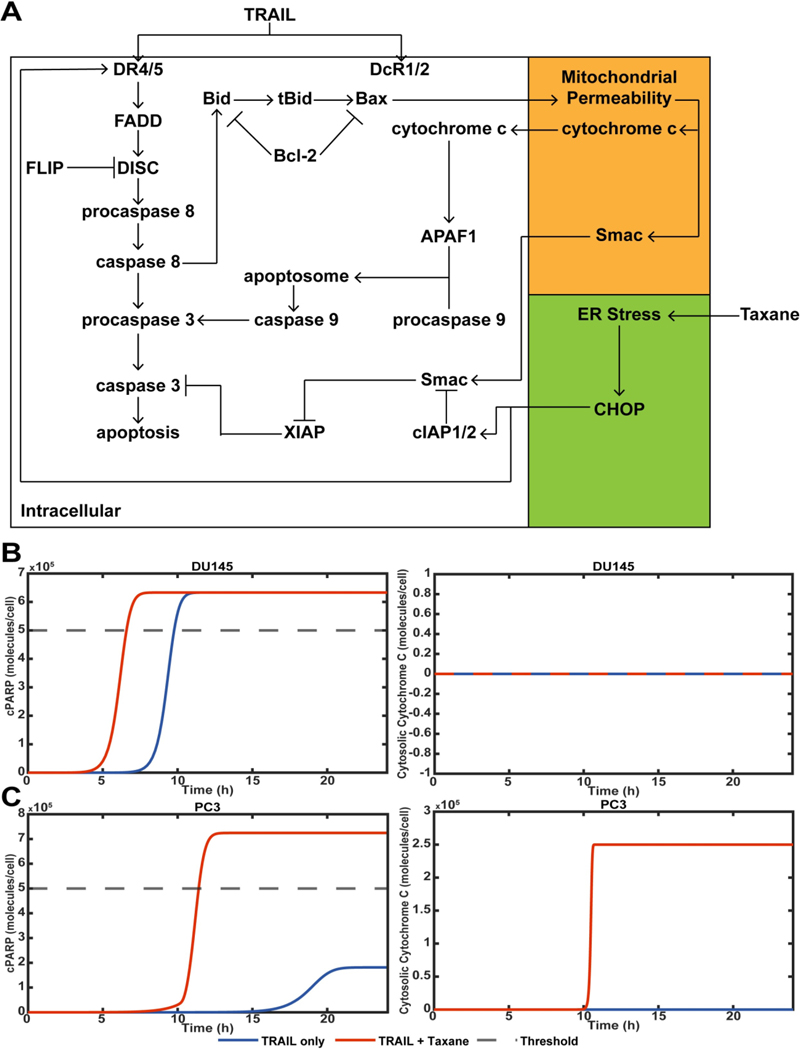
We further interrogated the observed mechanism in silico using an adapted computational model (Figure 6A).(46, 47) An ER-stressed condition was modeled for taxane-treated cells by altering the initial conditions of Bcl-2, DR4/5, and cIAP1/2 expression (Supplementary Table S1).(53)(54) DU145 and PC3 cells showed different modes of apoptosis in response to taxane plus TRAIL treatment. To account for this, DU145 and PC3 cells were given different initial conditions for Bax and XIAP (Supplementary Table S2). DU145 cells were modeled as having no Bax expression.(55) XIAP expression was also reduced for DU145 cells, as DU145 apoptosis was not dependent on cytochrome c or SMAC, indicating that DU145 cells undergo purely extrinsic apoptosis.
Figure 6.
Computational model of TRAIL-mediated apoptosis of DU145 and PC3 cells. A. Schematic of apoptosis signaling pathway of DU145 and PC3 cells in response to taxane and TRAIL treatment. B. cPARP concentration of DU145 cells treated with or without taxane therapy and TRAIL. The concentration of cytosolic cytochrome c to represent mitochondrial permeability. C. cPARP and cytochrome c concentrations for PC3 cells treated with or without taxane therapy and TRAIL.
Cleaved poly(ADP)-ribose polymerase (cPARP) was used as a measure of apoptosis, as its cleavage is indicative of late-stage apoptosis. When the cPARP concentration equaled half the initial condition of non-cleaved PARP, cancer cells were considered apoptotic. Cytochrome c was used to monitor mitochondrial permeability (Figure 6B and 6C). When DU145 cells were treated with combination therapy, the time until apoptosis was reduced. However, the overall amount of cPARP generated was not increased. This suggests that taxanes treatment enhances the potency of TRAIL. For Bax-deficient DU145 cells treated with TRAIL with or without taxane therapy, no mitochondrial permeability took place (Figure 6B).
PC3 cells treated with TRAIL and without taxane therapy did not pass the apoptotic threshold, whereas PC3 cells treated with combination therapy did. This suggests that taxane therapy had a substantial effect in increasing the potency of TRAIL applied to PC3 cells. PC3 cells are positive for Bax expression, allowing for mitochondrial permeability and the subsequent release of the pro-apoptotic protein cytochrome c. When taxanes were used in combination with TRAIL, mitochondrial permeability was observed, but not for cancer cells treated with TRAIL only. This simulation further suggests that taxanes enhance TRAIL-mediated apoptosis by enhancing the occurrence of mitochondrial permeability (Figure 6C).
The computational model was also used to probe how the differential expression of certain proteins will affect chemotherapy-induced sensitization of cancer cells to TRAIL. When mitochondrial outer membrane permeabilization (MOMP) or cell death did not occur, the time was plotted as 0 for visibility. For both cancer cell lines, increasing DR4/5 expression reduced the time until apoptosis occurred. For PC3 cells it also reduced the time until MOMP. Interestingly, MOMP preceded the time of cell death. This indicates that cell death continued to be dependent upon MOMP for PC3 cells. There was no MOMP for DU145 cells. To plot this, MOMP was set equal to 0 (Supplementary Figure S3A).
When XIAP expression was modified for DU145 cells, the time until cell death increased with increasing XIAP. At a certain point, XIAP prevented DU145 cells from undergoing apoptosis. This was represented by the time of death being set equal to 0 on the plot (Supplementary Figure S3B). This suggests that when XIAP reaches a certain level of expression, MOMP is necessary to induce apoptosis. However, due to DU145 cells being resistant to mitochondrial dysfunction, this is not possible. When XIAP expression was reduced for PC3 cells, MOMP would occur after the time of cell death. This shows that when XIAP is reduced, PC3 cells are no long reliant on MOMP for apoptosis to occur. As XIAP increased, MOMP no longer contributes to PC3 cells undergoing apoptosis (Supplementary Figure S3B).
Cytochrome c expression was varied as well for DU145 cells and PC3 cells. As expected, the cell death time of DU145 cells was not altered by cytochrome c expression (Supplementary Figure S3C). For PC3 cells, cytochrome c expression dramatically effected the time of cell death. When cytochrome c expression was low, PC3 cells would not undergo apoptosis in response to combination therapy. When cytochrome c expression was increased, the time of cell death was not significantly altered (Supplementary Figure S3C). Finally, when Smac expression was increased or reduced for PC3 and DU145 cells, there was no change in the time of cell death (Supplementary Figure S3D).
Discussion
In this study, we demonstrated that pretreatment with the clinically-used taxanes sensitizes PCa cells to TRAIL-induced apoptosis through JNK pathway activation and induction of DR4 and DR5 expression (Supplementary Figure S1 and Figure 1). These results are consistent with previous studies of human cancers where TRAIL-induced apoptosis was enhanced via DR4/DR5 regulation after exposure to DNA damage and chemotherapeutic agents.(56–61) For the first time, we established the role of the ER stress pathway in apoptosis induced by taxane and TRAIL combination in DU145 and PC3 cells accompanied by a new computational model that can predict responses to taxane plus TRAIL treatment. We used DTX and CBZ as first-line therapies for the sensitization of PCa cells to TRAIL. We found no significant difference in the TRAIL-mediated apoptotic response from either taxane pretreatment (Figure 1). This finding could potentially help bring clarity to the treatment sequencing in mCRPC based on patient-specific responses to each chemotherapeutic.
Current treatments target the bulk of the tumor and do not address the heterogeneity of cancer cells, including cancer stem cells.(62, 63) Previous studies using in vivo and in vitro models have demonstrated that cells positive for CD44, but lack CD24, are useful in identifying the CSC population.(10, 11, 40) Although there was a higher number of CSCs in PC3 cells, PC3 CSCs moderately responded to the combination therapy. DU145 CSCs appeared more resistant as the chemotherapy and combination treatments induced more phenotypical changes of non-CSCs to CSCs in the total population and viable subpopulation (Figure 2). There is a need for the devolopment of therapies that specifically target stem cell subpopulations to reduce their tumor-initiating potential, once more prostate stem cell markers are identified and standardized.
The mechanism by which taxanes increase the TRAIL sensitivity of PCa cells is still not completely understood and deserves further exploration. In this study, DU145 cells showed growth inhibition when exposed to taxanes or the combination. However, DU145 cells showed no pronounced effect when treated with apoptosis inhibitor, Z-VAD-FMK, suggesting that DU145 cells undergo a predominantly caspase-independent form of apoptosis.(64) On the other hand, PC3 cells displayed increased cell survival when treated with Z-VAD-FMK indicating the combination induces caspase-dependent apoptosis (Supplementary Figure S4). The activation of ER stress was seen in both cell lines when looking at GRP78 regulation specifically in DTX treatment groups (Supplementary Figures S4C and S4D).
Both DU145 and PC3 cells showed increased expression of DR5 compared to negative control cells when exposed to taxanes alone (Figure 4). In PC3 cells, there was a significant decrease in TRAIL sensitization in cells with knockdown cytochrome c, indicating that the intrinsic mitochondrial pathway is one of the moderators of apoptosis in these cells (Figure 3). The difference in TRAIL sensitization via the mitochondrial apoptotic pathway observed in this study further supports the idea that patient-specific therapy tailored to treatment response is needed and deserves further exploration in clinical trials.
Recent studies have shown that ER stress-mediated apoptosis is associated to autophagy through CHOP in breast cancer and human glioblastoma cells.(65)(66) Inhibiting CHOP in PCa cells reduced the apoptotic effect of the combination treatment in both cell lines (Figure 3). These findings suggest that each taxane may function at least partly through ER stress-induced apoptosis via CHOP. CHOP has been shown to activate other apoptotic pathways as a multifunctional transcription factor in the ER stress response.(67) CHOP can activate caspase-3 and BH3-only apoptotic proteins as well as inactivate BCL2 anti-apoptotic proteins through inhibition.(49) CHOP induces the downstream target gene GADD34 and dephosphorylates eIF2α which in turn leads to protein translation recovery increasing ER stress and apoptosis.(68) Production of reactive oxygen species and activation of cellular calcium signaling also leads to CHOP-mediated apoptosis.(67)
Since CHOP expression can be mediated by JNK, we explored the inhibitory response of SP600125 (JNK inhibitor).(39, 69) We observed an increase in cell viability that significantly differed from controls as well as pretreatment with the inhibitor (Figure 5). Furthermore, TRAIL sensitization was attenuated in the group treated with the JNK inhibitor after taxane exposure, suggesting that JNK must be activated in this ER stress-induced apoptotic pathway by each taxane. DR4 and DR5 were markedly reduced following a taxane then inhibitor treatment scheme (Figure 5). This finding suggests that JNK activation is one of the regulators of death receptor induction by taxanes. DU145 cells follow the ER stress-induced apoptotic pathway primarily via death receptor induction. In PC3 cells, death receptor induction is achieved by the cooperation of cell-intrinsic and cell-extrinsic pathways. In CHOP knockdown cells, PC3 but especially DU145 cells exhibited an increase in DR4/5 expression suggesting that CHOP is not an essential regulator of death receptor expression but has a major role in the ER stress apoptotic pathway. CHOP and JNK represent good targets to overcome TRAIL resistance and use in new drug therapies.
The experimental results from this study were used to inform the basis of the computational model. Both the model and the in vitro experiments showed that cytochrome c expression significantly affects chemotherapy-induced sensitization for PC3 cells (Figure 6). Using the simulation, DR4/5 expression was found to have a significant effect on cell death times for both DU145 and PC3 cells (Supplementary Figure S3). This model could be further used to examine the apoptotic response in different cell types based on cell-specific protein expression that affects apoptosis. In summary, we have demonstrated the synergistic effect of taxanes enhancing TRAIL-mediated apoptosis in two different PCa cells via ER stress and DR5 induction. Initially TRAIL resistant, the degree of apoptosis in these cells increased significantly when exposed to taxanes first, followed by TRAIL. Combination therapies and computer models such as these could help overcome tumor resistance mechanisms leading to better clinical outcomes for mCRPC patients.
Supplementary Material
Acknowledgements
KAG designed and performed all experiments, JMH researched and designed the computational model, KAG acquired, analyzed, and interpreted the data, KAG wrote the paper, WW and CAR-K contributed experimental expertise, and MRK supervised the research study and edited the manuscript.
Financial Support
This work was funded by NIH Grant No. R01CA203991 to M.R. King, NSF GRFP Grant No. DGE-1650441 to K.A. Grayson., and Alfred P. Sloan Fellowship to K.A. Grayson.
Footnotes
Conflict of Interest
The authors declare no potential conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019: Cancer Statistics, 2019. CA: A Cancer J Clin. 2019. Jan;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020. Jan;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002. May;2(5):389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagher R. Approval Summary: Docetaxel in Combination with Prednisone for the Treatment of Androgen-Independent Hormone-Refractory Prostate Cancer. Clin Can Res. 2004. Dec 15;10(24):8147–51. [DOI] [PubMed] [Google Scholar]
- 5.Slovin S. Chemotherapy and Immunotherapy Combination in Advanced Prostate Cancer. Clin Adv Hemat Onc. 2012. Feb;10(2);90–100. [PubMed] [Google Scholar]
- 6.Song P, Huang C, Wang Y. The efficacy and safety comparison of docetaxel, cabazitaxel, estramustine, and mitoxantrone for castration-resistant prostate cancer: A network meta-analysis. Int J Surgery. 2018. Aug 1;56:133–40. [DOI] [PubMed] [Google Scholar]
- 7.Tsao C-K, Cutting E, Martin J, Oh WK. The role of cabazitaxel in the treatment of metastatic castration-resistant prostate cancer. Ther Adv Urol. 2014. Jun;6(3):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001. Nov;414(6859):105–11. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Goncalves KA, Lyu B, Yuan L, Hu G. Chemosensitization of prostate cancer stem cells in mice by angiogenin and plexin-B2 inhibitors. Commun Biol. 2020. Dec;3(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44þ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. [DOI] [PubMed] [Google Scholar]
- 11.Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+CD24− prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008. Feb;98(4):756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003. Nov;22(53):8628–33. [DOI] [PubMed] [Google Scholar]
- 13.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999. Jul 15;104(2):155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell MJ, Wayne E, Rana K, Schaffer CB, King MR. TRAIL-coated leukocytes that kill cancer cells in the circulation. PNAS. 2014. Jan 21;111(3):930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wayne EC, Chandrasekaran S, Mitchell MJ, Chan MF, Lee RE, Schaffer CB, et al. TRAIL-coated leukocytes that prevent the bloodborne metastasis of prostate cancer. J Control Release. 2016. Feb 10;223:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jyotsana N, Zhang Z, Himmel LE, Yu F, King MR. Minimal dosing of leukocyte targeting TRAIL decreases triple-negative breast cancer metastasis following tumor resection. Sci Adv. 2019. Jul 1;5(7):eaaw4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trivedi R, Mishra DP. Trailing TRAIL Resistance: Novel Targets for TRAIL Sensitization in Cancer Cells. Front Oncol. 2015. Apr 2;5(69):1–20. t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005. Mar;12(3):228–37. [DOI] [PubMed] [Google Scholar]
- 19.van Dijk M, Halpin-McCormick A, Sessler T, Samali A, Szegezdi E. Resistance to TRAIL in non-transformed cells is due to multiple redundant pathways. Cell Death Dis. 2013. Jul;4(7):e702–e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori K, Kawahara T, Yoshida H, Yanagi H, Yura T. Signalling from endoplasmic reticulum to nucleus: transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway. Genes Cells. 1996. Sep;1(9):803–17. [DOI] [PubMed] [Google Scholar]
- 21.Schröder M, Kaufman RJ. The Mammalian Unfolded Protein Response. Ann Rev Biochem. 2005;74(1):739–89. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ. 2006. Mar;13(3):374–84. [DOI] [PubMed] [Google Scholar]
- 23.Schönthal AH. Endoplasmic Reticulum Stress: Its Role in Disease and Novel Prospects for Therapy. Scientifica. 2012;2012:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urra H, Dufey E, Lisbona F, Rojas-Rivera D, Hetz C. When ER stress reaches a dead end. BBA - Mol Cell Res. 2013. Dec;1833(12):3507–17. [DOI] [PubMed] [Google Scholar]
- 25.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012. Feb;13(2):89–102. [DOI] [PubMed] [Google Scholar]
- 26.Bourougaa K, Naski N, Boularan C, Mlynarczyk C, Candeias MM, Marullo S, et al. Endoplasmic Reticulum Stress Induces G2 Cell-Cycle Arrest via mRNA Translation of the p53 Isoform p53/47. Mol Cell. 2010. Apr;38(1):78–88. [DOI] [PubMed] [Google Scholar]
- 27.Voelkel-Johnson C, King DL, Norris JS. Resistance of prostate cancer cells to soluble TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) can be overcome by doxorubicin or adenoviral delivery of full-length TRAIL. Cancer Gene Ther. 2002. Feb;9(2):164–72. [DOI] [PubMed] [Google Scholar]
- 28.Kim SY, Kim J-H, Song JJ. c-Cbl shRNA-expressing adenovirus sensitizes TRAIL-induced apoptosis in prostate cancer DU-145 through increases of DR4/5. Cancer Gene Ther. 2013. Feb;20(2):82–7. [DOI] [PubMed] [Google Scholar]
- 29.Sah NK, Munshi A, Kurland JF, McDonnell TJ, Su B, Meyn RE. Translation Inhibitors Sensitize Prostate Cancer Cells to Apoptosis Induced by Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL) by Activating c-Jun N-terminal Kinase. J Biol Chem. 2003. Jun 6;278(23):20593–602. [DOI] [PubMed] [Google Scholar]
- 30.Shiraishi T, Yoshida T, Nakata S, Horinaka M, Wakada M, Mizutani Y, et al. Tunicamycin Enhances Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand–Induced Apoptosis in Human Prostate Cancer Cells. Can Res. 2005. Jul 15;65(14):6364–70. [DOI] [PubMed] [Google Scholar]
- 31.Shin D, Kwon H-Y, Sohn EJ, Nam MS, Kim JH, Lee JC, et al. Upregulation of Death Receptor 5 and Production of Reactive Oxygen Species Mediate Sensitization of PC-3 Prostate Cancer Cells to TRAIL Induced Apoptosis by Vitisin A. Cell Physiol Biochem. 2015;36(3):1151–62. [DOI] [PubMed] [Google Scholar]
- 32.Jung EJ, Chung KH, Kim CW. Identification of simvastatin-regulated targets associated with JNK activation in DU145 human prostate cancer cell death signaling. BMB Reports. 2017. Sep 30;50(9):466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei R-J, Zhang X-S, He D-L. Andrographolide sensitizes prostate cancer cells to TRAIL-induced apoptosis. Asian J Androl. 2018;20(2):200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lashinger LM, Zhu K, Williams SA, Shrader M, Dinney CPN, McConkey DJ. Bortezomib Abolishes Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand Resistance via a p21-Dependent Mechanism in Human Bladder and Prostate Cancer Cells. Cancer Res. 2005. Jun 1;65(11):4902–8. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell MJ, King MR. Fluid Shear Stress Sensitizes Cancer Cells to Receptor-Mediated Apoptosis via Trimeric Death Receptors. New J Phys. 2013. Jan 18;15:015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Sharkey CC, King MR. Piperlongumine and immune cytokine TRAIL synergize to promote tumor death. Sci Rep. 2015. May 18;5:9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly MM, Hoel BD, Voelkel-Johnson C. Doxorubicin Pretreatment Sensitizes Prostate Cancer Cell Lines to TRAIL Induced Apoptosis Which Correlates with the Loss of c-FLIP Expression. Cancer Biol Thera. 2002. Sep 13;1(5):520–7. [DOI] [PubMed] [Google Scholar]
- 38.Selimovic D, Hassan M, Haikel Y, Hengge UR. Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell Signal. 2008. Feb;20(2):311–22. [DOI] [PubMed] [Google Scholar]
- 39.Yoo J, Park S-S, Lee YJ. Pretreatment of docetaxel enhances TRAIL-mediated apoptosis in prostate cancer cells. J Cell Biochem. 2008. Aug 1;104(5):1636–46. [DOI] [PubMed] [Google Scholar]
- 40.Jaworska D, Szliszka E. Targeting Apoptotic Activity Against Prostate Cancer Stem Cells. Int J Mol Sci. 2017. Jul 29;18(8):1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nimmanapalli R, Perkins CL, Orlando M, O’Bryan E, Nguyen D, Bhalla KN. Pretreatment with paclitaxel enhances apo-2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levels. Cancer Res. 2001;61(2):759–63. [PubMed] [Google Scholar]
- 42.Gao F, Sun Z, Sun X, Zhang Y, Wang H, Zhong B, et al. Ulinastatin Exerts Synergistic Effects with Taxotere and Inhibits Invasion and Metastasis of Breast Cancer by Blocking Angiogenesis and the Epithelial–Mesenchymal Transition. Cancer Biother Radiopharm. 2013. Apr;28(3):218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul. 1984. Jan;22:27–55. [DOI] [PubMed] [Google Scholar]
- 44.Chou T-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol Rev. 2006. Sep 1;58(3):621–81. [DOI] [PubMed] [Google Scholar]
- 45.Chou T-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010. Jan 15;70(2):440–6. [DOI] [PubMed] [Google Scholar]
- 46.Hope JM, Lopez-Cavestany M, Wang W, Reinhart-King CA, King MR. Activation of Piezo1 sensitizes cells to TRAIL-mediated apoptosis through mitochondrial outer membrane permeability. Cell Death Dis. 2019. Nov;10(11):837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albeck JG, Burke JM, Spencer SL, Lauffenburger DA, Sorger PK. Modeling a Snap-Action, Variable-Delay Switch Controlling Extrinsic Cell Death. PLoS Biol. 2008. Dec;6(12):e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang DG, Li L, Chopra DP, Porter AT. Extended Survivability of Prostate Cancer Cells in the Absence of Trophic Factors: Increased Proliferation, Evasion of Apoptosis, and the Role of Apoptosis Proteins. Cancer Res.1998. Aug 1;58:3466–3479. [PubMed] [Google Scholar]
- 49.Yu S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front Immunol. 2019;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung EM. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through CHOP-independent DR5 upregulation. Carcinogenesis. 2006. Aug 2;27(10):2008–17. [DOI] [PubMed] [Google Scholar]
- 51.Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, et al. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014. Jul 4;345(6192):98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu S, Yalcin A, Lee GY, Li P, Fan J, Arruda AP, et al. Phenotypic assays identify a small molecule modulator of the unfolded protein response with anti-diabetic activity. Sci Transl Med. 2015. Jun 17;7(292):292ra98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamanaka RB, Bobrovnikova-Marjon E, Ji X, Liebhaber SA, Diehl JA. PERK-dependent regulation of IAP translation during ER stress. Oncogene. 2009. Feb 12;28(6):910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pihán P, Carreras-Sureda A, Hetz C. BCL-2 family: integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017. Sep;24(9):1478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hemmati PG, Gillissen B, von Haefen C, Wendt J, Stärck L, Güner D, et al. Adenovirus-mediated overexpression of p14 ARF induces p53 and Bax-independent apoptosis. Oncogene. 2002. May;21(20):3149–61. [DOI] [PubMed] [Google Scholar]
- 56.Wu GS, Burns TF, McDonald ER, Jiang W, Meng R, Krantz ID, et al. KILLER/DR5 is a DNA damage–inducible p53–regulated death receptor gene. Nat Genet. 1997. Oct;17(2):141–3. [DOI] [PubMed] [Google Scholar]
- 57.Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, et al. p53-dependent and -independent Regulation of the Death Receptor KILLER/DR5 Gene Expression in Response to Genotoxic Stress and Tumor Necrosis Factor α. Cancer Res. 1998. Apr 15;58(8):1593–8. [PubMed] [Google Scholar]
- 58.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000. Feb 15;60(4):847–53. [PubMed] [Google Scholar]
- 59.Guan B, Yue P, Clayman GL, Sun S-Y. Evidence that the death receptor DR4 is a DNA damage-inducible, p53-regulated gene. J Cell Physiol. 2001;188(1):98–105. [DOI] [PubMed] [Google Scholar]
- 60.Baritaki S, Huerta-Yepez S, Sakai T, Spandidos DA, Bonavida B. Chemotherapeutic drugs sensitize cancer cells to TRAIL-mediated apoptosis: up-regulation of DR5 and inhibition of Yin Yang 1. Mol Cancer Ther. 2007. Apr 1;6(4):1387–99. [DOI] [PubMed] [Google Scholar]
- 61.Wu X-X, Jin X-H, Zeng Y, Hamed AMAE, Kakehi Y. Low concentrations of doxorubicin sensitizes human solid cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-receptor (R) 2-mediated apoptosis by inducing TRAIL-R2 expression. Cancer Sci. 2007;98(12):1969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jordan CT. Cancer Stem Cells. N Engl J Medicine. 2006;9. [DOI] [PubMed] [Google Scholar]
- 63.Phi LTH, Sari IN, Yang Y-G, Lee S-H, Jun N, Kim KS, et al. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018;2018:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huisman C, Ferreira CG, Bröker LE, Rodriguez JA, Smit EF, Postmus PE, et al. Paclitaxel Triggers Cell Death Primarily via Caspase-independent Routes in the Non-Small Cell Lung Cancer Cell Line NCI-H460. Clin Cancer Res. 2002. Feb 1;8(2):596–606. [PubMed] [Google Scholar]
- 65.Zappavigna S, Cossu AM, Abate M, Misso G, Lombardi A, Caraglia M, et al. A Hydroquinone-Based Derivative Elicits Apoptosis and Autophagy via Activating a ROS-Dependent Unfolded Protein Response in Human Glioblastoma. Int J Mol Sci. 2019. Aug 6;20(15):3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mele L, la Noce M, Paino F, Regad T, Wagner S, Liccardo D, et al. Glucose-6-phosphate dehydrogenase blockade potentiates tyrosine kinase inhibitor effect on breast cancer cells through autophagy perturbation. J Exp Clin Cancer Res. 2019. Apr 12;38(160):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishitoh H. CHOP is a multifunctional transcription factor in the ER stress response. J Biochem. 2012. Mar 1;151(3):217–9. [DOI] [PubMed] [Google Scholar]
- 68.Iurlaro R, Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016. Jul;283(14):2640–52. [DOI] [PubMed] [Google Scholar]
- 69.Mhaidat NM, Thorne R, Zhang XD, Hersey P. Involvement of endoplasmic reticulum stress in Docetaxel-induced JNK-dependent apoptosis of human melanoma. Apoptosis. 2008. Dec;13(12):1505–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.