Current challenges in understanding chronic pain pathogenesis are being overcome by the creation of large consortia and biorepositories with a harmonised approach to phenotyping.
Keywords: UK Biobank, DOLORisk, PAINSTORM, Chronic pain, Neuropathic pain, Genetics, Epidemiology
Abstract
Chronic pain (CP) is a common and often debilitating disorder that has major social and economic impacts. A subset of patients develop CP that significantly interferes with their activities of daily living and requires a high level of healthcare support. The challenge for treating physicians is in preventing the onset of refractory CP or effectively managing existing pain. To be able to do this, it is necessary to understand the risk factors, both genetic and environmental, for the onset of CP and response to treatment, as well as the pathogenesis of the disorder, which is highly heterogenous. However, studies of CP, particularly pain with neuropathic characteristics, have been hindered by a lack of consensus on phenotyping and data collection, making comparisons difficult. Furthermore, existing cohorts have suffered from small sample sizes meaning that analyses, especially genome-wide association studies, are insufficiently powered. The key to overcoming these issues is through the creation of large consortia such as DOLORisk and PAINSTORM and biorepositories, such as UK Biobank, where a common approach can be taken to CP phenotyping, which allows harmonisation across different cohorts and in turn increased study power. This review describes the approach that was used for studying neuropathic pain in DOLORisk and how this has informed current projects such as PAINSTORM, the rephenotyping of UK Biobank, and other endeavours. Moreover, an overview is provided of the outputs from these studies and the lessons learnt for future projects.
1. Introduction
Chronic pain (CP), defined as pain lasting more than 3 months, represents a major global burden for years lived with disability and the associated economic impact due to health resources used and work absenteeism.128 As an example, nonspecific low back pain (LBP) is the largest single cause of years lived with disability globally,49 accounting for 11% of the entire disability burden from all diseases. In 2017, LBP was estimated to cost the UK up to 116 million lost workdays and approximately £12.3 billion through direct health care costs, production losses, and informal care https://www.gov.uk/government/publications/chronic-pain-in-adults-2017.
Pain has long been considered solely as a symptom, and it is only recently that CP has been recognised by the World Health Organization International Classification of Diseases (ICD)-11121 as a long-term condition in its own right.
Chronic pain prevalence increases with age as predisposing conditions such as obesity, arthritis, diabetes mellitus, and malignancy become more common.41 A recent meta-analysis of population-based epidemiological studies worldwide reported a pooled CP prevalence estimate of 31%,111 with an equivalent figure from UK studies of 43.5%,41 similar to data arising from UK Biobank (UKB, 42.9%).75 Of those reporting CP, a subset conservatively estimated at 20%20 have disabling CP that substantially interferes with activities of daily living. Similarly, there is an overlapping population who express a substantial need for health care. Such individuals are often characterised by comorbid depression, fear, cognitive dysfunction, avoidance of movement, and poor coping skills.15 From a public health perspective, the challenge is to prevent the progression of mild or transient pain to CP which becomes severe.14
The development and severity of CP involve a complex interaction between genetic, environmental, and clinical factors in vulnerable individuals.126 Chronic pain is heritable: Twin72,78,110 and extended family61 studies have provided estimates of 30% to 76%. Mutations in specific genes (most of which encode ion channels) cause rare (Mendelian) pain conditions in humans.12 This includes the disorder inherited erythromelalgia (characterized by pain and erythema of the extremities exacerbated by warming), which is caused by autosomal dominant and highly penetrant gain of function mutations in the gene SCN9A encoding the voltage-gated sodium channel NaV1.7.13 Human pain complex trait genetics (especially when combined with rich phenotypic data, biosamples, and large-scale brain imaging cohorts34) has the potential to revolutionise our understanding of CP pathogenesis, risk factors, and the determinants of treatment responses. Current significant challenges and limitations to this approach relate to (1) a lack of precision in CP phenotyping with respect to the duration, location, intensity, and quality of pain as well as the temporal relationship to predisposing factors and comorbidities such as anxiety and depression and (2) the size of the existing CP cohorts which are limited and, therefore, studies are relatively underpowered. Key to overcoming these challenges is large consortia with a harmonised approach to pain phenotyping and nationwide biorepositories with a wide range of genetic and nongenetic data. There is now an increasing international effort to harmonise data collection which is likely to further inform clinical practice. One example is “INTEGRATE-Pain”. This is a joint initiative between the US National Institute of Health (NIH) and Innovative Medicines Initiative-PainCare which is developing consensus on overarching core outcome domain sets for clinical pain trials and clinical pain research (https://www.comet-initiative.org/Studies/Details/2083). Newer cohorts now available to study CP include DOLORisk,93 and this has informed the approach taken by current studies such as the rephenotyped UKB Chronic Pain (UKB CP) Cohort and PAINSTORM.
This review introduces these cohorts, provides an overview of their main outputs, and outlines the key lessons learned.
2. UK Biobank (chronic pain cohort)
The scope of UKB, which comprises 500,000 volunteers enrolled between 2006 and 2010 at ages 40 to 69 from across the UK, provides a unique opportunity to examine the epidemiology and genetics of CP in a prospective population cohort. A brief assessment of CP was completed by all participants at booking (https://biobank.ndph.ox.ac.uk/showcase/field.cgi?id=6159) although it did not include validated questionnaires enabling categorisation of CP of diverse aetiologies. Using that initial data, we have previously demonstrated that the prevalence of CP and most regional (ie, site specific) musculoskeletal pains in UKB are similar to that found in other pain epidemiological studies. Our findings also reproduce known relationships from a range of socioeconomic and psychological factors.75,90
To address the lack of specificity when categorising CP, a UK academic consortium of clinicians and pain researchers, many of whom have experience of working with UKB75,86,130,133 and with a range of synergistic expertise in epidemiology, genomics, psychology, neuroimaging, and pain management, developed a UKB CP phenotyping survey (2017–2018). The pain phenotyping survey (https://biobank.ctsu.ox.ac.uk/crystal/ukb/docs/pain_questionnaire.pdf) was designed by a group of experts (including the authors B.H.S., D.W., and D.L.H.B.) and based on a series of validated questionnaires in routine use (Table 1), which were fully aligned with the CP cohorts described in this paper. These focused on the most prevalent causes of CP and associated comorbidities and risk factors.
Table 1.
Questionnaires used in DOLORisk, PAINSTORM, and UK Biobank.
| Category | Questionnaire | DOLORisk | PAINSTORM | UK Biobank | Reference | ||
|---|---|---|---|---|---|---|---|
| Core | Extended | Core | Extended | ||||
| Demographics | Age, gender, years in education, working status, weight, and height | X | X | X | X | X | |
| Ethnicity | X | X | X | ||||
| Household income | X | ||||||
| Characterisation of pain | Presence and duration of pain | X | X | X | X | X | |
| Family history | Family history of chronic pain | X | X | ||||
| Pain medication | Currently taking pain medication | X | X | X | X | ||
| Brief Pain Inventory—usefulness of medication | X | X | Cleeland and Ryan30 | ||||
| Adherence to medication | X | X | |||||
| Pain relief strategies other than medication | X | ||||||
| Pain severity | Chronic pain grade | X | X | X | X | Von Korf et al.127 | |
| Brief Pain Inventory—pain severity | X | Only the item on “pain on average” | X | X | Cleeland and Ryan30 | ||
| Pain quality | DN4 Questionnaire | X | X | X | X | X | Bouhassira et al.18 |
| DN4 Examination | X | X | |||||
| Neuropathic Pain Symptom Inventory | X | X | Bouhassira et al.17 | ||||
| PainDETECT | X | Freynhagen et al.47 | |||||
| Pain location | List of locations | X | X | X | X | ||
| Body map | X | X | |||||
| Pain interference | PROMIS Pain Interference 8a | X | Amtmann et al.3 | ||||
| PROMIS Ability to Participate in Social Roles and Activities | 8a | Hahn et al.54 | |||||
| Pain-related worrying | Pain Catastrophizing Scale | X | X | X | X | Sullivan et al.113 | |
| Health status and psychological assessment | EQ-5D-5L | X | X | X | X | X | Herdman et al.60 |
| Patient Health Questionnaire-9 | X | Kroenke et al.67 | |||||
| PROMIS Depression | 4a | 8a | 4a | 8a | Pilkonis et al.95 | ||
| PROMIS Anxiety | 4a | 8a | 4a | 8a | |||
| PROMIS Sleep Disturbance | 4a | 8a | 4a | 8a | Buysse et al.23 | ||
| PROMIS Fatigue | 8a | 8a | Lai et al.69 | ||||
| Fatigue Severity Scale | X | Krupp et al.68 | |||||
| PROMIS Emotional Support | 4a | 4a | 4a | 4a | Hahn et al.54 | ||
| PROMIS Instrumental Support | 4a | 4a | 4a | 4a | |||
| Trauma | X | X | X | X | |||
| Ten-item Personality Inventory | X | X | X | X | Gosling et al.51 | ||
| International Personality Item Pool (Emotional Stability) | X | Goldberg50 | |||||
| State Optimism Measure (SOM-7) | X | Millstein et al.87 | |||||
| Disease-specific (diabetic neuropathy) | Michigan Neuropathy Screening Instrument | X | X | Feldman et al.42 | |||
| Lifestyle | Smoking | X | X | X | X | Campbell et al.24 | |
| Alcohol | X | X | X | X | |||
| International Physical Activity Questionnaire | X | X | Craig et al.33 | ||||
| Bespoke questions | Financial situation and impact on pain management | X | |||||
| Description of pain in the participant's own words | X | ||||||
| Other chronic pain conditions | Fibromyalgia | X | Wolfe et al.131 | ||||
| Headache and migraine | X | Lipton et al.71 | |||||
DN4, Douleur Neuropathique en 4 Questions; EQ-5D-5L, EuroQol-5 dimensions-5 levels; PROMIS, Patient-Reported Outcomes Measurement Information System.
The CP phenotyping survey was then sent to ∼335,000 UKB participants who consented to recontact, had an email address, and were still actively participating as of May 2019 (ie, not deceased or withdrawn). The survey was (partially or fully) completed by ∼167,000 individuals (a response rate of 49.8%). Approximately 148,000 individuals either reported no CP (∼72,000), or CP (pain or discomfort that had been present for more than 3 months) and fully completed the Douleur Neuropathique en 4 (DN4) questionnaire (∼76,000; 51.1%). The data were released in early 2021 and are available to bona fide researchers worldwide. The UKB CP cohort has the added values of: (1) being by far the largest phenotyped CP cohort generated to date worldwide; (2) linkage to longitudinal GP records for >95% of respondents by 2024; (3) access to all the other rich datasets obtained by previous (and subsequent) UKB questionnaires completed by these individuals for imaging, depression, anxiety, cognition, multimorbidity, deprivation, etc; (4) the CP survey will be repeated in summer 2024 and extended to provide detailed outcome and treatment data on those with CP, thus allowing the identification over the intervening 5 years of those with newly reported CP; and (5) being of sufficient size and detail to allow data-derived categorisation of CP symptoms and risk factors.
The CP data demonstrate that 75% of subjects with CP reported pain having lasted for more than a year and about a third for more than 5 years. Using the Brief Pain Inventory (BPI) questionnaire, approximately 25% of subjects with CP reported severe or moderate pain whereas 20% reported severe or moderate interference in their activities of daily living. Over half of subjects with CP reported back or neck pain, over 40% had pain in one or more joints (the commonest being knee, then hip, hands, and feet), whereas 10% reported pain all over the body. The commonest self-reported CP diagnoses were osteoarthritis affecting one or more joints, followed by migraine, nerve damage or neuropathy, carpal tunnel, pelvic pain, and rheumatoid arthritis. Using the DN4 questionnaire, the prevalence of “possible” neuropathic pain (NeuP) was 9.2%, making up 18.1% of those with CP. Our recent analysis7 of those with NeuP demonstrated that this was significantly associated with worse health-related quality of life, having a manual or personal service type occupation and younger age compared with those without CP. As expected, NeuP was associated with diabetes and neuropathy but also with other pains (pelvic, postsurgical, and migraine) and musculoskeletal disorders (rheumatoid arthritis, osteoarthritis, and fibromyalgia). In addition, NeuP was associated with pain in the limbs and greater pain intensity and higher body mass index (BMI) compared with those with nonneuropathic pain.
2.1. Caveats/limitations
(1) Non-White ethnic backgrounds were rare in UKB (2.2%); 1.6% were from Black, Asian, and Minority ethnicities; 0.6% were mixed ethnicity; and the remaining 97.8% were White. This compares with 18.3% non-White in England and Wales in the 2021 Census.35 In the 2011 Census, which is closer to when the UKB cohort was recruited, 14% were non-White in England and Wales36 and 4% were non-White in Scotland28 (2022 census not yet available).
(2) There was an overrepresentation of participants who were female, of younger age, who had lower BMI, and who were less socially deprived in the group that completed the 2019 pain phenotyping questionnaire compared with the rest of the UKB cohort who did not. The overrepresentation of participants of younger age and who were less socially deprived could potentially be due to the fact that the questionnaire was only available online.
(3) The definition of NeuP relies on a self-completed screening tool, which does not meet the grading system for “probable” or “definite” NeuP. These necessitate clinical examination which is clearly not feasible in a large population survey.
2.2. Outputs
Because of the large sample size and range of data available compared with other cohorts, UKB allows associations to be quantified with greater precision and across different levels of demographics. As the data from the CP phenotyping survey was only released in early 2021, the results of studies using these data are only just beginning to emerge. Up to this point, studies have used the CP data that were collected at baseline recruitment. This has limited studies to specific single or multipain sites, without consideration for underlying aetiology. An extensive but nonexhaustive list of pain studies conducted either wholly or partially using UKB is provided in Table 2. These are intended to provide an overview of the kind of analyses that are possible using the cohort.
Table 2.
Publications on (chronic) pain using the UK Biobank cohort.
| Study | Pain phenotype | Design | Finding |
|---|---|---|---|
| Allen et al.1 | AP and CP | CS | Social exclusion and loneliness are associated with pain |
| Atkins et al.5 | CP | CS | Low cardiovascular disease score individuals had less chronic pain |
| Beasley et al.8 | CWP | CS | Relationship between alcohol consumption and reporting of CWP |
| Beasley et al.9 | CWP | MR | Protective effect of alcohol on CWP is not supported |
| Benavides et al.10 | CP | Genetic association | rs1045642 (ABCB1) effect on response to chronic pain treatment with nortriptyline or morphine combo |
| Bortsov et al.16 | BP (acute and chronic) | GWAS | 13 GWS loci for chronic BP, none for acute BP. SNP heritability 4.6% for chronic BP and 0.8% for acute BP |
| Broberg et al.21 | General pain and multisite CP | MR | Bidirectional causal relationship between insomnia and pain |
| Carvalho et al.25 | Musculoskeletal pain | CS and longitudinal | T2D is associated cross-sectionally and longitudinally with shoulder or neck, knee, or hip pain and longitudinally with neck or shoulder pain |
| Carvalho et al.26 | Musculoskeletal pain | CS | Metformin is protective of back, knee, neck or shoulder, and multisite pain |
| Cassidy et al.27 | Drugs prescribed for CP | CS | Opiate and NP medications taken with cardiometabolic medications associated with obesity, increased waist circumference, and hypertension compared with cardiometabolic medications alone |
| Chen et al.29 | Musculoskeletal pain | Longitudinal | Higher number of pain sites associated with risk of all-cause mortality |
| Cox et al.32 | Opioid cessation/CBP | GWAS | PRS for opioid cessation significantly associated with chronic back pain, being a former drinker, and being a former smoker |
| Faber et al.37 | Hip pain | CS | Cam morphology is associated with hip pain |
| Faber et al.38 | Hip pain | CS | Radiographic hip osteoarthritis, total osteocyte area, joint space narrowing and acetabular, and superior and inferior femoral osteophyte areas were all associated with hip pain |
| Faber et al.39 | Hip pain | CS | Osteophytes and joint space narrowing are associated with hip pain |
| Farrell et al.40 | CP | GWAS/PhWAS | Shared genetic signature across 8 chronic pain types and 1492 biopsychosocial traits. 488 traits with causal association with CP |
| Freidin et al.45 | BP | GWAS | 3 loci associated with BP. Pleiotropic effects of genetic risk factors for BP, height, and intervertebral disk problems. Genetic correlations between BP and depression symptoms, neuroticism, sleep disturbance, overweight, and smoking |
| Freidin et al.46 | Chronic BP | GWAS | 2 and 7 GWS loci associated with chronic BP in males and females, respectively |
| Green et al.53 | Frozen shoulder | GWAS/MR | 5 GWS loci associated with frozen shoulder. Diabetes but not obesity is a causal risk factor for frozen shoulder |
| Hanlon et al.55 | CWP | CS | Physical, sexual, and emotional childhood maltreatment and neglect associated with CWP |
| Hastie et al.56 | CP and CWP | Longitudinal | CP and CWP associated with hospital admission for COVID-19. CWP but not CP associated with COVID-19 mortality |
| Jin et al.62 | Oral inflammatory diseases (including mouth ulcer, painful gums, and toothache) | GWAS meta-analysis | 31, 4, and 4 GWS loci associated with mouth ulcer, painful gums, and toothache. 2 novel GWS loci associated with painful gums and toothache |
| Johnston et al.63 | Multisite CP | GWAS/MR | SNP heritability of 10.2%. 39 GWS loci associated with multisite CP. Genetic correlation with psychiatric, autoimmune, and anthropometric traits. Causal effect of multisite CP on MDD |
| Johnston et al.64 | Multisite CP | GWAS | 5 and 10 GWS loci associated with multisite chronic pain in men and women, respectively. Sex-specific gene associations and expression in dorsal root ganglion. Sex-specific association of multisite CP with MDD. Genetic correlation with a range of psychiatric and mood phenotypes |
| Kasher et al.65 | CBP | CS/MR | RA, OP, CRP, BMI, age, and gender associated with CBP. Genetic correlation between CBP and RA and CRP and BMI. CRP causally predicts CBP. Pleiotropy seems to explain relationship between CBP and RA/OP |
| Khoury et al.66 | Single and multisite CP | GWAS | 23 GWS loci associated with multisite CP (none with single site CP) and 9 replicated in HUNT cohort. Axonogenesis in brain tissues is a major contributing pathway |
| Larvin et al.70 | Painful gums | Longitudinal | Higher incidence of CVD and depression in painful gums compared with healthy controls. Increased risk of baseline → CVD → censor and baseline → metabolic → censor disease trajectory in painful gums. The former trajectory has increased the risk of mortality |
| Lobo et al.73 | Chronic multisite musculoskeletal pain | CS/genetic association | Interaction between FKBP5 rs3800373 risk variant and right hippocampal volume associated with chronic multisite musculoskeletal pain. This is mediated by severity of childhood trauma |
| Macfarlane et al.75 | ‘Any pain’, CP, and musculoskeletal pain | CS | Estimates of ‘any pain’, CP, and site-specific musculoskeletal pain prevalence similar between UK Biobank and MUSICIAN/NCDS cohorts |
| Macfarlane et al.76 | CWP | Longitudinal/meta-analysis | CWP associated with excess all-cause mortality as well as excess cancer, cardiovascular, and respiratory-related deaths |
| Macfarlane et al.77 | Opioid use | CS/longitudinal | 5.5% of UK Biobank regularly using opioids. Opioid use is most common in groups of low socioeconomic status. Weak and strong opioids were associated with excess mortality |
| Macfarlane et al.74 | (Chronic) Facial pain | CS | Overall prevalence of facial pain was 1.9%, of which 48% was chronic. Facial pain was more common in women, smokers and associated with psychological distress, low socioeconomic status, low alcohol consumption, and all types of regional pain |
| McIntosh et al.81 | CP | CS/genetic association | PRS for MDD associated with CP in UK Biobank |
| McQueenie et al.83 | CP/CWP | CS | Chronic pain is extremely common across a wide range of LTCs including migraine/headache, IBS, mental health conditions, and diseases of the digestive system. People with ≥4 LTCs 3 and 20 times more likely to have CP and CWP, respectively |
| Meng et al.84 | Knee pain | GWAS | 2 GWS loci (GDF5 and COL27A1) associated with knee pain |
| Meng et al.85 | Headache, facial, neck/shoulder, back, stomach/abdominal, hip and knee pain, and pain all over the body | Genetic association | Positive genetic correlation between all pain phenotypes and depressive symptoms, MDD, and neuroticism, except hip and knee pain |
| Meng et al.86 | Neck and shoulder pain | GWAS | 3 GWS loci associated with neck or shoulder pain. 2 loci (FOXP2 and LINC01572) weakly replicated in an independent cohort. Genetic correlation between neck or shoulder pain and depression, insomnia, and neuroticism |
| Muralidharan et al.88 | Multisite CP | Longitudinal/genetic association | Significant negative correlation between the number of chronic pain sites and age at death in men, but not women. TP53 significantly associated with the number of chronic pain sites in women but not men |
| Nicholl et al.89 | Multisite CP | CS | Individuals who report CP and multisite CP are more likely to have MDD and BD. Relationship between extent of CP and risk of MDD and BD |
| Nicholl et al.90 | CP | CS | CP is more common, and depression is less common in Black and Asian ethnic groups compared with White. Association between presence and extent of CP and depression strongest in minority ethnic groups |
| Pan et al.91 | Multisite musculoskeletal pain (hip, knee, back, and neck/shoulder pain) | CS | Greater number of painful sites consistently associated with poorer physical working capacity and low intensity physical activity compared with moderate or vigorous physical activity |
| Parisien et al.92 | Acute back pain | CS | Elevated risk of acute back pain persistence in subjects taking NSAIDs |
| Patasova et al.94 | Multisite CP and pain control medications (paracetamol, opioids, NSAIDs, and gabapentinoids) | CS/MR | Codeine, tramadol, paracetamol, ibuprofen, gabapentin, and pregabalin all individually associated with hyperopia. Causal effect of multisite CP on hyperopia |
| Rahman et al.96 | CWP | GWAS | 3 GWS loci (RNF123, ATP2C1, and COMT) associated with CWP. Partial genetic correlation between CWP and depressive symptoms, BMI, age of first birth, and years of schooling |
| Rönnegård et al.99 | Acute pain, chronic localised pain, and CWP | Longitudinal | Increasing risk of composite CVD (myocardial infarction, stroke, heart failure, and cardiovascular mortality) in people with increasing pain duration and widespreadness |
| Rosoff et al.100 | Prescription opioid use | MR | Evidence for potential causal associations between prescription opioid use and risk for MDD and ASRD. Genetic liability for MDD associated with increased risk of prescription opioid use |
| Shu et al.103 | LBP | MR | Genetic correlation between LBP and insomnia and daytime sleepiness. Insomnia significantly associated with increased risk of LBP. No reverse causation nor a causal effect of daytime sleepiness on LBP |
| Slade et al.106 | Facial pain | CS | Replication of considerable overlap of facial pain with pain in other parts of body. Greater association with headache and neck pain than pain below the neck |
| Suri et al.114 | CBP | GWAS meta-analysis | GWS locus at SOX5 associated and replicated with CBP. Two GWS loci (CCDC26/GSDMC and DCC) associated with CBP in meta-analysis |
| Tagliaferri et al.115 | Acute back pain, chronic localised back pain, and CBP with other pain sites | CS | People with acute, chronic localised, and CBP with other pain sites have significant differences on brain structure and psychosocial and physical health states than people without pain |
| Tamosauskaite et al.116 | CP | Genetic association | Homozygote HFE C282Y mutations (which is associated with excessive iron absorption) associated with CP in older men. HFE C282Y associated with knee, hip, and back pain in older women |
| Tang et al.117 | BP | MR | Evidence for causal associations between serum iron, ferritin, and transferrin saturation and risk of back pain |
| Verma et al.125 | Multisite CP | Genetic association | EREG (epiregulin) H2 haplotype protective for the presence of at least one CP site, H3 haplotype protective for chronic hip pain, and number of chronic pain sites |
| Walker-Bone et al.129 | CWP | CS | A history of bone fracture is associated with increased risk of CWP in men and women |
| Zhang et al.132 | Stomach/abdominal, multisite CP and neck/shoulder pain | GWAS | In patients with depression, TRIOBP associated with stomach/abdominal pain, SLC9A9 associated with multisite CP, and ADGRF1 associated with neck/shoulder pain |
| Zorina-Lichtenwalter et al.133 | Multisite CP | Genetic association | MC1R variants involved in red hair associated with reduced count of pain conditions |
(C)BP, (chronic) back pain; ABCB1, ATP binding cassette subfamily B member 1; ADGRF1, adhesion G protein-coupled receptor F1; AP, acute pain; ASRD, anxiety and stress-related disorders; ATP2C1, ATPase secretory pathway Ca2+ transporting 1; BD, bipolar disorder; BMI, body mass index; CCDC26, coiled-coil domain-containing 26; COL27A1, collagen Type XXVII alpha 1 chain; COMT, catechol-O-methyltransferase; COVID-19, coronavirus disease 2019; CP, chronic pain; CRP, C-reactive protein; CS, cross-sectional; CVD, cardiovasular disease; CWP, chronic widespread pain; DCC, deleted in colorectal cancer; EREG, epiregulin; FKBP5, FK506 binding protein 5; FOXP2, forkhead box protein P2; GDF5, growth differentiation factor 5; GSDMC, gasdermin C; GWAS, Genome-Wide Association Study; GWS, genome-wide significant; HFE, homeostatic iron regulator; HUNT, The Trøndelag Health Study; IBS, irritable bowel syndrome; LBP, low back pain; LINC01572, long intergenic nonprotein coding RNA 1572; LTC, long-term conditions; MC1R, melanocortin 1 receptor; MDD, major depressive disorder; MR, mendelian randomisation; MUSICIAN, managing unexplained musculoskeletal conditions using traditional and accessible new approaches; NCDS, National Child Development Study; NP, neuropathic pain; NSAID, nonsteroidal anti-inflammatory drug; OP, osteoporosis; PhWAS, Phenome-Wide Association Study; PRS, polygenic risk score; RA, rheumatoid arthritis; RNF123, ring finger protein 123; SLC9A9, solute carrier family 9 member A9; SNP, single nucleotide polymorphism; SOX5, SRY-box transcription factor 5; T2D, type 2 diabetes; TP53, tumor protein P53; TRIOBP, TRIO and F-actin binding protein.
Most pain studies conducted in UKB were cross-sectional because of the data being collected at a single time point. These have identified a wide range of associations with pain phenotypes including ethnicity,90 alcohol consumption,8,74 smoking,74 physical activity,91 low socioeconomic status,1,74 cardiovascular disease,5 type 2 diabetes (T2D),5 number of long-term comorbidities,83 adverse childhood experiences,55 depression,89,90 and bipolar disorder.89 They have also revealed that certain anatomical features are influential in pain. These include cam morphology (deformity of the femoral head–neck junction37), osteophytes38,39 and joint space narrowing38 with hip pain, bone fracture with chronic widespread pain (CWP),96,129 and differences in brain structure between acute back pain, chronic back pain, and chronic back pain occurring with other pain sites.115 UK Biobank has revealed that pain at specific sites, particularly facial pain, often overlaps with pain at other sites.106
The availability of self-reported medication (before UKB being linked to primary care records) has enabled the study of pain pharmacoepidemiology. Approximately 5.5% of people in UKB reported regular use of opioids (1.4% strong opioids and 4.2% weak opioids), which was found to be associated with low socioeconomic status and excess mortality.77 Strong opioid users (9.1%) were also more likely to die during follow-up than weak opioid users (6.9%) or nonusers (3.3%). The use of both opiate and NeuP pain medications with cardiometabolic medications was associated with obesity, increased waist circumference, and hypertension compared with taking cardiometabolic medications alone and the use of the antidiabetic drug metformin seemed to be protective of musculoskeletal pain.27 Common opioid (codeine and tramadol), nonsteroidal anti-inflammatory drug (NSAID) (ibuprofen), and NeuP medications were associated with far sightedness94 and people taking NSAIDs to treat back pain were more likely to report pain persistence than were those not treated with NSAIDs.92
Studies have also explored pain as a potential exposure for other clinical traits, particularly in longitudinal studies where the outcome has been measured at multiple time points. Using this approach, it has been demonstrated that CWP was associated with greater incidence of COVID-19 admission and mortality,56 as well as mortality relating to all-causes, cancer, respiratory disease, and cardiovascular disease.76 Chronic widespread pain and CP were also associated with cardiovascular disorders such as myocardial infarction, heart failure, and stroke,99 whereas a higher number of pain sites was associated with death at a younger age in men88 and all-cause mortality in both genders.29 The extensive data available in UKB allow researchers to adjust for a wide variety of potentially confounding factors, and this has been performed to a greater or lesser extent in the studies cited.
The availability of genome-wide genotyping data has advanced our understanding of genetic risk factors for pain and provided insights into the biological pathways involved. Novel genome-wide significant (GWS) genetic loci have been identified for back,16,45,46,114 knee,84 neck/shoulder,86,132 frozen shoulder,53 and stomach or abdominal pain132 as well as pain relating to oral inflammatory diseases,62 multisite CP,63,64,66,132 and CWP.96 These findings suggest a key role for genes involved in the central nervous system,16,45,63,66 dorsal root ganglion,64 and immune regulation.62 It has also highlighted some key differences in the genetics underpinning certain subsets of pain. For example, chronic back pain seems to be much more heritable than acute back pain (4.6% vs 0.8%).16 The same study identified 13 GWS loci associated with chronic back pain but none for acute back pain. Similarly, another study identified 23 GWS loci associated with multisite CP but none for single site CP.66 Sex-specific genetic risk factors have been identified in chronic back and multisite pain.46,64 Meanwhile, a separate study on chronic back pain identified 2 GWS loci in men but 7 in women.46
In addition to the identification of genetic risk factors, UKB genome-wide association study (GWAS) data have also been used to identify genetic correlation between different phenotypes. This is achieved by constructing polygenic risk scores (PRS) to summarise each participant's genetic predisposition for a given pain phenotype or by conducting linkage disequilibrium score regression (LDSR). These techniques have revealed, perhaps unsurprisingly, that there is strong genetic correlation between pain at different sites.40 Pain phenotypes also seem to have a shared genetic signature with a wide range of psychiatric and mood disorders such as depression, neuroticism, and sleep disorders,45,86 whereas shared genetic architecture seems to underpin the relationship between opioid cessation and CP, being a former drinker or being a former smoker.32
Finally, UKB GWAS data have been used to establish causal inference of nongenetic factors on pain phenotypes through Mendelian Randomisation. This technique uses known variation in a genetic marker and its influence on a particular trait (usually through GWAS) to interrogate the causal effect of an exposure on a particular outcome. As the genetic variants inherited by an individual are randomly assigned at conception and not subject to modification, it allows genetic markers to be used as a “proxy” for the exposure of interest, thus eliminating the risk of confounding or reverse causation. These studies have been able to establish a causal effect of C-reactive protein,65 insomnia103 and iron blood serum status117 with back pain, and diabetes with frozen shoulder.53 Furthermore, a bidirectional relationship was found to exist between insomnia and CP21 and between prescription opioid use and depression and anxiety disorders,100 whereas multisite CP was found to be a causative for major depressive disorder63 and far sightedness.94 By contrast, Mendelian Randomisation found no evidence for a causal effect of alcohol with CWP,8 obesity with frozen shoulder,53 or daytime sleepiness with lower back pain.103
3. DOLORisk
The approach to rephenotyping UKB participants for CP and NeuP was based on the experience of the DOLORisk consortium with phenotyping methods, in particular for population cohorts93 (Table 1).
DOLORisk, funded by EU Horizon 2020, was the starting point of an effort to expand and improve the development of NeuP cohorts across Europe (including 11 participating centres). The aim was to develop clinical cohorts of sufficient scale to study the multiple risk factors and determinants for NeuP (genetic, clinical, and psychosocial), to understand how such factors interact, and to identify those individuals most at risk of NeuP (Fig. 1). Observational in design, it consisted of cross-sectional cohorts and longitudinal cohorts and included both participants from the community in whom outcome measures were captured using questionnaires and more specialised cohorts from secondary care who had detailed phenotyping.93 Participant recruitment occurred between 2015 and 2019.
Figure 1.
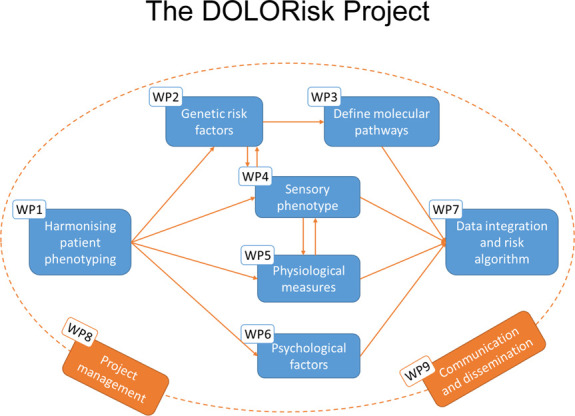
Illustration of the relationship between the different work packages in DOLORisk.
The main longitudinal branch of the study consisted of 2 existing population cohorts: Generation Scotland (a family-based study107) and GoDARTS (Genetics of Diabetes Audit and Research in Tayside Study—focused on diabetes57), whose participants were contacted by the University of Dundee to be rephenotyped for NeuP (approximately 9,000 respondents at baseline58). Aarhus University and INSERM recruited smaller cohorts of participants scheduled to undergo chemotherapy, thoracic surgery, or breast cancer surgery. These also had a longitudinal design, assessing patients before and after surgery or chemotherapy. The other branch of DOLORisk was cross-sectional and consisted of cohorts of participants with a neuropathy assessed (with clinical history, examination, and specialised tests such as quantitative sensory testing [QST]) in research centres. Aetiologies of neuropathy included diabetic neuropathy, other polyneuropathies, postsurgical neuropathy, small fibre neuropathy, chemotherapy-induced neuropathy, rare pain disorders, and traumatic nerve injury. The total number of participants included in this deeply phenotyped cohort was in the region of 1500. An effort was made to include participants with the relevant predisposition such as diabetic neuropathy but without pain as a control group.
One of the ambitions of DOLORisk was to set standards for data collection and deep phenotyping of NeuP. All centres followed a common protocol as a means of harmonisation. This was developed around a core set of self-report questionnaires, to be administered to the population cohorts by mail, and based on recent international consensus on NeuP phenotyping which had been developed through systematic review, Delphi survey, and expert consensus meetings.123 Detailed aspects of the protocol including further questionnaires, clinical measures, and specialised tests were developed by consensus and finalised at a dedicated consensus meeting between all participating centres. Proposals were made with members of the consortium leading on their area of expertise, reviewing the literature, and unpublished data (for instance exploring the performance of a shorter version of the QST protocol) and then working to achieve a group consensus. In addition to the core questionnaires, the deeply phenotyped cohorts had an extended set of questionnaires, neurological examination, and physiological tests. The questionnaires captured information on demographics; medication; the presence, characterisation, and intensity of pain; pain interference; psychological and lifestyle factors; and quality of life. The choice of questionnaires was based on validation in CP/NeuP and the availability of relevant translations (details of the questionnaires and specialised tests can be found in Tables 1 and 2 of Ref. 93). Every participant had (or had previously provided) blood samples taken to perform genetic analyses. A subset of participants also provided a serum sample and a skin biopsy sample. Additional investigations included QST using a slightly shortened version of the German NeuP Consortium protocol,98 electrophysiology (including nerve conduction studies and nerve excitability testing119), conditioned pain modulation (CPM), and electroencephalography (EEG). The diagnosis of NeuP was graded based on the NeuPSIG algorithm,44 which sorts participants into 4 groups (unlikely, possible, probable, and definite NeuP) and in those participants with neuropathy diagnostic criteria were based on the Tesfaye criteria.118 The use of the same core questionnaires was to enable comparison between large population cohorts which have the advantage of scale but lack of in-depth phenotyping and the smaller cohorts recruited in secondary care. For clinical examination and the specialised tests, standard protocols were used including training (both in person and using videos) as well as regular review and feedback of data quality.
3.1. Caveats/limitations
(1) Generation Scotland has an overrepresentation of females, affluence, older age, and lower BMI and underrepresentation of comorbidities compared with the Scottish population. For example, 32% reported CP (2.7% severe) compared with 46% (5.7%) nationally.
(2) GoDARTS has an underrepresentation of non–Anglo-American ethnicities (0.3% vs 9.2%) and people who have never smoked (41.5% vs 48.1%) in the diabetic part of the cohort, compared with the Scottish T2D population in 2020 (https://www.diabetesinscotland.org.uk/wp-content/uploads/2022/01/Diabetes-Scottish-Diabetes-Survey-2020.pdf).
(3) Self-reported ethnicity was not recorded in DOLORisk limiting exploration of the impact of ethnicity on CP (this was due to national laws in one participating country preventing the recording of ethnicity data).
(4) Most cohorts which underwent deep phenotyping in DOLORisk were cross-sectional rather than longitudinal limiting our ability to establish causality in the relationship between risk factor(s) and CP. This will be partly addressed by PAINSTORM (see below) which will collect follow-up data on these cohorts within the United Kingdom.
3.2. Outputs
DOLORisk ran from 2015 to 2020, and consequently studies are now beginning to be published (Table 3). The first study to emerge was a GWAS meta-analysis of Generation Scotland and GoDARTS (using a questionnaire-based phenotype identifying NeuP of any aetiology) together with UKB (using a phenotype based on self-reported medication124). This revealed a novel genome-wide significant locus at the mitochondrial phosphate carrier gene SLC25A3 and a suggestive locus at the calcium-binding gene CAB39L. In parallel with this study, the questionnaire-based data in Generation Scotland were used to construct longitudinal environmental risk models for onset and resolution of NeuP, which were then validated in GoDARTS.59 These models demonstrated the importance of psychological, social, lifestyle, and personality factors in predicting NeuP outcomes. The GoDARTS cohort was also used as a validation cohort in a study which trained machine learning models that can classify people with diabetic peripheral neuropathy into those with and without pain.6 These models were developed in deeply phenotyped cohorts recruited from the University of Oxford, Technion-Israel Institute of Technology, and Imperial College London and again highlighted the importance of personality, psychological, and quality of life factors in predicting pain. Other important predictors identified were levels of glycosylated haemoglobin (HbA1c), age, and BMI. It is hoped that eventually, these models can be used in a clinical setting to help improve prevention, diagnosis, and treatment for patients.
Table 3.
Publications on neuropathic pain from the DOLORisk study.
| Study | Pain phenotype | Design | Main finding |
|---|---|---|---|
| Baskozos et al.6 | Diabetic peripheral neuropathy | CS | Machine learning techniques demonstrated that HRQoL, personality traits, HbA1c, depression, anxiety, age, and BMI were the most powerful predictors of the presence of in pain people with diabetic peripheral neuropathy |
| Bennedsgaard et al.11 | Chemotherapy-induced peripheral neuropathy in patients with breast cancer referred for surgery | Longitudinal | Polyneuropathy and pain symptoms more common in patients treated with chemotherapy than those without. In people treated with chemotherapy, pain in the feet was less common than pain at the surgical site but was more intense |
| Granovsky et al.52 | Diabetic polyneuropathy | CS | Heat pain CPM was more efficient in people with painful vs painless diabetic polyneuropathy. Efficient heat pain CPM associated with greater pain intensity in previous 24 h and greater loss of mechanical sensation. |
| Hébert et al.59 | General NP | Longitudinal | Multivariable risk models demonstrate that psychosocial and lifestyle factors predict the onset and resolution of neuropathic pain. The models demonstrated adequate discrimination and clinical utility over a range of risk thresholds |
| Themistocleous et al.119 | Diabetic/chemotherapy-induced distal symmetrical polyneuropathy | CS | There were no significant differences in sensory and motor axonal excitability measures between patients with painful and painless diabetic/chemotherapy-induced peripheral neuropathy |
| Topaz et al.120 | Diabetic polyneuropathy | CS | Advanced predictive analysis demonstrated that resting-state electroencephalography–based functional brain activity (cortical functional connectivity) can discriminate between painful and painless diabetic polyneuropathy |
| Veluchamy et al.124 | General NP | GWAS meta-analysis | 1 GWS significant locus (SLC25A3) and one suggestive locus (CAB39L) associated with NP. Gene expression in NP-associated brain and DRG tissue. Previously reported genetic variants failed to replicate |
BMI, body mass index; CAB39L, calcium binding protein 39 like; CPM, conditioned pain modulation; CS, cross-sectional; DRG, dorsal root ganglion; GWAS, Genome-Wide Association Study; GWS, genome-wide significant; HbA1c, glycated haemoglobin; HRQoL, health-related quality of life; SLC25A3, NP, neuropathic pain; solute carrier family 25 member 3.
Further studies have been conducted on patients with diabetic polyneuropathy in more deeply phenotyped cohorts. For example, in a cohort recruited by Technion-Israel, both traditional and machine learning predictive techniques were used to analyse brain activity through EEG data.120 This analysis revealed that people with painful diabetic polyneuropathy had significantly greater resting-state cortical functional connectivity than people with painless diabetic polyneuropathy and that EEG-based brain activity could be a powerful biomarker than can accurately discriminate between the 2 groups. Another study using the cohort from Technion and a cohort recruited by Imperial College London found that people with painful diabetic polyneuropathy had more efficient CPM to heat stimuli applied to the forearm than those with painless diabetic polyneuropathy.52 This was the first comparison of CPM in painful vs painless diabetic neuropathy. Previous studies had compared groups of patients with CP to healthy controls and so would not take into account neuropathy induced damage to sensory afferents. Conditioned pain modulation heat stimuli efficiency was also correlated with greater pain intensity in the previous 24 hours and greater loss of mechanical sensation. One possible explanation for the more efficient CPM to heat stimuli in those with painful diabetic polyneuropathy may relate to neuropathy at the site of stimulation used in the protocol (and not only as a consequence of descending pain modulation). In light of this, new protocols in which stimuli are given to sites unaffected by neuropathy are needed. Finally, a study exploring the use of nerve excitability (using threshold tracking) as a biomarker in patients with diabetic and chemotherapy-induced peripheral neuropathy found that there was no difference in axonal excitability relating to large, myelinated fibers between those with pain and those without pain.119 However, because nociceptors are generally unmyelinated and, therefore, not assessed using this technique, these findings suggest that alternative techniques such as microneurography (which specifically examines small fibres) should be used to explore the relationship between neuron excitability and NeuP.
Separately, a longitudinal investigation of patients with breast cancer referred for surgery found that, in the group who had received chemotherapy, pain at the surgical site was more prevalent than pain in both feet (59% vs 30%).11 However, the pain in the feet was rated as more intense and with more daily life interference than pain in the surgical area. Furthermore, the prevalence of pain in both feet was greater in those who had pain in the surgical area, compared with those who did not have pain in the surgical area (40% vs 17%).
Analysis of the DOLORisk cohort is ongoing especially in relation to the deeply phenotyped cohorts including sensory profiles (determined using QST) and genomics.
4. PAINSTORM
Following on from DOLORisk, the PAINSTORM project (Partnership for Assessment and Investigation of Neuropathic Pain: Studies Tracking Outcomes, Risks and Mechanisms) was funded by the UK's Advanced Pain Discovery Platform (APDP),4 beginning in 2021. PAINSTORM will follow-up the DOLORisk diabetic population cohort in Dundee (GoDARTS), the diabetic and idiopathic neuropathy cohorts at Oxford and Imperial, and further expand the Oxford rare phenotypes cohort. It will also include new cohorts of people receiving chemotherapy, people with HIV and HTLV-1, and will use the newly available CP data in UKB. The aim of PAINSTORM is to collect more longitudinal data, especially in the deeply phenotyped cohorts, to define the risk factors and pathophysiological drivers of NeuP. Patient partners contributed directly to the consensus meeting and to the development of the PAINSTORM protocol and both of these are very similar to those in DOLORisk (Table 1). Based on feedback from patient partners and past experience, a few questionnaires were added (ethnicity, PROMIS Emotional Support, PROMIS Instrumental Support, bespoke items related to pain management, and the lived experience of having NeuP), substituted (PROMIS Pain Interference was replaced with PROMIS Ability to Participate in Social Roles and Activities; the IPIP items for Emotional Stability were replaced with the 7-item State Optimism Measure), or removed (PainDETECT). The inclusion of patient partners in PAINSTORM (form the application stage) has shaped our understanding of the issues that matter to people living with NeuP, the lived experience of NeuP, and the acceptability of measures to assess NeuP. The specialised investigation techniques in PAINSTORM differ slightly to DOLORisk: Nerve excitability testing (which we found did not discriminate painful from painless neuropathy in the DOLORisk study119) makes way for microneurography102,122; CPM was omitted as our data from the DOLORisk project suggest that an improved CPM protocol, to be applied and validated in the context of neuropathy, is required52; EEG was not included because as yet the technology for undertaking this at scale is not available; and some participants will take part in imaging studies of the brain, the spinal cord, and the peripheral nervous system. Genetic analysis is likely to include technology advances in both sequencing and analysis for much more comprehensive genomic assessment such as whole genome sequencing.
5. Caveats and lessons learned
Other important and relevant pain cohorts exist (such as OPPERA examining painful temporomandibular disorder43,104,105), and we have only described 3 in order that they can be discussed in detail. UK Biobank, DOLORisk, and PAINSTORM cohorts include a specific focus on (neuropathic) pain phenotypes. Some other cohorts include a few pain questions, but pain is not the focus, and these questions are almost incidental, although they can have value. For example, the English Longitudinal Study of Ageing (ELSA) included a single question about pain (“Are you often troubled by pain?”—yes/no), which allowed relatively detailed analysis of associations between pain and mortality.109 In one sense, this caveat could even be true of UKB at baseline, which used an untried, unvalidated, nonstandard set of relatively superficial questions. This has allowed a good number of studies to be published (Table 2), and their success may rely on sample size and consequent power, rather than on the precision or validity of the definitions. Not until the rephenotyping exercise, described above (UKB CP) were there validated, standard pain, and relevant associated questionnaires included.
The 3 cohorts we have described deliberately used similar, harmonised approaches to phenotyping pain. Generally, when looking at different research cohorts, we find that a lack of agreed approaches to phenotyping CP means that we cannot compare outputs from different cohorts. For example, in a systematic review of studies examining genetic factors associated with NeuP, we found 29 studies, identifying 28 genes, but none used the same approach to phenotyping, and few single genes were identified by more than one study. This means that we cannot understand whether differences between studies are the result of actual differences between study populations or artefacts of differential phenotyping. One study, for example, found that associations between CP and mortality depended on how the pain was phenotyped.108 This lack of harmonisation also prevents meta-analysis.
To surmount these phenotyping/case definition differences, we need the following:
(1) A series of studies exploring the effects that differences in case definition have in identifying subsamples with and without CP; eg, demographic and clinical differences/similarities between “cases” in different cohorts. Macfarlane et al.75 did explore this in relation to the original UKB pain phenotype, comparing prevalence and associations with those identified in other pain cohorts, and we have performed similar in relation to UKB CP (paper in press). Although the results were reassuring, more such analysis is called for.
(2) Harmonised case definitions/phenotyping moving forward, such as that agreed for genetic studies of NeuP (NeuroPPIC123). In addition to allowing comparison and meta-analysis (retrospective studies), this approach will also allow prospectively assembled collaborative cohorts, with enhanced power. This has been our philosophy with UKB CP, DOLORisk, and PAINSTORM.
A major issue with the cohorts we have described, and with every other cohort, is their representativeness. Even a very large cohort such as UKB can only reflect the population from which it was drawn. As noted above, in UKB's case, this population comprised adults aged 40 to 69 at recruitment,112 and underrepresents people from more socioeconomically deprived areas, as well as people who are obese, smoke, drink alcohol, self-report certain health conditions, and ethnic minorities.48 Although this allows assessment of relationships between exposures and outcomes, it limits findings relating to incidence and prevalence and to exposures/outcomes that are rare in the cohort. Similar constraints apply to the studies contributing to DOLORisk (including GS107 and GoDARTS57) and will also apply to PAINSTORM. A key is to focus on the strengths and unique selling points of the cohort; eg, a family study allows efficient measurement of heritability. It is also important to measure, understand, and, if possible, account or adjust for relevant differences between the cohort and target populations. Strategies also need to be developed to improve representation in groups that are traditionally underrepresented in research. Potential approaches for improving representativeness include expanding recruitment strategies, to include for example face-to-face, email, and postal invitations, through primary and specialist care. In addition, dedicating research resources into advertising through local/national business organisations, radio, newspapers, and social media can increase uptake and help to oversample these “hard-to-reach” groups. The involvement of people living with pain, for instance coworking with charities and community organisations can help with dissemination strategies and generate general awareness of studies. An example of an initiative that has successfully used these approaches in the Scottish Health Research Register (SHARE).82 However, there are certain situations in which a representative sample may not be necessary, for example, if an investigator wants to study a particular population subgroup.97
For practical reasons, large population studies generally only allow brief questionnaire measures, often purporting to represent complex multidimensional phenomena (such as pain) in summary numerical terms, often with continuous scales or categorical coding. Although these questionnaires are (1) usually validated in their development stages by comparison with more sophisticated and detailed assessments and (2) often supplemented in related studies by more detailed measurement/interviews with subsamples, they cannot tell the full story of what is being measured. This issue has long been recognised (eg, by Macnaughton79), and recent discussions with our patient partners on PAINSTORM confirm the issue, and the frustration it causes to people completing the questionnaires. Although we should continue to measure as accurately as possible, using questionnaires with maximum validity and reliability, we should also work with people living with pain to develop more realistic and satisfactory ways of assessing complex health and psychosocial issues at scale.
6. Future prospects
Alternative approaches to cohort recruitment and assessment include the use of routine clinical data, without the direct involvement of individuals (although with appropriate ethical and governance approvals in place). This approach has also been recommended for clinical trials in CP.101 For example, in the United Kingdom, GP-held primary care records offer the opportunity to identify relevant individuals through clinical diagnostic codes or prescribing. These have been used to recruit study participants with CP22,80 but require detailed validation and assessment of sensitivity, specificity, and positive/negative predictive values. The authors (BHS and DW) are currently developing this in 2 funded studies at the population level. Routine clinical data can also augment research-derived data arising from new and existing cohorts, through data linkage, noting the need for data security and confidentiality. As noted, UKB data are now linked to primary healthcare records for most participants. Advantages of linkage to routine data include comprehensiveness, representativeness, and low cost. Disadvantages include relatively poor-quality data and the complexity of data available, as well as the need to secure different approvals before data can be accessed and linked.
Development, harmonisation, meta-analysis, and data linkage of CP cohorts require, among other factors, high-quality data storage, management, and access. Also funded through the APDP, Alleviate is the Research Data Hub that will provide a platform for pain data for researchers around the world.2 Initially focusing on APDP consortia (including PAINSTORM) and projects, Alleviate will also store or allow access to other relevant datasets (including UKB CP and DOLORisk). These data sets will be findable, accessible, interoperable, and reusable (FAIR), and the Hub will be comparable with those already in existence for respiratory datasets (BREATHE19) and COVID-19 (CO-CONNECT31). Investment in data hubs such as Alleviate from researchers and funding bodies is key to expanding these cohorts on a global scale and integrating efforts around data harmonisation. Such investment can be encouraged and promoted through organisations such the International Association for the Study of Pain.
Meanwhile, we will continue our research with the above cohorts, both through further analysis of DOLORisk and UKB CP, and through development of PAINSTORM. Importantly, this will include collaboration with colleagues and cohorts elsewhere, including other APDP consortia and projects,4 with whom PAINSTORM is harmonising as much as possible. The scheduled follow-up of UKB CP phenotyping promises exciting approaches to longitudinal research on CP at large scale.
7. Conclusions
Large national level biobanks such as UKB have already provided important insights into the pathophysiology of CP; these are likely to become more robust with the greater precision of recently augmented pain phenotyping and longitudinal outcomes. There are exciting prospects ahead with the greater integration of GP records, new genetic technologies (such as whole genome sequencing), and the brain imaging of 100,000 participants in UKB. Making sense of such complex, multimodal data sets will require advanced analytics, including machine learning approaches. Although the scale of UKB has undoubted advantages, pain remains a subjective phenomenon which is difficult to capture using a limited number of questionnaires. This is particularly important in conditions such as NeuP where clinical assessment (including examination) is required to reach a robust case definition. This means that there is still an important place for smaller deeply phenotyped cohorts in which the link between a predisposing aetiology, CP, and biomarkers can be studied in detail. Such cohorts can also be used (while working with patient partners) to find new ways to assess pain and the functional impact of pain which can then be iteratively fed back to national level cohorts. The pharmaceutical industry is increasingly orienting analgesic drug discovery to targets in which there is human data validating the molecular target. Our hope is that integration of “big pain data” in humans with the rapid advances in cellular transcriptomics and neural circuit level approaches in animal models will facilitate the desperately needed development of novel analgesics, among other important advances.
Disclosures
The authors have no conflict of interest to declare.
Acknowledgements
B.H.S. and D.L.H.B. have received grants from the MRC, Versus Arthritis, Eli Lilly, and Astra Zeneca (as part of the Advanced Pain Discovery Platform) for PAINSTORM (MR/W002388/1) and from the European Union's Horizon 2020 research and innovation programme under grant agreement No 633491 (DOLORisk).
H.L.H. and M.M.V.P. are supported by PAINSTORM and have been supported by DOLORisk.
D.L.H.B. acknowledges grants from the Wellcome Trust, Diabetes UK, MRC, and the BBSRC and has acted as a consultant in the past 2 years for AditumBio, Amgen, Biointervene, Bristows, LatigoBio, GSK, Ionis, Lexicon therapeutics, Lilly, Neuvati, Olipass, Orion, Regeneron, Replay, and Theranexus on behalf of Oxford University Innovation (all paid to institution).
Data availability statement: Data sharing is not applicable as no new data were created or analysed in this article. All results presented in this article have been published previously and fully cited in the text.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
H. L. Hébert and M. M. V. Pascal contributed equally to this work.
References
- [1].Allen SF, Gilbody S, Atkin K, van der Feltz-Cornelis C. The associations between loneliness, social exclusion and pain in the general population: a N=502,528 cross-sectional UK Biobank study. J Psychiatr Res 2020;130:68–74. [DOI] [PubMed] [Google Scholar]
- [2].Alleviate—our advanced pain discovery platform (APDP) hub. Available at: https://www.hdruk.ac.uk/helping-with-health-data/health-data-research-hubs/alleviate/. [Google Scholar]
- [3].Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai JS. Development of a PROMIS item bank to measure pain interference. PAIN 2010;150:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Area of investment and support: advanced pain discovery platform (APDP): UK research and innovation, 2022. Available at: https://www.ukri.org/what-we-do/our-main-funds-and-areas-of-support/browse-our-areas-of-investment-and-support/advanced-pain-discovery-platform-apdp/ [Google Scholar]
- [5].Atkins JL, Delgado J, Pilling LC, Bowman K, Masoli JAH, Kuchel GA, Ferrucci L, Melzer D. Impact of low cardiovascular risk profiles on geriatric outcomes: evidence from 421,000 participants in two cohorts. J Gerontol A Biol Sci Med Sci 2019;74:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baskozos G, Themistocleous AC, Hebert HL, Pascal MMV, John J, Callaghan BC, Laycock H, Granovsky Y, Crombez G, Yarnitsky D, Rice ASC, Smith BH, Bennett DLH. Classification of painful or painless diabetic peripheral neuropathy and identification of the most powerful predictors using machine learning models in large cross-sectional cohorts. BMC Med Inform Decis Mak 2022;22:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baskozos G, Hébert HL, Pascal MMV, Themistocleous AC, Macfarlane GJ, Wynick D, Bennett DLH, Smith B. Epidemiology of neuropathic pain: an analysis of prevalence and associated factors in UK Biobank. Pain Rep 2023;8:e1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beasley MJ, Macfarlane TV, Macfarlane GJ. Is alcohol consumption related to likelihood of reporting chronic widespread pain in people with stable consumption? Results from UK Biobank. PAIN 2016;157:2552–60. [DOI] [PubMed] [Google Scholar]
- [9].Beasley M, Freidin MB, Basu N, Williams FMK, Macfarlane GJ. What is the effect of alcohol consumption on the risk of chronic widespread pain? A Mendelian randomisation study using UK Biobank. PAIN 2019;160:501–7. [DOI] [PubMed] [Google Scholar]
- [10].Benavides R, Vsevolozhskaya O, Cattaneo S, Zaykin D, Brenton A, Parisien M, Verma V, Khoury S, Gilron I, Diatchenko L. A functional polymorphism in the ATP-Binding Cassette B1 transporter predicts pharmacologic response to combination of nortriptyline and morphine in neuropathic pain patients. PAIN 2020;161:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bennedsgaard K, Grosen K, Attal N, Bouhassira D, Crombez G, Jensen TS, Bennett DL, Ventzel L, Andersen IS, Finnerup NB. Neuropathy and pain after breast cancer treatment: a prospective observational study. Scand J Pain 2022;23:49–58. [DOI] [PubMed] [Google Scholar]
- [12].Bennett DL, Woods CG. Painful and painless channelopathies. Lancet Neurol 2014;13:587–99. [DOI] [PubMed] [Google Scholar]
- [13].Bennett DL, Clark AJ, Huang J, Waxman SG, Dib-Hajj SD. The role of voltage-gated sodium channels in pain signaling. Physiol Rev 2019;99:1079–151. [DOI] [PubMed] [Google Scholar]
- [14].Blyth FM, Van Der Windt DA, Croft PR. Chronic disabling pain: a significant public health problem. Am J Prev Med 2015;49:98–101. [DOI] [PubMed] [Google Scholar]
- [15].Borsook D, Youssef AM, Simons L, Elman I, Eccleston C. When pain gets stuck: the evolution of pain chronification and treatment resistance. PAIN 2018;159:2421–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bortsov AV, Parisien M, Khoury S, Martinsen AE, Lie MU, Heuch I, Hveem K, Zwart JA, Winsvold BS, Diatchenko L. Brain-specific genes contribute to chronic but not to acute back pain. Pain Rep 2022;7:e1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, Boureau F. Development and validation of the neuropathic pain symptom inventory. PAIN 2004;108:248–57. [DOI] [PubMed] [Google Scholar]
- [18].Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lanteri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). PAIN 2005;114:29–36. [DOI] [PubMed] [Google Scholar]
- [19].BREATHE—our hub for respiratory health. Available at: https://www.ukri.org/what-we-do/our-main-funds-and-areas-of-support/browse-our-areas-of-investment-and-support/advanced-pain-discovery-platform-apdp/ [Google Scholar]
- [20].Breivik H. European pain management. In: Eccleston C, Wells C, Morlion B, editors. Oxford. New York, NY: Oxford University Press, 2018. pp. xix, 283 pages. [Google Scholar]
- [21].Broberg M, Karjalainen J, FinnGen, Ollila HM. Mendelian randomization highlights insomnia as a risk factor for pain diagnoses. Sleep 2021;44:zsab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bruhn H, Bond CM, Elliott AM, Hannaford PC, Lee AJ, McNamee P, Smith BH, Watson MC, Holland R, Wright D. Pharmacist-led management of chronic pain in primary care: results from a randomised controlled exploratory trial. BMJ Open 2013;3:e002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, Johnston KL, Shablesky-Cade MA, Pilkonis PA. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep 2010;33:781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Campbell A, Shona K, Porteous D. Generation Scotland SFHS data dictionary, 2006-2011 [dataset]. In: GaPHSIoGaMM University of Edinburgh. School of Molecular editor, 2018. [Google Scholar]
- [25].Carvalho ESAP, Ferreira ML, Ferreira PH, Harmer AR. Does type 2 diabetes increase the risk of musculoskeletal pain? Cross-sectional and longitudinal analyses of UK Biobank data. Semin Arthritis Rheum 2020;50:728–34. [DOI] [PubMed] [Google Scholar]
- [26].Carvalho ESAP, Harmer AR, Ferreira ML, Ferreira PH. The effect of the anti-diabetic drug metformin on musculoskeletal pain: a cross-sectional study with 21,889 individuals from the UK Biobank. Eur J pain 2021;25:1264–73. [DOI] [PubMed] [Google Scholar]
- [27].Cassidy S, Trenell MI, Anderson KN. The cardio-metabolic impact of taking commonly prescribed analgesic drugs in 133,401 UK Biobank participants. PLoS One 2017;12:e0187982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Census 2011: key results on population, ethnicity, identity, language, religion, health, housing and accommodation in Scotland—release 2A: National Records of Scotland. Available at: https://www.nrscotland.gov.uk/news/2013/census-2011-release-2a. [Google Scholar]
- [29].Chen L, Ferreira ML, Nassar N, Preen DB, Hopper JL, Li S, Bui M, Beckenkamp PR, Shi B, Arden NK, Ferreira PH. Association of chronic musculoskeletal pain with mortality among UK adults: a population-based cohort study with mediation analysis. EClinicalMedicine 2021;42:101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap 1994;23:129–38. [PubMed] [Google Scholar]
- [31].CO-CONNECT: COVID—curated and open analysis and research platform. Available at: https://co-connect.ac.uk/. [Google Scholar]
- [32].Cox JW, Sherva RM, Lunetta KL, Johnson EC, Martin NG, Degenhardt L, Agrawal A, Nelson EC, Kranzler HR, Gelernter J, Farrer LA. Genome-wide association study of opioid cessation. J Clin Med 2020;9. doi: 10.3390/jcm9010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sport Exer 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- [34].Elliott LT, Sharp K, Alfaro-Almagro F, Shi S, Miller KL, Douaud G, Marchini J, Smith SM. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 2018;562:210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ethnic group, England and Wales: Census 2021, released 29 November 2022. ONS website: Office for National Statistics (ONS). Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/bulletins/ethnicgroupenglandandwales/census2021. [Google Scholar]
- [36].Ethnicity and national identity in England and Wales: 2011, released 11 December 2012. ONS website: Office for National Statistics (ONS). Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/ethnicityandnationalidentityinenglandandwales/2012-12-11. [Google Scholar]
- [37].Faber BG, Ebsim R, Saunders FR, Frysz M, Gregory JS, Aspden RM, Harvey NC, Davey Smith G, Cootes T, Lindner C, Tobias JH. Cam morphology but neither acetabular dysplasia nor pincer morphology is associated with osteophytosis throughout the hip: findings from a cross-sectional study in UK Biobank. Osteoarthritis and cartilage/OARS. Osteoarthritis Res Soc 2021;29:1521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Faber BG, Ebsim R, Saunders FR, Frysz M, Lindner C, Gregory JS, Aspden RM, Harvey NC, Smith GD, Cootes T, Tobias JH. Osteophyte size and location on hip DXA scans are associated with hip pain: findings from a cross sectional study in UK Biobank. Bone 2021;153:116146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Faber BG, Ebsim R, Saunders FR, Frysz M, Lindner C, Gregory JS, Aspden RM, Harvey NC, Davey Smith G, Cootes T, Tobias JH. A novel semi-automated classifier of hip osteoarthritis on DXA images shows expected relationships with clinical outcomes in UK Biobank. Rheumatology (Oxford) 2022;61:3586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Farrell SF, Kho PF, Lundberg M, Campos AI, Renteria ME, de Zoete RMJ, Sterling M, Ngo TT, Cuellar-Partida G. A shared genetic signature for common chronic pain conditions and its impact on biopsychosocial traits. J Pain 2023;24:369–86. [DOI] [PubMed] [Google Scholar]
- [41].Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 2016;6:e010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–9. [DOI] [PubMed] [Google Scholar]
- [43].Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain 2013;14:T75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice AS, Serra J, Smith BH, Treede RD, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. PAIN 2016;157:1599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Freidin MB, Tsepilov YA, Palmer M, Karssen LC, Suri P, Aulchenko YS, Williams FMK, Group CMW. Insight into the genetic architecture of back pain and its risk factors from a study of 509,000 individuals. PAIN 2019;160:1361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Freidin MB, Tsepilov YA, Stanaway IB, Meng W, Hayward C, Smith BH, Khoury S, Parisien M, Bortsov A, Diatchenko L, Borte S, Winsvold BS, Brumpton BM, Zwart JA, Aulchenko YS, Suri P, Williams FMK, Pain HA-I. Sex- and age-specific genetic analysis of chronic back pain. PAIN 2021;162:1176–87. [DOI] [PubMed] [Google Scholar]
- [47].Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. [DOI] [PubMed] [Google Scholar]
- [48].Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 2017;186:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Goldberg LR. A broad-bandwidth, public domain, personality inventory measuring the lower-level facets of several five-factor models. European conference on personality. Vol. 7. Tilburg, the Netherlands: Tilburg University, 1999. pp. 7–28. [Google Scholar]
- [51].Gosling SD, Rentfrow PJ, Swann WB. A very brief measure of the Big-Five personality domains. J Res Pers 2003;37:504–28. [Google Scholar]
- [52].Granovsky Y, Shafran Topaz L, Laycock H, Zubiedat R, Crystal S, Buxbaum C, Bosak N, Hadad R, Domany E, Khamaisi M, Sprecher E, Bennett DL, Rice A, Yarnitsky D. Conditioned pain modulation is more efficient in patients with painful diabetic polyneuropathy than those with nonpainful diabetic polyneuropathy. PAIN 2022;163:827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Green HD, Jones A, Evans JP, Wood AR, Beaumont RN, Tyrrell J, Frayling TM, Smith C, Weedon MN. A genome-wide association study identifies 5 loci associated with frozen shoulder and implicates diabetes as a causal risk factor. PLoS Genet 2021;17:e1009577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hahn EA, DeWalt DA, Bode RK, Garcia SF, DeVellis RF, Correia H, Cella D, Group PC. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol 2014;33:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hanlon P, McCallum M, Jani BD, McQueenie R, Lee D, Mair FS. Association between childhood maltreatment and the prevalence and complexity of multimorbidity: a cross-sectional analysis of 157,357 UK Biobank participants. J Comorb 2020;10:2235042X10944344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hastie CE, Foster HME, Jani BD, O'Donnell CA, Ho FK, Pell JP, Sattar N, Katikireddi SV, Mair FS, Nicholl BI. Chronic pain and COVID-19 hospitalisation and mortality: a UK Biobank cohort study. PAIN 2023;164:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hebert HL, Shepherd B, Milburn K, Veluchamy A, Meng W, Carr F, Donnelly LA, Tavendale R, Leese G, Colhoun HM, Dow E, Morris AD, Doney AS, Lang CC, Pearson ER, Smith BH, Palmer CNA. Cohort profile: genetics of diabetes audit and research in Tayside Scotland (GoDARTS). Int J Epidemiol 2018;47:380–1j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hebert HL, Veluchamy A, Baskozos G, Fardo F, Van Ryckeghem DML, Pascal MMV, Jones C, Milburn K, Pearson ER, Crombez G, Bennett DLH, Meng W, Palmer CNA, Smith BH. Cohort profile: DOLORisk Dundee: a longitudinal study of chronic neuropathic pain. BMJ Open 2021;11:e042887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hebert HL, Veluchamy A, Baskozos G, Fardo F, Van Ryckeghem D, Pearson ER, Colvin LA, Crombez G, Bennett DLH, Meng W, Palmer CNA, Smith BH. Development and external validation of multivariable risk models to predict incident and resolved neuropathic pain: a DOLORisk Dundee study. J Neurol 2023;270:1076–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hocking LJ, Generation S, Morris AD, Dominiczak AF, Porteous DJ, Smith BH. Heritability of chronic pain in 2195 extended families. Eur J pain 2012;16:1053–63. [DOI] [PubMed] [Google Scholar]
- [62].Jin Y, Yang J, Zhang S, Shi X, Li J, Wang S. Identification of novel genome-wide pleiotropic associations with oral inflammatory traits. Mol Genet Genomics 2022;297:19–32. [DOI] [PubMed] [Google Scholar]
- [63].Johnston KJA, Adams MJ, Nicholl BI, Ward J, Strawbridge RJ, Ferguson A, McIntosh AM, Bailey MES, Smith DJ. Genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet 2019;15:e1008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Johnston KJA, Ward J, Ray PR, Adams MJ, McIntosh AM, Smith BH, Strawbridge RJ, Price TJ, Smith DJ, Nicholl BI, Bailey MES. Sex-stratified genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet 2021;17:e1009428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kasher M, Williams FMK, Freidin MB, Cherny SS, Malkin I, Livshits G, Group CIW. Insights into the pleiotropic relationships between chronic back pain and inflammation-related musculoskeletal conditions: rheumatoid arthritis and osteoporotic abnormalities. PAIN 2023;164:e122–34. [DOI] [PubMed] [Google Scholar]
- [66].Khoury S, Parisien M, Thompson SJ, Vachon-Presseau E, Roy M, Martinsen AE, Winsvold BS, Pain HA-I, Mundal IP, Zwart JA, Kania A, Mogil JS, Diatchenko L. Genome-wide analysis identifies impaired axonogenesis in chronic overlapping pain conditions. Brain 2022;145:1111–23. [DOI] [PubMed] [Google Scholar]
- [67].Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46:1121–3. [DOI] [PubMed] [Google Scholar]
- [69].Lai JS, Cella D, Choi S, Junghaenel DU, Christodoulou C, Gershon R, Stone A. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch Phys Med Rehabil 2011;92:S20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Larvin H, Kang J, Aggarwal VR, Pavitt S, Wu J. Multimorbid disease trajectories for people with periodontitis. J Clin Periodontol 2021;48:1587–96. [DOI] [PubMed] [Google Scholar]
- [71].Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:646–57. [DOI] [PubMed] [Google Scholar]
- [72].Livshits G, Popham M, Malkin I, Sambrook PN, Macgregor AJ, Spector T, Williams FM. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis 2011;70:1740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lobo JJ, Ayoub LJ, Moayedi M, Linnstaedt SD. Hippocampal volume, FKBP5 genetic risk alleles, and childhood trauma interact to increase vulnerability to chronic multisite musculoskeletal pain. Sci Rep 2022;12:6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Macfarlane TV, Beasley M, Macfarlane GJ. Self-reported facial pain in UK Biobank study: prevalence and associated factors. J Oral Maxillofac Res 2014;5:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Macfarlane GJ, Beasley M, Smith BH, Jones GT, Macfarlane TV. Can large surveys conducted on highly selected populations provide valid information on the epidemiology of common health conditions? An analysis of UK Biobank data on musculoskeletal pain. Br J Pain 2015;9:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Macfarlane GJ, Barnish MS, Jones GT. Persons with chronic widespread pain experience excess mortality: longitudinal results from UK Biobank and meta-analysis. Ann Rheum Dis 2017;76:1815–22. [DOI] [PubMed] [Google Scholar]
- [77].Macfarlane GJ, Beasley M, Jones GT, Stannard C. The epidemiology of regular opioid use and its association with mortality: prospective cohort study of 466 486 UK Biobank participants. EClinicalMedicine 2020;21:100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].MacGregor AJ, Andrew T, Sambrook PN, Spector TD. Structural, psychological, and genetic influences on low back and neck pain: a study of adult female twins. Arthritis Rheum 2004;51:160–7. [DOI] [PubMed] [Google Scholar]
- [79].Macnaughton RJ. Numbers, scales, and qualitative research. Lancet 1996;347:1099–100. [DOI] [PubMed] [Google Scholar]
- [80].McDermott ME, Smith BH, Elliott AM, Bond CM, Hannaford PC, Chambers WA. The use of medication for chronic pain in primary care, and the potential for intervention by a practice-based pharmacist. Fam Pract 2006;23:46–52. [DOI] [PubMed] [Google Scholar]
- [81].McIntosh AM, Hall LS, Zeng Y, Adams MJ, Gibson J, Wigmore E, Hagenaars SP, Davies G, Fernandez-Pujals AM, Campbell AI, Clarke TK, Hayward C, Haley CS, Porteous DJ, Deary IJ, Smith DJ, Nicholl BI, Hinds DA, Jones AV, Scollen S, Meng W, Smith BH, Hocking LJ. Genetic and environmental risk for chronic pain and the contribution of risk variants for major depressive disorder: a family-based mixed-model analysis. PLoS Med 2016;13:e1002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].McKinstry B, Sullivan FM, Vasishta S, Armstrong R, Hanley J, Haughney J, Philip S, Smith BH, Wood A, Palmer CN. Cohort profile: the Scottish Research register SHARE. A register of people interested in research participation linked to NHS data sets. BMJ Open 2017;7:e013351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].McQueenie R, Jani BD, Siebert S, McLoone P, McCowan C, Macdonald S, Mair FS, Nicholl BI. Prevalence of chronic pain in LTCs and multimorbidity: a cross-sectional study using UK Biobank. J Multimorb Comorb 2021;11:26335565211005870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Meng W, Adams MJ, Palmer CNA, andMe Research T, Shi J, Auton A, Ryan KA, Jordan JM, Mitchell BD, Jackson RD, Yau MS, McIntosh AM, Smith BH. Genome-wide association study of knee pain identifies associations with GDF5 and COL27A1 in UK Biobank. Commun Biol 2019;2:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Meng W, Adams MJ, Reel P, Rajendrakumar A, Huang Y, Deary IJ, Palmer CNA, McIntosh AM, Smith BH. Genetic correlations between pain phenotypes and depression and neuroticism. Eur J Hum Genet 2020;28:358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Meng W, Chan BW, Harris C, Freidin MB, Hebert HL, Adams MJ, Campbell A, Hayward C, Zheng H, Zhang X, Colvin LA, Hales TG, Palmer CNA, Williams FMK, McIntosh A, Smith BH. A genome-wide association study finds genetic variants associated with neck or shoulder pain in UK Biobank. Hum Mol Genet 2020;29:1396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Millstein RA, Chung WJ, Hoeppner BB, Boehm JK, Legler SR, Mastromauro CA, Huffman JC. Development of the state optimism measure. Gen Hosp Psychiatry 2019;58:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Muralidharan A, Sotocinal SG, Yousefpour N, Akkurt N, Lima LV, Tansley S, Parisien M, Wang C, Austin JS, Ham B, Dutra GM, Rousseau P, Maldonado-Bouchard S, Clark T, Rosen SF, Majeed MR, Silva O, Nejade R, Li X, Donayre Pimentel S, Nielsen CS, Neely GG, Autexier C, Diatchenko L, Ribeiro-da-Silva A, Mogil JS. Long-term male-specific chronic pain via telomere- and p53-mediated spinal cord cellular senescence. J Clin Invest 2022;132:e151817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Nicholl BI, Mackay D, Cullen B, Martin DJ, Ul-Haq Z, Mair FS, Evans J, McIntosh AM, Gallagher J, Roberts B, Deary IJ, Pell JP, Smith DJ. Chronic multisite pain in major depression and bipolar disorder: cross-sectional study of 149,611 participants in UK Biobank. BMC Psychiatry 2014;14:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Nicholl BI, Smith DJ, Cullen B, Mackay D, Evans J, Anderson J, Lyall DM, Fawns-Ritchie C, McIntosh AM, Deary IJ, Pell JP, Mair FS. Ethnic differences in the association between depression and chronic pain: cross sectional results from UK Biobank. BMC Fam Pract 2015;16:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Pan F, Byrne KS, Ramakrishnan R, Ferreira M, Dwyer T, Jones G. Association between musculoskeletal pain at multiple sites and objectively measured physical activity and work capacity: results from UK Biobank study. J Sci Med Sport 2019;22:444–9. [DOI] [PubMed] [Google Scholar]
- [92].Parisien M, Lima LV, Dagostino C, El-Hachem N, Drury GL, Grant AV, Huising J, Verma V, Meloto CB, Silva JR, Dutra GGS, Markova T, Dang H, Tessier PA, Slade GD, Nackley AG, Ghasemlou N, Mogil JS, Allegri M, Diatchenko L. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci Transl Med 2022;14:eabj9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Pascal MMV, Themistocleous AC, Baron R, Binder A, Bouhassira D, Crombez G, Finnerup NB, Gierthmuhlen J, Granovsky Y, Groop L, Hebert HL, Jensen TS, Johnsen K, McCarthy MI, Meng W, Palmer CNA, Rice ASC, Serra J, Sola R, Yarnitsky D, Smith BH, Attal N, Bennett DLH. DOLORisk: study protocol for a multi-centre observational study to understand the risk factors and determinants of neuropathic pain. Wellcome Open Res 2018;3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Patasova K, Khawaja AP, Tamraz B, Williams KM, Mahroo OA, Freidin M, Solebo AL, Vehof J, Falchi M, Rahi JS, Hammond CJ, Hysi PG. Association between medication-taking and refractive error in a large general population-based cohort. Invest Ophthalmol Vis Sci 2021;62:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, Group PC. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment 2011;18:263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rahman MS, Winsvold BS, Chavez Chavez SO, Borte S, Tsepilov YA, Sharapov SZ, Pain HA-I, Aulchenko YS, Hagen K, Fors EA, Hveem K, Zwart JA, van Meurs JB, Freidin MB, Williams FM. Genome-wide association study identifies RNF123 locus as associated with chronic widespread musculoskeletal pain. Ann Rheum Dis 2021;80:1227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Richiardi L, Pizzi C, Pearce N. Commentary: representativeness is usually not necessary and often should be avoided. Int J Epidemiol 2013;42:1018–22. [DOI] [PubMed] [Google Scholar]
- [98].Rolke R, Baron R, Maier C, Tolle TR, Treede DR, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. PAIN 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [99].Ronnegard AS, Nowak C, Ang B, Arnlov J. The association between short-term, chronic localized and chronic widespread pain and risk for cardiovascular disease in the UK Biobank. Eur J Prev Cardiol 2022;29:1994–2002. [DOI] [PubMed] [Google Scholar]
- [100].Rosoff DB, Smith GD, Lohoff FW. Prescription opioid use and risk for major depressive disorder and anxiety and stress-related disorders: a multivariable mendelian randomization analysis. JAMA Psychiatry 2021;78:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Rowbotham MC, Gilron I, Glazer C, Rice ASC, Smith BH, Stewart WF, Wasan AD. Can pragmatic trials help us better understand chronic pain and improve treatment? PAIN 2013;154:643–6. [DOI] [PubMed] [Google Scholar]
- [102].Serra J, Bostock H, Sola R, Aleu J, Garcia E, Cokic B, Navarro X, Quiles C. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. PAIN 2012;153:42–55. [DOI] [PubMed] [Google Scholar]
- [103].Shu P, Ji L, Ping Z, Sun Z, Liu W. Association of insomnia and daytime sleepiness with low back pain: a bidirectional mendelian randomization analysis. Front Genet 2022;13:938334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Slade GD, Fillingim RB, Sanders AE, Bair E, Greenspan JD, Ohrbach R, Dubner R, Diatchenko L, Smith SB, Knott C, Maixner W. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: implications and future directions. J Pain 2013;14:T116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, Dubner R, Diatchenko L, Meloto CB, Smith S, Maixner W. Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res 2016;95:1084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Slade GD, Rosen JD, Ohrbach R, Greenspan JD, Fillingim RB, Parisien M, Khoury S, Diatchenko L, Maixner W, Bair E. Anatomical selectivity in overlap of chronic facial and bodily pain. Pain Rep 2019;4:e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Smith BH, Campbell A, Linksted P, Fitzpatrick B, Jackson C, Kerr SM, Deary IJ, Macintyre DJ, Campbell H, McGilchrist M, Hocking LJ, Wisely L, Ford I, Lindsay RS, Morton R, Palmer CN, Dominiczak AF, Porteous DJ, Morris AD. Cohort Profile: Generation Scotland: Scottish Family Health Study (GS:SFHS). The study, its participants and their potential for genetic research on health and illness. Int J Epidemiol 2013;42:689–700. [DOI] [PubMed] [Google Scholar]
- [108].Smith D, Wilkie R, Croft P, McBeth J. Pain and mortality in older adults: the influence of pain phenotype. Arthritis Care Res (Hoboken) 2018;70:236–43. [DOI] [PubMed] [Google Scholar]
- [109].Smith D, Wilkie R, Croft P, Parmar S, McBeth J. Pain and mortality: mechanisms for a relationship. PAIN 2018;159:1112–8. [DOI] [PubMed] [Google Scholar]
- [110].Spector TD, MacGregor AJ. Risk factors for osteoarthritis: genetics. Osteoarthritis Cartilage 2004;12(suppl A):S39–44. [DOI] [PubMed] [Google Scholar]
- [111].Steingrimsdottir OA, Landmark T, Macfarlane GJ, Nielsen CS. Defining chronic pain in epidemiological studies: a systematic review and meta-analysis. PAIN 2017;158:2092–107. [DOI] [PubMed] [Google Scholar]
- [112].Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- [114].Suri P, Palmer MR, Tsepilov YA, Freidin MB, Boer CG, Yau MS, Evans DS, Gelemanovic A, Bartz TM, Nethander M, Arbeeva L, Karssen L, Neogi T, Campbell A, Mellstrom D, Ohlsson C, Marshall LM, Orwoll E, Uitterlinden A, Rotter JI, Lauc G, Psaty BM, Karlsson MK, Lane NE, Jarvik GP, Polasek O, Hochberg M, Jordan JM, Van Meurs JBJ, Jackson R, Nielson CM, Mitchell BD, Smith BH, Hayward C, Smith NL, Aulchenko YS, Williams FMK. Genome-wide meta-analysis of 158,000 individuals of European ancestry identifies three loci associated with chronic back pain. PLoS Genet 2018;14:e1007601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Tagliaferri SD, Fitzgibbon BM, Owen PJ, Miller CT, Bowe SJ, Belavy DL. Brain structure, psychosocial, and physical health in acute and chronic back pain: a UK Biobank study. PAIN 2022;163:1277–90. [DOI] [PubMed] [Google Scholar]
- [116].Tamosauskaite J, Atkins JL, Pilling LC, Kuo CL, Kuchel GA, Ferrucci L, Melzer D. Hereditary hemochromatosis associations with frailty, sarcopenia and chronic pain: evidence from 200,975 older UK Biobank participants. J Gerontol A Biol Sci Med Sci 2019;74:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Tang Y, Wu J, Xu M, Zhu T, Sun Y, Chen H, Wu L, Chen C. Causal associations of iron status and back pain risk: a Mendelian randomization study. Front Nutr 2022;9:923590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Themistocleous AC, Kristensen AG, Sola R, Gylfadottir SS, Bennedsgaard K, Itani M, Kroigard T, Ventzel L, Sindrup SH, Jensen TS, Bostock H, Serra J, Finnerup NB, Tankisi H, Bennett DLH. Axonal excitability does not differ between painful and painless diabetic or chemotherapy-induced distal symmetrical polyneuropathy in a multicenter observational study. Ann Neurol 2022;91:506–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Topaz LS, Frid A, Granovsky Y, Zubidat R, Crystal S, Buxbaum C, Bosak N, Hadad R, Domany E, Alon T, Meir Yalon L, Shor M, Khamaisi M, Hochberg I, Yarovinsky N, Volkovich Z, Bennett DL, Yarnitsky D. Electroencephalography functional connectivity—a biomarker for painful polyneuropathy. Eur J Neurol 2023;30:204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). PAIN 2019;160:19–27. [DOI] [PubMed] [Google Scholar]
- [122].Vallbo AB, Hagbarth KE. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol 1968;21:270–89. [DOI] [PubMed] [Google Scholar]
- [123].van Hecke O, Kamerman PR, Attal N, Baron R, Bjornsdottir G, Bennett DL, Bennett MI, Bouhassira D, Diatchenko L, Freeman R, Freynhagen R, Haanpaa M, Jensen TS, Raja SN, Rice AS, Seltzer Z, Thorgeirsson TE, Yarnitsky D, Smith BH. Neuropathic pain phenotyping by international consensus (NeuroPPIC) for genetic studies: a NeuPSIG systematic review, Delphi survey, and expert panel recommendations. PAIN 2015;156:2337–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Veluchamy A, Hebert HL, van Zuydam NR, Pearson ER, Campbell A, Hayward C, Meng W, McCarthy MI, Bennett DLH, Palmer CNA, Smith BH. Association of genetic variant at chromosome 12q23.1 with neuropathic pain susceptibility. JAMA Netw Open 2021;4:e2136560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Verma V, Khoury S, Parisien M, Cho C, Maixner W, Martin LJ, Diatchenko L. The dichotomous role of epiregulin in pain. PAIN 2020;161:1052–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012;73:638–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. PAIN 1992;50:133–49. [DOI] [PubMed] [Google Scholar]
- [128].Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Walker-Bone K, Harvey NC, Ntani G, Tinati T, Jones GT, Smith BH, Macfarlane GJ, Cooper C. Chronic widespread bodily pain is increased among individuals with history of fracture: findings from UK Biobank. Arch Osteoporos 2016;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wiberg A, Ng M, Schmid AB, Smillie RW, Baskozos G, Holmes MV, Kunnapuu K, Magi R, Bennett DL, Furniss D. A genome-wide association analysis identifies 16 novel susceptibility loci for carpal tunnel syndrome. Nat Commun 2019;10:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46:319–29. [DOI] [PubMed] [Google Scholar]
- [132].Zhang Z, Liu L, Zhang H, Li C, Chen Y, Zhang J, Pan C, Cheng S, Yang X, Meng P, Yao Y, Jia Y, Wen Y, Zhang F. The genetic structure of pain in depression patients: a genome-wide association study and proteome-wide association study. J Psychiatr Res 2022;156:547–56. [DOI] [PubMed] [Google Scholar]
- [133].Zorina-Lichtenwalter K, Maixner W, Diatchenko L. Detangling red hair from pain: phenotype-specific contributions from different genetic variants in melanocortin-1 receptor. PAIN 2020;161:938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


