Abstract
Amyloid light chain (AL) amyloidosis is a rare plasma cell dyscrasia with dismal prognosis. This study aims to investigate the T-cell immune checkpoint expression patterns in systemic AL amyloidosis and its relationship with clinicobiological traits. We examined the frequencies of V-domain immunoglobulin suppressor of T cell activation+ (VISTA+), programmed cell death 1+ (PD-1+), T cell immunoglobulin and mucin-domain-containing-3+ (Tim-3+), T cell immunoreceptor with Ig and ITIM domains+ (TIGIT+) T cells in peripheral blood (PB) and bone marrow (BM) from 19 patients with newly diagnosed AL amyloidosis. Patients with AL amyloidosis had significantly higher percentages of VISTA+ and PD-1+ T cells in PB than healthy individuals (HIs), with no statistical differences in BM. The percentages of some double-positive T cells in PB were also considerably higher in AL amyloidosis than those in HIs. Additionally, the patients with renal involvement had more PD-1+ and TIGIT+ T cells than the patients without, and PD-1+CD3+%, PD-1+CD4+%, PD-1+Treg% were positively correlated with 24-hour proteinuria levels. Furthermore, the AL amyloidosis patients had higher counts of PD-1+ Treg in PB than multiple myeloma (MM) patients, while the MM patients had higher counts of TIGIT+ T cells than AL amyloidosis patients. Collectively, this is the first report of elevated proportions of VISTA+ and PD-1+ T cells in PB of AL amyloidosis patients, indicating an immunosuppressive milieu, and the increased PD-1+ and TIGIT+ T cells were associated with renal damage. VISTA, PD-1, and TIGIT may be potential targets for reversing T-cell exhaustion in AL amyloidosis.
Keywords: AL amyloidosis, PD-1, TIGIT, Tim-3, VISTA
1. INTRODUCTION
Amyloid light chain (AL) amyloidosis is a hematologic malignancy characterized by an increase of clonal plasma cells in the bone marrow (BM). These aberrant plasma cells have the capacity to create monoclonal LCs, which aggregate into insoluble amyloid fibrils and deposit in tissues, leading to progressive organ damage.1 Left untreated, the prognosis for systemic AL amyloidosis is poor with a median survival of 8 months, even worse in patients with cardiac involvement,2 and the characteristics of multiorgan involvement will also challenge the tolerance of treatment, resulting in fatal outcomes.
The immunosurveillance system is crucial in preventing the cancerogenesis and metastasis. However, the cancer cells may develop various strategies to evade immune surveillance, and one of the mechanisms they employ is to induce T-cell exhaustion.3–5 Overexpression of T cell inhibitory receptors is a typical feature of exhausted T cells. These inhibitory receptors, also known as immune checkpoints, function to prevent T cell excessive activation, maintaining the immune system in balance under physiological conditions. While in pathological conditions, the up-regulation of these molecules may contribute to T-cell dysfunction and exhaustion.6 Programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4) are 2 of the earliest and best described immune checkpoints.7 Several hematologic malignancies, including leukemia, lymphoma, and multiple myeloma (MM), have been demonstrated to be associated with an increase in T lymphocytes expressing PD-1 or CTLA-4.8–11 In addition, numerous novel immune checkpoint molecules have also been identified including V-domain immunoglobulin suppressor of T cell activation (VISTA), T cell immunoglobulin and mucin-domain-containing-3 (Tim-3), T cell immunoreceptor with Ig and ITIM domains (TIGIT), etc.12–14 These immune checkpoints are regarded to exhibit distinct characteristics and functions in restraining antitumor immunity, and they have also been detected overexpression in multiple hematologic malignancies such as leukemia and MM.15–17 The strategies targeting immune checkpoints have been confirmed to be effective in reversing T cell dysregulation, and a number of immune checkpoint inhibitors (ICIs) used alone or in combination have demonstrated impressive success in treating multiple cancer types.7,18,19 Therefore, identifying immune checkpoint expression was of great value for both systematic investigation of cancer immunoregulatory networks as well as the selection of effective immunotherapy medications.
Contrary to other types of plasma cell disorder, such as MM, which has been the subject of extensive immune checkpoint researches,20–23 little is known about the alterations of immune checkpoints expression and their clinical implications in AL amyloidosis. In this study, we aimed to characterize the various distributions of VISTA, PD-1, Tim-3, and TIGIT on the T cell subsets in PB and BM from patients with newly diagnosed AL amyloidosis, and to further analyze potential correlations between the T-cell immunodeficiency status and clinicobiological characteristics.
2. MATERIALS AND METHODS
2.1. Patients
A total of 19 patients with confirmed newly diagnosed systemic AL amyloidosis between January 2021 and June 2022 at Guangdong Provincial People’s Hospital were enrolled in this study. The diagnosis of AL amyloidosis was based on the presence of amyloid-related systemic syndromes, positive amyloid staining by Congo red in any tissue, restricted LC deposition, and evidence of plasma cell clonality. The staging was made according to the 2012 Mayo staging system.24 Organ involvement was defined according to the updated consensus criteria for amyloid-related organ involvement.25 The clinical and laboratory characteristics of the patients with AL amyloidosis are listed in Table 1. Briefly, the median age was 54 years and 73.7% were males. The majority of patients had the λ LC isotype. For comparison with AL amyloidosis, 29 healthy individuals (HIs) and 36 newly diagnosed MM patients26 with matched age and sex were also included in this study. All patients and HIs gave informed consent in accordance with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of the Guangdong Provincial People’s Hospital (approval no.: KY-Q-2022-063-02).
Table 1.
Patient characteristics.
| Characteristics | Number of patients, n (%) or median (range) |
|---|---|
| Age, y, median (range) | 54 (30–72) |
| Male gender, n (%) | 14 (73.7) |
| NT-proBNP, ng/L (range) | 2582.0 (42.7–10,098.0) |
| hs-cTnT, µg/L, (range) | 0.07 (0.0067–0.2878) |
| 24-h urine protein, g (range) | 3.2 (0.08–9.15) |
| ALP, U/L (range) | 162 (40–1276) |
| dFLC > 180 mg/L, n (%) | 8 (42.1) |
| BMPCs, %, median (range) | 7 (2–22) |
| LC isotype, n (%) κ λ |
4 (21.1) 15 (78.9) |
| 2012 Mayo stage, n (%) I II III |
6 (31.6) 3 (15.8) 5 (26.3) |
| IV | 5 (26.3) |
| Organs involved, n (%) | |
| Cardiac involvement Renal involvement Liver involvement Gastrointestinal involvement Peripheral neuropathy Soft tissues |
13 (68.4) 12 (63.2) 2 (10.5) 2 (10.5) 2 (10.5) 9 (47.4) |
| Cytogenetic abnormalities, n (%) t(11;14) 1q21 gain t(14;16) |
7 (36.8) 5 (26.3) 1 (5.3) |
| Treatment regimen, n (%) VTD or VRD VCD VD Others |
7 (36.8) 5 (26.3) 1 (5.3) 6 (31.6) |
ALP = alkaline phosphatase, BMPCs = bone marrow plasma cells, dFLC = difference between involved and uninvolved free light chain, hs-cTnT = high-sensitivity cardiac troponin T, LC = light chain, NT-proBNP = N-terminal pro-B-type natriuretic peptide, VCD = bortezomib, cyclophosphamide, and dexamethasone, VD = bortezomib, dexamethasone, VRD = bortezomib, lenalidomide, and dexamethasone, VTD = bortezomib, thalidomide, and dexamethasone.
2.2. Blood and BM samples
Peripheral blood (PB; n = 74) and BM (n = 29) samples from patients or HIs were collected during routine diagnostic procedures and were processed within 12 hours of collection. Red blood cells were lysed first by 10 minutes of incubation at room temperature using 10 mL 1×lysis buffer (BD Biosciences, San Jose, California), and then the samples were washed with 1× PBS (Jingxin, Guangzhou, China), and harvested by centrifugation and prepared for flow cytometry staining.
2.3. Flow cytometry analysis
According to the manufacturer’s instructions, the freshly isolated target cells were stained with the following monoclonal antibodies: CD45-BV605 (clone HI30, BD Biosciences), CD3-APC-Cy7 (clone SK7, BD Biosciences), CD8-APC-R700 (clone RPA-T8, BD Biosciences), CD4-BV510 (clone SK3, Biolegend), CD25-BB515 (clone 2A3, BD Biosciences), Foxp3-BB700 (clone 236A/E7, BD Biosciences), PD-1-PE-Cy7 (clone EH12.1, BD Biosciences), Tim-3-BV421 (clone F38-2E2, Biolegend), VISTA-PE (clone B7H5DS8, eBioscience), and TIGIT-AF647 (clone A15153G, Biolegend). Nonreactive isotype-matched antibodies were used as controls. At least 30,000 CD45+CD3+ cells per sample were acquired for analysis with a BD Canto flow cytometer (BD Biosciences), and data were analyzed using the Flowjo software (Flowjo LLC).
2.4. Statistical analysis
The frequencies of the different T-cell subsets are presented as medians. The Mann–Whitney U test was used to analyze data between patients with AL amyloidosis and HIs for 2 independent samples. Spearman rank correlation coefficient (rs) was used to evaluate associations for continuous variables. All data analyses were performed using the SPSS software. Notably, differences with P < .05 were considered statistically significant.
3. RESULTS
3.1. Characteristics of VISTA/PD-1/Tim-3/TIGIT+ T cells in PB and BM from patients with AL amyloidosis
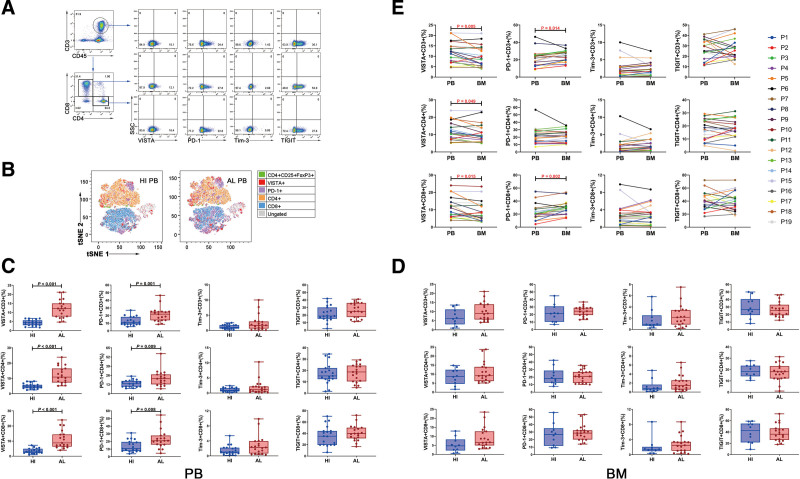
The percentages of VISTA+, PD-1+, Tim-3+, and TIGIT+ T cells in PB and BM collected simultaneously from 19 patients with AL amyloidosis were measured. PB samples from 19 HIs and BM samples from another 10 HIs were set as controls. The gating strategy is presented in Figure 1A. Overall, the global distribution and frequency of different phenotypes of T cells in the PB of patients with AL amyloidosis and HIs could be represented by t-distributed stochastic neighbor embedding (tSNE) clusters in which higher numbers of cells with VISTA or PD-1 expression were apparent in the patients (Fig. 1B). More specifically, as shown in Figure 1C, PB from patients with AL amyloidosis was shown to include considerably higher percentages of VISTA+ CD3+ and PD-1+ CD3+ T cells than HIs (median: 12.20 vs 4.38, P < .001; median: 21.90 vs 10.90, P = .001, respectively), and the VISTA+ and PD-1+ T cells were both increased in the CD4+ T and CD8+ T-cell subset (VISTA: P < .001 for CD4+ T and CD8+ T-cell; PD-1: P = .009 and P = .008 for CD4+ T and CD8+ T-cell, respectively). However, the frequency of the Tim-3+ or TIGIT+ subset in CD3+, CD4+, and CD8+ T cells did not differ statistically between the 2 groups. Because AL amyloidosis originates from BM, we also evaluated the frequency of VISTA+, PD-1+, Tim-3+, and TIGIT+ T-cell in BM samples, whereas none of the differences reached statistical significance between patients with AL amyloidosis and HIs (Fig. 1D).
Figure 1.
The percentage of VISTA/PD-1/Tim-3/TIGIT+ T cells in PB and BM from patients with AL amyloidosis and HIs. (A) The analytic logic of flow cytometry detection of VISTA/PD-1/Tim-3/TIGIT expression within CD3+, CD4+, and CD8+ T cell subsets in PB or BM. (B) tSNE clusters of the global distribution and frequency of different phenotypes of T cells in the PB of patients with AL amyloidosis and HIs. (C) Comparison of the percentage of VISTA/PD-1/Tim-3/TIGIT+ T cells in PB between patients with AL amyloidosis (n = 19) and HIs (n = 19). (D) Comparison of the percentage of VISTA/PD-1/Tim-3/TIGIT+ T cells in BM between patients with AL amyloidosis (n = 19) and HIs (n = 10). (E) Comparison of the percentage of VISTA/PD-1/Tim-3/TIGIT+ T cells between PB and BM from 19 patients (P1–P16) with AL amyloidosis. AL = amyloid light chain, BM = bone marrow, HIs = healthy individuals, PB = peripheral blood, PD-1 = programmed cell death-1, TIGIT = T cell immunoreceptor with Ig and ITIM domains, Tim-3 = T cell immunoglobulin and mucin-domain-containing-3, tSNE = t-distributed stochastic neighbor embedding, VISTA = V-domain immunoglobulin suppressor of T cell activation.
Moreover, we compared the frequencies of VISTA+, PD-1+, Tim-3+, and TIGIT+ T cells in the 19 PB and BM-paired samples from patients with AL amyloidosis. The percentages of VISTA+ CD3+/CD4+/CD8+ T cells were significantly higher in PB than that in BM (for CD3+ T: median: 12.20 vs 9.00, P = .005; for CD4+ T: median: 10.90 vs 9.09, P = .049; for CD8+ T: median: 8.79 vs 6.65, P = .015), and the percentages of PD-1+ CD3+ T cells were significantly higher in BM than that in PB (median: 24.50 vs 21.90, P = .014), primarily in the CD8+ T cell subset (median: 28.60 vs 20.90, P = .002), whereas the percentages of Tim-3+ or TIGIT+ T cells between PB and BM did not appear to be significantly different (Fig. 1E).
3.2. Characteristics of double-positive T cells expressing VISTA/PD-1/Tim-3/TIGIT in PB from patients with AL amyloidosis
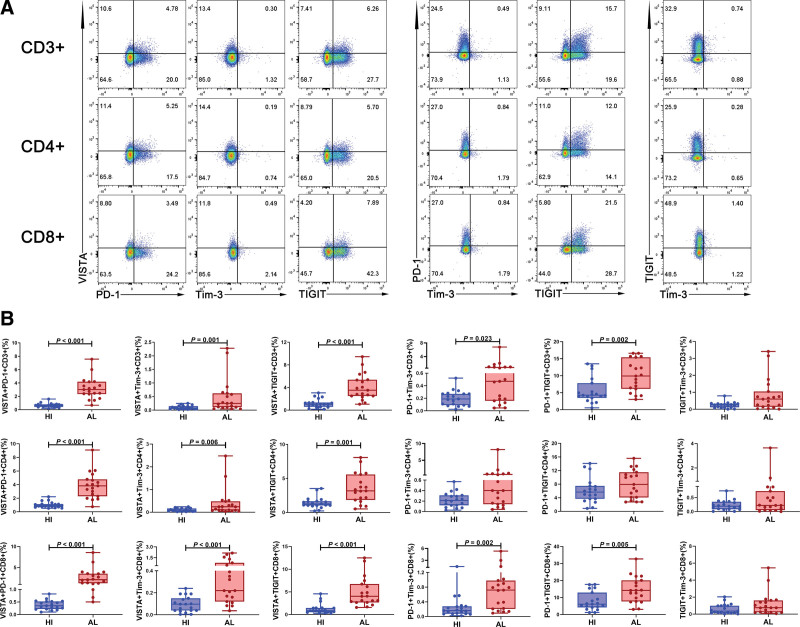
Combination of ICIs has the potential to improve responses for immunotherapy,27 so we further compared the characteristics of the double-positive T cells co-expressing VISTA/PD-1/Tim-3/TIGIT in PB between patients with AL amyloidosis and HIs (Fig. 2A). The results are represented in Figure 2B, we observed that the patients with AL amyloidosis contained considerably higher frequencies of VISTA+ PD-1+, VISTA+ Tim-3+, and VISTA+ TIGIT+ subsets within CD3+, CD4+, and CD8+ T cells compared with the HIs (for CD3+ T: P < .001, P = .001, P < .001; for CD4+ T: P < .001, P = .006, P = .001; for CD8+ T: P < .001, P < .001, P < .001, respectively); similarly, the patients contained significantly higher frequencies of PD-1+ Tim-3+ and PD-1+ TIGIT+ subsets within CD3+ and CD8+ T cells compared with the HIs (for CD3+ T: P = .023 and P = .002; for CD8+ T: P = .002 and P = .005, respectively), while within CD4+ T cells the same difference only appeared as a trend.
Figure 2.
The percentage of double-positive T cells expressing VISTA/PD-1/Tim-3/TIGIT in PB from patients with AL amyloidosis and HIs. (A) The analytic logic of flow cytometry detection of VISTA/PD-1/Tim-3/TIGIT co-expression within CD3+, CD4+, and CD8+ T cell subsets in PB. (B) Comparison of the double-positive T cells co-expressing VISTA/PD-1/Tim-3/TIGIT in PB between patients with AL amyloidosis (n = 19) and HIs (n = 19). AL = amyloid light chain, HIs = healthy individuals, PB = peripheral blood, PD-1 = programmed cell death-1, TIGIT = T cell immunoreceptor with Ig and ITIM domains, Tim-3 = T cell immunoglobulin and mucin-domain-containing-3, VISTA = V-domain immunoglobulin suppressor of T cell activation.
3.3. Characteristics of VISTA/PD-1/Tim-3/TIGIT+ Treg in PB and BM from patients with AL amyloidosis
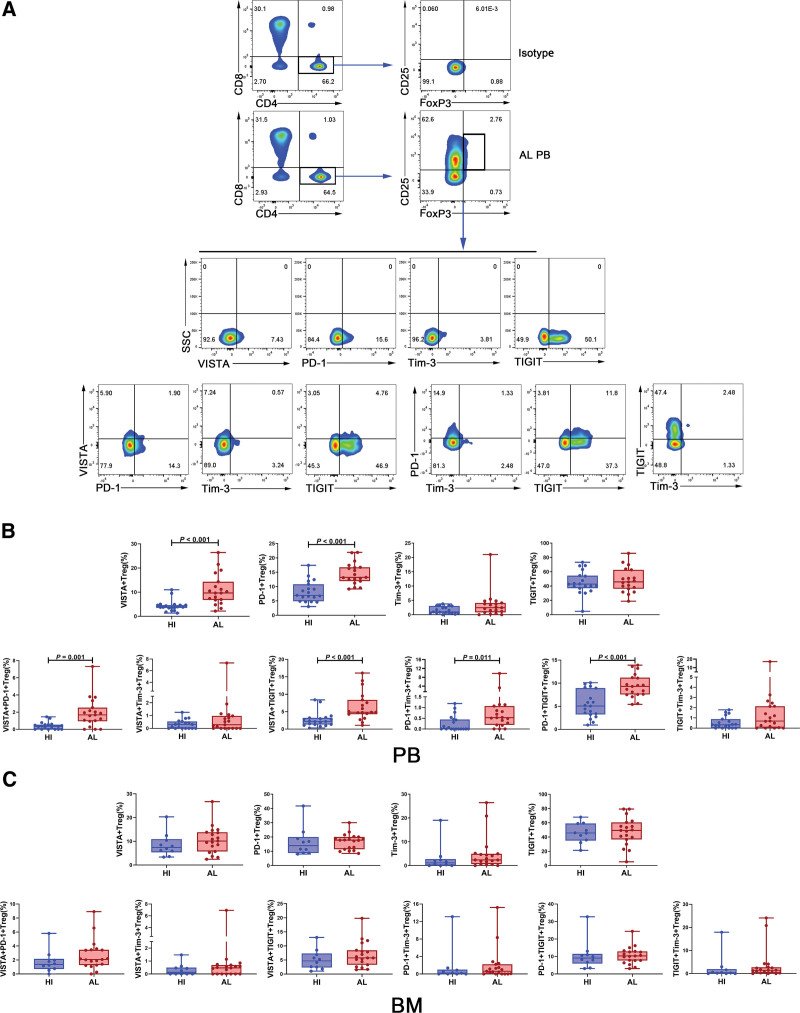
Regulatory T cell (Treg) is a major component of the immunosuppressive tumor microenvironment.28 Then we analyzed the proportions of VISTA+, PD-1+, Tim-3+, and TIGIT+ Tregs in PB from the same 19 patients with AL amyloidosis and HIs (Fig. 3A). Intriguingly, the proportions of Tregs expressing VISTA or PD-1 were likewise considerably higher in patients than those in HIs (for VISTA: median: 9.66 vs 3.79, P < .001; for PD-1: median: 13.20 vs 6.94, P < .001), whereas no differences of Treg subsets expressing Tim-3 or TIGIT were found between patients and HIs (Fig. 3B). Accordingly, in AL amyloidosis patients, the changes of the subsets expressing any one of the four checkpoint molecules in Tregs were the same as those in CD3+ T, CD4+ T, and CD8+ T cells. Regarding the double-positive Tregs co-expressing 2 of the 4 checkpoint molecules, we found that the patients contained significantly higher proportions of double-positive VISTA+ PD-1+, VISTA+ TIGIT+, PD-1+ Tim-3+, and PD-1+ TIGIT+ Tregs in comparison with the HIs (P = .001, P < .001, P = .011, and P < .001, respectively), while the proportions of the VISTA+ Tim-3+ Tregs were similar in both groups. This finding was inconsistent with those in CD3+ T, CD4+ T, and CD8+ T cells, perhaps because of the small sample size (Fig. 3B).
Figure 3.
The percentage of VISTA/PD-1/Tim-3/TIGIT+ Treg in PB and BM from patients with AL amyloidosis and HIs. (A) The analytic logic of flow cytometry detection of single positive (VISTA or PD-1 or Tim-3 or TIGIT) Treg or double-positive (co-expression with each other) Treg in PB or BM. (B) Comparison of the percentage of VISTA/PD-1/Tim-3/TIGIT+ Tregs in PB between patients with AL amyloidosis (n = 19) and HIs (n = 19). (C) Comparison of the percentage of VISTA/PD-1/Tim-3/TIGIT+ Tregs in BM between patients with AL amyloidosis (n = 19) and HIs (n = 10). AL = amyloid light chain, BM = bone marrow, HIs = healthy individuals, PB = peripheral blood, PD-1 = programmed cell death-1, TIGIT = T cell immunoreceptor with Ig and ITIM domains, Tim-3 = T cell immunoglobulin and mucin-domain-containing-3, VISTA = V-domain immunoglobulin suppressor of T cell activation.
When we further analyzed the distribution of VISTA+, PD-1+, Tim-3+, and TIGIT+ Tregs in BM from the patients with AL amyloidosis and HIs, we found no statistically significant differences between the 2 groups (Fig. 3C).
3.4. Correlations between T cell subsets expressing VISTA/PD-1/Tim-3/TIGIT in PB and clinical features of AL amyloidosis
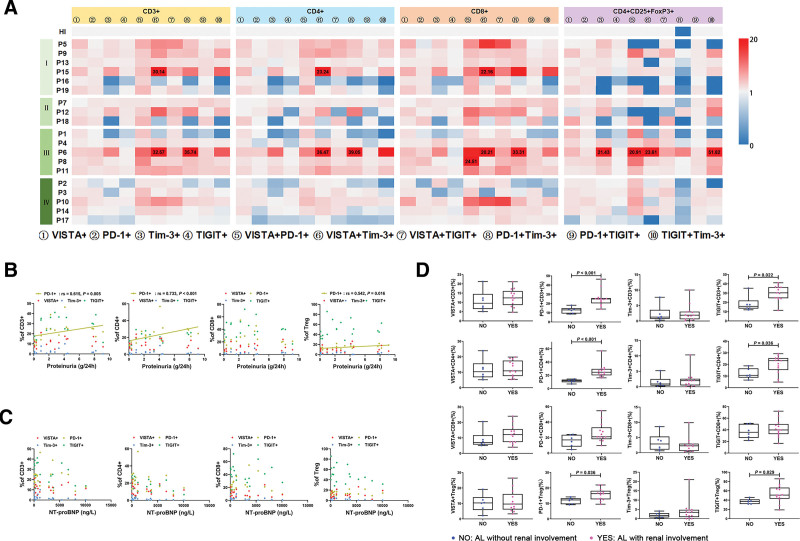
According to the aforementioned findings, the frequencies of T cell subsets expressing checkpoint molecules in PB of patients with AL amyloidosis changed prominently when compared to those of HIs, however, the frequencies of single positive (VISTA or PD-1 or Tim-3 or TIGIT) or double-positive (co-expression with each other) subsets within CD3+ T, CD4+ T, CD8+ T, and Tregs relatively varied among different AL amyloidosis patients (Fig. 4A). Notably, patient P6 had a larger percentage of T cell subsets expressing Tim-3 (Fig. 4A). When we divided them into stage I, II, III, and IV groups according to the 2012 Mayo staging system, the frequencies of these T cell subsets did not appear to be related with different stages of amyloidosis (Fig. 4A).
Figure 4.
Correlations between T cell subsets expressing VISTA/PD-1/Tim-3/TIGIT in PB and clinical features of AL amyloidosis. (A) Heatmap representing the frequency of single positive (VISTA or PD-1 or Tim-3 or TIGIT) subsets or double-positive (co-expression with each other) subsets within CD3+ T, CD4+ T, CD8+ T, and Treg from 19 patients with AL amyloidosis (stage I: 6 cases, stage II: 3 cases, stage III: 5 cases, and stage IV: 5 cases) compared with HIs. (B) Spearman correlation test between relative counts of VISTA/PD-1/Tim-3/TIGIT+ subsets within CD3+ T, CD4+ T, CD8+ T, Treg and 24-h proteinuria levels. (C) Spearman correlation test between relative counts of VISTA/PD-1/Tim-3/TIGIT+ subsets within CD3+ T, CD4+ T, CD8+ T, Treg, and NT-proBNP levels. (D) Comparison of the percentage of VISTA/PD-1/Tim-3/TIGIT+ T cell or Treg between patients with renal involvement (n = 13) and patients without renal involvement (n = 6). AL = amyloid light chain, HIs = healthy individuals, NT-proBNP = N-terminal pro-B-type natriuretic peptide, PB = peripheral blood, PD-1 = programmed cell death-1, rs = Spearman’s rank correlation coefficient, TIGIT = T cell immunoreceptor with Ig and ITIM domains, Tim-3 = T cell immunoglobulin and mucin-domain-containing-3, Treg = regulatory T cell, VISTA = V-domain immunoglobulin suppressor of T cell activation.
Considering heart and kidney are the most commonly involved organs in AL amyloidosis, we then analyzed the correlations between relative counts of different T cell subsets and cardiac or renal indicators. Interestingly, we observed a positive correlation between 24-hour proteinuria levels and PD-1+CD3+% (rs: 0.615, P = .005), PD-1+CD4+% (rs: 0.733, P < .001), and PD-1+Treg% (rs: 0.542, P = .016) (Fig. 4B). When we analyzed the correlations between relative counts of different T cell subsets with N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, we could not find any statistical differences (Fig. 4C). When we divided the patients into 2 groups based on the kidney involvement, we found that the frequencies of PD-1+ and TIGIT+ subsets within CD3+ T cells (for PD-1+: median: 24.90 vs 13.05, P < .001; for TIGIT+: median: 30.30 vs 15.70, P = .022), CD4+ T cells (for PD-1+: median: 24.60 vs 11.70, P < .001; for TIGIT+: median: 20.30 vs 10.65, P = .036), and Tregs (for PD-1+: median: 16.30 vs 12.25, P = .036; for TIGIT+: median: 50.80 vs 37.10, P = .029) were higher in patients with renal involvement (n = 13) compared with the individuals without renal involvement (n = 6) (Fig. 4D). However, we noted no differences between patients with and without renal involvement in the frequencies of VISTA+ or Tim-3+ T cells (Fig. 4D).
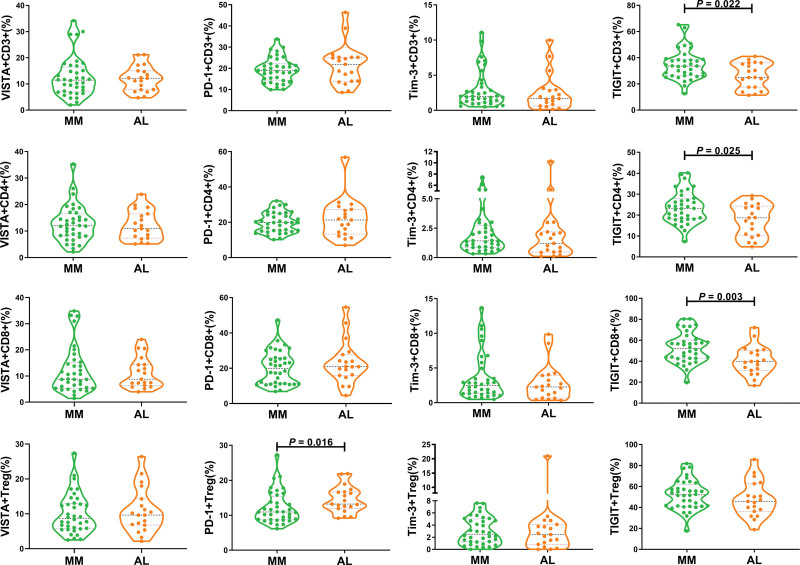
3.5. Comparison of T cell subsets expressing VISTA/PD-1/Tim-3/TIGIT in PB between AL amyloidosis and MM
Both MM and AL belong to malignant plasma cell proliferative diseases, subsequently, we compared the frequencies of T cell subsets expressing VISTA/PD-1/Tim-3/TIGIT in PB between 19 patients with AL amyloidosis and the data from our prior publications from 36 patients with MM.26,29 The findings demonstrated that, in comparison to AL amyloidosis, MM had significantly higher proportions of TIGIT+ CD3+ T cell (median: 33.65 vs 25.00, P = .022), TIGIT+ CD4+ T cell (median: 23.15 vs 18.70, P = .025), TIGIT+ CD8+ T cell (median: 52.15 vs 39.80, P = .003), as well as a higher propensity of TIGIT+ Treg. In contrast, AL amyloidosis had a higher tendency of PD-1+ Treg (median: 13.20 vs 10.95, P = 0.016) (Fig. 5). However, the frequency of VISTA+ or Tim-3+ T cell was statistically similar between the 2 groups (Fig. 5). These results implicated that the 2 malignant monoclonal gammopathies involved distinct immunological T cell subsets.
Figure 5.
Comparison of T cell subsets expressing VISTA/PD-1/Tim-3/TIGIT in PB between AL amyloidosis and MM. Increased TIGIT+ CD3+, TIGIT+ CD4+, TIGIT+ CD8+ T cells were found in the MM group, while increased PD-1+ Treg was found in the AL amyloidosis group. AL = amyloid light chain, MM = multiple myeloma, PB = peripheral blood, PD-1 = programmed cell death-1, Tim-3 = T cell immunoglobulin and mucin-domain-containing-3, TIGIT = T cell immunoreceptor with Ig and ITIM domains, Treg = regulatory T cell, VISTA = V-domain immunoglobulin suppressor of T cell activation.
4. DISCUSSION
AL amyloidosis is a rare plasma cell dyscrasia characterized by progressive, often devastating multiorgan dysfunction. In recent years, ICIs have shown striking benefit in the treatment of hematological malignancy.20,30,31 However, relatively little information regarding immune checkpoints in patients with AL amyloidosis has been discovered. In this study, we first analyzed the expression patterns of VISTA, PD-1, Tim-3, and TIGIT in the T cell populations of patients with newly diagnosed AL amyloidosis, and evaluated their relationship with clinicobiological characteristics.
Comparing with HIs, we discovered predominant higher frequencies of VISTA+ and PD-1+ T cells in PB of patients, with parallel increase in CD3+, CD4+, and CD8+ T cells. Although there were no significant differences in the numbers of Tim-3+ and TIGIT+ T cells between patients and HIs, the increased tendencies were observed in patients. PD-1, belonging to the CD28 immunoglobulin superfamily, is induced on activated T, B, natural killer T, and myeloid cells.32,33 It can bind to the B7-family ligands PD-L1 (B7-H1) and PD-L2 (B7-DC), and inhibit the proliferation and effector functions of immune cells, which are important causes of tumor immune escape.34,35 As the earliest and best-described immune checkpoint, PD-1 targeted inhibitors have demonstrated potentiality in multiple hematological malignancies.31 VISTA, Tim-3, and TIGIT are regarded as novel immune checkpoints, exhibiting distinct characters and functions. Their ligands are observed to express on cancer cells and/or various immune cells, and these inhibitory receptor–ligand interactions collaborate to achieve fine tuning of T-cell functions throughout the different phases of T-cell activation.13,35–37 Besides, these immune checkpoints can contribute to tumor resistance to PD-1/PD-L1 inhibitors, and combined blockade of ICIs can overcome the drug resistance to some extent.35 At present, the ICIs targeting these novel immune checkpoints are entering clinical practice. Overall, it can be summarized that the exhausted T cells are increased in PB of patients with AL amyloidosis even though the tumor load was generally very low, indicating a immunosuppressive microenvironment in PB of patients with AL amyloidosis, meanwhile, VISTA+ and PD-1+ T cells were main subpopulations of the exhausted T cells, indicating that their blockades could be promising approaches against this tumor.
Due to some patients giving up treatment or dying before or at the beginning of treatment, hematologic response was evaluable in only 11 patients (4 complete response, 5 very good partial response, 2 stable disease). Even so, initial analysis revealed that PB from patients with stable disease include considerably higher percentages of PD-1+ CD3+ T cells than the patients with complete response (median: 32.05 vs 14.46, P = .031). After a median follow-up period of 9 months (range, 1–31 months), no recurrence was observed, and 3 patients died of original disease. It is worth noting that all the 3 patients died within 2 months of diagnosis, and we found that PB from the early died patients include considerably higher percentages of PD-1+ CD3+ T cells than the survival patients (median: 36.73 vs 18.03, P = .003). These indicate that an immunosuppressive milieu may reduce treatment effectiveness and survival time in AL amyloidosis. Additional studies with larger groups of patients are needed to evaluate the T-cell immune checkpoint expression patterns in predicting treatment efficacy and long-term survival in AL amyloidosis.
In MM, BM represents an immunosuppressive microenvironment that can impair the function of T cells,38 and we have identified increased exhausted T cells in BM of MM patients in our previous studies,17,29 nevertheless T cell alterations within the BM microenvironment in AL are poorly understood. Thus we further evaluated the frequency of VISTA+, PD-1+, Tim-3+, and TIGIT+ T-cell in BM samples, however, no differences were found between patients with AL amyloidosis and HIs. Moreover, we discovered that the frequency of VISTA+ T-cell was higher in PB than that in BM, and the frequency of PD-1+ T-cell was higher in BM than that in PB. It should be pointed that in the HIs, the frequency of PD-1+ T-cell was also higher in BM than that in PB (data not shown). We are aware that the tumor burden in AL amyloidosis is relatively low with a median of 5% to 10% clonal plasma cells in BM (7% in this study),2,24 even lower in PB. Nevertheless, we observed more severe immunodeficiency of T cell in PB than that in BM (evidenced by the increased VISTA+ T-cell in PB), suggesting that the immunosuppression in PB may be more complicated in this disease. Investigating this result in a larger cohort might be intriguing.
Although current ICIs directed against CTLA-4 or PD-1 are exhibiting unprecedented efficacy in several malignant tumors, there are still many patients who do not respond to these drugs. For example, monotherapy of PD-1/PD-L1 inhibitors failed to show the anticipated clinical results in MM even with PD-1 overexpression on exhausted T cells.39,40 This failure indicated complicated T-cell immunosuppression in MM, and abnormal expression of multiple immune checkpoint molecules might be a major reason, in fact, exhausted T cells with PD-1 and Tim-3 or thymocyte selection-associated HMG BOX (TOX) double overexpression have been detected in MM.17,29 Therefore, to fully identify the state of T cell exhaustion, it is necessary to evaluate the characteristics of the co-expression of immune checkpoints on T cells. However, there has not been much study done on AL amyloidosis. Then, we dug deeper into our research and discovered that the patients with AL amyloidosis contained significantly higher levels of double-positive VISTA+ PD-1+, VISTA+ Tim-3+, VISTA+ TIGIT+, PD-1+ Tim-3+, and PD-1+ TIGIT+ T cells, suggesting that combinational approaches might be promising for better reversal of immune suppression and improving immunotherapies. Indeed, certain immune checkpoint molecules, such as Tim-3 or TIGIT, have distinctive signaling tails that provide the basis for both their unique regulatory functions and the synergistic effects of treatments targeting these molecules.36 And combinations of certain ICIs have demonstrated significant survival benefits when compared with single agent in some tumors.27,41,42
Treg, a critical subset of CD4+ T cell characterized by the CD4+CD25+FoxP3+ phenotype, play essential roles in immune homeostasis; however, infiltration of Treg into tumor microenvironment could promote tumor progression through impairing antitumor immune responses and supporting tumor immune escape.43 Therefore, it is essential to understand the Treg biology and the effect of ICIs on Treg for effective cancer immunotherapy.44 Several studies have reported that the immune checkpoint molecules including CTLA-4,45 PD-1,46 VISTA,47 Tim-3,48 and TIGIT49 on Tregs are critically important for their suppressive function, and the majority of intratumoral Tregs may upregulate immune checkpoint molecules to attenuate antitumor immune responses.47,50–52 In MM, it has been manifested that Tregs with PD-1 overexpression are associated with treatment failure or shorter survival.21 All these indicate that Treg can be key target for ICIs. Actually, there have been studies that shown that ICIs can impair the suppressive function and reduce the emergence of tumor-specific Tregs.47,53,54 In our study of Tregs expressing immune checkpoint molecules, we found that the patients contained considerably higher counts of VISTA+ and PD-1+ Tregs in PB than the HIs, suggesting that Tregs in AL amyloidosis may have stronger immunosuppressive function. However, further investigations are needed to characterize the function of the Tregs in AL amyloidosis pathogenesis, prognosis, and treatment.
Although higher VISTA+ and PD-1+ T cells were found in the PB of AL amyloidosis patients in our cohort, the quantities of these T cells relatively varied among patients. Thus, precise therapeutic strategies based on individual alterations in immune checkpoints can be anticipated to bring more satisfying effects to patients. Notably, higher numbers of T cell subsets expressing Tim-3 were found in patient P6 in this study. We found that this patient also suffered from psoriasis, an autoimmune disease, and that the patient died unexpectedly shortly after receiving just 1 cycle of treatment. Whether the patient’s history of immunosuppressive medication or some other causes that contributed to the overexpression of Tim-3 on his T cells remains an open question. In this case, coexisting AL amyloidosis and autoimmune disease can be extremely challenging to treat because the treatments are contradictory.
Although nearly any organ system can be affected in systematic AL amyloidosis, the heart and kidney are the most commonly involved organs. The 24-hour proteinuria and NT-proBNP levels are important indicators for kidney and heart involvement, respectively. Interestingly, in our cohort of patients, we observed that the 24-hour proteinuria levels were positively correlated with relative counts of PD-1+ T cells, while the NT-proBNP levels failed to correlate with any exhausted T cells. Furthermore, we found that the frequencies of PD-1+ and TIGIT+ T cells in patients with renal involvement were significantly higher than those in patients without renal involvement. In a word, we conclude that the quantity of exhausted T cells may be a risk factor for renal damage in AL amyloidosis, whereas exactly what role the T cells play in the renal damage is still uncertain.
MM and AL amyloidosis are the most common plasma cell disorder, and are thought to share a similar plasma cell clone. However, AL amyloidosis is typically associated with a lower indolent clonal plasma cell burden than MM,55 and mainly manifests as various forms of organ dysfunction rather than the CRAB symptoms typical of MM. These differences could be due to the different immune status in the tumor microenvironment. T-cell exhaustion has been implicated in MM pathogenesis,56 while similar studies are limited in AL amyloidosis. In our study, we observed that the MM patients contained significantly higher counts of TIGIT+ T cells in PB than the patients with AL amyloidosis, while the AL amyloidosis patients contained significantly higher counts of PD-1+ Treg than the MM patients. These findings indicated that, for different plasma cell disorder, the different immunosuppression patterns based on immune checkpoint alteration are relatively different.
To the best of our knowledge, we for the first time described the distributions of VISTA+, PD-1+, Tim-3+, and TIGIT+ T cells in PB and BM from patients with newly diagnosed AL amyloidosis. Limitations of this study include a limited patient sample size, a lack of analysis with regard to all coexisting immune checkpoints like CTLA-4 and lymphocyte activation gene-3, and a lack of analysis regarding treatment responses and survivals due to the short follow-up. Further investigations with more immune checkpoints and larger sample sizes will be needed to corroborate these findings and comprehensively evaluate the immune status of AL amyloidosis.
5. CONCLUSIONS
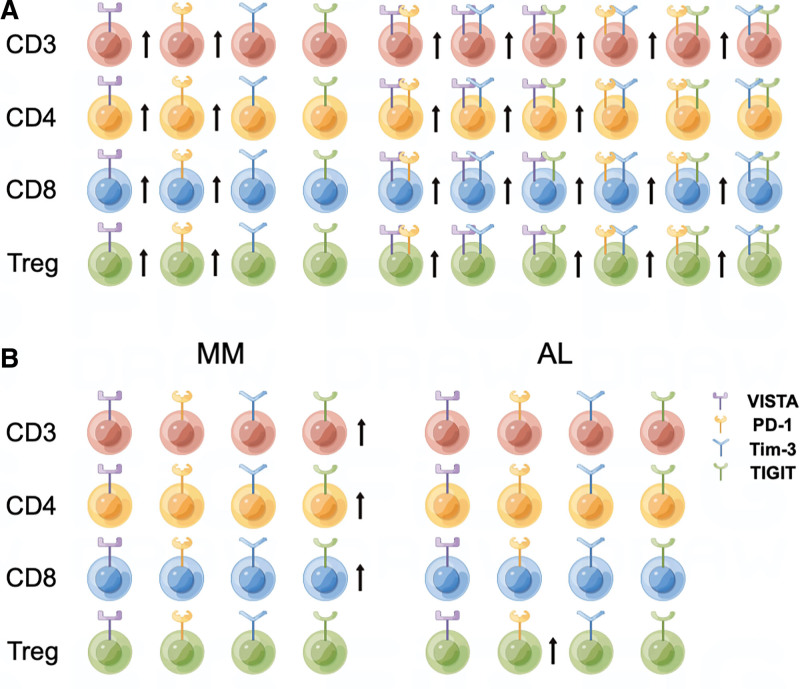
In summary, we for the first time observed elevated proportions of VISTA+ and PD-1+ subsets within CD3+, CD4+, CD8+ T cells, and Tregs in PB of AL amyloidosis patients, which are indicators of an immunosuppressive milieu (Fig. 6A). The increasing proportion of PD-1+ or TIGIT+ T cells were correlated with renal damage in AL amyloidosis. Additionally, there are significant differences in immunosuppressive patterns between MM and AL amyloidosis based on immune checkpoint alteration (Fig. 6B). These data suggest that VISTA, PD-1, and TIGIT are promising targets for reversing T cell exhaustion and enhancing T cell activity in AL amyloidosis.
Figure 6.
Characteristics of T cell subsets expressing VISTA/PD-1/Tim-3/TIGIT in PB of patients with AL amyloidosis or MM. AL = amyloid light chain, MM = multiple myeloma, PB = peripheral blood, PD-1 = programmed cell death-1, Tim-3 = T cell immunoglobulin and mucin-domain-containing-3, TIGIT = T cell immunoreceptor with Ig and ITIM domains, VISTA = V-domain immunoglobulin suppressor of T cell activation.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 82100238), the Science and Technology Program of Guangzhou (No. 202201011046), the High-level Hospital Construction Project of Guangdong Provincial People’s Hospital (No. DFJH201923), the Medical Scientific Research Foundation of Guangdong Province (No. A2019063).
Figure 6 was drawn by FigDraw.
ETHICAL APPROVAL
All patients and HIs gave informed consent in accordance with the Declaration of Helsinki, and the Ethics Committee of the Guangdong Provincial People’s Hospital approved the study protocol.
AUTHOR CONTRIBUTIONS
J.W. and Y.Z. acquired the data, performed the analysis, and wrote the manuscript. P.L. participated in data analysis. S.H. and Y.H. were responsible for data curation. L.Z., Y.L., and S.C. were involved in study design, supervision, and acquiring funding. All authors contributed to the study conception and designed and read and approved the final manuscript.
Footnotes
J.W., Y.Z., and P.L. contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (No. 82100238), the Science and Technology Program of Guangzhou (No. 202201011046), the High-level Hospital Construction Project of Guangdong Provincial People's Hospital (No. DFJH201923), the Medical Scientific Research Foundation of Guangdong Province (No. A2019063).
Conflict of interest: The authors declare that they have no conflict of interest.
All data generated or analyzed during this study are included in this published article.
REFERENCES
- [1].Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers 2018;4:38. [DOI] [PubMed] [Google Scholar]
- [2].Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med 1997;337:898–909. [DOI] [PubMed] [Google Scholar]
- [3].Wu Y, Biswas D, Swanton C. Impact of cancer evolution on immune surveillance and checkpoint inhibitor response. Semin Cancer Biol 2022;84:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells—mechanisms of immune surveillance and escape. Nat Rev Clin Oncol 2017;14:155–167. [DOI] [PubMed] [Google Scholar]
- [5].Guillerey C, Nakamura K, Vuckovic S, Hill GR, Smyth MJ. Immune responses in multiple myeloma: role of the natural immune surveillance and potential of immunotherapies. Cell Mol Life Sci 2016;73:1569–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wherry EJ, Kurachi M. Molecular and cellular insights into t cell exhaustion. Nat Rev Immunol 2015;15:486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Buchbinder EI, Desai A. Ctla-4 and pd-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016;39:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xu-Monette ZY, Zhou J, Young KH. Pd-1 expression and clinical pd-1 blockade in b-cell lymphomas. Blood 2018;131:68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tan J, Chen S, Lu Y, et al. Higher pd-1 expression concurrent with exhausted cd8+ t cells in patients with de novo acute myeloid leukemia. Chin J Cancer Res 2017;29:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zelle-Rieser C, Thangavadivel S, Biedermann R, et al. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J Hematol Oncol 2016;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gassner FJ, Zaborsky N, Catakovic K, et al. Chronic lymphocytic leukaemia induces an exhausted t cell phenotype in the tcl1 transgenic mouse model. Br J Haematol 2015;170:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ge Z, Peppelenbosch MP, Sprengers D, Kwekkeboom J. Tigit, the next step towards successful combination immune checkpoint therapy in cancer. Front Immunol 2021;12:699895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang X, Zhang X, Li E, et al. Vista: an immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J Hematol Oncol 2020;13:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alrasheed N, Lee L, Ghorani E, et al. Marrow-infiltrating regulatory t cells correlate with the presence of dysfunctional cd4(+)pd-1(+) cells and inferior survival in patients with newly diagnosed multiple myeloma. Clin Cancer Res 2020;26:3443–3454. [DOI] [PubMed] [Google Scholar]
- [15].Rezaei M, Tan J, Zeng C, Li Y, Ganjalikhani-Hakemi M. Tim-3 in leukemia; immune response and beyond. Front Oncol 2021;11:753677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mutsaers P, Balcioglu HE, Kuiper R, et al. V-domain Ig suppressor of t cell activation (vista) expression is an independent prognostic factor in multiple myeloma. Cancers (Basel) 2021;13:2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tan J, Chen S, Huang J, et al. Increased exhausted cd8(+) t cells with programmed death-1, t-cell immunoglobulin and mucin-domain-containing-3 phenotype in patients with multiple myeloma. Asia Pac J Clin Oncol 2018;14:e266–e274. [DOI] [PubMed] [Google Scholar]
- [18].Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase ii checkmate 205 trial. J Clin Oncol 2018;36:1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large b-cell lymphoma. Blood 2017;130:267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Le Calvez B, Moreau P, Touzeau C. Immune checkpoint inhibitors for the treatment of myeloma: novel investigational options. Expert Opin Investig Drugs 2021;30:965–973. [DOI] [PubMed] [Google Scholar]
- [21].Alkharabsheh O, Trisel Z, Badami S, Aljama MA, Sidiqi MH. Checkpoint inhibitors in multiple myeloma: intriguing potential and unfulfilled promises. Cancers (Basel) 2021;14:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hradska K, Kascak M, Hajek R, Jelinek T. Identifying and treating candidates for checkpoint inhibitor therapies in multiple myeloma and lymphoma. Expert Rev Hematol 2020;13:375–392. [DOI] [PubMed] [Google Scholar]
- [23].Costa F, Das R, Kini Bailur J, Dhodapkar K, Dhodapkar MV. Checkpoint inhibition in myeloma: opportunities and challenges. Front Immunol 2018;9:2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 2012;30:989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gillmore JD, Wechalekar A, Bird J, et al. Guidelines on the diagnosis and investigation of al amyloidosis. Br J Haematol 2015;168:207–218. [DOI] [PubMed] [Google Scholar]
- [26].Huang S, Zhao Y, Liao P, et al. Different expression patterns of vista concurrent with pd-1, tim-3, and tigit on t cell subsets in peripheral blood and bone marrow from patients with multiple myeloma. Front Oncol 2022;12:1014904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wu K, Yi M, Qin S, Chu Q, Zheng X, Wu K. The efficacy and safety of combination of pd-1 and ctla-4 inhibitors: a meta-analysis. Exp Hematol Oncol 2019;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tanaka A, Sakaguchi S. Regulatory t cells in cancer immunotherapy. Cell Res 2017;27:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhao Y, Liao P, Huang S, et al. Increased tox expression associates with exhausted t cells in patients with multiple myeloma. Exp Hematol Oncol 2022;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hatic H, Sampat D, Goyal G. Immune checkpoint inhibitors in lymphoma: challenges and opportunities. Ann Transl Med 2021;9:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kansara RR, Speziali C. Immunotherapy in hematologic malignancies. Curr Oncol 2020;27:S124–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Keir ME, Butte MJ, Freeman GJ, Sharpe AH. Pd-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Agata Y, Kawasaki A, Nishimura H, et al. Expression of the pd-1 antigen on the surface of stimulated mouse t and b lymphocytes. Int Immunol 1996;8:765–772. [DOI] [PubMed] [Google Scholar]
- [34].Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific cd8 t cells infiltrating the tumor express high levels of pd-1 and are functionally impaired. Blood 2009;114:1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Okazaki T, Honjo T. The pd-1-pd-l pathway in immunological tolerance. Trends Immunol 2006;27:195–201. [DOI] [PubMed] [Google Scholar]
- [36].Anderson AC, Joller N, Kuchroo VK. Lag-3, tim-3, and tigit: co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016;44:989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhao L, Cheng S, Fan L, Zhang B, Xu S. Tim-3: an update on immunotherapy. Int Immunopharmacol 2021;99:107933. [DOI] [PubMed] [Google Scholar]
- [38].Tu C, Zheng Y, Zhang H, Wang J. Exploration of the personalized immune checkpoint atlas of plasma cell dyscrasias patients using high-dimensional single-cell analysis. Oncol Rep 2020;44:224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol 2016;34:2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ribrag V, Avigan DE, Green DJ, et al. Phase 1b trial of pembrolizumab monotherapy for relapsed/refractory multiple myeloma: keynote-013. Br J Haematol 2019;186:e41–e44. [DOI] [PubMed] [Google Scholar]
- [41].Mayes PA, Hance KW, Hoos A. The promise and challenges of immune agonist antibody development in cancer. Nat Rev Drug Discov 2018;17:509–527. [DOI] [PubMed] [Google Scholar]
- [42].Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chen BJ, Zhao JW, Zhang DH, Zheng AH, Wu GQ. Immunotherapy of cancer by targeting regulatory t cells. Int Immunopharmacol 2022;104:108469. [DOI] [PubMed] [Google Scholar]
- [44].Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on t-regulatory cells. Immunol Cell Biol 2018;96:21–33. [DOI] [PubMed] [Google Scholar]
- [45].Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by cd25(+)cd4(+) regulatory t cells constitutively expressing cytotoxic t lymphocyte-associated antigen 4. J Exp Med 2000;192:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang B, Chikuma S, Hori S, Fagarasan S, Honjo T. Nonoverlapping roles of pd-1 and foxp3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model. Proc Natl Acad Sci U S A 2016;113:8490–8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Le Mercier I, Chen W, Lines JL, et al. Vista regulates the development of protective antitumor immunity. Cancer Res 2014;74:1933–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gao X, Zhu Y, Li G, et al. Tim-3 expression characterizes regulatory t cells in tumor tissues and is associated with lung cancer progression. PLoS One 2012;7:e30676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Joller N, Lozano E, Burkett PR, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory th1 and th17 cell responses. Immunity 2014;40:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ji M, Liu Y, Li Q, et al. Pd-1/pd-l1 pathway in non-small-cell lung cancer and its relation with egfr mutation. J Transl Med 2015;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Okamura T, Fujio K, Sumitomo S, Yamamoto K. Roles of lag3 and egr2 in regulatory t cells. Ann Rheum Dis 2012;71(suppl 2):i96–100. [DOI] [PubMed] [Google Scholar]
- [52].Syed Khaja AS, Toor SM, El Salhat H, Ali BR, Elkord E. Intratumoral foxp3(+)helios(+) regulatory t cells upregulating immunosuppressive molecules are expanded in human colorectal cancer. Front Immunol 2017;8:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-pd-1 antibody nivolumab, bms-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014;2:846–856. [DOI] [PubMed] [Google Scholar]
- [54].Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-ctla-4 antibodies of igg2a isotype enhance antitumor activity through reduction of intratumoral regulatory t cells. Cancer Immunol Res 2013;1:32–42. [DOI] [PubMed] [Google Scholar]
- [55].Merlini G, Stone MJ. Dangerous small b-cell clones. Blood 2006;108:2520–2530. [DOI] [PubMed] [Google Scholar]
- [56].Dosani T, Carlsten M, Maric I, Landgren O. The cellular immune system in myelomagenesis: Nk cells and t cells in the development of myeloma [corrected] and their uses in immunotherapies. Blood Cancer J 2015;5:e306. [DOI] [PMC free article] [PubMed] [Google Scholar]