Abstract
We examined the invasion of an established bovine mammary epithelial cell line (MAC-T) by a Staphylococcus aureus mastitis isolate to study the potential role of intracellular survival in the persistence of staphylococcal infections. S. aureus cells displayed dose-dependent invasion of MAC-T cells and intracellular survival. An electron microscopic examination of infected cells indicated that the bacteria induced internalization via a mechanism involving membrane pseudopod formation and then escaped into the cytoplasm following lysis of the endosomal membrane. Two hours after the internalization of S. aureus, MAC-T cells exhibited detachment from the matrix, rounding, a mottled cell membrane, and vacuolization of the cytoplasm, all of which are indicative of cells undergoing programmed cell death (apoptosis). By 18 h, the majority of the MAC-T cell population exhibited an apoptotic morphology. Other evidence for apoptosis was the generation of MAC-T cell DNA fragments differing in size by increments of approximately 180 bp and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling of the fragmented nuclear DNA of the infected host cells. These results demonstrate that after internalization S. aureus escapes the endosome and induces apoptosis in nonprofessional phagocytes.
Staphylococcus aureus is a pathogen with a broad host range and is a leading cause of infections in humans and domesticated animals worldwide. The rapidly increasing frequency of methicillin-resistant isolates and the looming threat of resistance to vancomycin by this organism have recently caused considerable alarm within the medical community. Infections associated with this organism are extremely common and often life threatening; therefore, there is serious potential for S. aureus to cause increased morbidity and mortality. S. aureus is also the leading cause of intramammary infection in ruminants, which is the most economically important disease to the dairy industry in the United States, with approximately one-half of dairy cows afflicted with some form of mastitis. This disease accounts for approximately 70% of the total expenses for dairy farmers (14) and results in the loss of billions of dollars each year (3). Thus, a thorough understanding of the pathogenesis of staphylococcal disease could have a profound impact on public health and agriculture in the United States and worldwide.
The mechanism of persistence of staphylococci in its hosts, despite the induction of seemingly sufficient levels of humoral and mucosal antibodies, remains unexplained. This is an important issue for both humans and animals, which can have repeated occurrences of staphylococcal infections and toxigenic diseases such as toxic shock syndrome (7). While there is some evidence that immunosuppression induced by superantigens is partially responsible for both the persistence of infection and reduced antibody levels against some staphylococcal products, not all human and animal cases are attributed to superantigen-producing organisms. For example, less than half of bovine mastitis staphylococcal isolates produce known superantigen exotoxins (26).
One confirmed mechanism employed by some bacteria to evade humoral immunity is to become internalized in host cells. For example, organisms such as Listeria monocytogenes and Mycobacterium tuberculosis are facultative intracellular pathogens that require cell-mediated immunity to be eliminated most efficiently; the presence of antibodies alone is ineffective during infection by these pathogens. S. aureus is generally not considered to be an intracellular pathogen of the magnitude associated with classical facultative intracellular pathogens (i.e., Listeria, Mycobacterium, Salmonella, and Shigella spp.). However, it is well documented that S. aureus can be internalized in epithelial cells (1) and endothelial cells (18, 37). Little is known regarding the mechanisms involved in internalization, the potential role of internalization of S. aureus by the host cell, or host cell responses.
The objective of this study was to investigate the invasion of mammary epithelial cells by a strain of S. aureus known to cause bovine mastitis. We now report that S. aureus cells successfully invade epithelial cells, exist free in the cytoplasm after effecting release from the endosome, and induce the host cells to undergo apoptosis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. aureus Novel (36) was isolated from cows with clinical mastitis and used throughout this study. Prior to each experiment, a single colony from a Todd-Hewitt (TH) (Difco Laboratories, Detroit, Mich.) agar plate was inoculated into 4 ml of TH broth and grown at 37°C with vigorous shaking for 6 to 8 h. From this 4-ml culture, 100 μl was transferred into 10 ml of TH broth and incubated overnight (16 h) at 37°C with vigorous shaking. The overnight culture was centrifuged, and the pellet was washed once with sterile phosphate-buffered saline (pH 7.2) and resuspended in 10 ml of invasion medium (see below) to give a cell density of 1010 CFU/ml.
Cell culture.
An established bovine mammary epithelial cell line, designated MAC-T (21), was used for all experiments. The MAC-T cell growth medium was Dulbecco’s modified Eagle medium (Gibco BRL, Grand Island, N.Y.) containing 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah), 5 μg of insulin/ml, 1 μg of hydrocortisone/ml, 44 mM NaHCO3 (Sigma, St. Louis, Mo.), 100 U of penicillin G/ml and 100 μg of streptomycin sulfate (Gibco BRL)/ml. Prior to use, cells were seeded at 6 × 104 cells/well and grown for 3 days at 37°C with 7% CO2. Cells were grown in 24-well tissue culture plates (Costar, Cambridge, Mass.) for invasion assays or 24-well tissue culture plates with 13-mm round Thermanox coverslip inserts (Nalge Nunc International; Naperville, Ill.) for electron microscopy. Lab-Tek four-chambered glass slides (Nalge Nunc International) were used for confocal microscopy.
Invasion assay.
Approximately 16 h prior to invasion experiments, the MAC-T cell growth medium was replaced with 1 ml of invasion medium (growth medium without antibiotics or fetal bovine serum). The morning of the experiment, the medium was removed and MAC-T cells were washed once with invasion medium and given 1 ml of fresh invasion medium. Appropriate wells of MAC-T cells were then inoculated with 107 CFU of washed S. aureus and incubated at 37°C with 7% CO2. After 2 h, supernatants of the cocultures were removed and replaced with 1 ml of invasion medium containing 100 μg of gentamicin (Sigma) per ml. After the incubation of cocultures at 37°C with 7% CO2 had continued for the specified times, the supernatants were removed and discarded. MAC-T cell monolayers were washed three times with sterile phosphate-buffered saline, treated with 0.25% trypsin in Hanks balanced salt solution (Gibco BRL), and further lysed with 0.025% Triton X-100 (United States Biochemicals, Cleveland, Ohio) in sterile distilled water. Cell lysates were serially diluted 10-fold and plated in triplicate on TH agar plates; the plates were incubated overnight at 37°C, and CFU were counted. To test the inhibition of S. aureus invasion by cytochalasin D, experiments were carried out as described for the invasion assays except that prior to inoculation with bacteria, MAC-T cell monolayers were incubated at 37°C with 7% CO2 for 2 h with invasion medium containing 0.5 μg of cytochalasin D (Sigma) per ml.
Assessment of apoptosis.
The occurrence of apoptosis in MAC-T cells was evaluated by three different methods: (i) DNA laddering, (ii) a terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay (12, 17, 33, 38), and (iii) microscopic observation of morphological changes in the cells.
For assessment of DNA laddering in infected MAC-T cells, coculture supernatants and trypsinized monolayers were collected and combined into a single sample. DNA was extracted once with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1 [vol/vol/vol]) and again with an equal volume of chloroform-isoamyl alcohol (24:1 [vol/vol]). DNA in the aqueous phase was precipitated overnight at −20°C in 1/10 volume of 3 M sodium acetate (pH 5.1) and 2 volumes of 100% ethanol. DNA was collected by centrifugation, and the pellet was washed once with 70% ethanol, dried, resuspended in sterile water, and treated with 20 μg of DNase-free RNase A (Sigma) per ml for 30 min at 37°C. DNA samples were separated by electrophoresis at 5 V/cm in 1.8% agarose, stained with ethidium bromide, visualized with UV light, and photographed. DNA fragments were sized by comparison with a 100-bp DNA ladder (Gibco BRL).
For the TUNEL assay, MAC-T cells were infected with S. aureus as described above for the invasion assay with the following modification. Two hours after inoculation with bacteria, gentamicin (100 μg/ml) was added to the cocultured cells, and the cells were allowed to incubate for 4 h. The TUNEL assay was performed with an ApoAlert DNA fragmentation assay kit (Clontech, Palo Alto, Calif.) according to the manufacturer’s directions for adherent cell samples for microscopic detection. The fluorescence of apoptotic cells was visualized with an MRC1000 confocal laser scanning microscope equipped with a krypton-argon mixed-gas multiline mode laser (Bio-Rad, Hercules, Calif.) and a Diaphot 200 inverted microscope (Nikon Inc., Melville, N.Y.).
To visualize the morphological changes occurring in the MAC-T monolayers during invasion by S. aureus, photographs were taken at hourly intervals with an IM microscope and a C-35AD camera (Olympus America Inc., Melville, N.Y.). Ultrastructural features of infected MAC-T cells were evaluated by transmission electron microscopy (TEM). Treatments for TEM were performed as for the invasion assay except that gentamicin was never added to the cocultures. Processing of tissues for TEM was done at Washington State University on a fee-for-service basis, and the procedure for tissue processing was modified from the standard procedure of Mukherjee et al. (30). After the appropriate incubation, the Thermanox coverslips were placed in 3% glutaraldehyde in 0.1 M cacodylate buffer containing 6% sucrose. Cells were postfixed with 2% osmium tetroxide for 1 h, rinsed with cacodylate-sucrose buffer, dehydrated in an acetone series, and embedded in an Epson/Spurrs resin mixture. Coverslips were removed from the resin with heat, and 100-nm-thick sections were cut with a Reichert Ultracut R microtome equipped with a diamond knife. Sections were collected onto either copper or nickel grids, stained with uranyl acetate followed by lead citrate, and viewed with a Joel 1200EX transmission electron microscope.
RESULTS
S. aureus invasion of MAC-T cells.
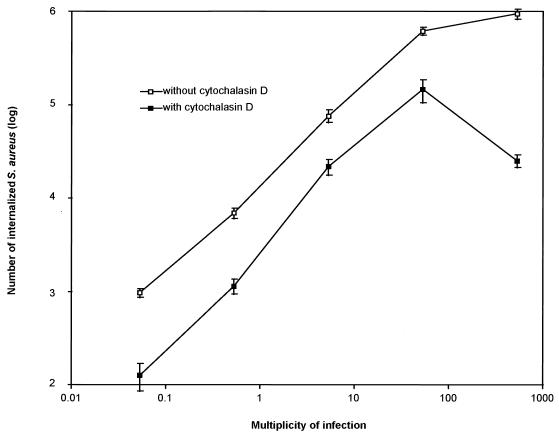
To study the internalization of S. aureus in more detail, we examined the ability of a highly transmissible S. aureus bovine mastitis isolate, designated Novel (36), to invade a well-established bovine mammary epithelial MAC-T cell line (21). The invasion assay employed is based on the principle that bacteria inside host cells are protected from the antimicrobial effects of gentamicin added to the medium (22). The Novel strain of S. aureus invaded the MAC-T cells in a dose-dependent fashion (Fig. 1). A linear increase in the number of surviving bacteria was observed up to a multiplicity of infection (MOI) of 53. Above this dose, substantial increases in bacterial survival were not observed, indicating that saturation of the internalization mechanism had occurred. We confirmed the results of other investigators working with different systems (1, 18) by demonstrating that treatment with 0.5 μg of cytochalasin D per ml reduced the survival of S. aureus approximately 10-fold (Fig. 1).
FIG. 1.
Invasion of MAC-T cells by S. aureus Novel. A dose response invasion assay was performed by exposing MAC-T cell monolayers to various concentrations of S. aureus cells so that the MOI was altered within the range indicated. Culture media were supplemented with either gentamicin alone (open squares) or gentamicin plus cytochalasin D (solid squares). Additional controls showed that overnight incubation of S. aureus cells in the presence of 0.5 μg of cytochalasin D per ml resulted in no loss in viability (data not shown). Data are from a representative experiment repeated four times. Error bars represent the means ± the standard errors of the means.
Cellular effects of invasion.
Several time course experiments were performed to evaluate the morphological events that were induced by the presence of intracellular S. aureus. MAC-T monolayers were infected with 107 bacteria (MOI = 34) prior to killing the extracellular bacteria with gentamicin. The number of intracellular bacteria increased continuously for 2 h, after which time the numbers began to decline gradually (data not shown). We attributed the initial increase in numbers to staphylococcal replication following the addition of gentamicin. Although many MAC-T cells were observed to contain large numbers of viable and dividing staphylococci for at least 3 days following the addition of S. aureus, an accurate assessment of the extent of intracellular replication was not possible, and numbers of viable bacteria eventually declined (data not shown).
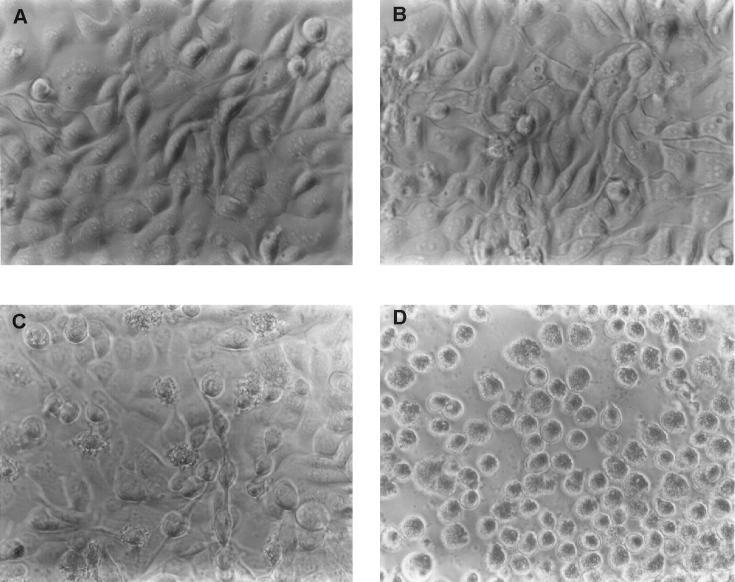
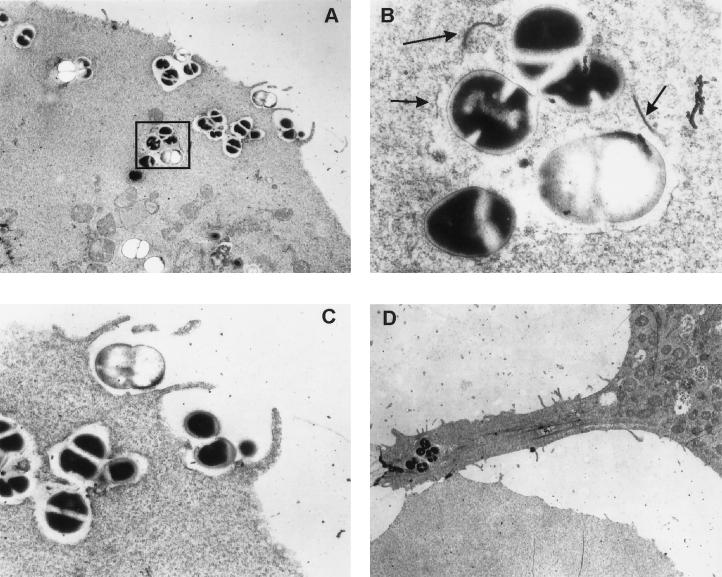
As shown in Fig. 2, a dramatic difference in the morphology of MAC-T cells between infected and uninfected cells was observed. Some infected cells detached from the plates within 2 h after staphylococci were added to the monolayers. Detachment from the substrate was accompanied by an overall rounding of the cells and a mottled appearance of the cell membrane (Fig. 2C and D), morphological changes consistent with apoptosis. By 18 h of incubation with gentamicin, the majority of the MAC-T cells (typically at least 80% of the population) were apoptotic. This correlated with the high percentage of infection, which was also estimated to be approximately 80%. The uninfected cells (to which gentamicin, but not S. aureus, had been added) maintained normal adherence and cellular morphology (Fig. 2A and B). An examination of electron micrographs of the infected cells (Fig. 3) also revealed several additional features of S. aureus invasion at the ultrastructural level. Bacteria that were in close contact with the MAC-T cell surface were associated with pseudopod-like structures (Fig. 3A and C); these structures were presumably involved in engulfing the bacteria, leading to the formation of membrane-bound endosomes. The membrane was clearly evident surrounding the bacteria in endosomes that had recently entered the cell (Fig. 3A and C). However, vacuoles farther from the cytoplasmic membrane of the host cell, presumably those internalized for longer periods of time, began to degrade. Some bacteria were observed to be partially surrounded by vacuolar fragments, while others were entirely free in the cytoplasm (Fig. 3A and B). Another interesting feature was the cytoplasmic membrane contortions that were associated with many of the infected cells (Fig. 3D). These contortions are also characteristic of apoptosis (35).
FIG. 2.
Effect of S. aureus Novel invasion on MAC-T cell morphology. MAC-T cell monolayers were either uninfected (controls) or were incubated in the presence of staphylococci for 2 h prior to the addition of gentamicin (experimental). Photomicrographs were taken at the times indicated below following the addition of gentamicin to uninfected and infected cultures. (A) Control, 2 h; (B) control, 18 h; (C) experimental, 2 h; (D) experimental, 18 h.
FIG. 3.
TEM analysis of MAC-T cells infected with S. aureus Novel. MAC-T cells were grown as described for invasion assays, but gentamicin was never added to the cocultures after inoculation with S. aureus. All panels represent a 3-h coculture of MAC-T cells with S. aureus. (A) Invasion of a MAC-T cell by S. aureus illustrating contact with the MAC-T cell surface, formation of pseudopod-like structures, engulfment of bacteria, phagosome formation, and degradation of the phagosome membrane in the interior of the cell (magnification, ×5,000). (B) Enlargement (magnification, ×30,000) of the boxed area shown in panel A. Arrows indicate fragments of the degraded phagosome membrane. (C) Enlargement (magnification, ×15,000) of pseudopod-like structures and engulfment of bacteria. (D) Cytoplasmic membrane contortion (magnification, ×4,000) associated with many of the infected MAC-T cells.
Infection by S. aureus induces apoptosis.
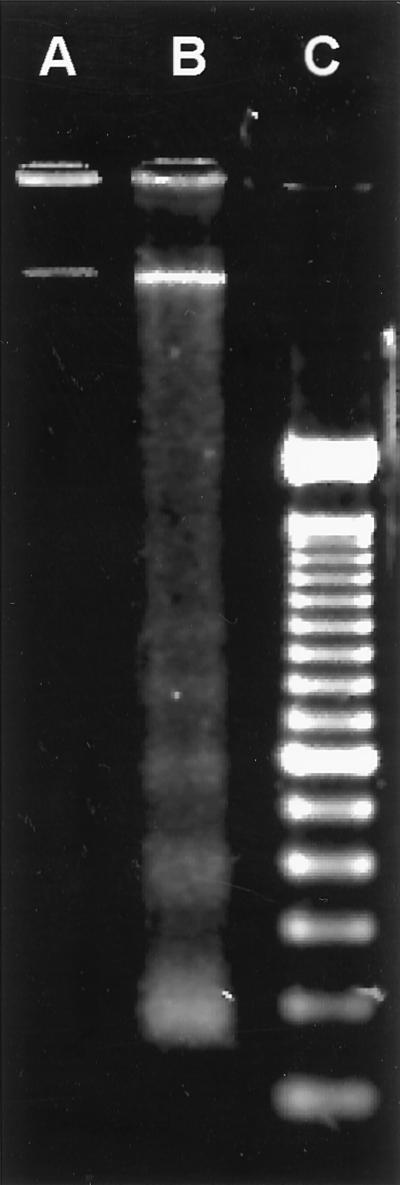
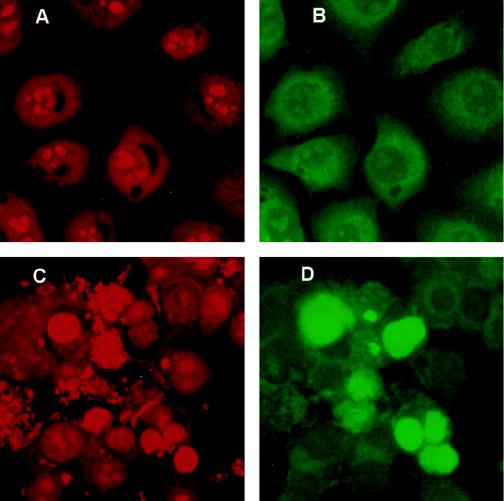
As described above, the morphological features of the infected MAC-T cells (Fig. 2 and 3) were reminiscent of those of cells undergoing apoptosis (27, 29). One hallmark of apoptotic cells is the fragmentation of the DNA by specific endonucleases (29, 32, 39). This phenomenon can be visualized as a characteristic ladder pattern where the size of each band increases by approximately 180 bp. As shown in Fig. 4, laddering of the DNA occurred in S. aureus-infected MAC-T cells (Fig. 4, lane B) but not in uninfected MAC-T control cells also treated with gentamicin (Fig. 4, lane A). In some experiments, samples of the growth media containing detached, rounded cells were collected separately from the remaining attached MAC-T cells and lysed and the DNA was visualized on agarose gels for fragmentation. DNA fragmentation laddering was observed predominately in samples from growth media containing detached cells (data not shown). This would be expected since it is characteristic of apoptotic cells to be released from the culture substrate into the growth medium (35). Additionally, some S. aureus-infected MAC-T cells were observed to exhibit an intense green positive fluorescence by using the TUNEL method (Fig. 5D), which is based on the specific staining of the 3′ hydroxyl ends of the fragmented DNA within apoptotic cells (12, 17, 33, 38). Figures 5A and C show uninfected and infected cells, respectively, visualized by red propidium iodide fluorescence. Panels B and D show the same fields as panels A and C, respectively, visualized by green fluorescein fluorescence. Apoptotic cells are visualized as intensely green fluorescent cells, easily recognized above background levels (Fig. 5D).
FIG. 4.
Agarose gel (1.8%) electrophoretic separation of DNA extracted from MAC-T cells. Lane A, uninfected control MAC-T cells treated with gentamicin for 3 h; lane B, MAC-T cells infected with S. aureus Novel for 2 h prior to the addition of gentamicin, then incubated for an additional 3 h; note the ladder of fragmented DNA bands in multiples of 180 bp; lane C, 100-bp DNA ladder. Data are from a representative experiment repeated four times in duplicate.
FIG. 5.
Evaluation of DNA fragmentation in MAC-T cells by the TUNEL method. MAC-T cells were grown in the absence (A and B) or presence (C and D) of S. aureus Novel for 2 h prior to the addition of gentamicin and then for an additional 4 h. Images were captured by confocal microscopy for both propidium iodide (PI; A and C) and fluorescein (B and D) fluorescence. PI was used to visualize all nuclear material. The incorporation of fluorescein-dUTP at the free 3′ hydroxyl ends of fragmented DNA is shown by intense green fluorescence. (A) Uninfected MAC-T cells; (B) the same microscopic field as for panel A; (C) MAC-T cells infected with S. aureus; (D) the same microscopic field as for panel C; note the intense green fluorescence over background. Data are from a representative experiment repeated three times in duplicate. Only those apoptotic bodies that remained adherent during the staining procedure are shown here.
DISCUSSION
While S. aureus has not traditionally been considered an intracellular pathogen, previous studies have revealed that S. aureus cells may be actively internalized by phagocytosis and are capable of intracellular survival (1, 18, 37). In characterizing S. aureus invasion further, we have observed that many of the internalization events are consistent with the cellular events that are induced by other well-established facultative intracellular pathogens (13). First, electron photomicrographs show that S. aureus cells interact or bind with the cell surface of the MAC-T host cell and induce membrane pseudopod formation. Which S. aureus adhesion factor(s) is responsible for the signal transduction initiating pseudopod formation and endocytosis is currently unknown. However, one or more of the group of specific cell surface proteins, collectively termed MSCRAMMs (for microbial surface components recognizing adhesive matrix molecules) (31) could be involved. Second, although the S. aureus cells were observed to be internalized initially within a membrane-bound endosome, the S. aureus cells eventually came to reside directly in the host cytoplasm, in this respect mimicking Rickettsia, Shigella, and Listeria cells.
It is noteworthy that not all intracellular parasites escape from the vacuole; Coxiella burnetii, for example, has evolved to thrive in the phagolysosome, while Salmonella, Mycobacterium, and Legionella spp. inhibit phagolysosomal fusion (15). Thus, it is of interest to establish the fate of S. aureus once it is within a host cell. The studies of Almeida et al. (1), who examined the invasion of S. aureus into primary and established bovine mammary epithelial cell lines, showed that the bacteria were localized in membrane-bound vacuoles. This apparent conflict with our observations might be explained as follows. First, it is possible that infected cells that were analyzed by TEM in their study were harvested at too early a time point to observe lysis of the endosomal membrane and access to the cytoplasm. Second, it has been shown that only a small fraction of internalized L. monocytogenes cells escape from the vacuoles (15). A similarly small fraction of S. aureus cells might escape from the vacuoles, making them difficult to locate. Regardless of this apparent conflict, our results are in agreement with those of Hudson et al. (20), who demonstrated that S. aureus cells were able to invade and appeared free in the cytoplasm of cultured chick osteoblast cells. Furthermore, our data are also consistent with the assumption of Proctor et al. (34) that intracellular S. aureus cells must reside in the host cell cytoplasm since this compartment would provide the biochemical environment that allows the survival of small-colony variants during persistent, recurring staphylococcal infections.
Since S. aureus was seen to escape from vacuoles in MAC-T cells, it becomes important to ask how this was achieved. Listeria and Shigella cells produce specific hemolysins (listeriolysin O and IpaB, respectively) that enzymatically degrade the endosomal membrane (15). The role of listeriolysin was elegantly demonstrated by expressing this hemolysin in the nonpathogenic species Bacillus subtilis, which allowed these bacteria to survive and replicate in the cytoplasm of phagocytic cells (2). We hypothesize that the release of S. aureus from the vacuole is mediated by one (or more) of at least four different staphylococcal hemolysins known to damage membranes (alpha-, beta-, gamma-, and delta-toxins) (4). Experiments are under way to test this hypothesis.
The ability of S. aureus to survive intracellularly could explain several aspects of host-pathogen relationships as they pertain to chronic recurrent staphylococcal diseases and long-term colonization. Internalization may be an important component of mastitis and other staphylococcal diseases, perhaps by providing protection against host defenses and antibiotic treatment. In fact, Craven and Anderson (8) demonstrated intracellular S. aureus in experimental mastitis in mice. As a species, S. aureus comprises a heterogeneous group of strains, each with the potential to elaborate its own set of virulence factors. Because of this diversity, many types of infectious diseases are associated with this organism. Although diseases such as toxic shock syndrome require the expression of immunomodulating superantigen exotoxins, the effect of the toxins must be preceded by colonization on mucosal surfaces. The most common types of staphylococcal diseases are pyogenic infections that are often a direct result of long-term colonization of the host by an endogenous organism. Furthermore, many types of staphylococcal infections that tend to become chronic, such as osteomyelitis and mastitis, are associated with multiple recurrences and do not resolve even in the presence of a seemingly adequate humoral immune response. Our results suggest that the chronic nature of S. aureus disease and/or its long-term persistence may be dependent on the ability of S. aureus to survive within host cells.
A further important finding of this study was that internalized S. aureus cells induced the bovine epithelial host cells to become apoptotic. Interestingly, the ability of Shigella flexneri to induce apoptosis in macrophages, T cells, and B cells has been shown (42). Invasion and cell killing were inhibited by cytochalasin D, indicating that the bacteria must be within the cell to induce apoptosis (41). It has been shown that the induction of apoptosis by ingested S. flexneri cells is mediated by the binding of IpaB invasin to interleukin-1-converting enzyme, which promotes apoptosis in macrophages (5). Although no IpaB-like invasin has been identified for S. aureus, it is important to note that in primary lymphocyte cultures, purified staphylococcal alpha-toxin and superantigens can induce apoptosis (24, 25). However, it is unlikely that a similar type of induction mechanism, mediated from the outside of the MAC-T cells, played a significant role in causing apoptosis in our cell cultures for several reasons. First, the S. aureus cells were carefully washed to remove extracellular proteins before infecting the MAC-T cells and the MAC-T cells were carefully washed after being infected with S. aureus. Second, the length of time that the MAC-T cells were exposed to viable staphylococcal prior to the addition of gentamicin was very short. Most secreted proteins are regulated by the products of the agr global transcriptional regulatory locus and are expressed late in the growth cycle (28). Finally, observations using microscopy revealed that following infection with S. aureus, MAC-T cells exhibiting normal (nonapoptotic) morphology were observed in the presence of apoptotic MAC-T cells at every time point; the presence of extracellular inducers of apoptosis would be expected to be able to affect all cells similarly.
An alternative hypothesis involves recent evidence implicating the sphingomyelin cycle as a key component in the induction of apoptosis (19). It has been demonstrated that certain extracellular agents that lead to apoptosis, such as tumor necrosis factor alpha, activate endogenous sphingomyelinase activity (10), cleaving membrane sphingomyelin to yield cellular ceramide. Evidence implicating ceramide as a mediator of apoptosis was reported by Jarvis et al. (23), who showed that apoptosis was induced in human leukemia and murine fibrosarcoma cell lines after the addition of exogenous sphingomyelinase or synthetic ceramide. Thus, the production of beta-toxin, a known sphingomyelinase (9, 11), by intracellular S. aureus cells could result in an increased level of ceramide, leading to the induction of apoptosis in the bovine mammary epithelial cells. It should be noted that we have demonstrated that beta-toxin is produced by S. aureus Novel (data not shown). Furthermore, other beta-toxin-producing S. aureus strains, including standard laboratory strains 8325-4 and RN6390, also invade MAC-T cells and induce apoptosis (data not shown). Interestingly, beta-toxin has been shown to be an important virulence factor for infection by using a mouse model for staphylococcal mastitis (16).
Does the ability of bacteria to induce apoptosis imply that there is some benefit to the bacteria? In some cases, as when S. flexneri cells are internalized and induce the death of macrophages by apoptosis (6), one can speculate that the induction of apoptosis in leukocytes is a mechanism for suppressing the immune response. Alternatively, the biological consequences of apoptosis were hypothesized to be the release of interleukin-1 prior to cell death (40), which in turn initiates an acute inflammatory response characteristic of bacillary dysentery. The ability of S. aureus to induce apoptosis in cultured bovine mammary epithelial cells also has profound implications for the possible role of programmed cell death during the pathogenesis of bovine mastitis. One hypothesis is that the induction of apoptosis produces a vehicle by which the bacteria could enter macrophages without stimulating bactericidal activities, while simultaneously being provided with a protective barrier against exogenous host immune defenses and/or antibiotics. This is supported by the observation that phagocytes isolated from infected udders often contain viable S. aureus cells (8). We are currently investigating this possibility.
This report demonstrates that S. aureus becomes intracellular following contact with the cell surfaces of bovine mammary epithelial cells, escapes the endosome to reside and possibly multiply in the host cell cytoplasm, and induces the host cell to become apoptotic. The observation that intracellular S. aureus induces programmed cell death in epithelial cells, orchestrating normal host cell processes for its own advantage, suggests that S. aureus might be a more versatile and adaptive pathogen than previously believed.
ACKNOWLEDGMENTS
We thank Raquel Brown and Chris Davitt for the electron microscopic examination of S. aureus invasion, Ann Norton for technical support with the confocal microscopy, and Katarzyna Dziewanowska for her characterization of S. aureus Novel.
This work was funded in part by NIH grant no. R29-AI38901 (K.W.B.), NRICGP USDA grant no. 9402399 (G.A.B.), Public Health Service grant no. AI28401 (G.A.B.), the United Dairymen of Idaho (G.A.B.), and an Organization for Economic Co-operation and Development sabbatical fellowship (W.R.T.).
REFERENCES
- 1.Almeida R A, Matthews K R, Cifrian E, Guidry A J, Oliver S P. Staphylococcus aureus invasion of bovine mammary epithelial cells. J Dairy Sci. 1996;79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- 2.Bielecki J, Youngman P, Connelly P, Portnoy D A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature (London) 1990;345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 3.Blosser T. Economic losses from and the national research program on mastitis in the United States. J Dairy Sci. 1979;62:119–127. doi: 10.3168/jds.S0022-0302(79)83213-0. [DOI] [PubMed] [Google Scholar]
- 4.Bohach G, Dinges M M, Mitchell D T, Ohlendorf D H, Schlievert P M. Exotoxins. In: Crossley K B, Archer G L, editors. The Staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 83–111. [Google Scholar]
- 5.Chen Y, Smith M R, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Zychlinsky A. Apoptosis induced by bacterial pathogens. Microb Pathog. 1994;17:203–212. doi: 10.1006/mpat.1994.1066. [DOI] [PubMed] [Google Scholar]
- 7.Chesney P J. Clinical aspects and spectrum of illness of toxic shock syndrome: overview. Rev Infect Dis. 1989;11:1–7. [PubMed] [Google Scholar]
- 8.Craven N, Anderson J C. The location of Staphylococcus aureus in experimental chronic mastitis in the mouse and the effect on the action of sodium cloxacillin. Br J Exp Pathol. 1979;60:453. [PMC free article] [PubMed] [Google Scholar]
- 9.Doery H M, Magnusson B J, Cheyne I M, Gulasekharam J. A phospholipase in staphylococcal toxin which hydrolyses sphingomyelin. Nature (London) 1963;198:1091–1092. doi: 10.1038/1981091a0. [DOI] [PubMed] [Google Scholar]
- 10.Dressler K A, Mathias S, Kolesnick R N. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992;255:1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- 11.Dziewanowska K, Edwards V M, Deringer J R, Bohach G A. Comparison of the beta-toxin from Staphylococcus aureus and Staphylococcus intermedius. Arch Biochem Biophys. 1996;335:102–108. doi: 10.1006/abbi.1996.0486. [DOI] [PubMed] [Google Scholar]
- 12.Facchinetti A, Tessarollo L, Mazzocchi M, Kingston R, Collavo D, Biasi G. An improved method for the detection of DNA fragmentation. J Immunol Methods. 1991;136:1251–1256. doi: 10.1016/0022-1759(91)90258-h. [DOI] [PubMed] [Google Scholar]
- 13.Falkow S, Isberg R R, Portnoy D A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 14.Ferrow J P. Subclinical mastitis: biology and economics. Compend Contin Educ Pract Vet. 1980;2:5223–5234. [Google Scholar]
- 15.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster T, O’Reilly M, Phonimdaeng P, Cooney J, Patel A, Bramley A. Genetic studies of virulence factors of S. aureus. Properties of coagulase and gamma-toxin and the role of alpha-toxin, beta-toxin and protein A in the pathogenesis of S. aureus infections. In: Novick R, editor. Molecular biology of the staphylococci. New York, NY: VCH Publishers, Inc.; 1990. pp. 403–417. [Google Scholar]
- 17.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamill R J, Vann J M, Proctor R A. Phagocytosis of Staphylococcus aureus by cultured bovine aortic endothelial cells: model for postadherence events in endovascular infections. Infect Immun. 1986;54:833–836. doi: 10.1128/iai.54.3.833-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannun Y A, Obeid L M. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci. 1995;20:73–77. doi: 10.1016/s0968-0004(00)88961-6. [DOI] [PubMed] [Google Scholar]
- 20.Hudson M C, Ramp W K, Nicholson N C, Williams A S, Nousiainen M T. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 21.Hyunh H T, Robitaille G, Turner J D. Establishment of bovine mammary epithelial cells (MAC-T): an in vivo model for bovine lactation. Exp Cell Res. 1991;197:191–199. doi: 10.1016/0014-4827(91)90422-q. [DOI] [PubMed] [Google Scholar]
- 22.Isberg R R, Voorhis D L, Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1978;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis W D, Kolesnick R N, Fornari F A, Traylor R S, Gewirtz D A, Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci USA. 1994;91:73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonas D, Walev I, Berger T, Liebetrau M, Palmer M, Bhakdi S. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal degradation. Infect Immun. 1994;62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature (London) 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 26.Kenny K, Reiser R F, Bastida-Corcuera F D, Norcross N L. Production of enterotoxins and toxic shock syndrome toxin by bovine mammary isolates of Staphylococcus aureus. J Clin Microbiol. 1993;31:706–707. doi: 10.1128/jcm.31.3.706-707.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerr J F R, Wylie A H, Currie A R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kornblum J, Kreiswirth B N, Projan S J, Ross H, Novick R P. Agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers, Inc.; 1990. pp. 373–402. [Google Scholar]
- 29.Majno G, Joris I. Apoptosis, oncosis and necrosis. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 30.Mukherjee T M, Dixon B. Diagnostic electron microscopy procedures. Microsc Soc Am Bull. 1993;23:200–205. [Google Scholar]
- 31.Patti J M, Allan B L, McGavin M, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissue. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 32.Peitsch M C, Mannherz H G, Tschopp J. The apoptosis endonucleases: cleaning up after cell death? Trends Cell Biol. 1994;4:37–41. doi: 10.1016/0962-8924(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 33.Piqueras B, Autran B, Debre P, Gorochov G. Detection of apoptosis at the single-cell level by direct incorporation of fluorescein-dUTP in DNA strand breaks. BioTechniques. 1996;20:634–640. doi: 10.2144/19962004634. [DOI] [PubMed] [Google Scholar]
- 34.Proctor R A, Balwit J M, Vesga O. Variant subpopulations of Staphylococcus aureus as cause of persistent and recurrent infections. Infect Agents Dis. 1994;3:302–312. [PubMed] [Google Scholar]
- 35.Smith C A, McCarthy N J, Williams G T. Cell recognition of apoptotic cells. In: Horton M A, editor. Blood cell biochemistry. Vol. 5. New York, N.Y: Plenum Press; 1993. pp. 393–421. [Google Scholar]
- 36.Smith, T. H., L. K. Fox, and J. R. Middleton. An outbreak of mastitis caused by a single strain of Staphylococcus aureus in a closed herd where strict milking time hygiene has been employed. J. Vet. Med. Assoc., in press.
- 37.Vann J M, Proctor R A. Ingestion of Staphylococcus aureus by bovine endothelial cells results in time- and dose-dependent damage to endothelial cell monolayers. Infect Immun. 1987;55:2155–2163. doi: 10.1128/iai.55.9.2155-2163.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wijsman J H, Jonker R R, Keijzer R, Van de Velde C J H, Cornelisse C J, Van Dierendonck J H. A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem. 1993;41:7–12. doi: 10.1177/41.1.7678025. [DOI] [PubMed] [Google Scholar]
- 39.Wyllie A H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature (London) 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 40.Zychlinsky A, Fitting C, Cavaillon J, Sansonetti P J. Interleukin-1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J Clin Invest. 1994;94:1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 42.Zychlinsky A, Thirumalai K, Arondel J, Cantey J R, Aliprantis A O, Sansonetti P J. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64:5357–5365. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]