Summary
Background
Although the antibody–drug conjugates (ADCs) have significantly improved the survival outcomes of patients with human epidermal receptor 2 (HER2)-expressing gastric or gastroesophageal junction (G/GEJ) cancer, the efficacy of ADC used as a single agent is limited. Therefore, it is necessary to investigate effective and safe combination regimens. Preclinical data indicated a synergetic antitumour effect of RC48 and programmed cell death protein 1 (PD-1) inhibitors. We aimed to evaluate the safety and efficacy of RC48 plus toripalimab in patients with HER2-expressing G/GEJ cancer and other solid tumours.
Methods
This was a open-label, multicentre, phase 1 trial performed at three hospitals in China. Eligible patients had advanced G/GEJ cancer or other solid tumours with HER2 IHC≥1 or ISH positivity and were refractory to at least one line of treatment, or standard treatment was intolerable or unavailable for these patients. This study followed a “3 + 3” design with predefined RC48 dosages of 2.0 mg/kg and 2.5 mg/kg plus toripalimab 3 mg/kg, once every 2 weeks (q2w). The primary objectives were to evaluate the safety and determine the recommended phase II dose (RP2D), and the secondary objectives included assessing the pharmacokinetics (PK) and preliminary efficacy. This study was registered with ClinicalTrials.gov, NCT04280341.
Findings
Between July 13, 2020 and August 30, 2022, 56 patients, including 30 patients with G/GEJ cancer and 26 patients with other solid tumours, were enrolled and received RC48 plus toripalimab (n = 7 for RC48 2.0 mg/kg, toripalimab 3 mg/kg, q2w; n = 49 for RC48 2.5 mg/kg, toripalimab 3 mg/kg, q2w). No dose-limiting toxic effects occurred. The RP2D was declared as RC48 2.5 mg/kg plus toripalimab 3 mg/kg, q2w. The most common grade 3 adverse events were a decreased neutrophil count (n = 13), and a decreased white blood cell count (n = 7). The efficacy assessment was completed for 52 patients. Among patients with G/GEJ cancer (n = 30), the confirmed objective response rate (ORR) was 43% (12/28, 95% CI 25, 63), median progression-free survival (PFS) was 6.2 months (95% CI 4.0, 6.9), median overall survival (OS) was 16.8 months (95% CI 7.2, NE). The ORR of patients with G/GEJ cancer receiving RP2D (n = 24) reached 50% (11/22, 95% CI 28, 72), with median PFS of 5.1 months (95% CI 1.4, 7.3) and median OS of 14.0 months (95% CI 6.3, NE). Among patients with G/GEJ cancer who received RP2D, a clinical benefit was observed in both HER2-positive and low HER2 expressing populations, with an ORR of 56% (5/9, 95% CI 21, 86) vs. 46% (6/13, 95% CI 19, 75), median PFS of 7.8 months (95% CI 0.9, NE) vs. 5.1 months (95% CI 1.2, 6.9), median OS of NE months (95% CI 4.3, NE) vs. 14.0 months (95% CI 5.1, NE), respectively. Antitumour activity was also observed for other solid tumours, including breast cancer (5/13) and endometrial carcinoma (1/1).
Interpretation
Our findings suggested that RC48 plus toripalimab had a manageable safety profile and showed encouraging efficacy in pretreated patients with HER2-positive and low HER2-expressing G/GEJ cancer. The findings of our phase 1 clinical trial support further investigation of HER2-targeted ADC plus immunotherapy in HER2-expressing G/GEJ cancer and pancancer treatment in the future.
Funding
Beijing Municipal Medical Research Institutes, Beijing Medical Research Institute (Z200015).
Keywords: Her2, ADC, PD-1, Gastric cancer, Solid tumours
Research in context.
Evidence before this study
We searched PubMed for articles published until Jul 25, 2023, with the terms ([“RC48” OR “HER2-targeted ADC”] AND [“PD-1” OR “PD-L1”] AND [“gastric cancer”]. We did not restrict our search by language or publication type. Our search retrieved no reports on patients with gastric cancer of the combination of HER2-targeted ADC plus an agent targeting PD-1 or PD-L1. Also, we searched the term "dose" or "dosage" and "RC48" or "totipalimab". Published data indicated that RC48 and toripalimab had been approved with fixed dose of 2.5 mg/kg q2w and 3.0 mg/kg q2w as monotherapy, respectively. However, there were no reports on combination therapy of the two drugs.
Added value of this study
To our knowledge, our study is the first clinical trial investigating the use of HER2-targeted ADC plus a PD-1 inhibitor in HER2-expresing advanced gastric cancer and other cancers. In this study, we investigated two dose levels for this combinaiton, RC48 2.0 mg/kg or 2.5 mg/kg plus toripalimab 3.0 mg/kg, q2w. We found both dose levels were tolerable, the higher dose elicited a higher response. Therefore, RC48 2.5 mg/kg plus toripalimab 3.0 mg/kg were declared as RP2D. A clinically meaningful improvement in the ORR and OS was noted in patients who received this combination compared with historical data on RC48 monotherapy, and no more adverse events were recorded for this combination regimen. Patients with low HER2-expressing gastric cancer gained equivalent benefit to those with HER2-positive gastric cancer. Exploratory analyses suggested that gastric cancer patients with PD-L1 CPS≥1 benefited more from RC48 plus toripalimab than those with CPS<1.
Implications of all the available evidence
Currently, the efficacy of HER2-targeted ADC monotherapy is limited with notable toxicities. There are unmet treatment needs in patients with HER2-positive gastric cancer that progressed after trastuzumab, also, there is no standard treatment for those with low HER2-expression. Our clinical trial demonstrated preliminary clinically meaningful response rates with an impressive duration of response, suggesting potential synergy between HER2-targeted ADCs and PD-1 inhibitors. Given these data, the potential role of HER2-targeted ADCs and PD-1 inhibitors in combination with other agents, including chemotherapy or anti-VEGF agents, deserves further investigation in patients with HER2-expressing G/GEJ cancer and other cancers in the future.
Introduction
An estimated 15–20% of advanced gastric or gastroesophageal junction (G/GEJ) cancers have overexpression or amplification of human epidermal growth factor receptor 2 (HER2). For two decades, trastuzumab was the only HER2-targeted drug available for this population. Until recently, the development of novel antibody–drug conjugates (ADCs), such as trastuzumab deruxtecan (T-Dxd), disitamab vedotin (RC48), and ARX788, has revolutionized the treatment landscape of HER2 positive G/GEJ cancer. With the regulatory approval of T-Dxd for the treatment of patients with HER2-positive advanced G/GEJ cancer in the USA and Japan, and RC48 for patients with HER2 Immunohistochemistry (IHC) 2+/3+ in China, the survival of patients with G/GEJ cancer has improved recently. Notwithstanding the success of HER2-targeted ADC, the challenges in treating patients with HER2-positive gastric cancer (GC) remain, as only a subset of patients can benefit from the use of ADC monotherapy, with the confirmed objective response rate (ORR) varying from 24.8% to 43.0%, and the median progression-free survival (PFS) from 4.1–5.6months.1, 2, 3 Additionally, the broader use of anti-HER2 ADC drugs is limited by notable toxicities, including dose-limiting toxicities (DLTs), such as myelosuppression, which are often shared by different ADCs that deliver the same cytotoxic payload, and off-site, on-target toxicities. For instance, interstitial lung disease (ILD)/pneumonitis is considered one of the adverse events (AEs) of special interest associated with T-Dxd, observed in 10% of patients in the DESTINY-Gastric 01 trial and severe ILD can be life-threatening.2 Both of these adverse events will lead to the dose modification, dose delay, or treatment discontinuation of the anti-HER2 ADCs. Therefore, investigation of rational combination drugs that might synergize with ADCs to augment their clinical efficacy without additional toxicity is urgently needed in the treatment of advanced HER2-overexpressing GC.4
Several translational studies have revealed a more inflamed immune microenvironment in HER2-positive than in HER2-negative advanced GC, supporting the combination of HER2-targeted drugs with immunotherapy.5,6 Moreover, the combination of programmed cell death protein 1 (PD-1) inhibitor and HER-2 targeted antibodies has entered clinical studies, demonstrating promising efficacy and good tolerability in HER2 positive GC.7,8 Furthermore, ADCs are designed not only to target cancer cells, but also to modulate the immune microenvironment, such as by eliciting immunogenic cell death, antibody-dependent cell-mediated cytotoxicity and dendritic cell activation, increasing T-cell activity, and upregulating Programmed death-ligand 1 (PD-L1) expression, ultimately providing potential synergism with immunotherapy.9, 10, 11 The synergistic antitumour effect of simultaneous blockade of PD-1 and HER2 targeted ADCs has been reported both in vitro and in vivo.12, 13, 14 In summary, these findings support further exploration of the synergetic efficacy of HER2-targeted ADC and PD-1 inhibitor combinations in the treatment of HER2-expression GC.
RC48 is a newly developed ADC drug targeting HER2 that comprises hertuzumab coupled with monomethyl auristatin E (MMAE) via a cleavable linker. In our previously reported studies, RC48 monotherapy showed a tolerable safety profile and promising antitumour activities against GC and other solid tumours with HER2-expression. Based on these results, RC48 has been approved in China for third line treatment in patients with HER2-overexpressing GC with a recommended dose of 2.5 mg/kg once every 2 weeks (q2w). Due to the excellent bystander effect, RC48 showed equivalent efficacy for low HER2-expressing and HER2-positive tumours.1,15 In addition, the good safety profile of RC48 also supports combination therapy; interstitial lung disease or pneumonitis was not observed with RC48 monotherapy, which is an AE of special interest associated with T-Dxd administration at a rate of 10% with a death rate of 2.6%.2 Toripalimab, also known as JS001, is a humanized IgG4 monoclonal antibody against PD-1. It is the first monoclonal anti-PD-1 antibody approved by the China National Medical Products Administration on the market. Toripalimab was generally well-tolerated and showed a promising anti-tumour effect in patients with G/GEJ cancer, whether in monotherapy or in combination with other drugs.16,17
Therefore, we initiated this multicentre, phase I study of RC48 plus toripalimab, in previously treated patients with HER2-expressing G/GEJ cancer and other solid tumours, which is to our knowledge the first study reporting the treatment of G/GEJ cancer with a HER2-targeted ADC in combination with a PD-1 inhibitor.
Methods
Ethics statement
The study protocol was approved by the Ethics Commission of Peking University Cancer Hospital and Institute (2019YJZ65). Written informed consent was obtained from all patients before study enrolment. This trial was performed in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines.
Study design and participants
This study was a phase 1, open-label, multicentre trial that included dose-escalation and dose-expansion parts. Patients were enrolled at hospitals in China. This study was registered with ClinicalTrials.gov, NCT04280341.
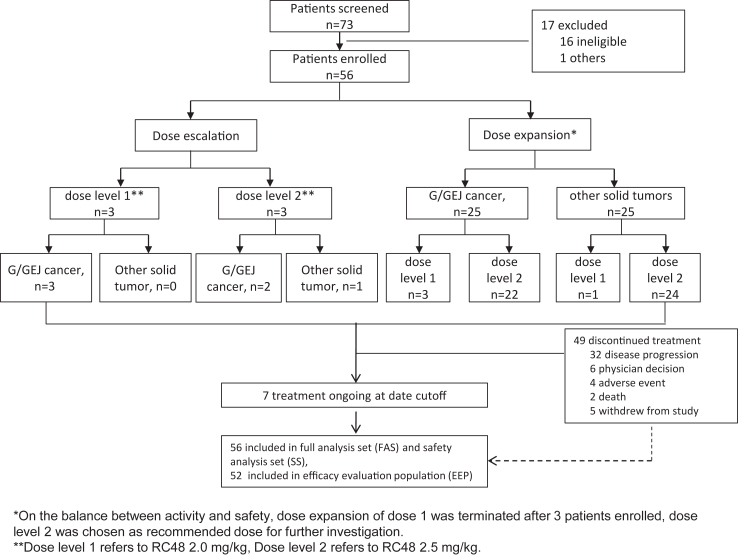
In the dose-escalation segment of the study, we assessed the DLT of RC48 plus toripalimab. The dose-escalation stage followed a “3 + 3” design and included two dose-level cohorts (Fig. 1). Considering that there is some overlap in the toxicity profiles of RC48 and toripalimab, we started from a dose level lower than the standard dose for RC48 combined with the clinically established fixed dose of toripalimab. For dose 1, three eligible patients were enrolled. If no DLTs were noted, the dose was increased to the next level. If no DLTs occurred at either of the two doses, the dose-expansion stage was initiated at the two dose levels to better assess safety and antitumour activity and determine the recommended phase II dose (RP2D).
Fig. 1.
Study design and patient disposition.
In the dose-expansion stage, patients were enrolled into two cohorts based on tumour types: cohort 1 included patients with locally advanced unresectable or metastatic G/GEJ adenocarcinomas, and cohort 2 included patients with advanced solid malignant tumours other than G/GEJ adenocarcinomas. We aimed to assess the safety and antitumour activity of RC48 plus toripalimab treatment.
Eligible patients were refractory to standard treatment, or standard treatment was intolerable or unavailable to them. For patients with G/GEJ cancer, considering that the ORR and PFS of the current recommended standard 2nd-line treatment are far from satisfactory and we have proven support for the efficacy of RC48 monotherapy in previous studies, those who had received 1st-line treatment were also allowed to be enrolled. Other key eligible criteria included HER2 IHC ≥ 1 or in site hybridization (ISH) positivity. Tumours were tested for HER2 status with IHC at local labs. HER2-positivity was defined as IHC 3+ or IHC 2+/ISH positiveity, and low HER2-expression was defined as IHC 2+/ISH negativity or IHC 1+ in this study. The combined positive score (CPS) for PD-L1 was the percentage of positive cells among all tumour cells. Measurable lesions were defined according to Response Evaluation Criteria in Solid Tumours (RECIST) 1.1, Eastern Cooperative Oncology Group performance status (ECOG) 0 or 1, and adequate kidney, hepatic, and bone marrow function. Key exclusion criteria included previous administration of anti–PD-1/PD-L1 antibodies, serious complications, such as active gastrointestinal bleeding, intestinal obstruction, or history of acute myocardial infarction, and congestive heart failure (NYHA) ≥ Grade 3. Full inclusion and exclusion criteria are listed in the protocol.
Determination of HER2 status
The HER2 test was performed at local laboratories. The positivity criteria are described in the Supplemental material.
Procedures
All patients received RC48 at either 2.0 mg/kg or 2.5 mg/kg plus toripalimab at 3.0 mg/kg once every two weeks. Patients could continue treatment until progressive disease (PD), intolerable AEs, or another protocol-specified discontinuation criterion was met. Toripalimab was administered for up to 24 months. Dose interruptions and reductions were implemented to manage AEs. Generally, the dose of RC48 would be delayed or reduced to allow the patients who already have or are likely to have clinical benefits to continue treatment while ensuring safety. No dose modifications were allowed for toripalimab during treatment, only dose interruptions were allowed. Detailed dose modification suggestions are listed in the protocol. Pharmacokinetic and pharmacodynamic profiling are described in the trial protocol. Safety assessments were performed at each study visit. All AEs were graded and monitored according to the Common Terminology Criteria for Adverse Events version 5.0. The clinical response was evaluated by an investigator according to RECIST version 1.1 at baseline and every 6 weeks, irrespective of dose delays or interruptions, until documented disease progression or death.
Outcomes
The primary objectives were to evaluate the safety and tolerability of RC48 in combination with toripalimab in patients with HER2-expressing locally advanced or metastatic solid tumours, to observe the possible DLTs, maximum tolerated dose (MTD), and to determine the RP2D. the definition of a DLT is included in the study protocol (appendix pp 20–21). The secondary objectives included assessing PK and preliminary efficacy. Efficacy endpoints included (per RECIST v1.1) the ORR, duration of response (DOR), disease control rate (DCR), PFS, and overall survival (OS).
Statistical analysis
This study was designed to assess the safety, tolerability, RP2D, and preliminary antitumour activity of RC48 plus toripalimab. Based on the safety evaluation results of previous studies, it was expected that 2 cohorts need to be evaluated in the dose escalation stage of this study. Patients not evaluable for DLT would be replaced, which might result in more patients actually enrolled than anticipated. The RP2D was decided after discussion of investigators on the balance of safety and efficacy of different dose levels. Selected indications from the first part of the study with observed antitumour activity entered the dose-expansion phase to further evaluate the efficacy. In the dose-expansion phase, we wanted to decide if the tumour response rate of the study medication on the selected indication was less than or equal to 15% or greater than or equal to 35% under a one-sided type I error of 0.025. A sample size of 20–50 patients per cohort was determined to provide a power over 86.7%. However, since the study remained exploratory, no formal hypothesis testing was performed in the final analysis. Demographic characteristics, baseline characteristics, adverse events, laboratory toxicities, and DLTs were summarized for patients in the safety analysis set (i.e., patients who received at least 1 dose of either RC48 or toripalimab) using descriptive statistics. The anti-tumour activity of the study intervention was summarized with descriptive statistics of ORR, PFS, OS and DOR for patients who received at least 1 dose of the study drug (either RC48 or toripalimab), and had at least 1 postdose tumour assessment. The percentage of patients with an objective response was summarized with the exact 95% confidence interval (CI) on the basis of Clopper–Pearson method. Estimates of median survival and 95% CI for time-to-event endpoints were calculated with the Kaplan–Meier method. Definitions for the assessment tools were as follows: An objective response was defined as the percent of patients documented to have a confirmed Complete Response (CR) or partial response (PR) as reported by the central review laboratory. PFS was defined as the time from the date of the first dose of the study treatment to the date of documented disease progression or death due to any cause. OS was defined as the time from the first dose until death or loss to follow-up. DOR was defined as the time from the first documentation of objective tumour response (CR or PR) to the first documented disease progression or to death due to any cause in the absence of documented PD. All statistical analyses were conducted using SAS, version 9.4 or later (SAS Institute).
Role of the funding source
This study was funded by Beijing Municipal Medical Research Institutes, Beijing Medical Research Institute (Z200015). The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors verified that this study was performed according to the protocol and was attested to the accuracy and completeness of the data. All authors had full access to the dataset. The corresponding authors had final responsibility for the decision to submit for publication.
Results
From July 13, 2020 to August 30, 2022, 56 patients (median age, 58 years [interquartile range (IQR) 50.5–64.0]) were enrolled at three hospitals in China. 28 (50%) of the 56 patients were male. The data cut-off for the analysis was Apr 28, 2023. Patient characteristics are detailed in Table 1.
Table 1.
Baseline patient characteristics.
| RC48 dose of 2.0 mg/kg (n = 7) |
RC48 dose of 2.5 mg/kg (n = 49) |
Total (n = 56) | |||||
|---|---|---|---|---|---|---|---|
| Gastric cancer (n = 6) | Other solid tumour (n = 1) | Total (n = 7) | Gastric cancer (n = 24) | Other solid tumour (n = 25) | Total (n = 49) | ||
| Age, years | 64.5 (59.0–71.0) | 67.0 (67.0–67.0) | 67.0 (59.0–71.0) | 60.5 (54.5–64.0) | 53.0 (47.0–60.0) | 58.0 (50.0–63.0) | 58.0 (50.5–64.0) |
| Sex | |||||||
| Female | 3 (50) | 1 (100) | 4 (57) | 5 (21) | 19 (76) | 24 (49) | 28 (50) |
| Male | 3 (50) | 0 | 3 (43) | 19 (79) | 6 (24) | 25 (51) | 28 (50) |
| ECOG performance status | |||||||
| 0 | 1 (17) | 0 | 1 (14) | 7 (29) | 5 (20) | 12 (25) | 13 (23) |
| 1 | 5 (83) | 1 (100) | 6 (86) | 17 (71) | 20 (80) | 37 (76) | 43 (77) |
| Primary site | |||||||
| Gastric | 4 (67) | 0 | 4 (57) | 20 (83) | 0 | 20 (41) | 24 (43) |
| Gastroesophageal | 2 (33) | 0 | 2 (29) | 3 (13) | 0 | 3 (6) | 5 (9) |
| Breast | 0 | 0 | 0 | 0 | 14 (56) | 14 (29) | 14 (25) |
| Intestine | 0 | 1 (100) | 1 (14) | 0 | 5 (20) | 5 (10) | 6 (11) |
| Other | 0 | 0 | 0 | 1 (4)a | 6 (24) | 7 (14) | 7 (13) |
| Burden of target tumour lesion | |||||||
| <5 cm | 4 (67) | 0 | 4 (57) | 13 (54) | 11 (44) | 24 (49) | 28 (50) |
| ≥5 cm–<10 cm | 1 (17) | 0 | 1 (14) | 9 (38) | 9 (36) | 18 (37) | 19 (34) |
| ≥10 cm | 1 (17) | 1 (100) | 2 (29) | 2 (8) | 5 (20) | 7 (14) | 9 (16) |
| Metastatic site | |||||||
| Lympha-node | 6 (100) | 1 (100) | 7 (100) | 17 (71) | 19 (76) | 36 (74) | 43 (77) |
| Lung | 1 (17) | 1 (100) | 2 (29) | 9 (38) | 12 (48) | 21 (43) | 23 (41) |
| Liver | 2 (33) | 1 (100) | 3 (43) | 12 (50) | 11 (44) | 23 (47) | 26 (46) |
| Bone | 2 (33) | 1 (100) | 3 (43) | 1 (4) | 12 (48) | 13 (27) | 16 (29) |
| Adrenal gland | 1 (17) | 1 (100) | 2 (29) | 1 (4) | 3 (12) | 4 (8) | 6 (11) |
| Other | 4 (67) | 0 | 4 (57) | 16 (67) | 15 (60) | 31 (63) | 35 (63) |
| Number of metastatic sites | |||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 1 (17) | 0 | 1 (14) | 5 (21) | 2 (8) | 7 (14) | 8 (14) |
| 2 | 2 (33) | 0 | 2 (29) | 5 (21) | 4 (16) | 9 (18) | 11 (20) |
| ≥3 | 3 (50) | 1 (100) | 4 (57) | 14 (58) | 19 (76) | 33 (67) | 37 (66) |
| Number of previous lines of therapy | |||||||
| 0 | 1 (17) | 0 | 1 (14) | 0 | 0 | 0 | 1 (2) |
| 1 | 2 (33) | 0 | 2 (29) | 16 (67) | 7 (28) | 23 (47) | 25 (45) |
| 2 | 2 (33) | 0 | 2 (29) | 7 (29) | 11 (44) | 18 (37) | 20 (36) |
| ≥3 | 1 (17) | 1 (100) | 2 (29) | 1 (4) | 7 (28) | 8 (16) | 10 (18) |
| Previously received anti-HER2 treatment | |||||||
| Non | 3 (50) | 1 (100) | 4 (57) | 17 (71) | 17 (68) | 34 (69) | 38 (68) |
| Trastuzumab | 2 (33) | 0 | 2 (29) | 6 (25) | 6 (24) | 12 (25) | 14 (25) |
| Pyrotinib | 1 (17) | 0 | 1 (14) | 1 (4) | 8 (32)b | 9 (18) | 10 (18) |
| Other | 0 | 0 | 0 | 0 | 2 (8)b | 2 (4) | 2 (4) |
| Clinical stage | |||||||
| IV | 5 (83) | 1 (100) | 6 (86) | 23 (96) | 24 (96) | 47 (96) | 53 (95) |
| Undetermined | 1 (17) | 0 | 1 (14) | 1 (4) | 1 (4) | 2 (4) | 3 (5) |
| HER2 status | |||||||
| IHC 3+ | 3 (50) | 0 | 3 (43) | 7 (29) | 6 (24) | 13 (27) | 16 (29) |
| IHC 2+/FISH + | 0 | 0 | 0 | 3 (13) | 4 (16) | 7 (14) | 7 (13) |
| IHC 2+/FISH - | 2 (33) | 1 (100) | 3 (43) | 8 (33) | 11 (44) | 19 (39) | 22 (39) |
| IHC 2+/FISH undertermined | 0 | 0 | 0 | 0 | 1 (4) | 1 (2) | 1 (2) |
| IHC 1+ | 1 (17) | 0 | 1 (14) | 6 (25) | 3 (12) | 9 (18) | 10 (18) |
| PD-L1 status | |||||||
| <1 | 4 (67) | 1 (100) | 5 (71) | 5 (21) | 6 (24) | 11 (22) | 16 (29) |
| ≥1 | 1 (17) | 0 | 1 (14) | 10 (42) | 1 (4) | 11 (22) | 12 (21) |
| Undetermined | 1 (17) | 0 | 1 (14) | 9 (38) | 18 (72) | 27 (55) | 28 (50) |
| MSI status | |||||||
| MSS | 6 (100) | 1 (100) | 7 (100) | 18 (75) | 12 (48) | 30 (61) | 37 (66) |
| MSI-L | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MSI-H | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Undetermined | 0 | 0 | 0 | 6 (25) | 13 (52) | 19 (39) | 19 (34) |
| Subsequential treatments | |||||||
| None | 1 (17) | 1 (100) | 2 (29) | 3 (13) | 3 (12) | 6 (12) | 8 (14) |
| Chemotherapy | 5 (83) | 0 | 5 (71) | 2 (8) | 6 (24) | 8 (16) | 13 (23) |
| Antiangiogenic drugs | 1 (17) | 0 | 1 (14) | 7 (29) | 5 (20) | 12 (24) | 13 (23) |
| Anti-HER2 treatment | 0 | 0 | 0 | 1 (4) | 3 (12) | 4 (8) | 4 (7) |
| Immunotherapy | 2 (33) | 0 | 2 (29) | 2 (8) | 1 (4) | 3 (6) | 5 (9) |
| Radiotherapy | 1 (17) | 0 | 1 (14) | 1 (4) | 2 (8) | 3 (6) | 4 (7) |
| Hormone therapy | 0 | 0 | 0 | 0 | 1 (4) | 1 (2) | 1 (2) |
| Others | 1 (17) | 0 | 1 (14) | 3 (13) | 3 (12) | 6 (12) | 7 (13) |
| NA | 0 | 0 | 0 | 9 (38) | 10 (40) | 19 (39) | 19 (34) |
Data are median (IQR) or n (%). ECOG = Eastern Cooperative Oncology Group. IRC = independent review Committee. HER2 = human epidermal receptor 2. IHC = immunohistochemistry. FISH = fluorescence in-situ hybridisation. MSI = Microsatellite Instability. MSS = Microsatellite stable. NA = not available. PD-L1 = Programmed death-ligand 1. CPS = combined positive score.
There is one case of gastric and intestine.
Five patients with breast cancer had previously received both trastuzumab and pyrotinib.
For the dose-escalation part of the study, 6 patients were enrolled and received the study drug following a “3 + 3” design. A total of three patients each initially received 2.0 or 2.5 mg/kg of RC48 plus 3 mg/kg of toripalimab; no DLT effects occurred for either of the two doses. In the dose-expansion stage, we predesigned two cohorts based on tumour types to investigate the preliminary efficacy in patients with GC and other solid tumours. Considering that the 3 + 3 sample size for the dose-escalation stage (n = 6) was too small to determine the RP2D, we started dose expansion in the two cohorts with two dose levels simultaneously. Patients in the two cohorts were alternatively allocated to receive RC48 dose levels of 2.0 mg/kg and 2.5 mg/kg. As expansion of the two cohorts proceeded, we performed analyses when seven patients were enrolled for each of the two dose levels (for dose escalation, N = 3 for 2.0 mg/kg dose, all with GC, N = 3 for the 2.5 mg/kg dose, 2 with GC, 1 with other solid tumour; for dose expansion, N = 6 for the GC cohort, 3 for the 2.0 mg/kg dose and 3 for the 2.5 mg/kg dose, N = 2 for the other solid tumour cohort, 1 for the 2.0 mg/kg dose and 1 for the 2.5 mg/kg dose). The ORR for the 2.5 mg/kg dose level was 2/7 while an ORR of 1/7 was found for the 2.0 mg/kg dose level. In patients with GC, the ORR was 2/5 and 1/6 at the 2.5 mg/kg and 2.0 mg/kg dose levels, respectively. Additionally, we found that both dose levels were well tolerated. Therefore, on the basis of the balance between activity and safety, dose expansion of 2.0 mg/kg RC48 was terminated, and the 2.5 mg/kg RC48 plus 3 mg/kg toripalimab dose was chosen as the recommended dose for further investigation (Fig. 1).
In total, seven patients were treated at a dosage of RC48 2.0 mg/kg q2w, six of whom were G/GEJ cancer patients and one had colorectal cancer; 49 patients were treated at a dosage of RC48 2.5 mg/kg q2w, including 24 patients with G/GEJ cancer and 25 patients with other solid tumours. Most patients failed on 1st-line therapy (n = 25, 45%), 30 (54%) had received at least two lines, and one (2%) patient had not previously received systemic therapy. HER2 expression was positive (IHC 3+ or 2+/ISH+) in 23 (41%) patients, 32 (57%) patients were had low HER2-expression (22 IHC 2+/ISH-, 10 IHC 1+), and only one (2%) patient had HER2 IHC 2+/ISH unknown. PD-L1 status were retrospectively collected from 28/56 (50%) of the patients, 12 (43%, 12/28) were positive, defined as CPS ≥1.
All enrolled patients (n = 56) had received at least one dose of RC48 plus toripalimab and were included in the safety assessment. The safety profile is summarized in Table 2. Treatment-related adverse events (TRAEs) of any grade occurred in all 56 patients, commonly including a decreased white blood cell count (n = 36, 64%), a decreased neutrophil count (n = 34, 61%), asthenia (n = 29, 52%), an increased AST level (n = 27, 48%), and an increased ALT level (n = 27, 48%). Thirty-three (59%) patients suffered grade 3 or worse TRAEs, with the most common observation being a decreased neutrophil count (n = 13, 23%), and a decreased white blood cell count (n = 7, 13%). Compared with patients with other solid tumours, we observed a much lower frequency of most TRAEs in patients with G/GEJ cancer at the recommended dose level of RC48 (Supplementary Table S1). Thirty-two patients (57%) developed immune-related adverse events (irAEs; Supplementary Table S2). The most common irAEs were rash (n = 15, 27%), increased blood creatine phosphokinase (n = 14, 25%) and hypothyroidism (n = 8, 14%). Of note, drug-related pneumonitis was reported in four patients, including three with G2 and one with G3 pneumonitis, all related to both RC48 and toripalimab. In total, 10 patients (18%) experienced treatment-related serious adverse events (TRSAE; Supplementary Table S3). TRAEs leading to drug discontinuation occurred in eight (14%) of 56 patients (including two patients due to hypoaesthesia and two patients due to infusion related reactions). There was one patient death reported due to sudden death.
Table 2.
Treatment-related adverse events.
| RC48 dose of 2.0 mg/kg (n = 7) |
RC48 dose of 2.5 mg/kg (n = 49) |
Total (n = 56) |
||||
|---|---|---|---|---|---|---|
| All grade n (%) | Grade 3–5 n (%) | All grade n (%) | Grade 3–5 n (%) | All grade n (%) | Grade 3–5 n (%) | |
| Any TRAE | 7 (100) | 5 (71) | 49 (100) | 28 (57) | 56 (100) | 33 (59) |
| White blood cell count decreased | 3 (43) | 0 | 33 (67) | 7 (14) | 36 (64) | 7 (13) |
| Neutrophil count decreased | 3 (43) | 0 | 31 (63) | 13 (27) | 34 (61) | 13 (23) |
| Asthenia | 3 (43) | 0 | 26 (53) | 5 (10) | 29 (52) | 5 (9) |
| Alanine aminotransferase increased | 3 (43) | 0 | 24 (49) | 2 (4) | 27 (48) | 2 (4) |
| Aspartate aminotransferase increased | 2 (29) | 0 | 25 (51) | 2 (4) | 27 (48) | 2 (4) |
| Hypoaesthesia | 2 (29) | 0 | 19 (39) | 4 (8) | 21 (38) | 4 (7) |
| Alopecia | 4 (57) | 0 | 15 (31) | 0 | 19 (34) | 0 |
| Constipation | 4 (57) | 0 | 14 (29) | 0 | 18 (32) | 0 |
| Decreased appetite | 2 (29) | 0 | 15 (31) | 0 | 17 (30) | 0 |
| Nausea | 4 (57) | 0 | 13 (27) | 0 | 17 (30) | 0 |
| Weight decreased | 0 | 0 | 17 (35) | 0 | 17 (30) | 0 |
| Anaemia | 3 (43) | 1 (14) | 13 (27) | 2 (4) | 16 (29) | 3 (5) |
| Blood creatine phosphokinase increased | 3 (43) | 1 (14) | 12 (25) | 0 | 15 (27) | 1 (2) |
| Pruritus | 1 (14) | 0 | 14 (29) | 0 | 15 (27) | 0 |
| Rash | 2 (29) | 2 (29) | 13 (27) | 1 (2) | 15 (27) | 3 (5) |
| Pyrexia | 2 (29) | 0 | 10 (20) | 0 | 12 (21) | 0 |
| Diarrhoea | 3 (43) | 0 | 7 (14) | 2 (4) | 10 (18) | 2 (4) |
| Vomiting | 4 (57) | 0 | 6 (12) | 0 | 10 (18) | 0 |
| Abdominal pain | 0 | 0 | 8 (16) | 0 | 8 (14) | 0 |
| Hypothyroidism | 1 (14) | 0 | 7 (14) | 0 | 8 (14) | 0 |
| Neurotoxicity | 0 | 0 | 8 (16) | 0 | 8 (14) | 0 |
| Hypokalaemia | 1 (14) | 0 | 6 (12) | 1 (2) | 7 (13) | 1 (2) |
| Hyponatraemia | 2 (29) | 0 | 5 (10) | 2 (4) | 7 (13) | 2 (4) |
| Bilirubin conjugated increased | 1 (14) | 0 | 5 (10) | 1 (2) | 6 (11) | 1 (2) |
| Infusion related reaction | 1 (14) | 0 | 5 (10) | 3 (6) | 6 (11) | 3 (5) |
This table shows all grade adverse events occurring in at least 10% of patients. TRAE = Treatment-related adverse event.
The cut-off date was Apr 28, 2023. Fifty-two of 56 patients (93%) had efficacy evaluations, including 28 patients with G/GEJ cancer and 24 patients with other solid tumours. Another four patients dropped out of the study by choice before the first efficacy evaluation. With a median follow-up of 18.4 months, 32 patients exhibited disease progression, and 28 patients died. The median treatment duration for RC48 was 12.1 (IQR 6.1–20.1) weeks and that for toripalimab was 13.4 (IQR 6.0–20.3) weeks.
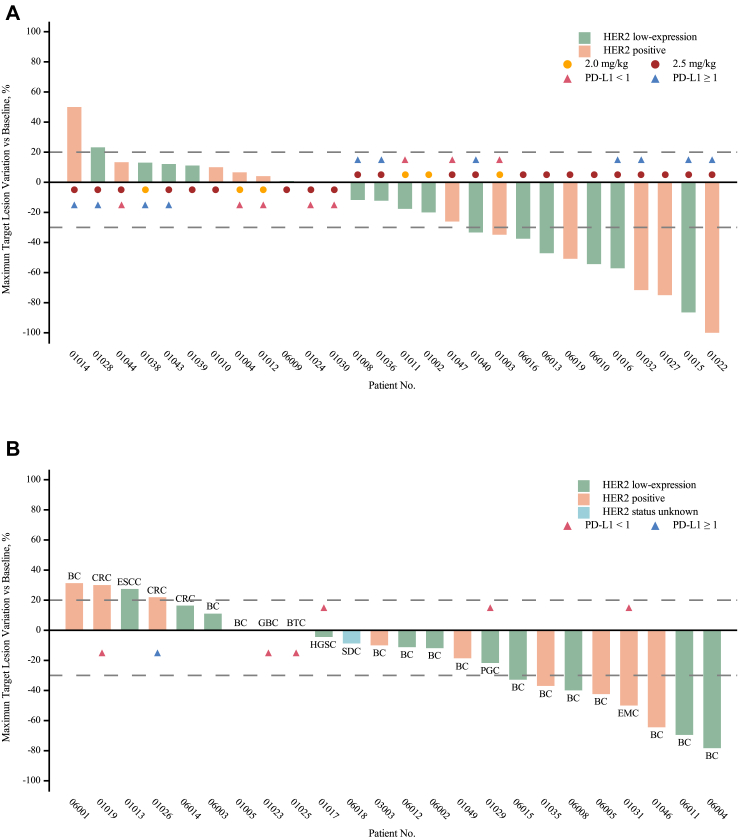
For the G/GEJ cancer cohorts, in total, 12 of the 28 evaluable participants were confirmed to have a partial response (PR; Table 3, Fig. 2A). Of them, only one patient received 2.0 mg/kg RC48, and 11 received 2.5 mg/kg RC48. The overall ORR was 43% (12/28, 95% CI 25, 63), with 17% (1/6, 95% CI 0, 64) in the 2.0 mg/kg RC48 cohort and 50% (11/22, 95% CI 28, 72) in the 2.5 mg/kg dose cohort. The DCR in the 2.0 mg/kg RC48 and 2.5 mg/kg RC48 groups was 100% (6/6, 95% CI 54, 100) and 68% (15/22, 95% CI 45, 86), respectively. In patients who received RC48 2.5 mg/kg, the mDOR was 5.4 months (95% CI 2.8, 18.8), we found that patients receiving RC48 plus toripalimab as 2nd-line therapy had a relatively longer DOR than those receiving ≥ 3rd-line (15.6 [95% CI 3.8, NE] vs. 3.6 months [95% CI 2.8, NE]), although the ORRs were equivalent between them (50% [7/14; 95% CI 23, 77]vs. 50% [4/8; 95% CI 16, 84]; Supplementary Fig. S1). Overall, the median PFS and OS were 6.2 months (95% CI 4.0, 6.9) and 16.8 months (95% CI 7.2, NE), respectively. In the 2.5 mg/kg RC48 dose group, the median PFS and OS were 5.1 months (95% CI 1.4, 7.3) and 14.0 months (95% CI 6.3, NE; Table 3), respectively. The ORR in patients with HER2-positivity and low HER2-expression was 56% (6/13; 95% CI 21, 86) and 46% (5/9; 95% CI 19, 75), respectively (Supplementary Figs. S2 and S3 and Table S4). Considering that prior anti-HER2 therapy would impact efficacy, we further compared efficacy based on previous anti-HER2 treatment. In the 2.5 mg/kg RC48 dose group, seven of 22 (32%) patients had previously received anti-HER2 therapy, six of whom were HER2-positive, and three of the seven patients (43%, 95% CI 10, 82) exhibited PR, while the ORR of patients who were anti-HER2 naïve was 53% (8/15; 95% CI 27, 79). Besides ORR, the anti-HER2 therapy-naive patients also showed longer median PFS (6.1 [95% CI 1.2, 7.3] vs. 4.2 [95% CI 0.9, 8.1] months) and median OS (24.7 [95% CI 6.1, NE] vs. 11.9 [95% CI 4.3, NE] months) than the patients previously treated with anti-HER2 therapy. In addition, the efficacy in those with G/GEJ cancer at the 2.5 mg/kg of RC48 dose level was further analysed according to PD-L1 expression (Supplementary Fig. S3 and Table S4). We retrospectively collected PD-L1 status from 14/22 (64%) of the patients. Patients with PD-L1 CPS≥1 had a relatively high ORR (50% [5/10; 95% CI 19, 81] vs. 25% [1/4; 95% CI 1, 81]), median longer PFS (6.8 [95% CI 1.1, 21.4] vs. 3.7 [95% CI 0.9, NE] months) and OS (24.7 [95% CI 5.0, NE] vs. 9.0 [95% CI 4.3, NE] months) than those with CPS<1, which is worth further exploration in the future. In the other solid tumour cohort, a total of 25 patients received the recommended dose, including 14 patients with breast cancer, and 11 patients with other solid tumours, and were evaluable for efficacy, and 24 patients had at least one assessment. In total, the confirmed ORR for the other solid tumour cohort was 25% (6/24, 95% CI 10, 47), the DCR was 75% (18/24, 95% CI 53, 90), and the mDOR was 8.2 months (95% CI 3.0, NE; Table 3, Fig. 2B). For patients with breast cancer, the ORR was 36% (5/14), the DCR was 86% (12/14). ORR in patients with HER2-positive and low HER2-expression breast cancer was 29% (2/7; 95% CI 4, 71) and 43% (3/7; 95% CI 10, 82), respectively (Supplementary Table S5). We also found that one patient with endometrial carcinoma exhibited PR, and five patients exhibited SD including one with biliary tract cancer, one with gallbladder carcinoma, one with parotid gland carcinoma, one with salivary duct carcinoma and one with high-grade serous carcinoma of the abdominal cavity (Fig. 2B, Supplementary Fig. S4).
Table 3.
Summary of response data and anti-tumour activity outcomes.
| Gastric cancer (n = 28) |
Other solid tumour (n = 24) |
|||
|---|---|---|---|---|
| RC48 2.0 mg/kg (n = 6) | RC48 2.5 mg/kg (n = 22) | Total (n = 28) | RC48 2.5 mg/kg (n = 24) | |
| Best overall response | ||||
| Complete response | 0 | 1 (5%) | 1 (4%) | 0 |
| Partial response | 1 (17%) | 10 (46%) | 11 (39%) | 6 (25%) |
| Stable disease ≥6 weeks | 5 (83%) | 4 (18%) | 9 (32%) | 12 (50%) |
| Progressive disease | 0 | 7 (32%) | 7 (25%) | 6 (25%) |
| Objective response rate, % (95% CI) | 1/6 (17%; 0, 64) | 11/22 (50%; 28, 72) | 12/28 (43%; 25, 63) | 6/24 (25%; 10, 47) |
| Disease control rate, % (95% CI) | 6/6 (100%; 54, 100) | 15/22 (68%; 45, 86) | 21/28 (75%; 55, 89) | 18/24 (75%; 53, 90) |
| Progression-free survival, months | ||||
| Median (95% CI) | 6.8 (4.0, NE) | 5.1 (1.4, 7.3) | 6.2 (4.0, 6.9) | 4.9 (2.3, 9.7) |
| Overall survival | ||||
| Median (95% CI) | 16.8 (6.8, NE) | 14.0 (6.3, NE) | 16.8 (7.2, NE) | 10.5 (9.4, 17.7) |
| Duration of response | ||||
| Median (95% CI) | 5.1 (NE, NE)a | 5.4 (2.8, 18.8) | 5.1 (2.8, 18.8) | 8.2 (3.0, NE) |
Data are n/n, n (%) or median (95% CI). Tumour response was assessed by investigators. NE = not evaluable.
Only one patient had response.
Fig. 2.
Waterfall plot in patients treated with RC48 plus toripalimab. (A) Waterfall plot for patients with G/GEJ cancer. (B) Waterfall plot for patients with other solid tumours, all patients received the dose of RC48 2.5 mg/kg. Dashed lines at −30% or 20% indicate the minimum change in tumour size for a partial response or progression disease respectively by RECIST 1.1. BC = breast cancer. BTC = biliary tract cancer, CRC = colorectal cancer, EMC = endometrial carcinoma, ESCC = oesophageal cancer, GBC = gallbladder carcinoma, HGSC = high grade serous carcinoma, PGC = parotid gland carcinoma, SDC = salivary duct carcinoma. RECIST = Response Evaluation Criteria in Solid Tumours. G/GEJ = gastric or gastroesophageal junction.
Intensive blood sampling was performed for PK analysis to measure the ADC, total antibody (TAb), free MMAE and JS001 in 11 patients. The mean serum concentrations of ADC, TAb, free MMAE and JS001 vs. time curves of patients who received RC48 at 2.0 and 2.5 mg/kg are shown in Supplementary Fig. S5. The PK characteristics of ADC and TAb showed that dose-proportional increases in both exposures and Peak Concentration (Cmax) at doses of 2.0 and 2.5 mg/kg. At the evaluated dose levels of 2.0 and 2.5 mg/kg, the serum concentrations of free MMAE for each dose group showed a low exposure level with a median Tmax of 48 h after the start of infusion. No evidence of accumulation in serum for ADC, TAb and free MMAE was observed after repeated dosing of the RC48-ADC drug at 2.0 and 2.5 mg/kg q2w. For JS001, the Cmax of JS001 was reached at the end of infusion (∼1.5 h) in combination with 2.0 and 2.5 mg/kg RC48. The PK characteristics of JS001 were similar in combination with 2.0 and 2.5 mg/kg RC48.
Discussion
This phase I study, to our knowledge, is the first to confirm the synergy of HER2-targeted ADC and blockade of PD-1 in G/GEJ cancer in a clinical trial, our findings show that the addition of toripalimab to RC48 results in a clinically meaningful improvement in efficacy in a subset of patients.
In the efficacy analysis, the confirmed ORR reached 50% in patients with G/GEJ cancer who received the RP2D, which is a clinically meaningful improvement compared with RC48 monotherapy. On the one hand, we need to note that in this study, more than half of the patients with G/GEJ cancer received only 1st-line treatment, which may have resulted in a higher ORR compared with historical data. On the other hand, the synergistic efficacy observed in our present study is consistent with several studies reported previously involving other anti-HER2 therapies and PD-1 inhibitors.7,18,19 Mechanically, HER2-positive G/GEJ cancer is a distinct tumour subtype that is characterized by a relatively active immune microenvironment compared with HER2-negative G/GEJ cancers.5,6 We speculate that the benefit from ADCs in combination with immunotherapy is considered to be dependent on preexisting immunity.9 This may explain why no discernible benefit with the addition of a PD-1 inhibitor compared with anti-HER2 ADC monotherapy was observed for HER2-positive breast cancer, both in our study and in a previous report on T-DM1.20
Another important finding in this study suggests that low HER2-expression patients with G/GEJ cancer also achieved an ORR of 46%, which is very encouraging compared to the currently available treatment regimen for this population. Mechanically, RC48 contains the novel humanized anti-HER2 antibody hertuzumab conjugated to monomethyl auristatin E (MMAE) via a cleavable linker. Compared to trastuzumab, hertuzumab has a higher affinity for HER2. The bystander effect of RC48 has been demonstrated in accumulating data, including preclinical models and clinical studies.15,21 Therefore, it is reasonable for HER2 IHC 2+/ISH- and HER2 IHC 1+ patients to benefit from this combination therapy. In addition, most of patients with low HER2-expression have not previously received anti-HER2 therapy, which may also have an impact on the efficacy to some extent. Furthermore, the efficacy results may be influenced by other factors, including tumour heterogeneity, changes in HER2 expression over time, and differences between local and central assessments of HER2 IHC status. Further studies with rebiopsy and central laboratory assessment will be needed to confirm the beneficial population. Finally, previous research demonstrated distinct TIME profiles with higher immunogenic profiles in HER2-low patients than in HER2-high patients,22 which may also help explain the efficacy we observed in patients with low HER2 expression using HER2-targeted ADC and immunotherapy combinations. The association between PD-L1 expression, a modest biomarker of PD-1 inhibitors and efficacy was also analysed in our study.23,24 In gastric cancer, we found that patients with PD-L1 positivity had a relatively higher ORR, longer median PFS and OS. However, we also need to notice that about 50% of PD-L1 status were missing in this study and the result should be interpreted carefully due to the limited samples. If confirmed, this exploratory observation may be helpful for patient selection in clinical trials in the future.
In addition to G/GEJ cancer and breast cancer, ADC drugs are beneficial for multiple other HER2-associated cancers.25 In our present study, meaningful and durable clinical responses (including CR, PR and SD for more than 6 months) were also observed across multiple HER2-expressing tumour types, including salivary gland cancer, endometrial cancer, and cholangiocarcinoma. Anti-HER2 therapy represents a therapeutic option for a small subgroup of these tumours.26,27 With the era of molecular typing, we consider this combination regimen to have great pantumour potential. Based on the preliminary promising efficacy in this pantumour cohort, the study protocol has been modified, and patients with other solid tumours, including cholangiocarcinoma and NSCLC, are continuing to be enrolled.
The combination of RC48 with toripalimab did not result in any unexpected adverse events. The patients with G/GEJ cancer tolerated the treatment better than those with other solid cancers, which is likely because most patients with G/GEJ cancer enrolled in the study received only 1st-line systemic therapy previously. Of note, treatment with RC48 monotherapy was reported with no lung toxicity in previous clinical trials. In this combination study, however, four patients (7%) had treatment-related pneumonitis including three patients with grade 2 pneumonitis and one patient with grade 3 pneumonitis. The potential lung damage induced by this combination warrants extensive caution.
Analysis of PK properties showed similar PK characterization of RC48 with monotherapy.11 The PK profile of JS001 was also similar between monotherapy13 and combination therapy.
This study had several limitations. In our trial, patients were recruited according to their documented HER2, which can dynamically change during cancer progression. Thus, this may interfere with the determination of the correlation between biomarkers and efficacy. Additionally, this study is limited by the small sample size. The promising antitumour outcome needs to be further confirmed in future clinical trials.
To our knowledge, this is the first study highlighting the potential for combining PD-1 inhibitors with HER2-targeted ADCs to treat patients with HER2-positivity or low HER2-expression G/GEJ cancer. ADCs are among the fastest growing drug classes in the HER2-positive G/GEJ cancer field. The synergistic efficacy of ADC combination therapy is being assessed for many types of cancers. The findings of our phase 1 clinical trial support further investigation of HER2-targeted ADC plus immunotherapy strategies for treating HER2-expressing G/GEJ cancer and other cancers in the future.
Contributors
LS, JG, and XZ were responsible for trial conception, design and protocol written. YW, AW, JW, ZP, XW, JZ, CQ, DL, JL, ML, ZL, YC, JY, and RZ recruited patients and collected data. YW, LS and JF did the statistical analysis. YW draft the manuscript and prepared the figures. JG and LS revised it. All authors confirm that they had full access to all the data in the study. JG, LS, XZ and JF verified all study data. LS and XZ had final responsibility for the decision to submit for publication.
Data sharing statement
The study protocol is provided in the Supplementary appendix. The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.
Declaration of interests
All the other authors declare no conflicts of interest.
Acknowledgements
This study was funded by Beijing Municipal Medical Research Institutes, Beijing Medical Research Institute (Z200015). All the coauthors would like to thank the patients and their families, the study investigators, coordinators, and research staff. RC48 was provided by Remegen Co., Ltd. Toripalimab was provided by Shanghai Junshi Biosciences Co., Ltd.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102415.
Contributor Information
Xiaotian Zhang, Email: zhangxiaotian@bjmu.edu.cn.
Lin Shen, Email: shenlin@bjmu.edu.cn.
Appendix ASupplementary data
References
- 1.Peng Z., Liu T., Wei J., et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun. 2021;41(11):1173–1182. doi: 10.1002/cac2.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shitara K., Bang Y.J., Iwasa S., et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–2430. doi: 10.1056/NEJMoa2004413. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y., Qiu M.Z., Wang J.F., et al. Phase 1 multicenter, dose-expansion study of ARX788 as monotherapy in HER2-positive advanced gastric and gastroesophageal junction adenocarcinoma. Cell Rep Med. 2022;3(11) doi: 10.1016/j.xcrm.2022.100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuentes-Antrás J., Genta S., Vijenthira A., Siu L.L. Antibody-drug conjugates: in search of partners of choice. Trends Cancer. 2023;9(4):339–354. doi: 10.1016/j.trecan.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y., Jia K., Sun Y., et al. Predicting response to immunotherapy in gastric cancer via multi-dimensional analyses of the tumour immune microenvironment. Nat Commun. 2022;13(1):4851. doi: 10.1038/s41467-022-32570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim R., An M., Lee H., et al. Early tumour-immune microenvironmental remodeling and response to first-line fluoropyrimidine and platinum chemotherapy in advanced gastric cancer. Cancer Discov. 2022;12(4):984–1001. doi: 10.1158/2159-8290.CD-21-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janjigian Y.Y., Kawazoe A., Yañez P., et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727–730. doi: 10.1038/s41586-021-04161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catenacci D.V.T., Kang Y.K., Yoon H.H., et al. Margetuximab with retifanlimab as first-line therapy in HER2+/PD-L1+ unresectable or metastatic gastroesophageal adenocarcinoma: MAHOGANY cohort A. ESMO Open. 2022;7(5) doi: 10.1016/j.esmoop.2022.100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber H.-P., Sapra P., Loganzo F., May C. Combining antibody–drug conjugates and immune-mediated cancer therapy: what to expect? Biochem Pharmacol. 2016;102:1–6. doi: 10.1016/j.bcp.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Tan X., Lam M.-H., Ragunathan S., Unsal-Kacmaz K., Loganzo F. Abstract 2757: antibody-drug conjugate payloads induce markers of immunogenic cell death in cancer cells. Cancer Res. 2018;78(13_Supplement):2757. [Google Scholar]
- 11.Nicolò E., Giugliano F., Ascione L., et al. Combining antibody-drug conjugates with immunotherapy in solid tumors: current landscape and future perspectives. Cancer Treat Rev. 2022;106 doi: 10.1016/j.ctrv.2022.102395. [DOI] [PubMed] [Google Scholar]
- 12.Huang L., Wang R., Xie K., et al. A HER2 target antibody drug conjugate combined with anti-PD-(L)1 treatment eliminates hHER2+ tumors in hPD-1 transgenic mouse model and contributes immune memory formation. Breast Cancer Res Treat. 2022;191(1):51–61. doi: 10.1007/s10549-021-06384-4. [DOI] [PubMed] [Google Scholar]
- 13.Iwata T.N., Ishii C., Ishida S., Ogitani Y., Wada T., Agatsuma T. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther. 2018;17(7):1494–1503. doi: 10.1158/1535-7163.MCT-17-0749. [DOI] [PubMed] [Google Scholar]
- 14.Müller P., Kreuzaler M., Khan T., et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015;7(315):315ra188. doi: 10.1126/scitranslmed.aac4925. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y., Wang Y., Gong J., et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer. 2021;24(4):913–925. doi: 10.1007/s10120-021-01168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F., Wei X.L., Wang F.H., et al. Safety, efficacy and tumour mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol. 2019;30(9):1479–1486. doi: 10.1093/annonc/mdz197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T., Yu D., Wang J., et al. 1403P Toripalimab combined with nab-paclitaxel/docetaxel as second-line treatment in patients with advanced gastric cancer: preliminary results from a single-arm, open-label phase II trial. Ann Oncol. 2021;32:S1057–S1058. [Google Scholar]
- 18.Lee K.W., Bai L.-Y., Jung M., et al. Zanidatamab (zani), a HER2-targeted bispecific antibody, in combination with chemotherapy (chemo) and tislelizumab (TIS) as first-line (1L) therapy for patients (pts) with advanced HER2-positive gastric/gastroesophageal junction adenocarcinoma (G/GEJC): preliminary results from a phase 1b/2 study. J Clin Oncol. 2022;40(16_suppl):4032. [Google Scholar]
- 19.Shen L., Gong J., Niu Z., et al. 1210P The preliminary efficacy and safety of KN026 combined with KN046 treatment in HER2-positive locally advanced unresectable or metastatic gastric/gastroesophageal junction cancer without prior systemic treatment in a phase II study. Ann Oncol. 2022;33:S1102. [Google Scholar]
- 20.Hamilton E.P., Shapiro C.L., Boni V., et al. 162O Primary analysis from DS8201-A-U105: a 2-part, open label, phase Ib trial assessing trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing advanced breast cancer. Ann Oncol. 2022;33:S196. [Google Scholar]
- 21.Chen Z., Yuan J., Xu Y., et al. From AVATAR mice to patients: RC48-ADC exerted promising efficacy in advanced gastric cancer with HER2 expression. Front Pharmacol. 2022;12 doi: 10.3389/fphar.2021.757994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alqahtani A., Baca Y., Xiu J., et al. Comparative analysis of the molecular profile and tumour immune microenvironment (TIME) of human epidermal growth factor receptor 2 (HER2) low (L)- versus high (H)-expressing gastroesophageal cancers (GEC) J Clin Oncol. 2023;41(4_suppl):287. [Google Scholar]
- 23.Wainberg Z.A., Fuchs C.S., Tabernero J., et al. Efficacy of pembrolizumab monotherapy for advanced gastric/gastroesophageal junction cancer with programmed death ligand 1 combined positive score ≥10. Clin Cancer Res. 2021;27(7):1923–1931. doi: 10.1158/1078-0432.CCR-20-2980. [DOI] [PubMed] [Google Scholar]
- 24.Xie T., Zhang Z., Zhang X., Qi C., Shen L., Peng Z. Appropriate PD-L1 cutoff value for gastric cancer immunotherapy: a systematic review and meta-analysis. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.646355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsurutani J., Iwata H., Krop I., et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 2020;10(5):688–701. doi: 10.1158/2159-8290.CD-19-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clauditz T.S., Reiff M., Gravert L., et al. Human epidermal growth factor receptor 2 (HER2) in salivary gland carcinomas. Pathology. 2011;43(5):459–464. doi: 10.1097/PAT.0b013e3283484a60. [DOI] [PubMed] [Google Scholar]
- 27.Meric-Bernstam F., Johnson A.M., Dumbrava E.E.I., et al. Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. Clin Cancer Res. 2019;25(7):2033–2041. doi: 10.1158/1078-0432.CCR-18-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.