Abstract
Aims/hypothesis
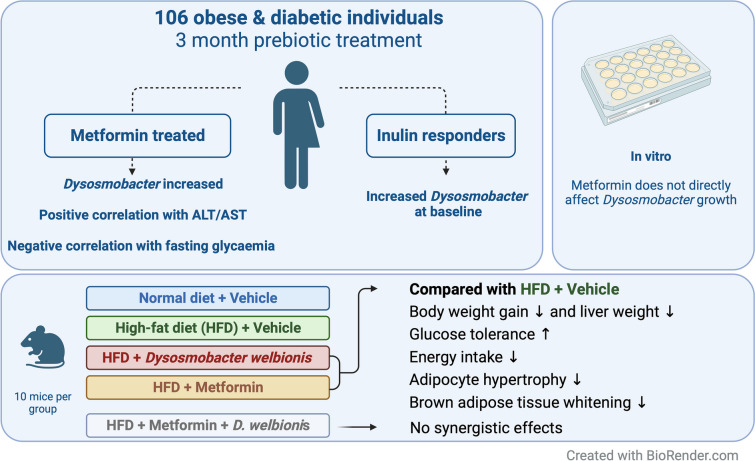
We aimed to investigate the association between the abundance of Dysosmobacter welbionis, a commensal gut bacterium, and metabolic health in human participants with obesity and diabetes, and the influence of metformin treatment and prebiotic intervention.
Methods
Metabolic variables were assessed and faecal samples were collected from 106 participants in a randomised controlled intervention with a prebiotic stratified by metformin treatment (Food4Gut trial). The abundance of D. welbionis was measured by quantitative PCR and correlated with metabolic markers. The in vitro effect of metformin on D. welbionis growth was evaluated and an in vivo study was performed in mice to investigate the effects of metformin and D. welbionis J115T supplementation, either alone or in combination, on metabolic variables.
Results
D. welbionis abundance was unaffected by prebiotic treatment but was significantly higher in metformin-treated participants. Responders to prebiotic treatment had higher baseline D. welbionis levels than non-responders. D. welbionis was negatively correlated with aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels and fasting blood glucose levels in humans with obesity and type 2 diabetes. In vitro, metformin had no direct effect on D. welbionis growth. In mice, D. welbionis J115T treatment reduced body weight gain and liver weight, and improved glucose tolerance to a better level than metformin, but did not have synergistic effects with metformin.
Conclusions/interpretation
D. welbionis abundance is influenced by metformin treatment and associated with prebiotic response, liver health and glucose metabolism in humans with obesity and diabetes. This study suggests that D. welbionis may play a role in metabolic health and warrants further investigation.
Clinical trial
Graphical Abstract
Supplementary Information
The online version contains peer-reviewed but unedited supplementary material available at 10.1007/s00125-023-06032-0.
Keywords: Adipose tissue, Dysosmobacter welbionis J115T, Glucose tolerance, Gut microbiota, Metformin, Obesity, Prebiotics, Type 2 diabetes
Introduction
In 2021, 537 million adults were living with type 2 diabetes worldwide. That same year, the IDF reported that, globally, another 541 million adults had impaired glucose tolerance and were therefore at high risk of developing type 2 diabetes [1]. With such alarming numbers, it is crucial to deepen our understanding of the physiopathology underlying this disease and to identify potential novel therapeutic targets. Among the various possible mediators, the gut microbiota is regarded as a pivotal factor influencing host physiology [2, 3]. Many observational studies are revealing various connections between the composition and/or functionality of the gut microbiota and diseases in the host [4]. Nonetheless, establishing a causal relationship for particular taxa remains challenging [5]. In the past 10 years, researchers have identified promising next-generation beneficial bacteria. Among these, the symbiotic bacterium Akkermansia muciniphila has undergone extensive investigation in preclinical research and has been recognised as a potential novel approach to address metabolic disorders and associated diseases [6]. Evidence suggests that A. muciniphila is directly influenced by different dietary regimens such as specific dietary fibres (e.g. the prebiotic inulin-type fructans) [7–9] and specific drug treatments such as the glucose-lowering drug metformin [10–13]. Inulin-type fructan treatments have been linked to changes in host metabolism (i.e. glucose and lipid metabolism and inflammation), gut microbiota and specific metabolites (including short-chain fatty acids [SCFAs]) [14–17]. We recently identified a novel butyrate-producing bacterium which we named Dysosmobacter welbionis J115T [18]. Using various human cohorts representing the general population (totalling about 12,000 individuals), we found that D. welbionis was detected in about 70% of the samples screened, making it a prevalent bacterium [19]. For comparison, A. muciniphila is detected in 70–80% of the general population when using part of the same cohorts [20]. Using a cohort of participants with obesity and type 2 diabetes, we previously discovered that D. welbionis negatively correlated with BMI, fasting glucose and HbA1c. We found no direct correlations with BMI and weight in non-diabetic participants, although we showed in preclinical studies that supplementation of D. welbionis strain J115T to high-fat diet (HFD)-fed mice reduced body weight and fat mass gain. This supplementation was associated with improved glucose homeostasis and increased non-shivering thermogenesis, coupled to a higher number of mitochondria in the brown adipose tissue (BAT) [19]; however, the molecular mechanisms are still unknown. Whether specific dietary interventions or metformin treatments influence the abundance of D. welbionis also remains unknown.
In this study, we combined human intervention studies, in vitro studies and in vivo mouse experiments to (1) investigate whether individuals with obesity receiving high doses of the prebiotic inulin (16 g/d as a supplement, coupled to dietary advice to consume inulin-rich vegetables for 3 months) exhibited specific changes in faecal D. welbionis abundance compared with individuals with obesity receiving supplements of maltodextrin, a starch-derived carbohydrate with poor prebiotic capacities, and advised to consume inulin-poor vegetables; (2) examine if metformin-treated individuals have a different abundance of D. welbionis in their gut compared with non-treated individuals; and (3) compare the effects of D. welbionis J115T supplementation vs metformin administration on glucose intolerance and fat mass in mice on HFD.
Methods
For detailed methods and human and animal ethics, please refer to the electronic supplementary material (ESM) Methods.
Human cohort
The Food4Gut study was a 3-month-long, multicentric, single-blind, placebo-controlled trial. Recruitment, enrolment, randomisation, sample size determination, inclusion and exclusion criteria, and outcomes were previously described [16]. Male and female participants (106 individuals) were recruited in three university hospitals in Belgium (Cliniques universitaires Saint-Luc and Hôpital Erasme in Brussels, and Centre Hospitalier Universitaire in Liège) as previously described [14–16]. Details of the inclusion and exclusion criteria and sampling methods are shown in the ESM Methods.
Quantitative PCR of D. welbionis and total bacteria in human samples
Absolute quantification of total bacterial load and D. welbionis was performed by quantitative PCR (qPCR) using a QuantStudio3 (Applied Biosystems, the Netherlands) with SYBR Green (GoTaq qPCR mix, Promega, USA) for detection and using the QuantStudio software (version 1.4.3, Applied Biosystems, the Netherlands).
Culture and preparation of D. welbionis for mouse experiments
D. welbionis J115T was cultured anaerobically in a modified yeast extract, casitone and fatty acids (YCFA) medium supplemented with 10 g/l inositol. Cells were harvested, immediately frozen in anaerobic vials and stored at −80°C.
Mouse experiments
Mouse model
Sets of 7-week-old C57BL/6J male mice (Janvier Laboratories, Le Genest-Saint-Isle, France) were housed in pairs in SOPF (specific opportunistic and pathogen free) conditions, in a controlled environment with free access to sterile (irradiated) food and sterile (autoclaved) water.
A set of 50 mice was randomly divided into five groups of ten mice (housed two per cages). The mice were fed either control diet (CT) (AIN93Mi) or HFD (D12492, Research Diets, USA), with a daily oral supplementation of D. welbionis J115T, metformin, D. welbionis J115T + metformin or vehicle solution. The treatment continued for 10 weeks. Mice were killed and dissected by experimenters masked to the treatment groups after a 6 h fasting period.
OGTT
One week before the end of the experiment, fasted mice were given an oral gavage glucose load. Blood glucose was regularly measured 30 min before to 2 h after oral glucose load. Plasma insulin concentration was also measured.
Mouse tissue sampling
Blood samples and tissue samples (liver, BAT, subcutaneous adipose tissue, mesenteric adipose tissue) were dissected and stored at −80°C for further analysis. Part of the adipose tissue was fixed for histological analysis.
The insulin resistance index
The insulin resistance index was determined by multiplying the area under the curve (from −30 to 15 min) of blood glucose and plasma insulin obtained during the OGTT.
Mouse histological analyses
Brown and white adipose tissues were fixed in 4% paraformaldehyde for 24 h at room temperature. Samples were then processed for paraffin embedding and stained for further analysis using a Panoramic ScanII slide scanner (3DHistech, Hungary) and visualised with the Cytomine web platform (https://cytomine.com, Belgium).
Gene expression analysis by real-time qPCR
Real-time PCR was performed with the StepOnePlus real-time PCR system and software (Applied Biosystems, Den Ijssel, the Netherlands) using GoTaq qPCR mix (Promega, USA) for detection, according to the manufacturer’s instructions. Primer sequences for the mouse genes are shown in ESM Table 1.
Mouse plasma multiplex analysis
Portal vein plasma levels of total glucagon-like peptide-1 (GLP-1), peptide YY (PYY), ghrelin, leptin and glucagon were measured by multiplex assay kits based on chemiluminescence detection, following the manufacturer’s instructions (Meso Scale Discovery [MSD], Rockville, MD, USA). Analyses were performed using a QuickPlex SQ 120 instrument (MSD) and DISCOVERY WORKBENCH 4.0 software (MSD).
Animal ethics
All mouse experiments were approved by the Ethical Committee for Animal Care of the Health Sector of the Université catholique de Louvain (UCLouvain) and were performed in accordance with the guidelines of the Local Ethics Committee and in accordance with the Belgian Law of 29 May 2013 regarding the protection of laboratory animals (agreement number LA1230314).
GLP-1 stimulation on murine L cells (GLUTag cells)
GLUTag cells (donated by D. Drucker [University of Toronto]) were plated on 1% Matrigel-coated 24-well plates. D. welbionis J115T cells were suspended in the cell line medium at increasing concentrations and incubated in the presence of dipeptidyl peptidase IV (DPP-IV) inhibitor. Total GLP-1 levels in collected supernatants and cell lysates were measured using an MSD ELISA kit.
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 9.4.0 (GraphPad Software, Boston, MA, USA, http://www.graphpad.com/) and RStudio program (RStudio 2022.07.1 for MacOS, PBC, Boston, MA, USA, http://www.rstudio.com/). Details about the statistical test used can be found in the figure legends. Data are presented as mean±SEM. Outliers were tested and removed using the ROUT test when p<0.01 (Prism 9.4v).
Results
D. welbionis is not affected by prebiotic treatment but is highly abundant in metformin-treated individuals
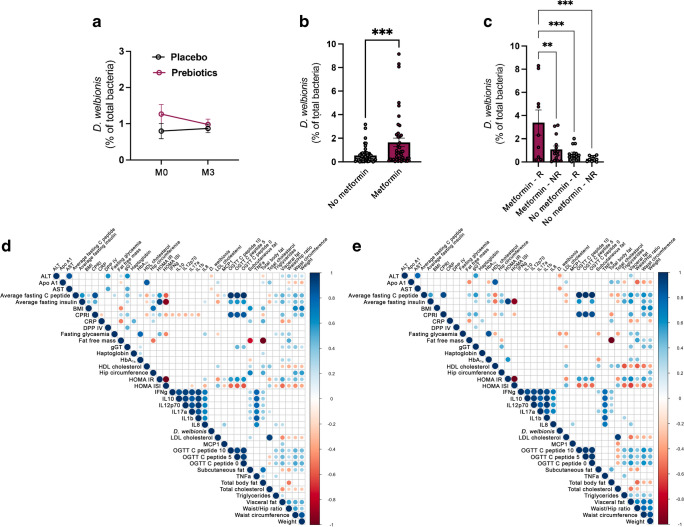
In total, 106 participants (placebo n=55, prebiotics n=51) were included in the analysis. As previously reported, the groups were similar in terms of age, sex, weight and BMI at baseline [16]. We first aimed to identify whether the abundance of D. welbionis was affected by treatment with prebiotics. We found that D. welbionis was unaffected by the dietary intervention (Fig. 1a).
Fig. 1.
D. welbionis levels are not affected by prebiotics but are higher in metformin-treated individuals and responders. (a) Faecal D. welbionis levels (% of total bacteria) before (M0) and 3 months after (M3) supplementation with prebiotics or a placebo. (b) Faecal D. welbionis levels (% of total bacteria) at baseline in participants treated with metformin (metformin) or not (no metformin). (c) Faecal D. welbionis levels (% of total bacteria) at baseline in participants treated or not with metformin and responders (R) or non-responders (NR) to the prebiotic treatment for BMI decrease. (d) Correlation analysis between anthropometric and metabolic variables and D. welbionis measured in the gut microbiota of all individuals included in the cohort prior intervention (baseline, M0). (e) Correlation analysis between anthropometric and metabolic variables and D. welbionis measured in the gut microbiota of metformin-treated individuals included in the cohort prior intervention (baseline, M0). Results are presented as bar plots with mean±SEM for parts (a), (b) and (c). Data were analysed using unpaired t test for part (b) and one-way ANOVA followed by Tukey’s post hoc test for part (c). **p<0.01; ***p<0.001. For parts (d) and (e), the presence of a circle indicates that the correlation is significant, p<0.05 (Spearman’s correlation test). The circle size relates to the absolute value of correlation. The bigger the circle size, the higher the correlation. Blue indicates positive correlations and red negative correlations. Apo, apolipoprotein; CPRI, C-peptide to glucose ratio; CRP, C-reactive protein; DPP IV, dipeptidyl peptidase IV; gGT, γ-glutamyl transferase; MCP1, monocyte chemoattractant protein 1
Given that metformin has previously been shown to be a confounding factor when assessing gut microbiota composition in type 2 diabetic individuals [10–13], we analysed the baseline data separately in metformin-treated (metformin) and metformin-naive (no metformin) participants [16]. Of 106 individuals, 59 were metformin-naive (prebiotics n=27, placebo n=32) and 47 were metformin-treated (all diabetic, prebiotics n=24, placebo n=23). Using this stratification, we discovered that metformin-treated individuals had significantly higher levels of D. welbionis in their faeces (Fig. 1b) at baseline, strongly suggesting that the abundance of D. welbionis is influenced by this glucose-lowering drug.
D. welbionis at baseline is more abundant in individuals responding to prebiotic treatment
In our previous study, we observed that prebiotic treatment did not result in a reduction of BMI for all individuals, indicating individual variability in the response to this intervention [14]. After 3 months of prebiotic treatment, some participants exhibited a significant decrease of 2 BMI units (responders) whereas others showed no change (non-responders). We formerly linked this observation with a specific composition of the gut microbiota at baseline and were able to confirm a causal role for the microbiota in a mouse model (transplantation of the gut microbiota from individuals with obesity to HFD-fed mice) [14]. However, at the time of these analyses, D. welbionis was still unknown. In this study, we investigated whether the abundance of D. welbionis could be linked with the status of responders vs non-responders to the prebiotic treatment on BMI decrease. Strikingly, we found that individuals who responded to the prebiotic treatment had almost three times higher faecal levels of D. welbionis at baseline. This effect persisted even after stratification of individuals by metformin treatment. Thus, non-responders had significantly lower D. welbionis in their gut at baseline, a difference that was even more pronounced when comparing metformin-treated vs non-metformin-treated individuals (Fig. 1c).
D. welbionis is negatively correlated with AST, ALT and fasted blood glucose levels in humans with diabetes and obesity
At baseline, we found that D. welbionis was significantly inversely correlated with alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the entire cohort, but not with any other variables (Fig. 1d). When considering metformin treatment, this correlation was lost in the non-metformin group, whereas in the metformin group D. welbionis was not only significantly inversely correlated with ALT and AST, but also inversely correlated with fasted blood glucose levels (Fig. 1e).
Metformin does not directly affect growth of D. welbionis J115T in vitro
Taken together, these observations suggest that D. welbionis is strongly influenced by metformin use. Metformin has been shown to alter the composition and metabolic activity of gut microbiota, with some bacteria being able to utilise it as a carbon source and grow in its presence [21]. Therefore, we explored in vitro whether metformin directly influences the growth of D. welbionis J115T. We found that the growth curves of D. welbionis J115T were affected by the presence or absence of its unique carbon source (i.e. inositol [18]) but not by metformin (ESM Fig. 1). These data suggest that the increase in D. welbionis observed in human gut is likely explained by a specific interaction in a complex environment and not by the direct utilisation of metformin as an energy source.
Metformin and D. welbionis J115T reduce diet-induced obesity, fat mass accumulation and liver weight without synergistic effects
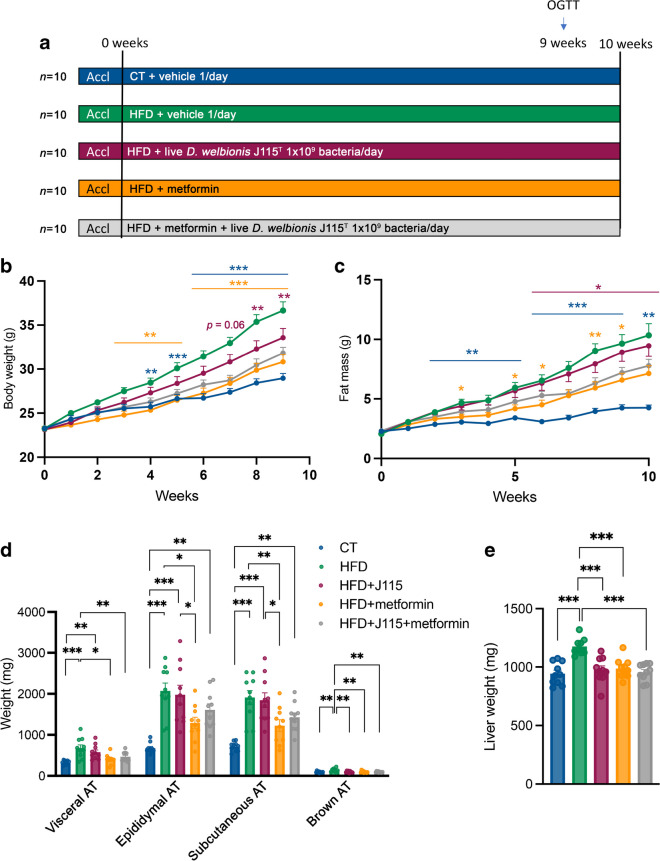
To further explore whether the effects of metformin and D. welbionis are intertwined, we designed an in vivo experiment in mice. We treated mice with HFD supplemented or not with metformin and with either D. welbionis J115T or vehicle for 10 weeks (Fig. 2a). We confirmed that the administration of D. welbionis J115T lowered HFD-induced body weight gain (Fig. 2b). Metformin was more efficient to limit HFD-induced body weight gain compared with D. welbionis J115T (Fig. 2b), and the combination of metformin + D. welbionis J115T had no additive effect. Except for a significantly lower BAT weight, the effects of D. welbionis J115T on the reduction of fat mass gain were not statistically significant, whereas fat mass was significantly reduced in metformin-treated mice and visceral fat was significantly reduced when the combination was used (Fig. 2c,d). Untreated HFD-fed mice were characterised by a 20% higher liver weight compared with CT-fed mice (Fig. 2e). Treatment with D. welbionis J115T, metformin or the combination of both significantly lowered liver weight, which was normalised and equivalent to that of control lean mice (Fig. 2e). The lower liver weight was not explained by a change in total triacylglycerol or cholesterol content in livers of treated mice (ESM Fig. 2a,b). In addition, using qPCR we measured several markers of hepatic inflammation such as monocyte chemoattractant protein 1 (MCP1), LPS binding protein (LBP) and IL-1β (ESM Fig. 3a–c). HFD-treated mice did not display specific signs of hepatic inflammation.
Fig. 2.
Metformin and D. welbionis J115T treatment reduces diet-induced obesity, fat mass accumulation and liver weight without synergistic effects. (a) Experimental design of the study. (b, c) Body weight and fat mass evolution during 10 weeks of mice being fed a normal diet (CT) or HFD, treated with either a daily oral gavage of vehicle or live D. welbionis J115T (HFD+J115) (1×109 colony forming units [CFUs]), or of HFD mice treated with metformin (HFD+metformin) or a combination of metformin and live D. welbionis J115T (HFD+J115+metformin). (d) Weight of the white adipose tissues (ATs) at the end of the 10 weeks: visceral AT, epididymal AT, subcutaneous AT and brown AT. (e) Liver weight. Number of mice per group: 9 (HFD) or 10 (other groups). Data were analysed using one-way ANOVA followed by Tukey’s post hoc test for parts (d) and (e) and two-way ANOVA followed by Tukey’s post hoc test for parts (b) and (c). *p<0.05; **p<0.01; ***p<0.001. Results are presented as bar plots with mean±SEM for parts (d) and (e). In parts (b) and (c), blue asterisks are for comparisons between the HFD and CT groups, orange asterisks are for comparisons between the HFD and HFD+metformin groups and maroon asterisks and the maroon p value are for comparisons between the HFD and HFD+J115 groups groups. Accl, acclimatisation
D. welbionis J115T improves glucose tolerance to a similar extent to metformin
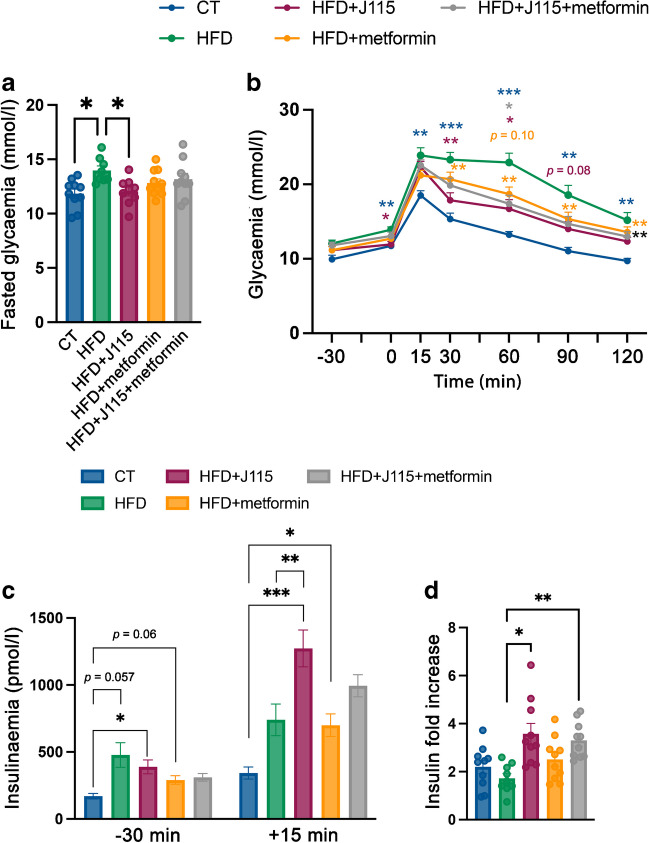
To further explore whether the lower body weight, liver mass and fat mass were associated with changes in glucose metabolism, we performed an OGTT. First, we found that D. welbionis J115T-treated mice exhibited significantly lower fasted blood glucose levels comparable to that of control lean mice, whereas this effect was not observed in metformin-treated groups (Fig. 3a). Mice receiving D. welbionis J115T had significantly reduced glycaemia and a better OGTT profile than metformin-treated mice (Fig. 3b). To further explore the mechanism by which D. welbionis J115T led to an improved glucose profile despite higher fat mass, we measured insulinaemia at baseline and 15 min after glucose administration. Fasting insulinaemia was higher in mice treated with HFD (280%), D. welbionis J115T (220%) and metformin (160%) compared with CT mice (Fig. 3c). As expected, insulin levels were significantly increased 15 min after the oral glucose challenge in CT mice (>twofold increase) and to a lower extent in HFD mice (<twofold increase), although these results were not significant (Fig. 3d). This effect was significantly higher (about 3.5-fold increase) for mice receiving D. welbionis J115T (HFD+J115 and HFD+J115+metformin). Consequently, D. welbionis J115T treatment may partially resolve HFD-induced impaired glucose tolerance by stimulating glucose-induced insulin secretion. It is noteworthy that these effects were observed despite a higher insulin resistance index in all groups except metformin-treated mice (ESM Fig. 4).
Fig. 3.
D. welbionis J115T improves glucose tolerance to a similar extent to metformin. (a) Fasted glycaemia. (b) Plasma glucose profile during a 2 h OGTT. (c) Plasma insulin levels 30 min before and 15 min after glucose administration. (d) Insulin fold increase between +15 and −30. Number of mice per group: 9–10. Data were analysed using one-way ANOVA followed by Tukey’s post hoc test for parts (a), (c) and (d). *p<0.05; **p<0.01; ***p<0.001. Data were analysed using two-way ANOVA followed by Tukey’s post hoc test for part (b). *p<0.05; **p<0.01; ***p<0.001. Blue asterisks are for comparisons between the HFD and CT groups, maroon asterisks or maroon p value are for comparisons between the HFD and HFD+J115 groups, grey asterisks are for comparisons between the HFD and HFD+J115+metformin groups, orange p value is for comparison between the HFD and HFD+metformin groups, orange asterisks are for comparisons between the metformin and CT groups and black asterisks are for comparisons between HFD+J115 and CT groups. Results are presented as mean±SEM for parts (a), (c) and (d)
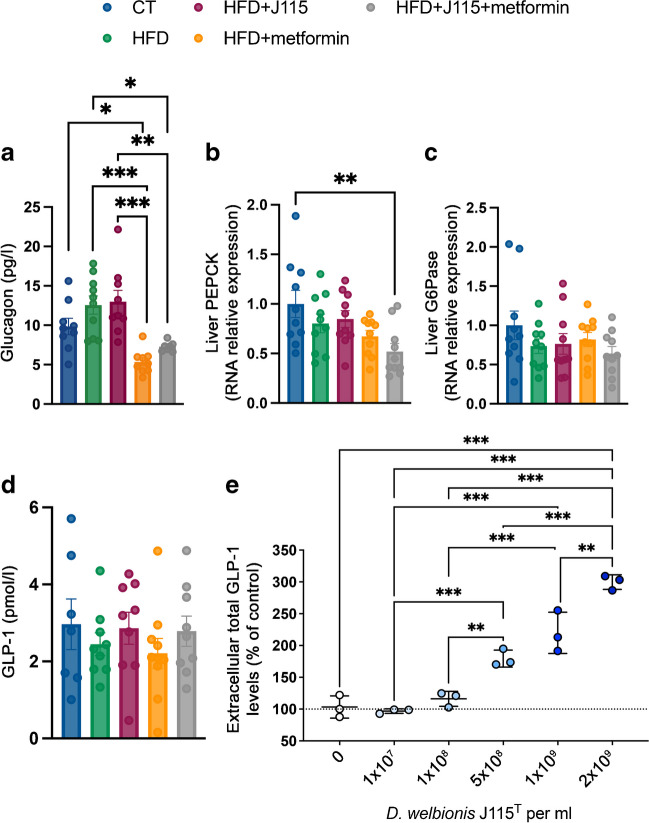
D. welbionis J115T reduces fasting glycaemia by mechanisms independent of glucagon levels and gluconeogenesis markers
To further decipher the potential mechanisms by which D. welbionis J115T acts on fasting glycaemia, glucose tolerance and insulin levels, we measured hormones involved in glucose metabolism. The lower fasting glycaemia in D. welbionis J115T-treated mice was explained neither by fasting glucagon levels (Fig. 4a) nor by the mRNA expression of key liver gluconeogenic enzymes, that is, PEPCK and glucose 6-phosphatase (G6Pase) (Fig. 4b,c, ESM Table 1). Metformin significantly lowered fasting glucagon levels in HFD mice (Fig. 4a), independently of PEPCK and G6Pase mRNA expression (Fig. 4b,c). We asked whether the higher insulin secretion upon oral glucose load is related to the incretin GLP-1. We found that portal plasma levels of GLP-1 were not significantly different between groups (Fig. 4d). However, these levels were measured in portal vein blood of fasted mice at the end of the experiment; we did not have access to the portal vein during the OGTT.
Fig. 4.
D. welbionis J115T reduces fasted glycaemia by mechanisms independent of glucagon levels and hepatic gluconeogenesis markers. (a) Portal vein glucagon levels. (b, c) mRNA expression of PEPCK and G6Pase genes relative to the control group. (d) Portal vein GLP-1 levels. (e) Effect of different doses of live D. welbionis J115T (HFD+J115) (from 1×107 to 2×109 colony forming units [CFUs] per ml) on GLP-1 stimulation in GLUTag cells (murine L cells). Data were analysed using one-way ANOVA followed by Tukey’s post hoc test for all figure parts. *p<0.05; **p<0.01; ***p<0.001. Results are presented as mean±SEM
To circumvent this, we used enteroendocrine L cells in vitro to assess whether D. welbionis J115T directly promotes GLP-1 secretion (Fig. 4e). D. welbionis J115T significantly stimulates secretion of GLP-1 in a dose-dependent manner, suggesting that the bacterium can communicate with enteroendocrine cells. It is worth noting that the levels of the active form of GLP-1 were also positively correlated with the levels of D. welbionis in the human cohort (ESM Fig. 5).
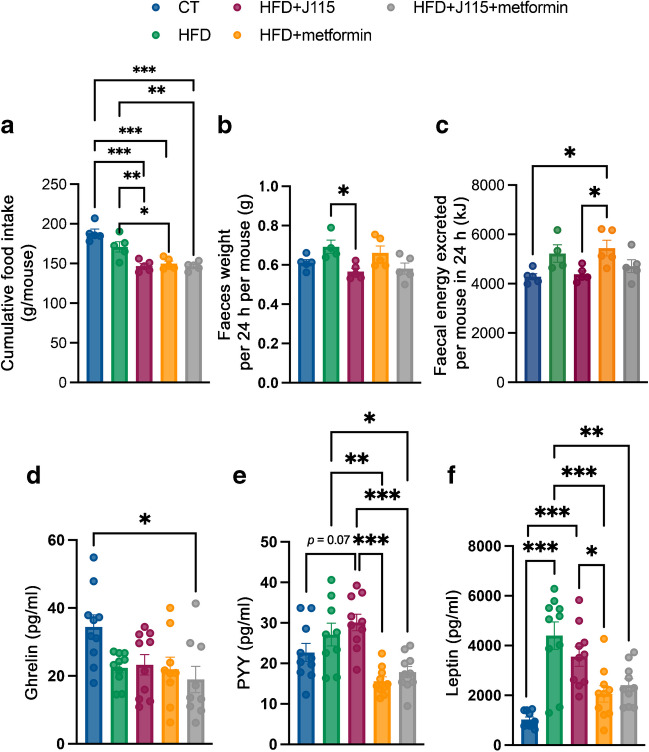
D. welbionis J115T and metformin reduce cumulative energy intake
Next, we assessed whether the observed decreases in body weight and fat mass were associated with changes in energy intake. We found that both D. welbionis J115T- and metformin-treated mice consumed less food throughout the experimental period; the combination of both did not amplify this effect (Fig. 5a). Because metformin-treated mice gained less weight and fat mass than D. welbionis J115T-treated mice despite similar energy intake, we investigated whether part of the energy consumed was excreted via the faeces. We found that D. welbionis J115T-treated mice excreted less faeces per 24 h than HFD mice (Fig. 5b), while metformin did not affect this. Using bomb calorimetry, we determined that total energy excreted in the faeces was lower in D. welbionis J115T-treated mice compared with HFD-fed metformin-treated animals (Fig. 5c). To explain the lower food intake observed in both D. welbionis J115T- and metformin-treated mice, we measured hormones involved in the regulation of appetite such as the orexigenic hormone ghrelin and anorexigenic PYY. We found that ghrelin was decreased similarly in all mice on HFD (Fig. 5d), but that PYY levels were highest in D. welbionis J115T-treated mice not receiving metformin (Fig. 5e). Leptin is considered an anorexigenic hormone but mostly reflects the adiposity of the mice. Leptin levels were significantly increased in HFD mice compared with CT. Metformin reduced leptin levels, while D. welbionis J115T did not (Fig. 5f).
Fig. 5.
D. welbionis J115T and metformin treatment reduces cumulative energy intake and changes gut peptides involved in appetite regulation. (a) Cumulative food intake. (b) Total faeces weight per 24 h per cage of two mice. (c) Faecal energy excreted per 24 h measured using bomb calorimetry. (d) Portal vein ghrelin levels. (e) Portal vein PYY levels. (f) Portal vein leptin levels. Number of mice per group: 9–10. Data were analysed using one-way ANOVA followed by Tukey’s post hoc test. *p<0.05; **p<0.01; ***p<0.001. Results are presented as mean±SEM
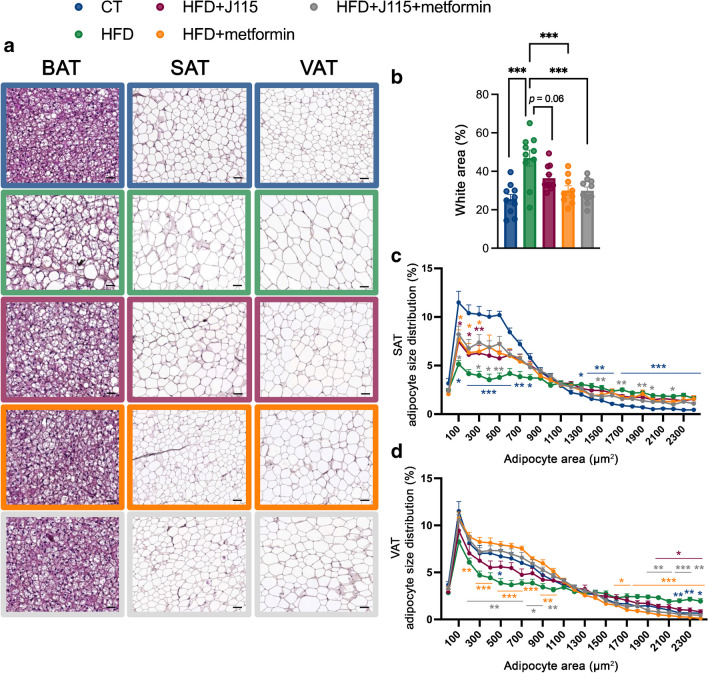
D. welbionis J115T abolishes HFD-induced adipocyte hypertrophy and whitening of BAT
We found previously that D. welbionis J115T decreases whitening of BAT, thereby contributing to a better functionality of BAT and to oxidation of nutrients [19]. Here, D. welbionis J115T also reduced whitening of BAT, but this did not reach significance (p=0.06) (Fig. 6a,b). D. welbionis J115T does not decrease fat mass to the same extent as metformin (Fig. 2c,d). Despite this limited impact on total fat mass, it had major effects on fasting glycaemia, OGTT glycaemic profile and insulin secretion. Given that smaller adipocytes are known to be more insulin sensitive and to contribute to lower glucose levels compared with hypertrophic adipocytes [22, 23], we measured mean adipocyte size as well as the adipocyte size distribution in subcutaneous and visceral (white) adipose tissues. We found that HFD mice had more large adipocytes and fewer small adipocytes in both white fat depots when compared with CT mice (Fig. 6a,c,d), suggesting that HFD-induced fat cell hypertrophy. Conversely, despite harbouring almost the same total fat mass as HFD mice (Fig. 2b), D. welbionis J115T-treated mice had a significantly different size distribution profile compared with HFD-fed mice, with lower frequency of large adipocytes and higher occurrence of small adipocytes (Fig. 6a,c,d) in both subcutaneous and visceral fat depots. Similar profiles were observed in mice treated with metformin.
Fig. 6.
D. welbionis J115T abolishes HFD-induced adipocyte hypertrophy and the whitening of BAT. (a) Representative H&E-stained pictures of BAT and subcutaneous and visceral adipose tissues (SAT and VAT, respectively). (b) Percentage of white area on the slices, corresponding to lipid droplets, in the BAT. (c) Adipocyte diameter (μm) distribution in the SAT. (d) Adipocyte diameter (μm) distribution in the VAT. Number of mice analysed per group: 9–10. Number of pictures per animal per tissue: 5. Data were analysed using one-way ANOVA followed by Tukey’s post hoc test. *p<0.05; **p<0.01; ***p<0.001. Blue asterisks are for comparisons between the HFD and CT groups, grey asterisks are for comparisons between HFD and HFD+metformin+J115 groups, orange asterisks are for comparisons between the metformin and CT groups and maroon asterisks are for comparisons between HFD+J115 and CT groups. Results are presented as mean±SEM. SAT and VAT: scale bar, 50 µm; BAT: scale bar, 25 µm
Discussion
In this study, we present novel findings regarding the abundance of D. welbionis and its relationship with prebiotic treatment and metformin administration. In a cohort involving 106 participants with obesity, including both men and women, in which we investigated the abundance of D. welbionis in faeces before and after prebiotic treatment and in the presence or absence of metformin, we found that D. welbionis was unaffected by prebiotic treatment, but was significantly more abundant in metformin-treated participants. Additionally, D. welbionis was negatively correlated with hepatic toxicity markers in humans with diabetes and obesity, and with fasting glycaemia in individuals treated with metformin. Our study further revealed that the abundance of D. welbionis was linked to response to prebiotic treatment. Responders had nearly three times higher levels of D. welbionis in their faeces before starting the treatment, suggesting a correlation between microbiota composition and efficacy of prebiotic treatment.
In vitro experiments showed that the growth of D. welbionis J115T was not directly affected by metformin, suggesting that the increase in D. welbionis observed in the human gut is likely due to a complex microbial interaction, such as crossfeeding. To demonstrate causality, we performed in vivo experiments, showing that D. welbionis J115T reduced diet-induced obesity and liver weight. The combination of metformin and D. welbionis J115T did not show additive or synergistic effects. Nevertheless, in accordance with previous studies [19], mice treated with D. welbionis J115T exhibited an improved glucose profile which we linked to increased glucose-induced insulin secretion. The lower fasting glycaemia was explained neither by fasting glucagon levels nor by key gluconeogenic enzymes in the liver. D. welbionis J115T significantly and dose-dependently stimulated GLP-1 secretion in vitro, suggesting that the bacterium communicates with enteroendocrine cells and could thereby affect food intake. Indeed, we found that D. welbionis J115T-treated mice consumed less food. Interestingly, mice receiving metformin alone consumed equal amounts of food as D. welbionis J115T-treated mice but excreted more energy via the faeces, probably explaining some of the differences in fat mass gain. However, this is not sufficient to explain all the differences in fat mass gain, as mice receiving both treatments did not excrete more energy in kJ.
Similarly, we found in humans that D. welbionis abundance was positively correlated with PYY and GLP-1 levels (ESM Fig. 5). We previously discovered that D. welbionis J115T produces SCFAs such as butyrate and propionate. These SCFAs have been shown to stimulate the secretion of gut peptides such as PYY and GLP-1 by activating G protein-coupled receptors such as G protein-coupled receptor 41 (GPR41) and GPR43. Whether the higher GLP-1 and PYY in humans is due to a possible SCFAs–D. welbionis J115T-dependent mechanism remains speculative. Of note, in enteroendocrine cells we found a direct dose-dependent stimulation of GLP-1 secretion, suggesting that the effects could be due to bacterial compounds rather than the presence of SCFAs in the culture medium. Further investigations are needed to decipher the exact molecule or group of molecules produced by D. welbionis J115T, as previously done for other beneficial bacteria [6, 24–26].
In this study, the lower body weight gain observed in D. welbionis J115T-treated mice was not fully reflected by a lower fat mass. One possible explanation would be a difference in fat-free mass. However, we have not observed any difference between metformin- and D. welbionis J115T-treated mice for this variable (not shown). Another possible explanation could be a lower glycogen content in the bodies of D. welbionis J115T-treated mice. This hypothesis warrants further investigation.
Despite the differences in fat mass, both metformin and D. welbionis J115T significantly decreased the whitening of BAT. We previously discovered that D. welbionis J115T also increases the number and activity of mitochondria in this tissue, an effect associated with lower inflammation [19]. Although metformin and D. welbionis J115T similarly reduced the formation of white-like adipocytes in BAT, the impact on total white fat mass was discrepant between treatments. We cannot rule out that the lowering of white fat mass does not fully rely on BAT activity. For instance, metformin has been shown to modulate gut microbiota in favour of specific bacteria such as Subdoligranulum and A. muciniphila [5, 13, 27, 28]. Whether treatment with D. welbionis J115T or metformin differentially affects the gut microbiota was not explored in this study.
Besides metformin, smoking and physical activity might be additional confounding factors. In this study, no relationship between the levels of Dysosmobacter and smoking status were observed. Interestingly, in this group of individuals, a specific set of participants (n=61) were recruited from two of the three hospitals [29]. They completed an International Physical Activity Questionnaire (IPAQ) at both the beginning and the conclusion of the protocol to assess their physical activity levels throughout the study. The participants were not given any guidance to alter their physical activity habits during the research period. At the conclusion of the study, no relationship with Dysosmobacter was observed for low-, moderate- or high-intensity or overall physical activity level for the participants in either cohort (with 31 individuals in the maltodextrin group and 30 individuals in the inulin group).
Overall, this study contributes to our understanding of the relationship between gut microbiota composition and prebiotic and metformin treatments. The strengths of this study rely on the translational aspects of our work. We obtained data using in vitro and in vivo approaches in mice, humans and cell lines. Moreover, the current data highlight that specificities of the gut microbiota drive the metabolic and microbial responses to inulin.
Among the limitations, we were unable to fully dissect the direct role of metformin on Dysosmobacter levels; this will require an appropriate design using samples obtained before and after supplementation with metformin. As discussed, other possible confounding factors exist in human studies such as smoking, the use of specific drugs (e.g. metformin, proton pump inhibitors, antibiotics), physical activity, food intake and others. In this study, some of these variables were controlled either during the selection or after statistical correction. However, whether Dysosmobacter could be influenced directly by smoking status, specific drugs or dietary items other than inulin is unknown at this stage. Finally, the mouse study was performed in male mice because they are more prone to developing metabolic disorders than female mice. However, further studies should be done to delineate possible sex differences.
In conclusion, our data show that D. welbionis J115T was more potent than metformin, the gold standard comparator in the field, for glucose tolerance and had beneficial effects on insulin secretion. These findings may have implications for the development of personalised approaches for the treatment of obesity and diabetes.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BAT
Brown adipose tissue
- CT
Control diet
- GLP-1
Glucagon-like peptide-1
- G6Pase
Glucose 6-phosphatase
- HFD
High-fat diet
- MSD
Meso Scale Discovery
- PYY
Peptide YY
- qPCR
Quantitative PCR
- SCFA
Short-chain fatty acid
Acknowledgements
We thank A. Puel, H. Danthinne, M. Olivier, B. Es Saadi, L. Gesche and R. M. Goebbels (at UCLouvain, Université catholique de Louvain) for their excellent technical support and assistance. AB is a research associate and PDC is honorary research director at FRS-FNRS (Fonds de la Recherche Scientifique). We thank T. Le Roy (formerly a WELBIO post doc at UCLouvain) for help during the culture used for the GLP-1 in vitro cell assay.
Data availability
All the data contained within this article are available within the paper. All the participant characteristics and markers measured in the human trial are available in Rodriguez et al [14] and Hiel et al [16]. The trial protocol was published on protocols.io (dx.doi.org/10.17504/protocols.io.baidica6). The raw data on mice will be shared upon reasonable request: Contact Patrice D. Cani (UCLouvain, patrice.cani@uclouvain.be).
Funding
Food4Gut was supported by the competitive cluster Wagralim from Wallonia (Food4Gut 518 project, convention 1318148). NMD is a recipient of grants from the Fonds de la Recherche Scientifique (FRS-FNRS, convention PINT-MULTI R.8013.19 [NEURON, call 2019] and convention PDR T.0068.19). PDC is a recipient of grants from the FNRS (Projet de Recherche PDR-convention: FNRS T.0030.21, CDR-convention: J.0027.22, FRFS-WELBIO: WELBIO-CR-2022A-02, EOS: program no. 40007505), ARC (Action de recherche concertée: ARC19/24-096) and La Caixa (NeuroGut). The study funders were not involved in the design of the study; the collection, analysis and interpretation of data; or writing of the report; and did not impose any restrictions regarding the publication of the report.
Authors’ relationships and activities
PDC and EMdH are inventors on patent applications dealing with the use of specific bacteria and components in the treatment of different diseases. PDC was co-founder of The Akkermansia Company SA and Enterosys. The other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
All authors contributed to the design of the clinical study, collection of samples and interpretation of the results. EMdH, PDC, MVH, AMN and NMD contributed to data management and data analyses of the human study. EMdH, PDC and MVH contributed to data collection, management and data analyses of the mouse experiments. PDC and MVH wrote the first draft of the manuscript. All authors reviewed and edited the first draft of the manuscript. All authors approved the final version of the manuscript. PDC and MVH are the guarantors of this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Matthias Van Hul, Email: Matthias.vanhul@uclouvain.be.
Patrice D. Cani, Email: patrice.cani@uclouvain.be
References
- 1.International Diabetes Federation . IDF Diabetes Atlas. 10. Brussels: International Diabetes Federation; 2021. [PubMed] [Google Scholar]
- 2.de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71:1020–1032. doi: 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Hul M, Cani PD. The gut microbiota in obesity and weight management:microbes as friends or foe? Nat Rev Endocrinol. 2023;19:258–271. doi: 10.1038/s41574-022-00794-0. [DOI] [PubMed] [Google Scholar]
- 4.Cani PD. Gut microbiota - at the intersection of everything? Nat Rev Gastroenterol Hepatol. 2017;14:321–322. doi: 10.1038/nrgastro.2017.54. [DOI] [PubMed] [Google Scholar]
- 5.Van Hul M, Le Roy T, Prifti E, et al. From correlation to causality: the case of Subdoligranulum. Gut microbes. 2020;12:1–13. doi: 10.1080/19490976.2020.1849998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cani PD, Depommier C, Derrien M, Everard A, de Vos WM. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol. 2022;19:625–637. doi: 10.1038/s41575-022-00631-9. [DOI] [PubMed] [Google Scholar]
- 7.Everard A, Lazarevic V, Derrien M, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everard A, Lazarevic V, Gaïa N, et al. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. I S M E J Multidiscip J Microb Ecol. 2014;8:2116–2130. doi: 10.1038/ismej.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojo O, Wang X, Ojo OO, et al. The effect of prebiotics and oral anti-diabetic agents on gut microbiome in patients with type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials. Nutrients. 2022;14:5139. doi: 10.3390/nu14235139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Hu N. Effects of metformin on the gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2020;13:5003–5014. doi: 10.2147/DMSO.S286430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosario D, Benfeitas R, Bidkhori G, et al. Understanding the representative gut microbiota dysbiosis in metformin-treated type 2 diabetes patients using genome-scale metabolic modeling. Front Physiol. 2018;9:775. doi: 10.3389/fphys.2018.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez J, Hiel S, Delzenne NM. Metformin: old friend, new ways of action-implication of the gut microbiome? Curr Opin Clin Nutr Metab Care. 2018;21:294–301. doi: 10.1097/MCO.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez J, Hiel S, Neyrinck AM, et al. Discovery of the gut microbial signature driving the efficacy of prebiotic intervention in obese patients. Gut. 2020;9:1975–1987. doi: 10.1136/gutjnl-2019-319726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neyrinck AM, Rodriguez J, Zhang Z, et al. Prebiotic dietary fibre intervention improves fecal markers related to inflammation in obese patients: results from the Food4Gut randomized placebo-controlled trial. Eur J Nutr. 2021;60:3159–3170. doi: 10.1007/s00394-021-02484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiel S, Gianfrancesco MA, Rodriguez J, et al. Link between gut microbiota and health outcomes in inulin -treated obese patients: Lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin Nutr (Edinburgh, Scotland) 2020;39:3618–3628. doi: 10.1016/j.clnu.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 18.Le Roy T, Van der Smissen P, Paquot A, et al. Dysosmobacter welbionis gen. nov., sp. nov., isolated from human faeces and emended description of the genus Oscillibacter. Int J Syst Evol Microbiol. 2020;70:4851–4858. doi: 10.1099/ijsem.0.003547. [DOI] [PubMed] [Google Scholar]
- 19.Le Roy T, Moens de Hase E, Van Hul M, et al. Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice. Gut. 2022;71:534–543. doi: 10.1136/gutjnl-2020-323778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q, Zhang Y, Wang X, et al. Gut bacteria Akkermansia is associated with reduced risk of obesity: evidence from the American Gut Project. Nutr Metab (Lond) 2020;17:90. doi: 10.1186/s12986-020-00516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 22.Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurencikiene J, Skurk T, Kulyte A, et al. Regulation of lipolysis in small and large fat cells of the same subject. J Clin Endocrinol Metab. 2011;96:E2045–2049. doi: 10.1210/jc.2011-1702. [DOI] [PubMed] [Google Scholar]
- 24.Cani PD, Knauf C. A newly identified protein from Akkermansia muciniphila stimulates GLP-1 secretion. Cell Metab. 2021;33:1073–1075. doi: 10.1016/j.cmet.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Qian K, Chen S, Wang J, Sheng K, Wang Y, Zhang M. A β-N-acetylhexosaminidase Amuc_2109 from Akkermansia muciniphila protects against dextran sulfate sodium-induced colitis in mice by enhancing intestinal barrier and modulating gut microbiota. Food Funct. 2022;13:2216–2227. doi: 10.1039/D1FO04094D. [DOI] [PubMed] [Google Scholar]
- 26.Lenoir M, Martin R, Torres-Maravilla E, et al. Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3. Gut Microbes. 2020;12:1–16. doi: 10.1080/19490976.2020.1826748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin NR, Lee JC, Lee HY, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 28.de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, et al. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40:54–62. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez J, Neyrinck AM, Van Kerckhoven M, et al. Physical activity enhances the improvement of body mass index and metabolism by inulin: a multicenter randomized placebo-controlled trial performed in obese individuals. BMC Med. 2022;20:110. doi: 10.1186/s12916-022-02299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data contained within this article are available within the paper. All the participant characteristics and markers measured in the human trial are available in Rodriguez et al [14] and Hiel et al [16]. The trial protocol was published on protocols.io (dx.doi.org/10.17504/protocols.io.baidica6). The raw data on mice will be shared upon reasonable request: Contact Patrice D. Cani (UCLouvain, patrice.cani@uclouvain.be).