Abstract
Objective
Spinal muscular atrophy (SMA) is an autosomal recessive disorder mainly affecting the neuromuscular system, which seriously threatens the life and health of patients. But few studies have reported the acceptance rate of SMA gene screening and SMA carrier rate in China. The present study aimed to clarify the two issues in China through a retrospective analysis of 18,818 reproductive age women in Wuhan area of China.
Methods
The copy number (CN) of exons 7 and 8 in survival motor neuron 1 (SMN1) gene was detected by real-time quantitative PCR, and the results were verified by multiplex ligation-dependent probe amplification.
Results
Carrier screening was offered to 44,953 women of childbearing age in our medical center from March, 2018, to February, 2022, of whom 18,818 were enrolled in the program. A total of 336 women were identified as carriers (1.73%; 326/18,808; without fertility history of the children with SMA). Among 18,818 reproductive age women, 286 spouses (85.12%; 286/336) were successfully recalled for screening. The results showed 17 couples at high risk of having children with SMA, of whom prenatal diagnosis was implemented in 11, and 6 fetuses were identified with SMA. All the 5 pregnant women bearing the 6 SMA fetuses chose to terminate the pregnancy by artificial abortion.
Conclusion
Reproductive age women and their spouses in Wuhan area showed a positive attitude toward general screening for SMA carriers. Given the high early mortality of children with SMA, screening for SMA carriers in women of reproductive age is necessary and feasible.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02991-w.
Keywords: Spinal muscular atrophy, SMN1, Carrier screening, Prenatal genetics
Introduction
Spinal muscular atrophy (SMA) is a neurodegenerative disease characterized by progressive proximal muscle weakness and paralysis. It is the most common autosomal recessive disease with an estimated incidence of about 1 in 5000 to 1 in 10,000 in newborns and a carrier rate of 1 in 25 to 1 in 50 in the general population [1]. SMA is mainly caused by mutations of the SMN1 gene, which is located on chromosome 5q13 and identical to a centromeric pseudogene [2]. In humans, the SMN1 gene differs from the SMN2 gene by five nucleotides of exon 7. The SMN1 gene contributes to the functionality of the SMN protein. It is reported that mutations in SMN1 decrease functioning of the SMN protein, resulting in discrepant clinical severities of SMA [3, 4]. About 95% of typical SMA patients have homozygous absence of SMN1 gene, which occurs by deletion or conversion to SMN2 gene, with the absence of exon 7 or both exons 7 and 8 [5]. SMN2 is associated with variable phenotypes and severity of the disease; however, its defects alone do not seem to cause the disease [6].
According to the disease severity (maximum motor function achieved) and the onset age, SMA is divided into five subtypes [7, 8]. Type 0 SMA is characterized by antenatal onset with progressively reduced movements, or stillbirth, requiring mechanical ventilation support at birth; most patients died before 6 months of age. Type 1 SMA is another severe but the most frequent subtype that initiates within the first few months requiring aided sitting; most patients died before 2 years. Type II SMA is intermediate with an onset age of 6–18 months; patients can sit independently and have a near normal lifespan but may be perplexed by respiratory complications throughout their lives. Type III SMA is usually diagnosed after 7 or 8 years of age; most patients can stand and walk unaided, and their lifespan is usually normal. Type IV SMA onset usually occurs during adulthood at 20–30 years of age, presenting as progressive muscle weakness and ultimately being unable to walk. All five subtypes are caused by functional loss of the SMN1 gene.
Spinraza is the first pharmacologic therapy for SMA approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) [9, 10]. It has also recently been approved by the Chinese State Drug Administration and National Health Commission. When it was first introduced in China, the price was nearly 700,000 yuan per dose. In December, 2021, after its inclusion in the medical insurance list, the price was reduced to 33,000 yuan per dose. Despite the dramatic price reduction, it is still unaffordable to most families. Therefore, the current information on SMA carrier frequency becomes even more critical. In 2008, the American College of Medical Genetics and Genomics (ACMG) recommended screening for SMA carriers in the general population, regardless of ethnicity or race [11]. In 2017, the American College of Obstetricians and Gynecologists (ACOG) recommended that SMA carrier screening be offered to all pregnant women [12]. Carrier screening for SMA has been available in several countries [13–15]. However, there is limited data on China mainland.
This retrospective cohort study aimed to explore the SMA carrier rate, acceptance rate of SMA carrier screening, and regional differences in SMA prevalence by screening 18,818 reproductive-age women in Wuhan area of China.
Materials and methods
Sample collection
A total of 44,953 reproductive-age women received SMA carrier screening in the fetal medicine center at Tongji Hospital in Huazhong University of Science and Technology from March, 2018, to February, 2022. Of them, 18,818 patients and their 286 spouses were enrolled in this study for analysis. The study was approved by the Ethics Committee of the said hospital (retrospectively registered: TJ-IRB202303145). All the participants of the study provided their informed consent orally.
Genomic DNA extraction
A total of 2 ml peripheral blood was collected aseptically from each subject. When both spouses were carriers or one carrier and one patient, amniocentesis was further recommended for prenatal diagnosis at 18–24 weeks of gestation. Genomic DNA was extracted from the peripheral blood and amniotic fluid using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Real-time quantitative PCR (qRT-PCR) for SMN1 gene
qRT-PCR for SMN1 gene was performed on CFX96 Real-Time System (Bio-Rad Laboratories, USA) using the SMN1 exon deletion detection kit (Medicare Technology Co., LTD, Shanghai, China) under the conditions of 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 58 °C for 60 s. Each reaction was performed in a 20 μl volume. The data obtained were evaluated using cycle thresholds (CT). △CT and △△CT were calculated according to CT values to determine exon 7 of SMN1.
Multiple ligation-dependent probe amplification (MLPA) for SMN1 gene
The copy number (CN) variations of exons 7 and 8 of SMN1 were verified using a SALSA MLPA P460 kit (MRC-Holland, Amsterdam, the Netherlands), according to the manufacturer’s protocol. The samples were tested using the ABI 3500 genetic analyzer (ABI Corporation, USA), and the initial data were analyzed on the Coffalyser version 8.0 (MRC-Holland). The CN of the target gene segment was determined based on the relative peak ratio (RPR) of the probe.
Results
Characteristics of preconception or prenatal SMA carrier screening patients
From March, 2018, to February, 2022, a total of 44,953 reproductive age women underwent SMA screening, of whom 18,818 women accepted SMA carrier screening. The characteristics of these 18,818 women are shown in Table 1. All the participants were from mainland China. Women from 26 to 34 years of age were the most willing majority to this genetic screening (73.08%; 95% credibility interval (CI), 72.44–73.71%), and most of them were pregnant women before 14 weeks of gestational age (64.21%; 95%CI, 63.52–64.89%) (Table 1).
Table 1.
The characteristics of 18,818 reproductive age women involved in the screening program
| 2018.03–2019 | 2019–2020 | 2020–2021 | 2021–2022.03 | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 2292 | 95%CI | n = 8718 | n = 3243 | n = 4565 | n = 18,818 | ||||||||||
| Groups | n | % | n | % | 95%CI | n | % | 95%CI | n | % | 95%CI | n | % | 95%CI | |
| Age, years | |||||||||||||||
| ≤ 25 | 388 | 16.93 | 15.45–18.52 | 1303 | 14.95 | 14.22–15.71 | 390 | 12.03 | 10.96–13.19 | 417 | 9.13 | 8.32–10.01 | 2498 | 13.27 | 12.79–13.76 |
| 26–34 | 1609 | 70.20 | 68.29–72.04 | 6290 | 72.15 | 71.19–73.09 | 2412 | 74.38 | 72.85–75.85 | 3442 | 75.40 | 74.13–76.63 | 13,753 | 73.08 | 72.44–73.71 |
| ≥ 35 | 295 | 12.87 | 11.56–14.30 | 1125 | 12.90 | 12.21–13.62 | 441 | 13.60 | 12.46–14.82 | 706 | 15.47 | 14.45–16.55 | 2567 | 13.64 | 13.16–14.14 |
| Gestational age, weeks | |||||||||||||||
| Preconception | 395 | 17.23 | 15.74–18.83 | 1299 | 14.90 | 14.17–15.66 | 422 | 13.01 | 11.90–14.21 | 486 | 10.65 | 9.79–11.58 | 2602 | 13.83 | 13.34–14.33 |
| < 14 | 1306 | 56.98 | 54.94–58.99 | 5459 | 62,62 | 61.60–63.63 | 2090 | 64.45 | 62.79–66.08 | 3228 | 70.71 | 69.37–72.01 | 12,083 | 64.21 | 63.52–64.89 |
| 14–23 | 494 | 21.55 | 19.92–23.28 | 1548 | 17.76 | 16.97–18.58 | 541 | 16.68 | 15.42–18.02 | 699 | 15.31 | 14.29–16.38 | 3282 | 17.44 | 16.90–17.99 |
| ≥ 23 | 97 | 4.23 |
3.48– 5.13 |
412 | 4.73 | 4.30–5.20 | 190 | 5.86 | 5.09–6.74 | 152 | 3.33 | 2.85–3.89 | 851 | 4.52 | 4.23–4.83 |
The preconception or prenatal carrier screening rate in Wuhan area of China
Among the 18,818 childbearing-age women with the normal phenotype, 336 were identified as carriers with heterozygous deletion (CN 1) of SMN 1, with a carrier rate of 1.73% (10 carriers with a fertility history of children with SMA were excluded), and no affected women with homozygous deletion were detected (Table 2). Of the 336 carriers identified, 286 (85.12%; 95%CI, 80.92–88.53) accepted further carrier screening and the remaining 50 (14.88%) refused (Table 2). The result showed 16 heterozygous deletion carriers of SMN 1 exon 7 and one homozygous deletion patient. After providing genetic counseling services and fertility guidance to these 17 high-risk couples who may have children with SMA, 11 of the 14 pregnant couples received prenatal amniocentesis for further diagnosis. Artificial reproduction technology (ART)/preimplantation genetic testing (PGT) as pre-pregnancy screening or second-trimester amniocentesis after natural pregnancy was recommended for three couples.
Table 2.
The uptake and outcome of population-based SMA carrier screening
| Variable | 2018.03–2019 | 2019–2020 | 2020–2021 | 2021–2022.02 | Total |
|---|---|---|---|---|---|
| Total women, n | 12,688 | 15,971 | 10,664 | 14,630 | 44,953 |
| Screened women, n | 2292 | 8718 | 3243 | 4565 | 18,818 |
| Acceptance rate, % | 18.06 | 54.59 | 30.41 | 31.2 | 41.86 |
| 95% CI, % | 17.40–18.74 | 53.82–55.36 | 29.54–31.29 | 30.45–31.96 | 41.40–42.32 |
| Carriers, n | 47 | 138 | 65 | 86 | 336 |
| Carrier rate, % | 2.05 | 1.58 | 2 | 1.88 | 1.79 |
| 95% CI, % | 1.55–2.72 | 1.34–1.86 | 1.57–2.54 | 1.52–2.32 | 1.61–1.99 |
| Carriers, n (without SMA offspring in the past) | 42 | 136 | 64 | 84 | 326 |
| Carrier rate, % (without SMA offspring in the past) | 1.84 | 1.56 | 1.97 | 1.84 | 1.73 |
| 95% CI, % | 1.36–2.48 | 1.32–1.84 | 1.55–2.51 | 1.49–2.27 | 1.55–1.93 |
| Partner, n | 41 | 115 | 55 | 75 | 286 |
| Recall rate, % | 87.23 | 83.33 | 84.62 | 87.21 | 85.12 |
| 95% CI,% | 74.82–94.01 | 76.23–88.63 | 73.95–91.43 | 78.53–92.71 | 80.92–88.53 |
| Carrier couples, n | 6 | 3 | 4 | 3 | 16 |
| Homozygous partner, n | 1 | 0 | 0 | 0 | 1 |
| Carrier rate of partner, % | 14.63 | 2.7 | 5.97 | 5.17 | 5.94 |
| Carrier couples, n (without SMA offspring in the past) | 2 | 1 | 3 | 1 | 7 |
| Carrier rate of partner, % (without SMA offspring in the past) | 5.56 | 0.87 | 5.56 | 1.35 | 2.54 |
| Prenatal diagnoses, n | 4 | 1 | 3 | 3 | 11 |
| Affected cases, n | 1 | 1 | 2 (twins) | 2 | 6 |
| Pregnancies terminated, n | 1 | 1 | 2 | 2 | 6 |
| Termination rate, % | 100 | 100 | 100 | 100 | 100 |
Outcomes of the couples with positive results of SMA carrier screening
Of the 17 couples at high risk of SMA, 14 were pregnant and received regular prenatal care, of whom 11 received a further prenatal diagnosis, and the other three were non-pregnant women who are still under follow-up observation. The CNs of SMN1 of the fetuses are detailed in Table 3. Five full-term fetuses have normal phenotype, including three fetuses with one copy and two fetuses with two copies of SMN1 exon 7 or 8. One 27-week preterm infant with a normal genotype (2 copies of SMN1) had severe neonatal asphyxia at birth and was phenotypically normal at 3 years of age. The remaining fetus was confirmed as having SMA, with no copies of the SMN1 gene, and its mother was advised to terminate her pregnancy by artificial abortion. Three pregnant women who declined the prenatal diagnosis were followed up, and all of them had full-term delivery and their neonates were all with a normal phenotype.
Table 3.
Pregnant follow-up in 17 high-risk couples
| Case | Female SMN1 exon 7 |
Male SMN1 exon 7 |
Prenatal diagnosis SMN1 exon 7/8 |
Pregnancy outcome |
|---|---|---|---|---|
| P1 | 1 | 0 | / | Unpregnancy |
| P2 | 1 | 1 | / | Unpregnancy |
| P3 | 1 | 1 | / | Unpregnancy |
| P4 | 1 | 1 | / | Delivery |
| P5 | 1 | 1 | / | Delivery |
| P6 | 1 | 1 | / | Delivery |
| P7 | 1 | 1 | 0 | Terminate |
| P8 | 1 | 1 | 0 | Terminate |
| P9 | 1 | 1 | 0 | Terminate |
| P10 | 1 | 1 | 0 | Terminate |
| P11 | 1 | 1 | 0/0, 0/0 (twins) | Terminate |
| P12 | 1 | 1 | 1 | Delivery |
| P13 | 1 | 1 | 1 | Delivery |
| P14 | 1 | 1 | 1 | Delivery |
| P15 | 1 | 1 | 2 | Delivery |
| P16 | 1 | 1 | 2 | Delivery |
| P17 | 1 | 1 | 2 | Premature birth |
Note: / indicates that the prenatal diagnosis was not performed
Discussion
Acceptability for SMA carrier screening in childbearing-age women in Wuhan area of China
The aim of preconception or prenatal counseling, screening, and diagnosis is to reduce the number of children born with hereditary diseases. SMA is an autosomal recessive genetic disease caused by SMN1 mutation. Given the severe lethality and disability of SMA, the American College of Obstetricians and Gynecologists recommends that SMA carrier screening be performed in all preconception or pregnant women [12]. So far, Europe, the USA, Australia, Israel, and other Western countries have carried out SMA screening as a routine for pregnant women [16–20]. However, the prevalence information of SMA in China is unclear. Although SMA carrier screening has gradually been carried out in some provinces of China in recent years [21–23], the significance of SMA carrier screening remains poorly understood in the general population of mainland China.
In this study, we carried out a popularization education about SMA in 44,953 women of childbearing age in Wuhan area of China from 2018 to 2022, of whom 18,818 women agreed to participate in the SMA screening program with an overall acceptance rate of 41.86%, which is higher than that in China’s Taiwan but lower than that in Nanjing of China [21, 23]. However, the large population screening in the USA showed that 98.7% were screened for SMA carriers [24]. In addition, 93% of women in Israel request to be tested for SMA carriers [25]. We started the SMA screening program in 2018, and the acceptance rate peaked in 2019. But as Wuhan was hit by the COVID-19 pandemic in 2020, the SMA carrier screening acceptance decreased sharply with the sharp decrease of normal medical visits. It was surprising to find that the SMA screening acceptance rate in 2020 was still higher than that in 2018, and showed a slow but steady increase after 2020.
Of the 18,818 included subjects, 16,216 pregnant women (86.17%) participated in the screening program, and the proportion of pregnant women receiving screening continued to increase slowly during the pandemic. Most of the SMA carrier screening tests were performed on an outpatient basis. As the number of pregnant women who seek prenatal examinations on an outpatient basis was by far greater than that of reproductive-age women who seek preconception counseling, it is necessary to further design SMA carrier screening programs for childbearing-age women, and more clearly reveal the acceptance of SMA screening for pregnant and preconception women.
Initially, many women in this study denied any family history of SMA in their preconception and prenatal counseling visits and chose not to participate in SMA carrier screening, but after detailed genetic education, most women (87.31%) who participated in the screening program were confirmed as not having a family history of adverse pregnancies and birth defects (Supplement 1), indicating that improving genetic knowledge has a positive effect on the popularization of SMA screening. Overall, our findings indicate that women of childbearing age in Wuhan area of China exhibit a positive attitude toward SMA carrier screening, which lays a good foundation for implementing a comprehensive carrier screening program in this geographical region.
Acceptability of spouses to recall screening in Hubei Province
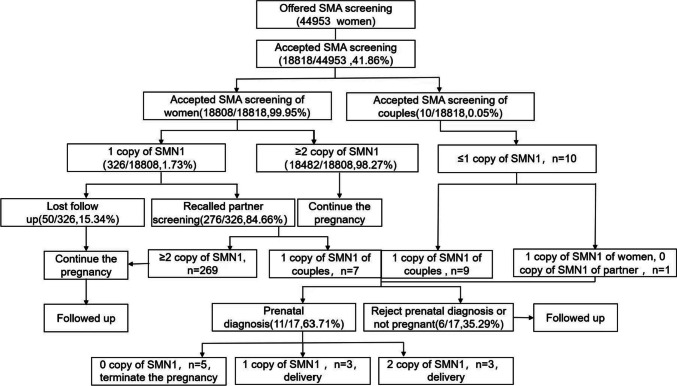
Given the genetic mechanism and serious consequences of SMA, we adopted two screening modes of informed selection in this study. One is the sequential screening model, in which women were screened individually; if a woman was suspected as a carrier, her spouse was recalled for screening; genetic counseling and prenatal diagnosis would be suggested if both spouses were carriers. The other is the simultaneous screening model, in which both couples had a reproductive or family history of SMA (Fig. 1).
Fig. 1.
Flow chart of spinal muscular atrophy (SMA) carrier screening and prenatal diagnosis
In the present investigation, 336 of the 18,818 included women were identified as SMA carriers, with an SMA carrier rate of 1.73% (10 carriers with a fertility history of children with SMA were excluded), which is consistent with the SMA carrier rates reported in other regions of China [23, 26]. In addition, we conducted a recall of spouses for carrier screening, achieving an overall recall rate of 85.12% (286/336). The rate of spouse recall from 2018 to 2021 is 87.23%, 83.33%, 83.62%, and 87.21%, respectively, showing a fluctuating trend due to the outbreak of the COVID-19 pandemic. Archibald et al. reported that 94.68% of the spouses accepted carrier screening for SMA in the Australia [14], whereas 90.01% of spouses in Taiwan requested the SMA carrier test [21].
Following genetic counseling, several couples in our study declined to undergo further screening, saying that they would not terminate a pregnancy only based on a screening result. Another reason for refusing the recall is that the women investigated were not in a pregnant state. While we acknowledge and respect individual autonomy and decision-making, we must strive to increase public awareness and understanding of SMA to mitigate the incidence of birth defects and the consequent effects on future fertility.
Acceptance of genetically imperfect offspring by high-risk couples
Among 286 male spouses recalled for screening, 16 were found to be carriers and one was found to be homozygous. In the present study, we identified a total of 16 couples with both carriers and one couple with a female carrier/male patient and further conducted detailed genetic counseling services for these 17 high-risk couples, including the genetic mechanism and the population carrier rate of SMA disease, clinical presentation and treatment, prenatal diagnosis, and recommendations for pedigree screening of SMA patients.
After receiving comprehensive genetic counseling services, the reproductive decisions of the 17 high-risk couples have undergone significant changes, particularly those with a previous history of SMA children. The birth of a child with congenital defects often imposes enormous emotional and social burdens on the parents. In this study, many parents with previous experience of SMA-related complications were unwilling to risk having a second generation of children with genetic defects. To mitigate the risk of SMA-related complications, some families opted for informed choices, such as amniotic fluid puncture during the second trimester or ART/PGT. However, some families declined amniotic fluid puncture during the second trimester, irrespective of the pregnancy outcome. However, two serious cases deserve attention. Both families had two previous births of SMA children and never received genetic counseling. In this genetic counseling, the mother in the P1 family was diagnosed as a carrier, while the father was asymptomatic. After careful consideration, the couple made an informed choice not to conceive. Both husband and wife are carriers in the P2 family. Due to personal factors, they refused to receive pregnancy follow-up. Among the seven families with no history of SMA children, most chose to receive a prenatal diagnosis. The remaining families accepted the possibility of an imperfect child and refused prenatal diagnosis. Based on the concept of medical ethics, we respect the couple’s conception of fertility and the right to make an independent choice about pregnancy. However, we also believe that to reduce major birth defects, the region needs to strengthen health education for the entire population and establish a more scientific view of family and fertility.
The limitations of our data are based on the one-center study, so the sample size is still limited. Another limitation of the study is that our test cannot detect “2 + 0” carriers and some small mutations in genes. For individuals with two or more copies of the SMN1 gene, there is still a residual risk of becoming a carrier. Therefore, families with a history of SMA fertility without detection of SMN1 gene deletion will need to further rule out the possibility of gene mutation through Sanger DNA sequencing and other methods [27].
Conclusion
This study provided SMA carrier screening for preconception and pregnant people in Wuhan area of China and preliminarily explained the acceptability, feasibility, and necessity of the screening in this area. We hope that our work can provide data support for further carrying out a large-sample, multi-center, and multi-ethnic investigation of the carrier rate in China and strengthen the publicity and popularization of SMA-related knowledge.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the individuals for participation in the screening program. We are grateful to Berry Genomics Corporation (Beijing, China) and Zhixian Zhao (Berry Genomics Corporation, Beijing, 102200, China), for their technical assistance and data analysis.
Author contribution
S.C. participated in and designed the study. Y.S, S.M, J.X, J.W, Y.W, X.S, S.L, and L.F collected medical records and interpreted data. Y.S. and S.C. drafted the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported in part by grants from the National Natural Science Foundation of China (Grant No. 81200354), the National Science and Technology Major Project of China (2017ZX09304022), and the National Key Research and Development Program of China (2018YFC1002900).
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Yanan Sun and Songyan Ma are co-first authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lunn MR, Wang CH. Spinal muscular atrophy. Lancet. 2008;371(9630):2120–2133. doi: 10.1016/S0140-6736(08)60921-6. [DOI] [PubMed] [Google Scholar]
- 2.Melki J, Lefebvre S, Burglen L, Burlet P, Clermont O, Millasseau P, Reboullet S, Bénichou B, Zeviani M, Le Paslier D, et al. De novo and inherited deletions of the 5q13 region in spinal muscular atrophies. Science. 1994;264(5164):1474–1477. doi: 10.1126/science.7910982. [DOI] [PubMed] [Google Scholar]
- 3.Lorson CL, Hahnen E, Androphy EJ, Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci USA. 1999;96(11):6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaytow H, Huang YT, Gillingwater TH, Faller KME. The role of survival motor neuron protein (SMN) in protein homeostasis. Cell Mol Life Sci. 2018;75(21):3877–3894. doi: 10.1007/s00018-018-2849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldkötter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70(2):358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calucho M, Bernal S, Alías L, March F, Venceslá A, Rodríguez-Álvarez FJ, Aller E, Fernández RM, Borrego S, Millán JM, Hernández-Chico C, Cuscó I, Fuentes-Prior P, Tizzano EF. Correlation between SMA type and SMN2 copy number revisited: an analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul Disord. 2018;28(3):208–215. doi: 10.1016/j.nmd.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Finkel R, Bertini E, Muntoni F, Mercuri E. 209th ENMC International Workshop: Outcome Measures and Clinical Trial Readiness in Spinal Muscular Atrophy 7–9 November 2014, Heemskerk, The Netherlands. Neuromuscul Disord. 2015;25(7):593–602. doi: 10.1016/j.nmd.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Wang CH, Finkel RS, Bertini ES, Schroth M, Simonds A, Wong B, Aloysius A, Morrison L, Main M, Crawford TO, Trela A. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22(8):1027–1049. doi: 10.1177/0883073807305788. [DOI] [PubMed] [Google Scholar]
- 9.Li Q. Nusinersen as a therapeutic agent for spinal muscular atrophy. Yonsei Med J. 2020;61(4):273–283. doi: 10.3349/ymj.2020.61.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu J, Wu L, Qu R, Jiang T, Bai J, Sheng L, Feng P, Sun J. History of development of the life-saving drug “Nusinersen” in spinal muscular atrophy. Front Cell Neurosci. 2022;16:942976. doi: 10.3389/fncel.2022.942976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prior TW. Carrier screening for spinal muscular atrophy. Genet Med. 2008;10(11):840–842. doi: 10.1097/GIM.0b013e318188d069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ACOG committee opinion No 2009 432: spinal muscular atrophy, Obstet Gynecol 113(5):1194 1196 [DOI] [PubMed]
- 13.Boardman FK, Sadler C, Young PJ. Newborn genetic screening for spinal muscular atrophy in the UK: the views of the general population. Mol Genet Genomic Med. 2018;6(1):99–108. doi: 10.1002/mgg3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Archibald AD, Smith MJ, Burgess T, Scarff KL, Elliott J, Hunt CE, McDonald Z, Barns-Jenkins C, Holt C, Sandoval K, Siva Kumar V, Ward L, Allen EC, Collis SV, Cowie S, Francis D, Delatycki MB, Yiu EM, Massie RJ, Pertile MD, du Sart D, Bruno D, Amor DJ. Reproductive genetic carrier screening for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy in Australia: outcomes of 12,000 tests. Genet Med. 2018;20(5):513–523. doi: 10.1038/gim.2017.134. [DOI] [PubMed] [Google Scholar]
- 15.Ross LF, Clarke AJ. A historical and current review of newborn screening for neuromuscular disorders from around the world: lessons for the United States. Pediatr Neurol. 2017;77:12–22. doi: 10.1016/j.pediatrneurol.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Boemer F, Caberg JH, Dideberg V, Dardenne D, Bours V, Hiligsmann M, Dangouloff T, Servais L. Newborn screening for SMA in Southern Belgium. Neuromuscul Disord. 2019;29(5):343–349. doi: 10.1016/j.nmd.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Miskovic M, Lalic T, Radivojevic D, Cirkovic S, Ostojic S, Guc-Scekic M. Ten years of experience in molecular prenatal diagnosis and carrier testing for spinal muscular atrophy among families from Serbia. Int J Gynaecol Obstet. 2014;124(1):55–58. doi: 10.1016/j.ijgo.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Vill K, Schwartz O, Blaschek A, Gläser D, Nennstiel U, Wirth B, Burggraf S, Röschinger W, Becker M, Czibere L, Durner J, Eggermann K, Olgemöller B, Harms E, Schara U, Kölbel H, Müller-Felber W. Newborn screening for spinal muscular atrophy in Germany: clinical results after 2 years. Orphanet J Rare Dis. 2021;16(1):153. doi: 10.1186/s13023-021-01783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aharoni S, Nevo Y, Orenstein N, Basel-Salmon L, Ben-Shachar S, Mussaffi H, Sagi-Dain L, Cohen R, Singer A. Impact of a national population-based carrier-screening program on spinal muscular atrophy births. Neuromuscul Disord. 2020;30(12):970–974. doi: 10.1016/j.nmd.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Sanchis-Juan A, French CE, Connell AJ, Delon I, Kingsbury Z, Chawla A, Halpern AL, Taft RJ, Bentley DR, Butchbach MER, Raymond FL, Eberle MA. Spinal muscular atrophy diagnosis and carrier screening from genome sequencing data. Genet Med. 2020;22(5):945–953. doi: 10.1038/s41436-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su YN, Hung CC, Lin SY, Chen FY, Chern JP, Tsai C, Chang TS, Yang CC, Li H, Ho HN, Lee CN. Carrier screening for spinal muscular atrophy (SMA) in 107,611 pregnant women during the period 2005–2009: a prospective population-based cohort study. PLoS One. 2011;6(2):e17067. doi: 10.1371/journal.pone.0017067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan V, Yip B, Yam I, Au P, Lin CK, Wong V, Chan TK. Carrier incidence for spinal muscular atrophy in southern Chinese. J Neurol. 2004;251(9):1089–1093. doi: 10.1007/s00415-004-0487-z. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Wang Y, Ma D, Sun Y, Li Y, Yang P, Luo C, Jiang T, Hu P, Xu Z. Carrier screening and prenatal diagnosis for spinal muscular atrophy in 13,069 Chinese pregnant women. J Mol Diagn. 2020;22(6):817–822. doi: 10.1016/j.jmoldx.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Prior TW, Snyder PJ, Rink BD, Pearl DK, Pyatt RE, Mihal DC, Conlan T, Schmalz B, Montgomery L, Ziegler K, Noonan C, Hashimoto S, Garner S. Newborn and carrier screening for spinal muscular atrophy. Am J Med Genet A. 2010;152a(7):1608–16. doi: 10.1002/ajmg.a.33474. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Shachar S, Orr-Urtreger A, Bardugo E, Shomrat R, Yaron Y. Large-scale population screening for spinal muscular atrophy: clinical implications. Genet Med. 2011;13(2):110–114. doi: 10.1097/GIM.0b013e3182017c05. [DOI] [PubMed] [Google Scholar]
- 26.Huang Z, Yang Q, Ye J, Huang J, Lin J, Chen J, Liang Z, Cao Z. Screening and prenatal diagnosis of survival motor neuron gene deletion in pregnant women in Zhaoqing city, Guangdong Province. BMC Med Genomics. 2023;16(1):39. doi: 10.1186/s12920-023-01468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao YY, Zhang WH, Qu YJ, Bai JL, Jin YW, Wang H, Song F. Diagnosis of spinal muscular atrophy: a simple method for quantifying the relative amount of survival motor neuron gene 1/2 using Sanger DNA sequencing. Chin Med J (Engl) 2018;131(24):2921–2929. doi: 10.4103/0366-6999.247198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.