Abstract
Purpose
Patients with polycystic ovarian morphology (PCOM) make up 20% cases for assisted reproductive technology (ART). Folliculogenesis is impaired in PCOS. Signaling molecules are involved in follicle development. Dysregulations of intrafollicular environment and signaling molecules are observed in PCOS. Granulosa cells (GCs) and oocytes secrete molecules into follicular fluid by exocytosis of SNAREs. The aim of this study is to evaluate vesicle transport and vesicle fusion proteins (SNAREs) in GCs from PCOS patients who have undergone IVF treatment.
Methods
Follicular fluids were collected from patients who undergo IVF/ICSI with the diagnosis of male factor (n = 10) and PCOS (n = 10) patients. GCs were separated and cultured. Each group of GCs was stimulated with FSH-hCG. The cells were examined under electron microscope. Immunofluorescent labeling was performed on cells for Stx6, SNAP25, StxBP1, FSHr, and KITL. Integrated density was analyzed from images of Stx6, SNAP25, StxBP1, FSHr, and KITL.
Results
Intercellular communication occurs by signal molecules; Stx6, SNAP25, and StxBP1 fusion proteins involved in exocytosis were decreased in the GCs of PCOS. There was no increase in in vitro stimulation with FSH-hCG either. In the electron microscope, it was observed that exocytosis of the vesicles was disrupted.
Conclusions
Exocytosis and vesicular dynamics are among the basic physiological functions of human steroidogenic granulosa cells. Follicle development is necessary for production of competent oocytes and ovulation. Understanding the pathophysiology of PCOS at follicular level is important for disease management. According to our findings, deficits in vesicular dynamics of human granulosa cells in may be central to the treatment strategy for PCOS patients.
Keywords: Exocytosis, SNAREs, PCOS, SNAP25, Stx6, StxBP1, KITL
Introduction
Polycystic ovary syndrome (PCOS) is an ovarian dysfunction syndrome with hyperandrogenism (such as clinical hirsutism, and/or biochemical hyperandrogenemia) and polycystic ovary morphology on ultrasonography. Clinical symptoms of PCOS may consist of menstrual irregularities, obesity, and signs of androgen excess [1]. PCOS is an endocrinopathy in reproductive-aged women affecting 5–20% worldwide [2] whose etiology remains unclear [3]. According to the consensus at the conference held in 2003 known as the Rotterdam criteria, PCOS diagnosed the presence of at least two of the criteria such as oligo‐ and/or anovulation, clinical and/or biochemical signs of hyperandrogenism, and polycystic ovaries. Also, other related etiologies should be excluded such as congenital adrenal hyperplasia, androgen-secreting tumors, and Cushing’s syndrome [1]. Considering those 55–91% of women with anovulation [4] and 20% of women who applied to ART clinics exist polycystic ovarian morphology (PCOM) [5], it is an important cause of infertility/subfertility in women of reproductive age.
In anovulatory women with PCOS, the later-stage follicular development fails to become a Graafian follicle. These follicles are arrested before they reach the mature stage to ovulate. This anomaly may be due to the hormonal environment of the follicles; however, there are intrinsic abnormalities of ovarian folliculogenesis that remain unclear [6]. Especially in the early follicle stages, the follicle environment is under the control of oocyte and GCs due to the presence of the blood follicle barrier [7]. During folliculogenesis and ovulation, GCs secrete many factors by exocytosis [8]. For example, KITL secreted from GCs maintains follicle survival, so it may also have roles in thecal steroidogenesis [9].
Vesicle trafficking within the cell and vesicular transport are responsible for communication between the cells, the intracellular compartments, and extracellular compartment [10]. Some of the proteins are responsible for the fusion of the vesicle membrane and the plasma membrane. Soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins are tail-anchored proteins, which represent 3–5% of membrane proteins. SNAREs are in charge for membrane fusion events within cells. Synaptic vesicle fusion is a well-studied example of SNARE activity. Synaptic vesicle carries on vesicle SNARE (v-SNARE) such as synaptobrevin; target membrane carries on target SNAREs (t-SNARE) such as SNAP-25 [11]. SNAP-25 is important for the mechanism of regulated exocytosis in the nervous system. Neuroendocrine cells also express SNAP-25. In addition to these, endocrine cells of the ovary such as follicular cells and the cells of corpus luteum express SNAP-25 in a rat [12]. The expression of SNAP-25 by granulosa cells is regulated by gonadotropins. Gonadotropin (FSH) stimulation increases SNAP-25 expression [13]. Most essential regulator of SNAREs is α-SNAP [14]. It has been reported that SNAP-25 and α-SNAP regulate cholesterol movement and support steroidogenesis within the GCs [15]. SNAP-25 plays an important role in exocytosis from the GCs [8]. Moreover, the defective function of α-SNAP in mice is related to increased atretic follicle, elongated cycle length, decreased fecundability, and decreased corpora lutea ratio [13].
Stx-6 mediates the regulation of lipid and protein composition of the cellular membrane. It maintains the maturation of immature secretory granules. It is involved in regulated secretion in pancreatic β-cells [16]. Stx-6 provides internalization and endosomal sorting of GLUT4 in adipocytes [17]. Moreover, it has a role in the retrograde transport of membrane-associated glycosphingolipids and low-density lipoprotein (LDL)-derived cholesterol to the trans-Golgi network (TGN) [18]. It acts as a cholesterol-binding protein in intracellular cholesterol traffic [19]. SNARE proteins can be adequate to remain membrane fusion. Yet, exocytosis requires some additional factor due to temporal and spatial restrictions [20]. StxBP-1, which is also called Munch18-1, is one of the additional factors of membrane fusion [20, 21]. StxBP1 provides the regulation of insulin secretion and GLP secretion from intestinal epithelial cells. The expression of StxBP1 decreases under high fatty acid conditions [22, 23]. The aim of this study is to reveal the changes in vesicle transport proteins in the granulosa cells of PCOS patients who are undergoing IVF treatment and how they are affected by FSH-LH induction.
Materials and methods
Participant and study design
For this study, we used the GCs within the excess follicular fluid from women who underwent oocyte pick-up (OPU) at the ART clinic of Hacettepe University Hospital. Study groups were designed as control group composed of healthy women (n = 10) of couples who applied to the ART clinic because of male infertility and PCOS group consisting of diagnosed as PCOS women (n = 10) according to the Rotterdam criteria who applied to the ART clinic were included in the study between 2020 and 2021. Participants were selected from the individuals with a body mass index of 18.5–24.9 kg/m2. The individuals with metabolic syndrome, diabetes mellitus, thyroid diseases, endometriosis, adenomyosis, chromosomal abnormalities, patients undergoing preimplantation genetic testing (PGT) for genetic reasons, or who AMH levels under 1.2 ng/ml were not included in the study [24]. There are four subgroups in the study as in vitro stimulated (with FSH (0.5 IU/ml-hCG (10 IU/ml)) and in vitro unstimulated groups.
Ovulation induction
Routine IVF treatment protocols were used, which involved primarily gonadotropin-releasing hormone (GnRH) antagonist protocol. Women commenced FSH with a daily dose of 125–200 IU (Gonal F, Merck, Turkey) on the third day of the menstrual cycle. The GnRH antagonist (Cetrotide, Merck, Turkey) was began on the fifth or sixth day of menses. Then human chorionic gonadotrophin (500 mcg) trigger (Ovitrelle, Merck, Turkey) was applied. The HCG trigger was applied when two or more follicles were observed to be ≥ 17 mm in diameter.
Isolation and culture of the GCs
The excess follicular fluid which remains after the OPU and cumulus cells from the oocyte denudation (Hyase-10X, Vitrolife, Sweden) were transferred to the research laboratory for isolation. GCs were separated from the follicular fluid using gradient-based protocol that we have published before [25]. Separated cells were cultured in DMEM-F12 and Bio-Amf 1 medium (Biological Industries, USA) 1:1 mixture with 4% L-Glutamine (Capricorn Scientific, Germany) and 1% penicillin-streptomycin-amphotericin B (Biowest, France) at optimum culture conditions (5% CO2, 37 °C, and humidified incubator). DMEM F-12 with 4% L-Glutamine and 1% penicillin-streptomycin-amphotericin B was used for subsequent feeds [25]. When cells reached 80% confluency, they were subcultured by using 0.25% trypsin (Trypsin, Capricorn Scientific, Germany).
Stimulation of GCs by FSH-hCG
After the cells reached the sufficient number for immunofluorescence labeling and electron microscopic examination, the part of the cells from both PCOS and control groups were incubated in a medium containing FSH (0.5 IU/ml) (Sigma, F4021-10UG, lot#MKCK7637, USA) and hCG (10 IU/ml) (Sigma, C1063-1VL, lot#SLBS9309, USA) for 24-h stimulation [12]. Simultaneously, non-stimulated granulosa cells were also incubated with medium without FSH and hCG. The cells were stimulated on a cell culture slide (Cell Culture Slide 8well, SPL Life Sciences, South Korea) and labeled for immunofluorescence imaging.
Immunofluorescent labeling of GCs
The immunolabeling and imaging were performed for all groups. After the incubation for 24 h, the culture medium was removed, and then the granulosa cells on the cell culture slide were washed with PBS (phosphate-buffered saline) at room temperature (RT). The cells were fixed by methanol (Extra pure methanol, Merck, Germany) and the cells were permeabilized by using 0.1% Tween20 (Sigma, Germany). Antibodies for FSHr (FSH receptor Rabbit Polyclonal Antibody, cat: bs-0895R, Bioss Antibodies, USA), Stx6 (Syntaxin 6 Rabbit Polyclonal Antibody, cat: bs-11261R, BiossAntibodies, USA), SNAP25 (SNAP25 Rabbit Polyclonal Antibody, cat: bs-1131R, Bioss Antibodies, USA), StxBP1 (STXBP1/Munc18 Rabbit Polyclonal Antibody, cat: bs-3954R, Bioss Antibodies, USA), and KITL (SCF Rabbit Polyclonal Antibody, cat: bs-0545R, Bioss Antibodies, USA) were used at 1/300 dilution as primary antibody. The cells after the incubation with primary antibodies for 1 h 30 min at R were washed and incubated with secondary antibody (Alexa Fluor® 488 AffiniPure Goat Anti-Rabbit IgG (H + L), cat:111-545-144, Jackson Immunoresearch, USA) for 1 h according to instructions. The slides were mounted by antifade mounting medium with DAPI (Antifade mounting medium, Vectashield, cat: H-1200-10, USA). The cells were imaged by fluorescent microscope (DM6B, DFC7000T, Leica, Germany). Approximately 150–200 cells were evaluated for each participant. Fluorescent intensity was measured for cell level, and background intensity was removed from the analysis. Intensities analyzed from raw data. All images were shot under the same acquisitions.
Transmission electron microscopy
The stimulated and unstimulated cells of each group (PCOS and control) were centrifuged at 260 G for 5 min after being removed from the culture dish. The pellet was fixed by 2% glutaraldehyde for 30 min then 1% osmium tetroxide (Osmium Tetroxide, 1 g crystal, EM grade, Ted Pella, Sweden) for secondary fixation for 1 h at dark and RT. After washing and removing osmium tetroxide, the pellet was embedded in 2% agarose gel. The cell pellet is dehydrated by passing through a series of ethanol solutions in ascending concentrating up to 100%. Dehydrated specimens were incubated twice in propenoxide (Propenoxid 99%, Acros Organics, Thermo Fisher Scientific, USA), then were infiltrated and embedded in araldite (Araldite 6005 embedding kit, cat: #13920, Electron Microscopy Sciences, USA) according to manufacturer instructions. Polymerization was performed at 60 °C for 48 h. The 60-nm-thick sections were stained with uranyl acetate (Ultrostain 1, Leica, Germany) and lead citrate (Ultrostain 2, Leica, Germany). Approximately 100–150 cells were examined in an electron microscope for each participant (Jeol JEM1400, Japan).
Statistical analysis
Statistical analysis and visualization of data were performed using the SPSS software v.23 software licensed by Hacettepe University. The sample size needed for the study was calculated according to parametric tests. The study must have 10 individuals for each group to have 80% power with 5% type 1 error level to detect a minimum significant difference of 0.5 units.
The variables were investigated using histogram, probability plots, and Shapiro-Wilk tests to determine whether they are normally distributed. Descriptive analyses were presented using medians, minimum, and maximum for non-normally distributed data. The Mann-Whitney U test was used to compare two independent groups. Cases where the p value was below 0.05 were considered statistically significant. The Kruskal-Wallis test was used to compare more than two independent groups. Bonferroni correction was performed after the Kruskal-Wallis test. For statistical significance, the total type 1 error level was taken as 5%.
Results
The demographic features of the participants were evaluated according to their ages. There was no significant difference (Mann–Whitney test, p = 0.068) between the ages of the participants in the control (median = 27, min = 22, max = 35) and PCOS (median 32, min = 25, max = 39) groups. The total FSH doses of all patients were compared, and there were no significant differences between PCOS and control patients (Mann–Whitney test, p = 0.095). It was observed that isolated GCs adhere to the culture plate at 48th hours. The adherent GCs had epithelioid morphological features with their similar patterns that were both within and between groups in phase contrast microscopy (Figs. 1 and 2). FSHr, Stx6, SNAP25, StxBP1, and KITL expressions were observed in stimulated and unstimulated conditions in all groups in fluorescence labeling (Figs. 3 and 4).
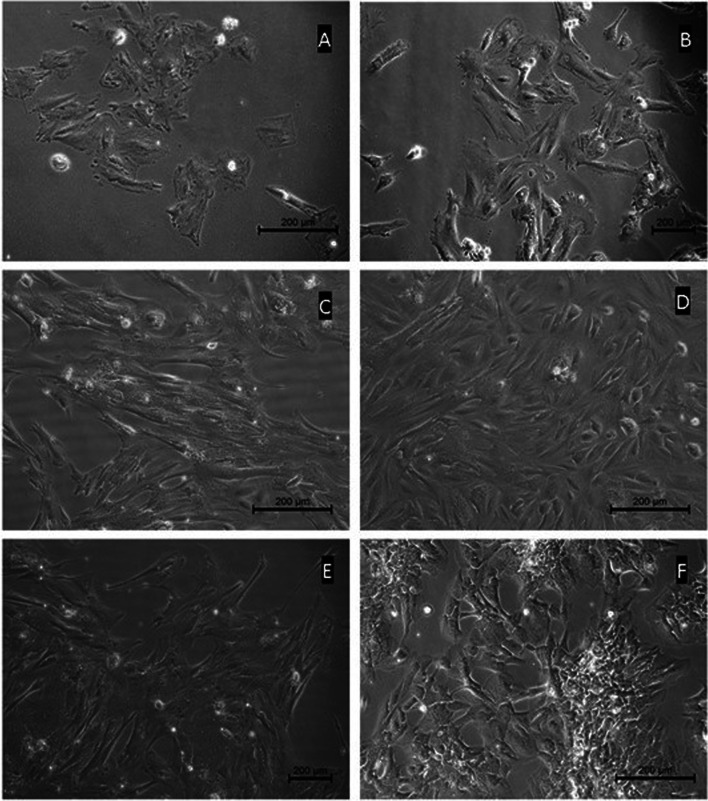
Fig. 1.
Primary culture micrographs of granulosa cells from healthy women (control group). The morphologies of the culture cells show homogeneous epithelial cell characteristics. Almost all cells are contacted with their extensions
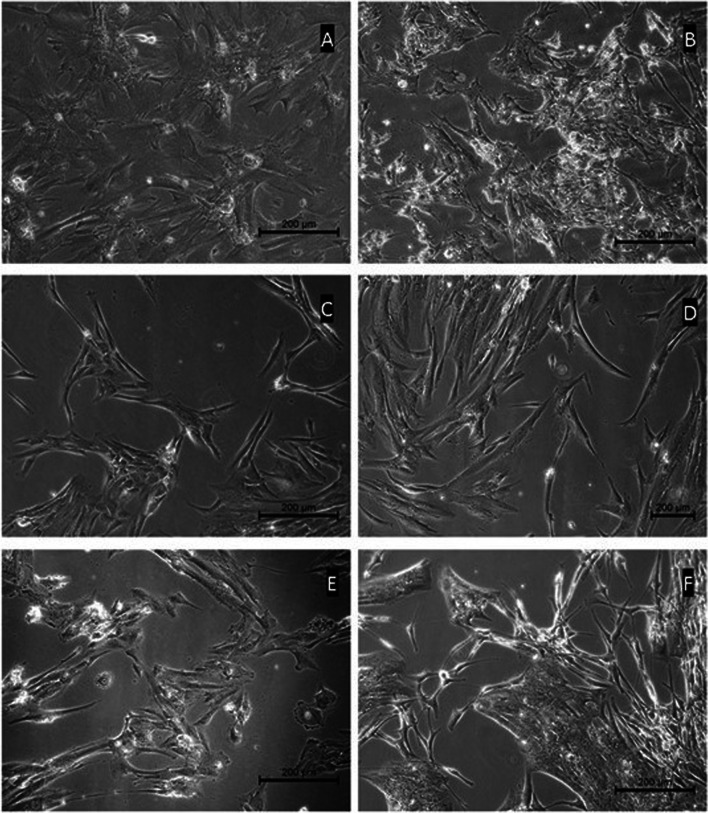
Fig. 2.
Primary culture micrographs of granulosa cells of women diagnosed with PCOS (PCOS group). Phase contrast microscope images were taken from different individuals in the group. Cells are morphologically homogeneous. It was observed that the cells tended to contact with their cytoplasmic extensions
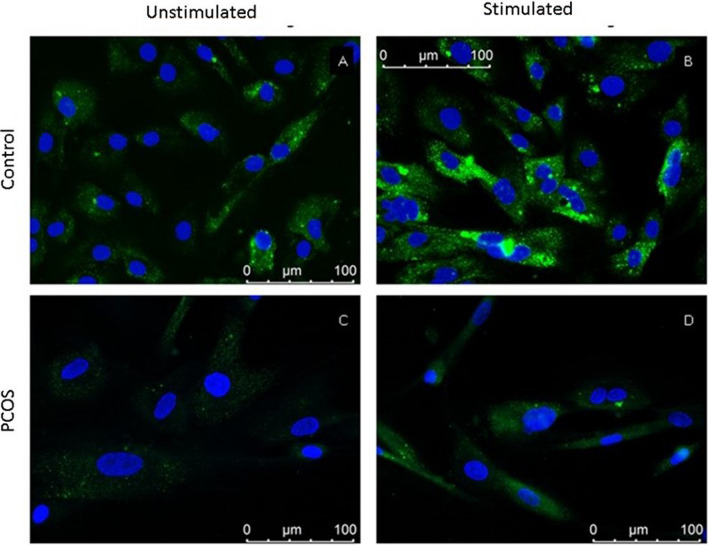
Fig. 3.
Representative images of FSHr immunofluorescence in granulosa cells. Micrographs were taken at × 40 objective magnification. A Unstimulated control group. B Control group with stimulation. C Unstimulated PCOS group. D PCOS group with stimulation
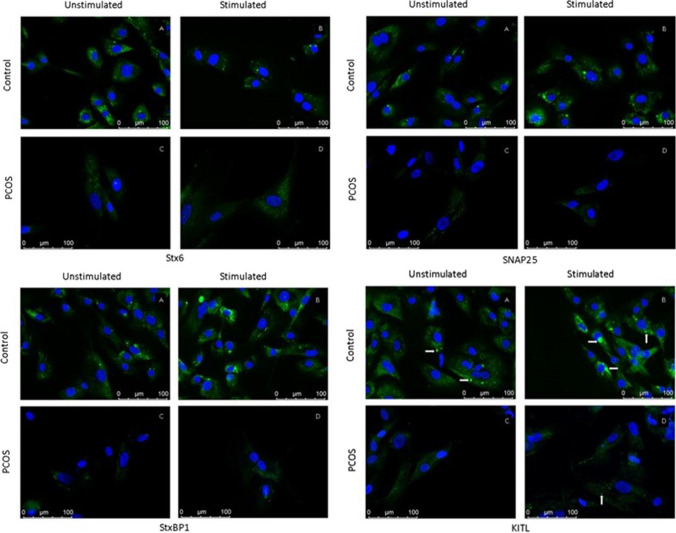
Fig. 4.
Stx6, SNAP25, StxBP1, and KITL immunofluorescence representative micrographs in granulosa cells. Arrows indicate fluorescence expressions. Micrographs were taken at × 40 objective magnification
Under stimulated conditions, FSHr expression was located towards the central cytoplasm of the cell in control group. Also, FSHr intensity was higher in the stimulated condition than unstimulated condition (Fig. 3). The labeling intensity was not as high as control group in PCOS group for both conditions. The integrated density of FSHr at control group for both conditions was higher than PCOS group (p < 0.001). Density was higher in stimulated conditions than unstimulated conditions in both PCOS (p = 0.002) and control (p < 0.001) groups (Graph 1).
Graph 1.

FSHr immunofluorescence intensities in granulosa cell samples of control and PCOS groups. Asterisk (*) indicates a statistically significant difference. Error bars represent confidence interval of differences
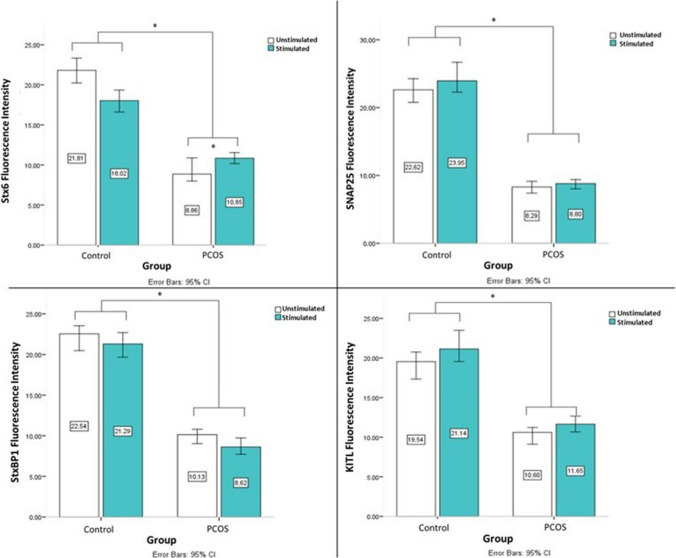
Stx6, SNAP25, StxBP1, and KITL labeling were observed in the cytoplasm of GCs for both groups and conditions. Besides, Stx6, SNAP25, StxBP1, and KITL densities were higher than PCOS in control group for each condition (Graph 2). The Stx6 labeling was observed mainly concentrated in the cytoplasm around the nucleus in control group at unstimulated condition. Labeling localization was distributed towards the cell periphery with FSH-hCG stimulation. A similar staining pattern was not observed in the PCOS group. The SNAP25 labeling was observed as different-sized granular pattern. Labeling localization was mainly distributed towards the cell periphery with FSH-hCG stimulation. The diffuse granular labeling pattern of StxBP1 was mainly distributed towards the cell periphery with FSH-hCG stimulation as well. Scattered granular staining pattern and clusters in the cytoplasm were observed for KITL labeling. It was observed that the clustering tendency increased with FSH-hCG stimulation in control group (Fig. 4).
Graph 2.
Stx6, SNAP25, StxBP1, and KITL immunofluorescence intensities in the granulosa cell samples of control and PCOS groups. Asterisk (*) indicates a statistically significant difference. Error bars represent confidence interval of differences
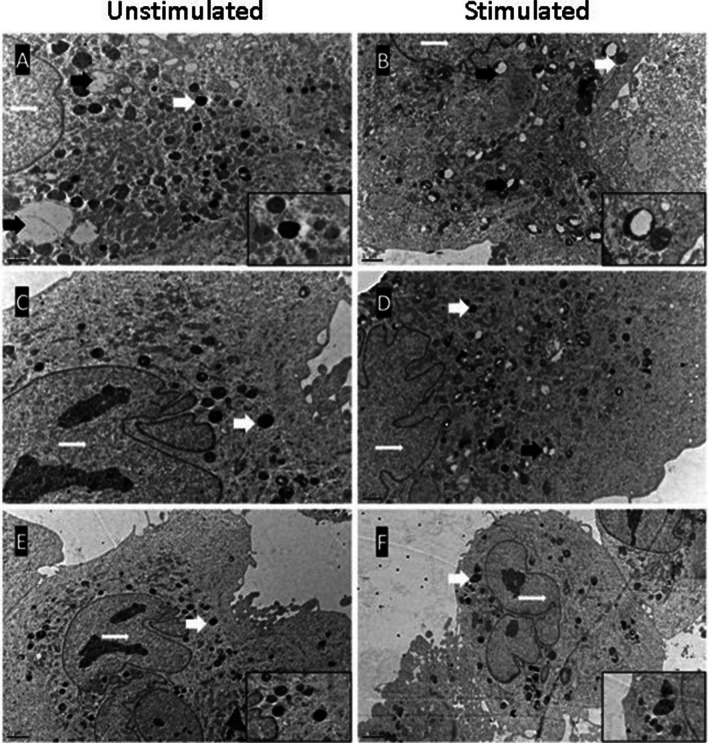
The GCs were examined under the electron microscope with their polyhedral to oval-shaped cytoplasm and loosely packed chromatin material in the nucleus. The nuclei of the cells were mostly rounded and minimal eccentrically located with one to two prominent nucleoli. Organelles populated within the cytoplasm with tubular-vesicular mitochondria. They were elongated or rod-shaped and showed varied longitudinally oriented cristae. Membranes of smooth endoplasmic reticulum, cisternae of rough endoplasmic reticulum, rare free ribosomes, Golgi stacks, and vesicles scattered in the cytoplasm. Granules suggesting as related to secretion besides the lysosomes and lipid droplets were also seen in the cytoplasm. GC provided with ultra structural characteristics of typical healthy, metabolically active steroidogenic cell. The nucleus with their chromatin distribution and the cytoplasm with the cytoplasmic membrane structures of the cells in the control and PCOS groups were observed with similar ultrastructural characteristics. There were many lipid droplets in the cytoplasm of control group in unstimulated conditions (Figs. 5 and 6).
Fig. 5.
Representative micrographs from electron microscopy of in vitro cultured granulosa cells from healthy (control) women. Thick white arrows indicate high electron density vesicles, white stars indicate lipid droplets, thin white arrows indicate nuclei, and black arrows indicate empty or partially empty vesicles. (A, B, and D micrographs × 20,000; C, E, and F micrographs × 8000)
Fig. 6.
Representative micrographs from electron microscopy of in vitro cultured granulosa cells from women with PCOS. Vesicles with high electron density, which continue to exist under stimulated conditions, are seen. In unstimulated conditions, the vesicle content of the cytoplasm is decreased compared to the control group. Thick white arrows indicate high electron density vesicles, thin white arrows indicate nuclei, and black arrows indicate empty or partially empty vesicles. (Micrographs A, B, C, and D were taken at 12,000 magnifications; E and F micrographs were taken at 6000 magnifications)
However, it was noted that lipid droplets decreased in stimulated conditions of control group. In the PCOS group, lipid droplets were not as rich as the control group in both conditions. Also, membranous organelles were lesser than the control group. It was observed that the cells of control group were rich in dense vesicles under unstimulated conditions. Dense vesicles decreased and appeared empty which was suggesting being a released vesicles increased by stimulation in the control group. These intuitive vesicles in the GCs of women with PCOS were much less in unstimulated conditions compared to the control group. Despite stimulated conditions, vesicles with high electron density were found to persist in the GCs of women with PCOS. There were no changes in the number of dense vesicles and partially empty vesicles with stimulation in the PCOS group.
Discussion
The women of the PCOS group were slightly older than the male factor group when the ages of the women of the couples admitted to the ART clinic with the diagnosis of PCOS and male factor were compared. However, the presence of an older median age in the PCOS group may be related to the time lost because of referring to IVF treatment after treatments such as lifestyle changes, clomiphene citrate or metformin, intrauterine insemination, and the others in PCOS [26]. Overall, there were no statistically significant differences at ages between the groups.
GCs of both healthy and PCOS women who have undergone IVF treatment, express FSHr in vitro. It was observed that FSHr labeling pattern was shifted towards the nucleus which is suggesting the internalization receptor of GCs of healthy women by FSH-hCG stimulation. This indicates that FSHr is activated by stimulation in healthy GCs. Higher FSHr intensity under stimulated conditions in GCs obtained from healthy women may be due to the upregulation effect of hCG [27]. Like the control group, the labeling intensity of FSHr increased with stimulation in the PCOS group. The receptor internalization was not distinct in PCOS as in the control group. It has been reported that women with PCOS are more sensitive to exogenous gonadotropins than healthy women during ovulation induction [28]. However, in our in vitro findings, FSHr level and internalization in GCs of women with PCOS were observed to be quite low compared to healthy individuals. Also, Wu et al. reported decreased FSHr expression in granulosa cells of PCOS [29]. Sensitivity to exogenous gonadotropins in PCOS appears to be related to the FSHr polymorphism at PCOS [30].
Stx6 performs homotypic fusion of immature secretory vesicles [10, 18], recycling of the plasma membrane [31], joining of coated vesicles into the secretory pathway [32]. According to our findings, the labeling pattern of Stx6 in the granulosa cells of healthy women in unstimulated conditions is frequently in the cytoplasm adjacent to the nucleus and migrated towards the cell periphery under stimulated conditions. In PCOS, both in FSH-hCG stimulated and unstimulated conditions, Stx6 was less common than in healthy GCs and did not show any difference in its intracellular location with stimulation. When the fluorescent intensities of Stx6 labeling of all participants were compared, the detection of a statistically significant decrease in PCOS compared to healthy GCs in all conditions. This finding is also consistent with the lack of FSHr internalization of the PCOS group to FSH-hCG stimulation.
SNAP25 is the main SNARE molecule of the regulated secretory pathway, playing a role both in the formation of the vesicle fusion complex that takes place in the process of exocytosis regulated by intracellular calcium [33] and in the determination of the amount of intracellular calcium [34–36]. Morphologically different granular SNAP25 labeling was observed in the cells of both groups. Observation of SNAP25 labeling signal as clustered, large sizes, and intense with FSH-hCG stimulation in GCs obtained from healthy women suggests the vesicle maturation process. The fact that SNAP25 aggregation in PCOS is not as large and dense as in healthy GCs may suggest that the maturation process of secretory vesicles is disrupted in PCOS. The decrease of SNAP25 labeling signal in PCOS compared to the control group in both unstimulated and stimulated conditions and the absence of vesicular response to FSH-hCG stimulation in PCOS granulosa cells in electron microscopy findings support each other. Impairment of the vesicular response in PCOS detected in electron microscopic examination may be associated with SNAP25.
StxBP1 is an auxiliary protein for vesicle fusion in the exocytosis of secretory vesicles [37]. Shim et al. reported that StxBP1 was significantly associated with PCOS and discussed their findings to the role of StxBP1 in insulin secretion in PCOS [38]. Olesen et al. found that the expression level of StxBP1 increased in the time period when meiosis started in embryonic mouse ovaries [39]. Human GCs also express granular StxBP1 in the cytoplasm. StxBP1 was observed as a dense and increasing signal to cluster near the cytoplasm plasma membrane with FSH-hCG stimulation in healthy GCs. The low level of StxBP1 in PCOS granulosa cells in FSH-hCG stimulated and unstimulated conditions confirms to Shim et al.’s finding about StxBP1 in PCOS. In our study, the findings of Shim et al. were also supported by another endocrine secretory cell, granulosa cells. However, in addition to the inference of Shim et al., our findings revealed that the association of StxBP1 in PCOS is led by granulosa cells. The change of StxBP1 in GCs in PCOS was reported for the first time in our study.
KITL released from GCs is the main growth factor in the proliferation and survival of cells within the follicle [40–42]. KITL fluorescent labeling was observed as large clusters in the cytoplasm of GCs from healthy women when examined morphologically. In contrast to the control group, no clustered granular marking was observed in the PCOS group. As in the results of Tan et al. [43], the fluorescence intensity was decreased in both of the stimulated and unstimulated groups in the PCOS group compared to the control group. When GCs obtained from healthy participants were examined under unstimulated conditions by electron microscopy, there were vesicles with high electron density, different sizes, and heterogeneous contents in the cytoplasm. It was determined that the number and density of vesicles decreased after stimulation with FSH-hCG for 24 h. Toonen et al. changed the expression of StxBP1 in their study. They observed that when the expression of StxBP1 is decreased, the electron-dense vesicles remain in the cytoplasm, and when the expression of StxBP1 is increased, the electron-dense vesicles decrease in the cytoplasm [44]. Electron-dense vesicles decreased in control group cells by FSH-hCG stimulation as well. The amount of vesicle in unstimulated conditions in PCOS was not as much as in the control group. In addition, it was observed that electron-dense vesicles persisted with FSH-hCG stimulation in PCOS. This suggests that the vesicular response to FSH-hCG stimulation is impaired in PCOS. When the impairment in Stx6, SNAP25, and StxBP1 expressions in PCOS is evaluated with electron microscopy findings, it can be concluded that vesicle fusion and exocytosis are disrupted in GCs from PCOS patients.
According to our findings in this study, the decrease in factors such as KITL in the follicle in PCOS patients who have undergone IVF treatment is due to the disruption in its synthesis as well as its deterioration in exocytosis in GCs. Vesicle fusion proteins did not increase with in vitro FSH-hCG stimulation, and electron-dense vesicles were not released with stimulation in electron microscopic examination. In addition, it has been observed that the FSH receptor internalization is impaired in PCOS patients in ART. Even though it is not possible to clearly reflect the findings of this study to the population because it was conducted on patients who received in vivo stimulation treatment, as mentioned in the limitations section, PCOS represents an important patient group in ART clinics. Therefore, our findings are important for the understanding of the biology of PCOS patients undergoing IVF treatment. It has been demonstrated for the first time in our study under in vitro conditions that receptor internalization and exocytosis, which are functions of vesicle fusion proteins, are impaired and do not improve with in vitro FSH-hCG stimulation in PCOS patients in ART.
Demonstration of these results in a large patient cohort will improve our knowledge in the field of fertility treatment in PCOS patients. Additionally, investigating granulosa cells obtained through the natural cycle will increase our knowledge on the molecular pathogenesis of PCOS.
Limitations
The first and perhaps the most important limitation of the study is the narrow sample size combined with the endocrine and metabolic variability of PCOS. In this study, which was completed with human material, the sample size had to be limited. Moreover, elective treatments, including ART, were stopped in our country due to the COVID 19 pandemic during the study. Thus, the sample size of groups could not be enlarged. The statistical power of the analysis was calculated as 80% with this sample size for the study. Considering the heterogeneity in PCOS, increasing the sample size will strengthen the study in reflecting the findings to the population.
Secondly, all patients in whom granulosa cells were obtained were cycles of IVF with ovulation induction. There was no natural cycle IVF. Cells were cultured and passaged to avoid the effects of in vivo stimulation. However, it is not possible to predict how much the in vivo stimulation effect is avoided.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SE, SZ, LKS, GB, and SFM. The first draft of the manuscript was written by SE and SFM. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. GB: data collection; SE: project development, protocol, data analysis, manuscript writing; SFM: supervision, project development, protocol, data management and analysis, manuscript editing; LKS: protocol, data collection; SZ: protocol, data analysis.
Funding
This study was supported by Hacettepe University Scientific Research Projects Coordination Unit (Grant numbers: TSA-2019-18196).
Data Availability
The data that support the findings of this study are available on request from the corresponding author, [SFM]. The data are not publicly available due to the privacy of research participants.
Declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study. Non-interventional Clinical Research Ethics Board of Hacettepe University provided approval for conducting this study (GO 19/678, 2019/16-09).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fauser BCJM, Tarlatzis F, et al. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/HUMREP/DEH098. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Prim. 2016;2:16057. doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- 3.Waldman IN, Legro RS. Polycystic ovary syndrome. In: LEUNG PCK, ADASHI EY (eds) The Ovary: Third Edition, Third Edit. Elsevier; 2019. pp 415–435.
- 4.Torpy JM, Lynm C, Glass RM. Polycystic ovary syndrome. J Am Med Assoc. 2007;297:554. doi: 10.1001/jama.297.5.554. [DOI] [PubMed] [Google Scholar]
- 5.Andreeva P, Milachich T, Shterev A. ART treatment of infertile patient with PCO. In: Polycystic Ovarian Syndrome Conference. Endocrinol Metab Syndr. 2015;4(4):85. [Google Scholar]
- 6.Webber LJ, Stubbs S, Stark J, et al. Formation and early development of follicles in the polycystic ovary. Lancet (London, England) 2003;362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 7.Irving-Rodgers HF, Rodgers RJ. Extracellular matrix of the developing ovarian follicle. Semin Reprod Med. 2006;24:195–203. doi: 10.1055/s-2006-948549. [DOI] [PubMed] [Google Scholar]
- 8.Shimada M, Yanai Y, Okazaki T, et al. Synaptosomal-associated protein 25 gene expression ıs hormonally regulated during ovulation and ıs ınvolved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol. 2007;21:2487–2502. doi: 10.1210/me.2007-0042. [DOI] [PubMed] [Google Scholar]
- 9.Tuck AR, Robker RL, Norman RJ, et al. Expression and localisation of c-kit and KITL in the adult human ovary. J Ovarian Res. 2015;8:31. doi: 10.1186/s13048-015-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wendler F, Tooze S. Syntaxin 6: The promiscuous behaviour of a SNARE protein. Traffic. 2001;2:606–611. doi: 10.1034/j.1600-0854.2001.20903.x. [DOI] [PubMed] [Google Scholar]
- 11.Pollard TD, Earnshaw WC, Lippincott-Schwartz J, Johnson GT. Secretory membrane system and Golgi apparatus. In: Cell Biology, Third Edit. Elsevier; 2017. pp 351–376.
- 12.Grosse J, Bulling A, Brucker C, et al. Synaptosome-associated protein of 25 kilodaltons in oocytes and steroid-producing cells of rat and human ovary: molecular analysis and regulation by gonadotropins1. Biol Reprod. 2000;63:643–650. doi: 10.1095/biolreprod63.2.643. [DOI] [PubMed] [Google Scholar]
- 13.Arcos A, de Paola M, Gianetti D, et al. α-SNAP is expressed in mouse ovarian granulosa cells and plays a key role in folliculogenesis and female fertility. Sci Rep. 2017;7:11765. doi: 10.1038/s41598-017-12292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong W. SNAREs and traffic. Biochim Biophys Acta - Mol Cell Res. 2005;1744:120–144. doi: 10.1016/J.BBAMCR.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Hou X, Shen W-J, et al. SNARE-mediated cholesterol movement to mitochondria supports steroidogenesis in rodent cells. Mol Endocrinol. 2016;30:234–247. doi: 10.1210/me.2015-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuliawat R, Kalinina E, Bock J, et al. Syntaxin-6 SNARE involvement in secretory and endocytic pathways of cultured pancreatic beta-cells. Mol Biol Cell. 2004;15:1690–1701. doi: 10.1091/MBC.E03-08-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perera HKI, Clarke M, Morris NJ, et al. Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes. Mol Biol Cell. 2003;14:2946. doi: 10.1091/MBC.E02-11-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung JJ, Inamdar SM, Tiwari A, Choudhury A. Regulation of intracellular membrane trafficking and cell dynamics by syntaxin-6. Biosci Rep. 2012;32:383–391. doi: 10.1042/BSR20120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reverter M, Rentero C, Garcia-Melero A, et al. Cholesterol regulates syntaxin 6 trafficking at trans-Golgi network endosomal boundaries. Cell Rep. 2014;7:883–897. doi: 10.1016/j.celrep.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 20.Graham ME, Prescott GR, Johnson JR, et al. Structure-function study of mammalian Munc18–1 and C. elegans UNC-18 implicates domain 3b in the regulation of exocytosis. PLoS One. 2011;6. 10.1371/journal.pone.0017999. [DOI] [PMC free article] [PubMed]
- 21.STXBP1 - Syntaxin-binding protein 1 - Homo sapiens (Human) - STXBP1 gene & protein. https://www.uniprot.org/uniprot/P61764. Accessed 21 Feb 2021.
- 22.Zhang K, Wang T, Liu X, et al. CASK, APBA1, and STXBP1 collaborate during insulin secretion. Mol Cell Endocrinol. 2021;520:111076. doi: 10.1016/j.mce.2020.111076. [DOI] [PubMed] [Google Scholar]
- 23.Campbell JR, Martchenko A, Sweeney ME, et al. Essential role of syntaxin-binding protein-1 in the regulation of glucagon-like peptide-1 secretion. Endocrinol (United States). 2020;161. 10.1210/endocr/bqaa039. [DOI] [PMC free article] [PubMed]
- 24.İnfertilite ve Genetik Yönü. https://dijitalakademi.turkiyeklinikleri.com/flippage/tibbi-genetik-ozel/4-3/tr-index.html#p=20. Accessed 25 Apr 2021
- 25.Zırh S, Erol S, Zırh EB, et al. A new isolation and culture method for granulosa cells. Cell Tissue Bank. 2021 doi: 10.1007/s10561-021-09929-5. [DOI] [PubMed] [Google Scholar]
- 26.Costello MF, Misso ML, Balen A, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Hum Reprod Open. 2019;2019:1–24. doi: 10.1093/hropen/hoy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Velasco JA, Rodríguez S, Agudo D, et al. FSH receptor in vitro modulation by testosterone and hCG in human luteinized granulosa cells. Eur J Obstet Gynecol Reprod Biol. 2012;165:259–264. doi: 10.1016/j.ejogrb.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Szilágyi A, Bártfai G, Mánfai A, et al. Low-dose ovulation induction with urinary gonadotropins or recombinant follicle stimulating hormone in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2004;18:17–22. doi: 10.1080/09513590310001651731. [DOI] [PubMed] [Google Scholar]
- 29.Wu L, Fang Q, Wang M, et al. Effect of weight loss on pregnancy outcomes, neuronal-reproductive-metabolic hormones and gene expression profiles in granulosa cells in obese infertile PCOS patients undergoing IVF-ET. Front Endocrinol (Lausanne). 2022;13. 10.3389/FENDO.2022.954428. [DOI] [PMC free article] [PubMed]
- 30.Laven JSE. Follicle stimulating hormone receptor (FSHR) polymorphisms and polycystic ovary syndrome (PCOS) Front Endocrinol (Lausanne) 2019;10:23. doi: 10.3389/FENDO.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peak TC, Su Y, Chapple AG, et al. Syntaxin 6: A novel predictive and prognostic biomarker in papillary renal cell carcinoma. Sci Rep. 2019;9. 10.1038/s41598-019-39305-z. [DOI] [PMC free article] [PubMed]
- 32.Martín-Martín B, Nabokina SM, Blasi J, et al. Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis. Blood. 2000;96:2574–2583. doi: 10.1182/blood.V96.7.2574. [DOI] [PubMed] [Google Scholar]
- 33.Kádková A, Radecke J, Sørensen JB. The SNAP-25 protein family. Neuroscience. 2018 doi: 10.1016/J.NEUROSCIENCE.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Wiser O, Trus M, Hernández A, et al. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc Natl Acad Sci U S A. 1999;96:248–253. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcheva B, Perelis M, Weidemann BJ, et al. A role for alternative splicing in circadian control of exocytosis and glucose homeostasis. Genes Dev. 2020;34:1089–1105. doi: 10.1101/GAD.338178.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang T, Qin T, Kang F, et al. SNAP23 depletion enables more SNAP25/calcium channel excitosome formation to increase insulin exocytosis in type 2 diabetes. JCI Insight. 2020;5. 10.1172/jci.insight.129694. [DOI] [PMC free article] [PubMed]
- 37.Wang X, Fu G, Wen J, et al. Membrane location of syntaxin-binding protein 1 is correlated with poor prognosis of lung adenocarcinoma. Tohoku J Exp Med. 2020;250:263–270. doi: 10.1620/tjem.250.263. [DOI] [PubMed] [Google Scholar]
- 38.Shim U, Kim HN, Lee H, et al. Pathway analysis based on a genome-wide association study of polycystic ovary syndrome. PLoS One. 2015;10. 10.1371/journal.pone.0136609. [DOI] [PMC free article] [PubMed]
- 39.Olesen C, Nyeng P, Kalisz M, et al. Global gene expression analysis in fetal mouse ovaries with and without meiosis and comparison of selected genes with meiosis in the testis. Cell Tissue Res. 2007;328:207–221. doi: 10.1007/s00441-006-0205-5. [DOI] [PubMed] [Google Scholar]
- 40.Liu K, Zhang H, Risal S, et al. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24:2501–2508. doi: 10.1016/j.cub.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Lim JJ, Lima PDA, Salehi R, et al. Regulation of androgen receptor signaling by ubiquitination during folliculogenesis and its possible dysregulation in polycystic ovarian syndrome. Sci Rep. 2017;7. 10.1038/s41598-017-09880-0. [DOI] [PMC free article] [PubMed]
- 42.Williams CJ, Erickson GF. Morphology and Physiology of the Ovary. [Updated 2012 Jan 30]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278951/. [PubMed]
- 43.Tan J, Wen X-Y, Su Q, et al. Reduced expression of SCF in serum and follicle from patients with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2016;20:5049–5057. [PubMed] [Google Scholar]
- 44.Toonen RF, Kochubey O, de Wit H, et al. Dissecting docking and tethering of secretory vesicles at the target membrane. EMBO J. 2006;25:3725–3737. doi: 10.1038/sj.emboj.7601256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, [SFM]. The data are not publicly available due to the privacy of research participants.