Abstract
Extracellular regulated protein kinases 1/2 (ERK1/2) are key members of multiple signaling pathways, including the ErbB axis. Ectopic ERK1/2 activation contributes to various types of cancer, especially drug resistance to inhibitors of RTK, RAF and MEK, and specific ERK1/2 inhibitors are scarce. In this study, we identified a potential novel covalent ERK inhibitor, Laxiflorin B, which is a herbal compound with anticancer activity. However, Laxiflorin B is present at low levels in herbs; therefore, we adopted a semi-synthetic process for the efficient production of Laxiflorin B to improve the yield. Laxiflorin B induced mitochondria-mediated apoptosis via BAD activation in non-small-cell lung cancer (NSCLC) cells, especially in EGFR mutant subtypes. Transcriptomic analysis suggested that Laxiflorin B inhibits amphiregulin (AREG) and epiregulin (EREG) expression through ERK inhibition, and suppressed the activation of their receptors, ErbBs, via a positive feedback loop. Moreover, mass spectrometry analysis combined with computer simulation revealed that Laxiflorin B binds covalently to Cys-183 in the ATP-binding pocket of ERK1 via the D-ring, and Cys-178 of ERK1 through non-inhibitory binding of the A-ring. In a NSCLC tumor xenograft model in nude mice, Laxiflorin B also exhibited strong tumor suppressive effects with low toxicity and AREG and EREG were identified as biomarkers of Laxiflorin B efficacy. Finally, Laxiflorin B-4, a C-6 analog of Laxiflorin B, exhibited higher binding affinity for ERK1/2 and stronger tumor suppression. These findings provide a new approach to tumor inhibition using natural anticancer compounds.
Keywords: laxiflorin B, NSCLC, ERK1/2, AREG, EREG, natural compound
Introduction
Extracellular regulated protein kinases (ERKs), which belong to the mitogen-activated protein kinase (MAPK) family, play pivotal roles in regulating multiple effectors in cell proliferation and survival pathways under both physiological and pathological conditions [1]. The MAPK pathway is always activated in cancer development and progression, with abnormal ERK1/2 activation induced by upstream signals caused by events such as mutation or amplification in receptor tyrosine kinases (RTKs), RAS, proto-oncogene serine/threonine-protein kinases (RAFs) and mitogen-activated protein kinases (MAPK)/ERK kinases (MEKs) [1]. These changes have been reported to result in malignancy and poor prognosis [2, 3].
Due to the prevalence of ERK-involved MAPK pathway activation in various types of malignancies, kinase inhibitors have become a common strategy for clinical treatment. For example, constitutively active epidermal growth factor receptor (EGFR) mutants, which account for 15%–20% of all lung cancer cases, can spontaneously activate the ERK1/2-involved MAPK pathway, thereby promoting cell growth and survival, as well as leading to poor prognosis and tumor recurrence in patients. Although RTK inhibitors have been developed and applied in the clinic for more than two decades, the incidence of drug resistant lung cancer is increasing due to a variety of newly discovered mechanisms. Several studies have shown that prolonged administration of inhibitors of RTK, RAF and MEK result in drug resistance and re-activation of ERK1/2 in cancer cells [4]. Therefore, an effective strategy for specific targeting of ERK is urgently required to improve the prognosis of numerous cancer patients. A greater understanding of the ERK-involved MAPK pathway will not only aid in the identification and development of ERK1/2 inhibitors, but may also overcome resistance to its upstream kinase inhibitors in the treatment of a variety of cancers.
Compared with its upstream RTKs (RAS, RAF and MEK), ERK1/2 has a more conserved amino acid sequence and structure, with very few mutations in cancer cell genomes [5]. Through phosphorylation, ERK1/2 regulates widely diverse substrates responsible for evolutionarily conserved cellular processes, such proliferation, differentiation, growth, metabolism, migration and survival. The ERK1/2-associated signaling cascade is strictly controlled by a series of negative regulators, including dual-specificity phosphatases (DUSPs) [1]. Among the current strategies for ERK1/2 inhibition in malignancies, ATP analogs, such as pyridine-pyrrole, indazole-pyrrolidine and pyrazole amino-pyrimidine derivatives, have been demonstrated to inhibit ERK1/2 effectively by blocking the ATP-binding pocket [6]. Compared to RAF or MEK inhibitors, Ulixertinib was shown to inhibit ERK1/2 with high specificity and delayed drug resistance [7]. Moreover, Ulixertinib was found to overcome RAF inhibitor resistance in a patient-derived xenograft model and also a phase I clinical trial [8]. In phase I clinical trials, Ravoxertinib, FR180204 and LY3214996 effectively inhibited ERK1/2 [9–11], with synergistic effects achieved in RAF- or KRAS-mutant malignancies by using a RAF inhibitor compared to monotherapy [12, 13]. In addition, robust efficacy of ERK inhibitors, such as SCH7779684, MK8353 and LY3214996, was reported for KRAS- or RAF-mutant cancer cells in xenograft models [11, 14, 15]. However, effective ERK1/2 inhibitors have rarely been reported in clinical trials [6].
Isodon eriocalyx (I. eriocalyx var. laxiflora) is a widely used herbal medicine in China. Research has implicated ent-kaurene diterpenes isolated from Isodon plants as novel anticancer drugs with broad potential [16]. One such example, Laxiflorin B, was shown to inhibit cancer cell viability [17]. However, comprehensive studies of this compound are hindered by its low (0.00061%) abundance [18].
Here, we developed a new strategy to improve to the yield of Laxiflorin B through semi-synthetic production from its analog, Eriocalyxin B. We demonstrated that Laxiflorin B exerts strong anticancer activity by inhibiting the proliferation and promoting apoptosis of non-small-cellular lung cancer (NSCLC) cells expressing a constitutively active EGFR. Moreover, Laxiflorin B application primarily influenced the ErbB pathway and ERK1/2 was identified as a novel potential target of Laxiflorin B. The cystine (Cys) residues at positions 183 and 178 of ERK1 were identified as the covalent binding sites for Laxiflorin B. Subsequently, we found that BAD, an initiator of mitochondria-mediated apoptosis regulated by the ERK-RSK axis, was activated by Laxiflorin B-induced ERK inhibition, and this effect was rescued by BAD knockdown. The ERK-driven secretion of growth factors, such as AREG and EREG, was also largely abolished by Laxiflorin B-targeted ERK suppression, resulting in a positive feedback loop of ErbB signaling induced by autocrine AREG and EREG downregulation. Our in vivo study revealed that Laxiflorin B exerted strong antitumor effects with low toxicity, implying a wide range of possibilities for modification and therapeutic applications.
Materials and methods
General compound synthesis
Unless otherwise noted, all air- and water-sensitive reactions were carried out under nitrogen and with dry solvents under anhydrous conditions, respectively. Reactions were monitored by thin-layer chromatography (TLC) on 0.25 mm silica gel plates (60F-254) that were analyzed by measuring fluorescence at 254 nm or by staining with KMnO4 (200 mL H2O containing 1.5 g KMnO4, 10 g K2CO3, and 1.25 mL 10% aqueous NaOH). Silica gel 60 (particle size 0.040–0.063 mm) was used for flash column chromatography.
All chemicals were purchased commercially and used without further purification. Anhydrous solvents were distilled according to standard procedures. Details of general methods, compound characterization, HPLC analysis, and 1H and 13C NMR spectra of the compounds prepared in this study are provided in the Supplementary Materials and Methods.
Reagents, enzymes, and antibodies
Chemical reagents and reaction buffers were obtained from Sigma–Aldrich (St. Louis, MO, USA). Q5® High-Fidelity DNA Polymerase, DNA ligase and the restriction enzymes were purchased from New England BioLabs (NEB, Ipswich, MA, USA). A list of antibodies used is provided in the Supplementary Materials and Methods.
Construction of plasmids and mutagenesis
pBABEpuro-HA-MEK1 (#49328), pMM9-MAPKK2-WT (MEK2, #40805), pFLAG-CMV-hERK1 (#49328), pHAGE-MAPK1 (ERK2, #116760) were purchased from Addgene (MA, USA). Point mutations were introduced using Q5 DNA polymerase to generate the ERK1C178A, ERK1C183A and ERK1C178A/C183A mutants. The primary DNA template was digested with DpnI for 2 h at 37 °C before DH5α competent cells were transformed with mutant ERK1 to amplify the construct. The sequences were confirmed by Sanger sequencing. Details of the primers used for mutagenesis are provided in the Supplementary Materials and methods.
Cell culture and stable pool selection
HEK293T cells were obtained from the Cell Bank of the Chinese Academy of Sciences and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies) supplemented with 10% fetal bovine serum (FBS; PAN Seratech, Aidenbach, Germany) and 100 U/ml penicillin-streptomycin (Life Technologies). The human NSCLC cell lines PC9, HCC827, A549, NCI-H1975 and NCI-H1650 were obtained from the American Type Culture Collection (ATCC) and cultured in RPMI-1640 (Life Technologies) containing 10% FBS (PAN Seratech) and 100 U/mL penicillin-streptomycin (Life Technologies). HEK293T, PC9, HCC827, A549, NCI-H1975, NCI-H1650 and stable pools were incubated at 37 °C under 5% CO2. All cell lines were authenticated by short tandem repeat (STR) profiling (Guangdong Huaxi Forensic Physical Evidence Judicial Appraisal Institute, Guangdong, China). The cell lines were passaged fewer than 10 times or for a maximum of 6 months after initial revival from frozen stocks; all cell lines tested negative for mycoplasma.
For generation of stable pools with BAD or ERK1/2 knockdown, NSCLC cell lines were infected with lentiviruses expressing BAD or ERK1/2 shRNA and selected by culturing in RPMI-1640 complete medium containing 10 μg/mL puromycin (Selleck, TX, USA) for 1 week. BAD or ERK1/2 expression was evaluated by qPCR and Western blot analyses. Functional assays were performed after confirmation of gene expression. Details of the shRNA oligonucleotides are provided in the Supplementary Materials and Methods.
Real-time PCR analysis
Total RNA was extracted using TRIzol reagent (Life Technologies) and cDNA was synthesized using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA) according to the manufacturers’ instructions. All real-time PCRs were performed using the CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) with the ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China). Each real-time PCR reaction was repeated three times, and target gene expression data were normalized using GAPDH as an internal reference. Details of the primers used for real-time PCR are shown in the Supplementary Materials and methods.
Immunoprecipitation, pull-down assays and Western blot analysis
For immunoprecipitation (IP), HEK293T cells transfected with the indicated plasmids were cultured in a 100-mm dishes. Cells were collected at 80% confluence using IP lysis buffer. Following clarification, the supernatant fractions were immunoprecipitated by incubation with magnetic beads conjugated with anti-HA or anti-Flag antibodies at 4 °C overnight with rotation at 5 rpm (MedChemExpress, Monmouth Junction, NJ, USA). After washing the magnetic beads several times with wash buffer (25 mM Tris–HCl, pH 7.5, 100 mM NaCl, 0.1% NP-40), the immune-precipitant was finally eluted with sample buffer containing 1% SDS.
For pull-down assays, Laxiflorin B-biotin was conjugated with streptavidin magnetic beads and then incubated with total lysate generated from HEK293T or PC9 cells harvested at 80% confluence using IP lysis buffer. After washing the magnetic beads several times with wash buffer (25 mM Tris–HCl, pH 7.5, 100 mM NaCl, 0.05% Triton-X100), the immune-precipitant was finally eluted with sample buffer containing 1% SDS.
Western blotting was performed following IP. The protein concentration was determined using a BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai, China). All protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Burlington, MA, USA), and hybridized with the corresponding antibodies. IP bands were visualized using Pierce ECL Western blotting substrate (Thermo Scientific) and detected by Fluor Chem Q (ProteinSimple, USA). Raw Western blot data were quantified using AlphaEaseFC version 4.0 software.
In vitro kinase assay
PC9 cells overexpressing pBABEpuro-HA-MEK1 were lysed by hypoosmotic lysis buffer containing protease inhibitors. The lysate was incubated with anti-HA magnetic beads (MedChemExpress, Monmouth Junction, NJ, USA) at 4 °C for 16 h. After washing three times with cold PBS to remove impurities, the beads were incubated at 30 °C for 30 min with 3xFlag-MEK1, GST-purified ERK1 protein (400 ng each), or reaction buffer (working concentration: 20 mM Tris–HCl, pH 7.4, 10 mM MgCl2, 50 mM KCl, 0.5 mM KH2PO4, 0.05 mg/mL BSA, 5 mM DTT, ATP 400 μM), and various concentrations of Laxiflorin B (0, 1, 10, 50 μM). The beads were then washed three times with PBS before the levels of ERK1 and phospho-ERK1 were detected by Western blot analysis.
Mass spectrometry (MS) analysis of Laxiflorin B binding with ERK1
HEK293T cells overexpressing a Flag-ERK1 fusion protein were purified using magnetic beads conjugated with an anti-Flag antibody (MedChemExpress, Monmouth Junction, NJ, USA) and then incubated with 10 µM Laxiflorin B for 16 h at 4 °C. The bead samples were then washed three times with cold PBS containing 0.05% TritonX-100 before incubation with the reaction buffer (1% SDC/100 mM Tris–HCl, pH 8.5/10 mM TCEP/40 mM CAA) at 95 °C for 10 min for protein denaturation, cysteine reduction and alkylation. The eluates were diluted with an equal volume of H2O and digested with 1 μg trypsin overnight at 37 °C. The peptide was purified using self-made SDB desalting columns. The eluate was vacuum-dried and stored at −20 °C for later use.
Mass spectrometry was performed using a TripleTOF 5600 + LC–MS/MS system (SCIEX). The peptide sample was injected using an autosampler and bound to a C18 capture column (5 μm, 5 × 0.3 mm), followed by elution to the analytical column (300 μm × 150 mm, 3 μm particle size, 120 Å pore size, Eksigent) for separation. Two mobile phases (mobile phase A: 3% DMSO, 97% H2O, 0.1% formic acid and mobile phase B: 3% DMSO, 97% ACN, 0.1% formic acid) were used to establish the analytical gradient. The flow rate of the liquid phase was set to 5 μL/min. For mass spectrometry IDA mode analysis, each scan cycle involved a full MS scan (m/z range 3,501,250, ion accumulation time 250 ms), followed by 40 MS/MS scans (m/z range 100–1,500, ion accumulation time 50 ms). The conditions for MS/MS acquisition were set to a parent ion signal >120 cps and a charge number of +2 to +5. The exclusion time for ion repeat acquisition was set to 18 s.
Raw data generated by the TripleTOF 5600 were analyzed with ProteinPilot (V4.5) using the Paragon database search algorithm and the integrated false discovery rate (FDR) analysis function. Spectra files were searched against the UniProt Human reference proteome database using the following parameters: sample type, identification; Cys alkylation, iodoacetamide; digestion, trypsin; search effort, rapid ID. Search results were filtered with unused ≥1.3. Decoy hits and protein contaminants were removed; the remaining identifications were used for further analysis.
Cell growth, 2-D clonogenic and soft agar assays
For analysis of cell growth, cells were seeded in 96-well plates at 5 × 103 cells/well in a final volume of 100 µL culture medium and incubated at 37 °C. Cell viability was evaluated at 0, 24, 48, and 72 h after cell attachment. Cell Counting Kit-8 (CCK-8, Meilun Biotechnology, China) reagent (10 µL) was added to each well, and the plates were incubated for 2 h at 37 °C. The absorbance was measured at 450 nm by Maestronano spectrophotometer (TriStar2LB942, Berthold, Germany).
For 2-D clonogenic assays, cells were seeded in 6-well plates at 500 cells/well in a final volume of 4 mL culture medium containing various concentrations (0, 0.05, 0.1, 0.2 μM) of Laxiflorin B, SCH772984 or Ulixertinib. After incubation at 37 °C for 12–20 days, the 6-well plates were washed with 1× PBS before fixation in 100% methanol at 4 °C for 10 min. The cells were then stained with 0.5% crystal violet for 10 min at room temperature before being washed twice in double-distilled (dd) H2O.
For soft agar assays, cells were seeded in 0.4% low-melting agarose (Sigma–Aldrich) in 6-well plates at 5 × 103 cells per well. The cells were incubated at 37 °C for 2–3 weeks, then stained with 0.005% crystal violet for 1 h at room temperature before being de-stained in ddH2O.
Molecular docking and molecular dynamic simulation
In this study, the HADDOCK server [19] was used to simulate covalent docking of Laxiflorin B analogs onto ERK1/2. Both the “in” and “out” conformations of Laxiflorin B analogs were studied. The A chain of crystal structures 6GES [20] and 6G54 [20] were used as the templates of ERK1 and ERK2 for docking with Laxiflorin B analogs. During docking, the clustering method was based on RMSD and the cutoff was set to 1 Å. For scoring parameters, Evdw 1 and Eelec 3 were changed to 1.0 and 0.1, respectively. Several advanced sampling parameters were reset. The initial temperatures for the second and third TAD cooling steps with flexible side-chain and full flexibility at the interface were set to 500 K and 300 K, respectively. The numbers of molecular dynamic (MD) steps for rigid body high temperature TAD and during the first rigid body cooling stage were both set to 0. All other parameters were unchanged. For each docked complex, the representative structure was selected as the initial structure for MD simulation.
All MD simulations were performed in the AMBER package [21]. The force field FF14SB [22] was selected to build the force field of standard amino acids in proteins. The parameters for Laxiflorin B analogs were prepared based on the general AMBER force field (GAFF) [23]. The atomic charges of Laxiflorin B analogs were fitted based on the electrostatic potential (ESP) determined by quantum calculation. In accordance with the atomic charge from the FF14SB force field, the HF-6–31G* basis set was selected to obtain the ESP. Each docked complex was then immersed in a TIP3P water box [24] (size 10 Å). The counter ions were added to neutralize the system, which included approximately 50,000 atoms in total. The whole system was first minimized to convergence. After minimization, all heavy atoms were restrained by force constant 100 kcal/(mol rad2), and the temperature was gradually increased to 300 K in the NVT ensemble. The whole system was then relaxed in the NPT ensemble without restraint. The duration of the simulation was 200 ns. A snapshot was collected every 1 ps. All analyses were conducted using AMBER in-built modules and in-house code. The cluster analysis was performed using the linkage method to select the representative structure. The cutoff was set to 0.8 Å.
The noncovalent binding free energy was calculated using the molecular mechanics generalized born surface area (MM-GBSA) method [25]. The entropic contribution was not considered in the total binding energy; therefore, the total binding free energy between Laxiflorin B analogs and ERK1/2 included only the electrostatic contribution (EEL), the van der Waals (VDW) interaction contribution in the gaseous phase and the solvation free energy equal to the sum of the polar (EGB) and nonpolar (ESURF) contributions.
Cell apoptosis analysis
PC9 and HCC827 cells were seeded into 6-well plates (5 × 105/well) and cultured for 24 h at 37 °C. The attached cells were then treated with Laxiflorin B at 0, 1, 2 and 4 μM for 48 h. The cells were then stained with Annexin V and PI using the Annexin V-FITC Apoptosis Detection Kit (BioVision, USA) and analyzed using the FACSCalibur platform (BD Biosciences).
Xenografts in nude mice
All animal research procedures were performed according to the rules of the Animal Care and Use Ethics Committee of Shenzhen University Health Science Center, and all animals were treated in strict accordance with protocols approved by the Institutional Animal Use Committee of the Health Science Center, Shenzhen University. Animal experiments were performed in the Animal Center of Peking University Shenzhen Graduate School (approval number AP0023012). Female nu/nu nude mice (aged 4–6 weeks) were subcutaneously injected in the dorsal region with 5 × 106 PC9 cells. When the tumor volume reached 50 mm3 (after 7 days), saline and Laxiflorin B-Ala (5, 10, 20 mg/kg) were injected intraperitoneally once per day. Tumor size and mouse body weight were measured once every two days. Tumor volume was calculated according to the formula: volume = length × width2/2. After 3 weeks of Laxiflorin B treatment, mice were euthanized, and their tumor xenografts were harvested and weighed before immunohistochemical staining for phospho-RSK (1:50), AREG (1:50), EREG (1:50), Ki-67 (1:100).
Evaluation of apoptosis by TUNEL staining
The sections were deparaffinized twice using xylene, dehydrated in a gradient series of ethanol solutions and washed with distilled water. Apoptotic cells were then detected using the TUNEL staining kit (Servicebio Technology, Wuhan, China) according to the manufacturer’s instructions. Nuclei were counterstained with hematoxylin staining solution, differentiate solution and bluing solution then washed by pure water. Finally, the sections were dehydrated in 100% alcohol, soaked in n-butanol and xylene for at least 5 min to achieve transparency and then dried before adding resin mounting solution.
Statistical analyses
All in vitro experiments were performed at least in triplicate. Data were presented as the mean ± standard deviation and analyzed using Microsoft Excel 2010 Professional Plus (Version 14.0.7237.5000) and GraphPad Prism 6 (Version 6.01). Two-tailed, unpaired Student’s t-tests were used to compare differences between two groups with similar variances. For all tests, a P-value < 0.05 was considered to indicate a statistically significant difference.
Results
Semi-synthetic production of Laxiflorin B from its natural analog, Eriocalyxin B
Eriocalyxin B and Laxiflorin B were isolated from dried Isodon eriocalyx. laxiflora leaves with 0.084% and 0.00061% yields [18], respectively. To obtain large amounts of Laxiflorin B, we designed a semi-synthetic route illustrated in Fig. 1. The synthetic process was initiated by oxidative cleavage of the C6–C7 carbon–carbon bond of Eriocalyxin B by treatment with Dess–Martin periodinane. The aldehyde functional group of the oxidation product was then selectively reduced with NaBH4 under acidic conditions. Column chromatography showed that the total yield of Laxiflorin B was 70% (Fig. 1).
Fig. 1. Semi-synthetic production of Laxiflorin B from Eriocalyxin B.
Laxiflorin B was prepared at scale from Eriocalyxin B through oxidative cleavage of the C6–C7 carbon-carbon bond with Dess–Martin periodinane, followed by selective reduction of aldehyde with NaBH4 under acidic conditions.
Laxiflorin B inhibits the progression and promotes apoptosis of NSCLC cells
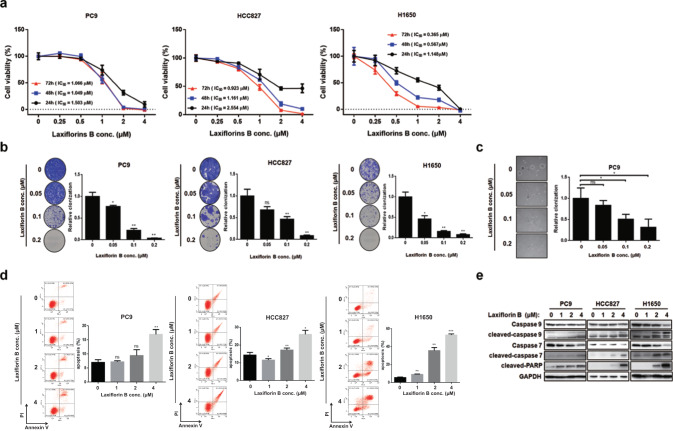
Laxiflorin B has been shown to inhibit cancer cell viability [17], whereas, the detail mechanism and antitumor efficacies in NSCLC still remained unclear. Therefore, we first examined the anticancer efficacy of Laxiflorin B in NSCLC cell lines. The cell growth of constitutive active EGFR mutated cells, such as PC9, HCC827, H1650 (Fig. 2a), H1975, and KRAS12S cell, including A549 (Fig. S1a and Table 1) was inhibited by Laxiflorin B in a dose-dependent manner. This dose-dependent inhibition of cell viability was confirmed in 2-D clonogenic assays using PC9, HCC827, H1650 (Fig. 2b), A549 and H1975 (Fig. S1b) cells. Laxiflorin B also inhibited the anchorage-independent growth of PC9 cells (Fig. 2c). These results indicated that Laxiflorin B is a natural compound with strong anticancer effects at relative low concentration.
Fig. 2. Inhibitory effects of Laxiflorin B on non-small cellular lung cancer cells.
a Viability of PC9, HCC827 and H1650 cells after Laxiflorin B treatment. b Clonogenic assays of PC9, HCC827 and H1650 cell growth after Laxiflorin B treatment for 2–3 weeks. c Anchorage-independent growth of PC9 cells after Laxiflorin B treatment for 31 days. d Flow cytometric analysis of apoptosis of PC9, HCC827 and H1650 cells following Laxiflorin B treatment for 48 h. e Western blot analysis of the expression of the apoptosis-related proteins, caspase 9, 7 and PARP, following Laxiflorin B treatment for 48 h. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 1.
IC50 of Laxiflorin B in non-small cellular lung cancer cell lines.
| Status | PC9 | HCC827 | H1650 | H1975 | A549 | |
|---|---|---|---|---|---|---|
| EGFR | Ex19del | Ex19del | Ex19del | L858R, T790M | WT | |
| KRAS | WT | WT | WT | WT | KRAS (G12S) | |
| IC50 (μM) | 24 h | 1.503 | 2.554 | 1.148 | 3.147 | 2.298 |
| 48 h | 1.049 | 1.161 | 0.5669 | 1.023 | 1.596 | |
| 72 h | 1.066 | 0.9231 | 0.3647 | 0.716 | 1.878 | |
To investigate the mechanism underlying the anticancer effects of Laxiflorin B, we assessed the ability of Laxiflorin B to induce apoptosis in the NSCLC cell lines PC9, HCC827 and H1650. Flow cytometric analysis showed that Laxiflorin B treatment for 48 h induced apoptosis in these cell lines in a dose-dependent manner (Fig. 2d). Next, we showed that the apoptotic markers, cleaved-caspase 7, 9 and PARP (Fig. 2e), were upregulated following Laxiflorin B treatment, while cleaved-caspase 3 was not (Fig. S1c, d), suggesting that Laxiflorin B induced apoptosis via the mitochondrial pathway. Taken together, these results clearly demonstrate the inhibitory effects of Laxiflorin B on the growth and survival of NSCLC cell lines.
ERK1/2 were identified as novel targets of Laxiflorin B
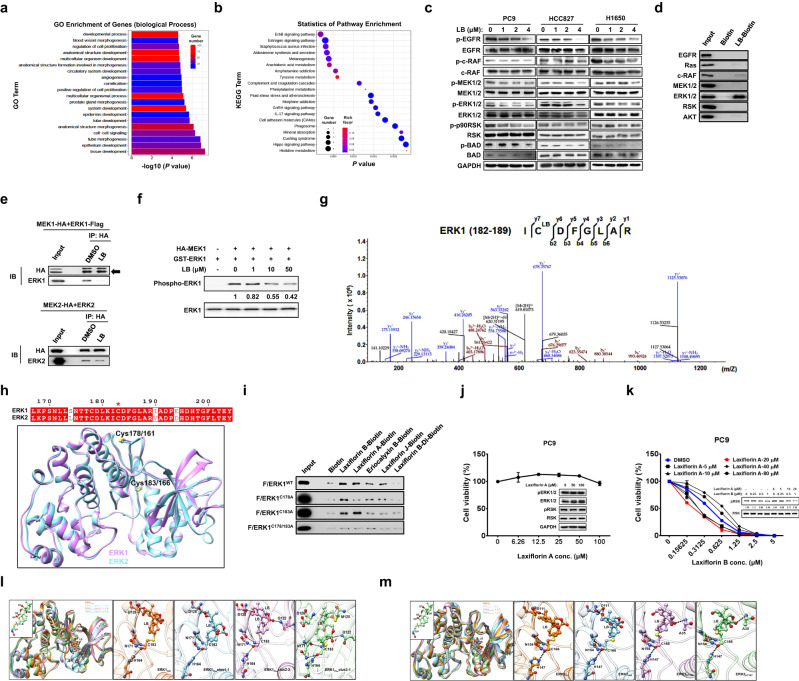
We next focused on the signaling pathways and molecular mechanism underlying the effects of Laxiflorin B by analyzing the changes in gene expression in Laxiflorin B-treated PC9 by transcriptome analysis (Fig. S2a–c). The enrichment of biological processes (Fig. 3a) and associated pathways (Fig. 3b) in PC9 cells after Laxiflorin B treatment were classified by gene ontology (GO) and KEGG pathway analyses, respectively. Genes associated with EGFR/ErbB-related signaling were significantly (P < 0.005) downregulated after Laxiflorin B administration (Fig. 3b and Table S1). Thus, we screened the status of EGFR/ErbB-associated molecules in NSCLC cells after Laxiflorin B treatment using DMSO treatment as a negative control (Fig. S3a). The levels of phospho-ERK1/2 and phospho-RSK, which are downstream components of the ErbB signaling pathway, were consistently and distinctly reduced in PC9, HCC827 and H1650 cells after Laxiflorin B treatment (Figs. 3c and S3b). The level of phosphorylated BAD, an apoptosis-induced factor [1] regulated by the ERK-RSK axis, was also reduced (Fig. 3c). In contrast, the stability of ERK1/2 proteins was not influenced by Laxiflorin B treatment (Fig. S3c). We also monitored the status of the MAPKp38 and STAT3 of EGFR downstream in NSCLC cells after Laxiflorin B treatment (Fig. S3d).
Fig. 3. ERK1/2 as a potential novel target of Laxiflorin B.
a, b GO and KEGG pathway analyses of the effects of 4 µM Laxiflorin B treatment for 48 h on the transcription of genes related to cellular functions and signaling pathways in PC9 cells. c Western blot analysis of EGFR-associated pathway proteins in PC9, HCC827 and H1650 cells following Laxiflorin B treatment for 24 h. d Pull-down assays of the interaction of Laxiflorin B with EGFR pathway-associated molecules. e Co-immunoprecipitation analysis of the inhibitory effect of Laxiflorin B on the interaction of MEK1 with ERK1 or MEK2 with ERK2. f In vitro kinase assay of the inhibitory effect of Laxiflorin B on MEK1-induced phosphorylation of ERK1. g HPLC–MS/MS analysis showing that Laxiflorin B (molecular weight: 344.1624) binds covalently with ERK1 on Cys-183. h Structural models of ERK1 and ERK2. The sequences of the conserved Cys-178/161 and Cys-183/166 in ERK1 and ERK2 are aligned at the top of model. i Pull-down assays of the interaction of Laxiflorin B analogs with wild-type and mutated ERK1. j CCK-8 assay and Western blot analysis of the cytotoxic effects of Laxiflorin A on PC9 cells. k CCK-8 assay and Western blot analysis of drug sensitivity and phospho-RSK expression, respectively, of PC9 cells co-treated with Laxiflorin A and B. l The predicted interactional model of EKR1 and Laxiflorin B from MD simulations. All representative structures are superimposed in the first panel. m The predicted interactional structures of EKR2 and Laxiflorin B from MD trajectories. The representative structures are superimposed in the first panel and models of the interactions between Laxiflorin B and EKR2 are shown in the following four panels. The fourth and fifth panels illustrate the interactions between Laxiflorin B and phosphorylated EKR2. The hydrogen bonds in k and l are represented by dotted lines.
Laxiflorin B binds directly to ERK1/2
These results showed that Laxiflorin B treatment significantly affected components of the ErbB signaling pathway, indicating that Laxiflorin B directly targets key molecules involved in ErbB signal transduction. To identify these targets, we investigated the binding of Laxiflorin B with ErbB downstream molecules using biotin pull-down assays. The results showed that Laxiflorin B binds ERK1/2 with high affinity compared with other molecules (Fig. 3d). ERK is the direct downstream effector of MEK in the MEK-ERK axis. Therefore, we investigated the influence of Laxiflorin B on this interaction by performing immunoprecipitation assays using HA-tagged MEK1 and MEK2. These studies showed that Laxiflorin B blocked the interaction between MEK1 and ERK1, and partially blocked the MEK2-ERK2 interaction (Fig. 3e). However, the interaction of MEK1 with ERK2 or MEK2 with ERK1 was not influenced by Laxiflorin B treatment (Fig. S3e). These results showed that Laxiflorin B binds directly to ERK1/2. To elucidate the effects of this interaction on ERK1/2, we also performed in vitro kinase activity assays, which showed that phospho-ERK1 levels decreased in a dose-dependent manner following Laxiflorin B treatment (Fig. 3f).
We further investigated the specificity with which Laxiflorin B targets ERK1/2 in NSCLC cells through knockdown (KD) mediated by sh-ERK1, sh-ERK2 and sh-ERK1/2. Laxiflorin B treatment decreased the viability of PC9 and HCC827 cells in a dose-dependent manner. In contrast, the sensitivities of PC9 and HCC827 cells to the Laxiflorin B-induced decrease in viability were inhibited by knockdown of ERK1, ERK2 or ERK1/2 (Fig. S3f–i), implicating ERK1/2 as potential targets of LB. Furthermore, Cysteine 178 (Cys-178, Fig. S4a) and Cysteine 183 (Cys-183, Fig. 3g) of ERK1 were identified as potential binding sites for Laxiflorin B by HPLC–MS/MS analysis. This was consistent with a previous report that Cys-183 is a covalent targeting site on ERK1 [26]. Cys-161 and Cys-166 of ERK2 were also identified as potential binding sites for Laxiflorin B (Fig. 3h). Similarly, Cys-166 was previously identified as binding site for FR148083, an irreversible covalent inhibitor of ERK1/2 [27]. The amino acid sequence homology of ERK1 and ERK2 reached 86% (Fig. S4b). Furthermore, the amino acid sequence from Cys-161 to Cys-166 of ERK2 was identical to the sequence from Cys-178 to Cys-183 of ERK1 (Fig. 3h). More importantly, both Cys-166 of ERK2 and Cys-183 of ERK1 were located within the ATP-binding pockets of ERK1 and ERK2, respectively (Fig. 3h). These results implicated ERK1/2 as potential target proteins in the mechanism by which Laxiflorin B inhibits the MEK-ERK axis.
The D-ring of Laxiflorin B links covalently with the side-chain of Cys-183 in the ATP-binding pocket of ERK1
To investigate the significance of the chemical structure of Laxiflorin B, we next analyzed the binding of the Laxiflorin B analogs, Laxiflorin A, Eriocalyxin B and Laxiflorin J to its target proteins [17, 18, 28]. Structurally, Eriocalyxin B retains both the A- and D-rings of Laxiflorin B, although the ring junctions differ. In Laxiflorin A, the enone functional group of the D-ring was selectively reduced. Laxiflorin J has a similar stereochemical structure to Eriocalyxin B, although the enone functional group of the A-ring is displaced. In Laxiflorin B-Di, which was synthesized by hydrogenation of Laxiflorin B, the enone functional groups of the A- and D-rings were displaced (Fig. S5). After biotin-labeling Laxiflorin B and the four analogs (Laxiflorin A, Eriocalyxin B, Laxiflorin J and Laxiflorin B-Di), we performed pull-down assays to investigate their binding to wild-type (ERK1WT-Flag), and mutant forms (ERK1C178A-Flag, ERK1C183A-Flag, ERK1C178A/C183A-Flag) of ERK1 overexpressed in HEK293T cells (Fig. 3i). The results indicated that Laxiflorin B interacted strongly with ERK1WT, ERK1C178A and ERK1C183A due to its functional A- and D-rings. Laxiflorin A interacted only with ERK1WT and ERK1C183A due to the targeting of Cys-178 on ERK1 by the A-ring. Eriocalyxin B and Laxiflorin J, which share similar ring junctions, but differ from Laxiflorin B, interacted with ERK1WT and ERK1C178A, but not ERK1 C183A, indicating that the D-rings play a significant role in targeting Cys-183 on ERK1. In addition, disruption of both the A- and D-rings of the Laxiflorin B-Di, or dual mutation of Cys-178 and Cys-183 on ERK1, resulted in the dissociation of Laxiflorin B analogs from ERK1 (Fig. 3i). Taken together, these results revealed that the D-ring of Laxiflorin B links covalently with the side-chain of Cys-183 in the ATP-binding pocket of ERK1, resulting in inhibition of ERK1 activity in the MEK-ERK-RSK axis of NSCLC cells.
Binding of Cys-178 by the A-ring of Laxiflorin A facilitated efficient targeting of Cys-183 of ERK1 by the D-ring of Laxiflorin B to promote cellular sensitivity
In accordance with previous findings, Laxiflorin A, the analog of Laxiflorin B without the D-ring (Fig. S6a), showed low levels of cytotoxicity against PC9 NSCLC cells (Figs. 3j and S6b, c) [17]. The molecular docking and molecular dynamic (MD) simulations show that Laxiflorin A occupies the small cavity around Cys-178. Leu132, His 142 and Phe146 of ERK1 provide a hydrophobic environment that favors Laxiflorin A binding (Fig. S6d). Gln136, Gln149, Asn175 and Thr176 form stable hydrogen bonds with the backbone and side-chain atoms in Laxiflorin A (Fig. S6e, f). However, the drug sensitivity and inhibition of the ERK-RSK axis were promoted in PC9 cells by co-treatment with Laxiflorin A and Laxiflorin B (Fig. 3k), which confirmed that binding of Cys-178 by the A-ring of Laxiflorin A facilitated efficient targeting of the Cys-183 of ERK1 by the D-ring of Laxiflorin B.
Laxiflorin B inhibited ERK activity by covalent binding to Cys-183/Cys-166 of ERK1/ERK2 and displacement of ATP from the binding pocket
Finally, we used MD simulation to generate a model of the interaction between ERK1/ERK2 and Laxiflorin B. Due to the free rotation of Laxiflorin B, Laxiflorin B exists in “in” and “out” conformations (Fig. S7a). Regardless of the conformation, the representative interactional models based on docking were found to be stable during the 200-ns unbiased MD simulation (Fig. S7b, c) in ERK1 and ERK2. The overlapped representative structures from cluster analysis showed that the interactions between Laxiflorin B and ERK1/ERK2 were almost identical (Fig. 3l, m). Laxiflorin B covalently binds to Cys-183 in ERK1 and Cys-166 in ERK2 to occupy the ATP-binding pocket and inhibit ERK activity. The probability that the backbone atoms of Cys-183/Cys-166 form several hydrogen bonds with His-164/His-147, Asn-171/Asn-154 was found to exceed 80% (Fig. S7b, c). The oxygen atom labeled O7 in the A-ring also has the ability to form conserved hydrogen bonds with Asp-128/Asp-111 in ERK1/2. Furthermore, the interactions of Laxiflorin B with ERK1 are dynamic, with the O7 atom of the A-ring and O4 atom of the D-ring also able to form hydrogen bonds with Met-125 and Gln-122, respectively. We also examined the well-known phosphorylated site at Thr185 and Tyr-187 of ERK2 on the binding of Laxiflorin B. We found that the hydrogen bonds between the O7 atom of the A-ring of Laxiflorin B and Asp-111 of ERK2 were disrupted by phosphorylation at Thr185 and Tyr-187 of ERK2, while the O5 atom of the D-ring formed hydrogen bonds with the backbone atoms of Ala-35 of ERK2 (Fig. 3m). Overall, we showed that Laxiflorin B binds covalently to Cys-183/Cys-166 of ERK1/ERK2 and expels the ATP molecule from the binding pocket, thereby inhibiting the activity of ERKs in the MEK-ERK-RSK axis of NSCLC cells.
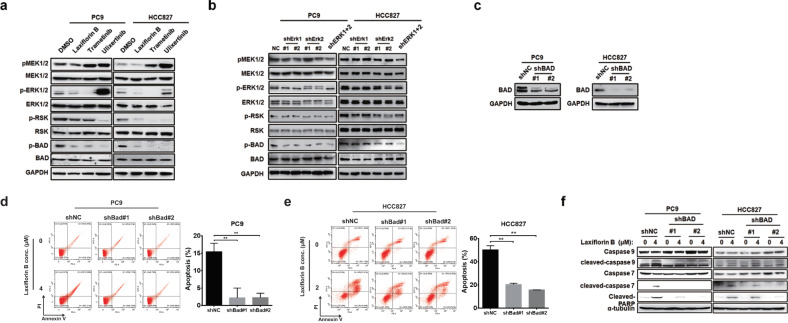
Laxiflorin B-induced apoptosis was mediated by BAD via ERK1/2 inhibition
Our previous data implied that Laxiflorin B-induced apoptosis in a caspase 3-independent and caspase 7-dependent manner (Figs. S1c, d and 2e), and the level of phosphorylated BAD was also reduced (Fig. 3c). Unphosphorylated BAD functions as a critical mediator of ERK-related apoptosis by promoting mitochondria-mediated cell death [1]. We validated the role of BAD in the inhibition of the MEK-ERK axis by Laxiflorin B using chemical and molecular biological approaches. Treatment of NSCLC cells with Laxiflorin B (1 µM), Trametinib (50 nM, MEK inhibitor) or Ulixertinib (1 µM, ERK inhibitor) resulted in similar reduction in the levels of phosphorylated BAD (Fig. 4a). Similar effects were also observed following dual knockdown (KD) of ERK1 and ERK2 by specific shRNAs in both PC9 and HCC827 cells (Figs. S8a, b and 4b). However, KD of either ERK1 or ERK2 was insufficient to diminish the level of BAD phosphorylation (Fig. 4b) in NSCLC cells.
Fig. 4. Laxiflorin B-induced apoptosis was mediated by BAD via ERK1/2 inhibition.
a Western blot analysis of the MEK-BAD axis in PC9 and HCC827 cells after treatment with Laxiflorin B, Trametinib (a MEK inhibitor, 50 nM), and Ulixertinib (an ERK1/2 inhibitor, 1 µM) for 24 h. b Western blot analysis of the MEK-BAD axis-related proteins in PC9 and HCC827 cells after Laxiflorin B treatment and ERK-knockdown (KD). c Western blot confirmation of the efficiency of BAD-KD in PC9 and HCC827 cells. d, e Flow cytometric analysis of apoptosis induced in PC9 and HCC827 with stable BAD-KD cells by Laxiflorin B treatment for 48 h. f Western blot analysis of apoptotic proteins (caspase 9, 7 and PARP) in PC9 and HCC827 cells with stable BAD-KD after Laxiflorin B treatment for 48 h. **P < 0.01.
We next investigated the significance of BAD in Laxiflorin B-induced apoptosis by specific shRNA-mediated KD of BAD. The efficiency of BAD-KD was confirmed at the mRNA (Fig. S8c) and protein (Fig. 4c) levels. In PC9 and HCC827 cells, BAD-KD significantly inhibited the Laxiflorin B-mediated reduction in cell viability in a dose- and time-dependent manner (Fig. S8d–f and Table S2). BAD-KD also significantly inhibited the Laxiflorin B-mediated reduction in colony formation capacity of PC9 and HCC827 cells (Fig. S8g, h). Expression of cleaved-caspase 7, 9 and PARP in PC9 and HCC827 cells was effectively induced by Laxiflorin B treatment, whereas BAD-KD significantly blocked Laxiflorin B-induced apoptosis and expression of cell death markers in both PC9 and HCC827 cells (Fig. 4d–f). Taken together, our results indicate that BAD plays a key role in the induction of apoptosis following Laxiflorin B-mediated inhibition of ERK1/2.
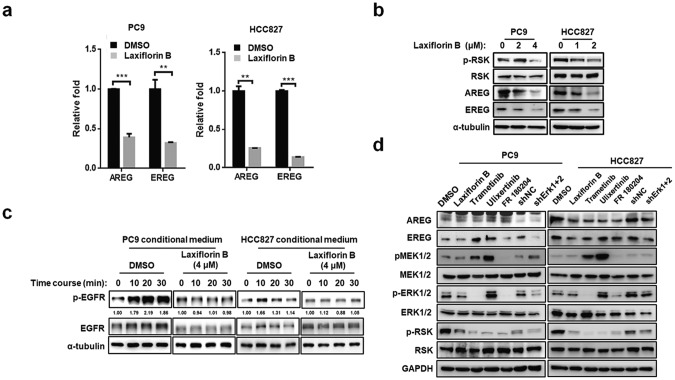
Autocrine production of ERK1/2-downstream growth factors was abolished by Laxiflorin B
Our data indicated that Laxiflorin B treatment influenced the phosphorylation status of ERK upstream signaling molecules, such as phospho-EGFR, phospho-RAF and phospho-MEK1/2 (Fig. 3c). KEGG pathway analysis of gene differential gene expression in Laxiflorin B-treated PC9 cells (Fig. S2a–c) showed that the genes encoding AREG and EREG were downregulated (4.41- fold and 2.40-fold, respectively) after Laxiflorin B administration (Fig. S9a and Table S1). AREG and EREG encode the ERK-regulated growth factors ARGE and EREG, respectively, which are ligands of the ErbB family signaling proteins and have been shown to promote cancer progression [10, 29]. The expression of AREG and EREG was significantly repressed by Laxiflorin B treatment at both the mRNA (Fig. 5a and Fig. S9b) and protein (Fig. 5b) levels in NSCLC cells. In addition, conditioned culture medium collected from Laxiflorin B-treated NSCLC cells did not induce EGFR activation by phosphorylation (Fig. 5c). Moreover, ERK-specific shRNAs suppressed the expression of both AREG and EREG by PC9 cells in a manner that resembled the inhibitory effect of Laxiflorin B (Fig. 5d). These results demonstrated that the positive feedback loop of the ERK-driven autocrine pathway is impaired by Laxiflorin B treatment.
Fig. 5. Autocrine production of ERK1/2-downstream growth factors was abolished by Laxiflorin B.
a Real-time PCR analysis of the expression of ERK1/2 downstream growth factors after Laxiflorin B treatment for 48 h. b Western blot analysis of the expression of ERK downstream growth factors after Laxiflorin B treatment for 48 h. c Western blot analysis of phosphorylated and unphosphorylated EGFR expression in starved PC9 or HCC827 cells treated with conditioned medium collected from cultures of PC9 or HCC827 cells treated with DMSO or Laxiflorin B for 48 h. d Western blot analysis of components of the MEK-ERK axis in starved PC9 cells treated with conditioned medium collected from cultures of PC9 or HCC827 cells treated with Laxiflorin B, ERK shRNA, and ERK or MEK inhibitors. **P < 0.01; ***P < 0.001.
Laxiflorin B exerted anticancer effects in vivo
To investigate the anticancer effects of Laxiflorin B in a xenograft model in vivo, we generated modifications of Laxiflorin B to increase the hydrophilic characteristics required to improve its solubility in normal saline for peritoneal injection.
Compared with the conserved structure of Eriocalyxin B, Laxiflorin B has widely modifiable space due to its flexible structure and the presence of a primary alcohol at C-6 position (Fig. 1). Laxiflorin B-PI and Laxiflorin B-Ala were modified by the addition of piperazine and an alanine group, respectively, at the C-6 position of Laxiflorin B to increase solubility (Fig. S10a). Although cell viability assays showed the inhibitory effect of Laxiflorin B-Ala was lower than those of Laxiflorin B and Laxiflorin B-PI (Fig. S10b and Table S3), the ERK-related axis was suppressed effectively by Laxiflorin B-Ala (Fig. S10c). Based on the in vivo toxicity profiles, Laxiflorin B-Ala was selected as the most promising compound due to the release of the non-toxic alanine group as a result of metabolism in mice.
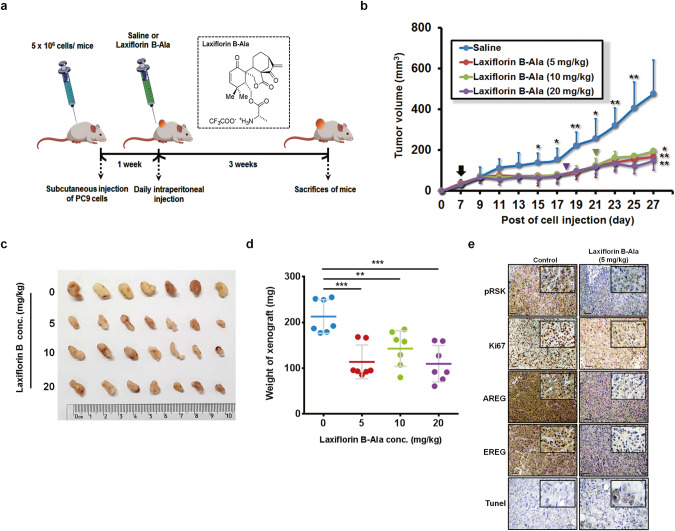
We next evaluated the in vivo effects and therapeutic potential of Laxiflorin B-Ala in a PC9 cell xenograft model established in nude mice. A schematic diagram of the experimental protocol is shown in Fig. 6a. In the groups receiving 10 and 20 mg/kg Laxiflorin B-Ala, treatment was terminated on days 15 and 12, respectively, due to a slight decrease in body weight. Subsequently, the reduction in body weight of the mice in these groups was reversed (Figs. 6b and S10d). Tumor growth was significantly suppressed by Laxiflorin B-Ala treatment (Fig. 6b–d), which was also found to be associated with low toxicity (Fig. S10e). IHC staining of the xenografts showed that expression of the cell proliferation marker Ki-67 and the ERK downstream markers, phospho-RSK, AREG and EREG, were inhibited by Laxiflorin B-Ala treatment in vivo, whereas, TUNEL staining revealed increased apoptosis (Fig. 6e). These findings indicate the potential value of AREG and EREG levels as appropriate biomarkers for evaluating the efficacy of Laxiflorin B treatment in vivo.
Fig. 6. Anticancer effect of Laxiflorin B in vivo.
a Schematic diagram of the experiment designed to evaluate the in vivo efficacy of Laxiflorin B-Ala and its therapeutic potential in the PC9 cell xenograft model established in nude mice. b Tumor volume was measured once every two days post-injection of Laxiflorin B-Ala for 3 weeks. Black arrow indicates the initiation of Laxiflorin B administration, green and purple inverted triangles indicate the termination of administration in the 10 and 20 mg/kg groups on days 15 and 12, respectively. c Tumors harvested from the control and Laxiflorin B-Ala-treated groups. d Tumor weight was measured after 3 weeks of Laxiflorin B treatment. e Immunohistochemical staining of the expression of phospho-RSK, Ki-67, AREG, EREG and TUNEL staining in xenografts. *P < 0.05; **P < 0.01; ***P < 0.001.
Evaluation of the modifiable capacity and efficacy of Laxiflorin B analogs
As a 6,7-seco-ent-kauranoid, the reduced spatial hindrance of the characteristic functional group on C6 renders Laxiflorin B suitable for modification. Therefore, we next modified the C-6 position hydroxyl group of Laxiflorin B to obtain more effective compounds for further investigation (Fig. 7a). Compared with unmodified Laxiflorin B, Laxiflorin B-4 and Laxiflorin B-5 had lower IC50 values in both PC9 (Fig. 7b and Table S4) and HCC827 (Fig. S11a) cells.
Fig. 7. The modifiable capacity of Laxiflorin B.
a Molecular structures of Laxiflorin B analogs modified by the addition of different functional groups. b IC50 of Laxiflorin B analogs against PC9 cells in CCK-8 assays. c Ball-stick model of the stable interaction between ERK1/2 and Laxiflorin B/Laxiflorin B-4. ERK1/2 is in cartoon model; the key residues interacting with Laxiflorin B and Laxiflorin B-4 are labeled. d The proposed free energy profile of the reaction pathway for Laxiflorin B and Laxiflorin B-4 was represented. e Western blot analysis of the inhibition of phosphorylated-RSK by Laxiflorin B and Laxiflorin B-4. f CCK-8 assay of the inhibitory effects of commercial ERK inhibitors, Laxiflorin B and Laxiflorin B-4 on PC9 cell viability. g Western blot analysis of the status of the MEK-ERK-RSK axis after treatment with commercial ERK inhibitors and Laxiflorin B analogs at 1 µM in PC9 cells.
We also used computational methods to investigate the interactions between Laxiflorin B-4 and ERK1/2 (Figs. 7c, S11b, c). We found that the additional benzene heterocycle in Laxiflorin B-4 occupied the adenosine-binding pocket of ERK1/2 that remains vacant in the interaction with Laxiflorin B. These findings were consistent with previous reports that Laxiflorin B only partially occupies the ATP-binding pocket of ERK1/2 [30, 31]. Chatterjee et al. proposed that the binding of a covalent inhibitor occurs in a stepwise process, which includes noncovalent and covalent binding stages [32]. While similar covalent bonds are formed during the binding of Laxiflorin B and Laxiflorin B-4 with ERKs, we focused on the relative binding free energy of the noncovalent binding step. As shown in Figure S11d, the free energy of noncovalent binding of Laxiflorin B-4 with both ERK1 and ERK2 was found to be approximately 5 kcal/mol greater than that of the corresponding interactions with Laxiflorin B. The additional benzene heterocycle in Laxiflorin B-4 contributes more than 20 kcal/mol hydrophobic energy. Interestingly, the free energy of the binding of both Laxiflorin B and Laxiflorin B-4 with ERK2 was approximately 5 kcal/mol greater than that of the interactions with ERK1. These findings were consistent with the proposed free energy changes during the noncovalent binding of Laxiflorin B and Laxiflorin B-4 with ERKs depicted in Fig. 7d. Moreover, compared with Laxiflorin B, Laxiflorin B-4 prolonged the inhibition of RSK phosphorylation (Fig. 7e) and enhanced the efficiency of cell growth inhibition (Fig. 7b), indicating that the additional functional group on Laxiflorin B-4 enhances the stability of the interaction with ERK1/2 (Fig. 7c). Considering the small size of Laxiflorin B, the large binding pocket of ERK1/2 offers the opportunity for the addition of chemical groups, indicating the enormous potential for modification of Laxiflorin B to improve the binding affinity with ERK1/2.
Next, we selected several commercial ERK inhibitors as standards to evaluate the inhibitory effects of Laxiflorin B and Laxiflorin B-4 in NSCLC cells. Interestingly, compared with the commercial ERK inhibitors, both Laxiflorin B and Laxiflorin B-4 exhibited superior efficacy in suppressing NSCLC cell viability (Figs. 7f, S11e and Table S5). Laxiflorin B also enhanced capacities to inhibit colony formation and promote apoptosis compared with well-known ERK1/2 inhibitors (Figs. 2b–d, and S11g, h). Furthermore, pathway screening of NSCLC cells implied that Laxiflorin B and Laxiflorin B-4 not only mediated marked suppression of RSK phosphorylation, but also abolished AKT compensation due to lower levels of AREG and EREG than those induced by other ERK inhibitors at the same concentration (Figs. 7g and S11f).
Proposed mechanism of the anticancer effects of Laxiflorin B in NSCLC
Taken together, our findings provide evidence that, in cancer progression, the MEK-ERK-RSK axis is activated to inhibit apoptosis via the EGFR signaling cascade through BAD phosphorylation and increased production of ErbB ligands, such as AREG and EREG, leading to the maintenance of cell growth and survival. Our results also indicate that Laxiflorin B inhibits cancer cell viability by stimulating a primary response involving either downregulation of AREG/EREG expression or blockade of BAD phosphorylation through covalent targeting of ERK1/2. The dephosphorylated BAD then initiates cell death via the BAD-caspase 7-PARP axis. The secondary response is then driven by downregulation of AREG and EREG to shut down the autocrine feedback loop resulting in inhibition of EGFR signaling in NSCLC. Furthermore, Laxiflorin A, an analog of Laxiflorin B, synergized with Laxiflorin B to enhance drug sensitivity of cancer cells by non-inhibitory competition (NIC) (Fig. 8).
Fig. 8. Laxiflorin B inhibits the EGFR-related pathway in non-small-cell lung cancer by BAD activation and blockage of EREG/AREG-EGFR axis via a positive feedback loop.

Laxiflorin B is improved its yield by semi-synthetic procedure from its analogue, Eriocalyxin B, which is isolated from Isodon eriocalyx. Mechanically, Laxiflorin B directly and covalently targets ERK1/2 and represses downstream RSK activation, promoting cell apoptosis through Bad activation. By secondary response, cell growth is suppressed by blockage of EREG/AREG-EGFR axis due to ERK1/2 inhibition by Laxiflorin B. Furthermore, Laxiflorin A, an analogue of Laxiflorin B, serves as a sensitizer in Laxiflorin B treatment. Laxiflorin B-4 and Laxiflorin B-Ala, the modified Laxiflorin B analogs, exhibit more efficacious and increases solubility to facilitate in vivo utilization, respectively.
Discussion
In our study, analysis of the LB-treated transcriptome in PC9 cells revealed the highest negative fold-change in expression of components of the ErbB pathway, which is consistent with the genetic background PC9 (EGFR ex19 del, constitutively active mutant). This information highlighted the role of the EGFR pathway in the effects of LB. According to this clue, we screened EGFR pathway effective proteins from up- to downstream, and found that LB downregulated EGFR signaling by reduction of the phosphorylated levels of effectors, as well as detecting the effectors of EGFR pathway binding with LB by biotin-pull-down assay. Finally, we identified ERK1/2 as the major target of LB among these effectors. Furthermore, we elucidated LB directly and covalently bound Cys-183 on ERK1 (corresponding to Cys-166 on ERK2) in the ATP pockets of ERK1/2 by LC–MS/MS, which abolished ERK1/2 kinase activity. Based on our multi-disciplinary investigations and data, we suggest Laxiflorin B as the first ERK1/2 inhibitor derived from a natural plant.
The docking and MD simulation results show that Laxiflorin B occupies the ATP-binding pocket and forms stable interactions with ERK1. However, ERK1 point mutation studies revealed that approximately 50% of Laxiflorin B is degraded at the Cys-178 residue outside the ATP-binding pocket of ERK1, which highlights the potential for structural modification of Laxiflorin B to enhance pharmacologic efficacy. Simulation of the potential binding mode of the ERK2 crystal structure has also shown that Cys-166 residues (corresponding to Cys-183 on ERK1) are located in a relatively inaccessible region of the ATP-binding pocket [33]. In contrast, sulfonamides may contribute to the formation of appropriate bonds, thus highlighting the significance of Cys-166 in the ERK2 binding model. For example, FR148083 [27] and CC-90003 [26] were reported as irreversible covalent inhibitors of Cys-166 on ERK2 [34] and Cys-183/184 on ERK1/2, respectively. Interestingly, due to the lack of D-ring activity, Laxiflorin A binds strongly to Cys-178/161 of ERK1/2 through the A-ring, which is non-toxic to cells. We applied the reported isolation ratio of Laxiflorin A and B from herbs of 16:1 [18] in our co-treatment-experiment, and found that the sensitivity to Laxiflorin B treatment was enhanced by non-toxic Laxiflorin A via non-inhibitory competition in NSCLC cells. The ERK-targeting ability and lack of toxicity indicate that Laxiflorin A has important potential for the development for ERK-related applications.
Human ERK1/2 are composed of 379/360 amino acids with molecular weights of 44/42 kDa and a sequence similarity of approximately 85% (higher at the ATP-binding sites). In previous studies, ERK1/2 activation was found to be highly negatively correlated with the prognosis of cancer patients [35]. Currently, the designation of ERK1/2 inhibitors is based mainly on modification of the existing compound skeleton. By combining a high-throughput screening platform with molecular dynamics simulation to rank a range of lead compounds for further research, a detailed model of the binding mode can be resolved at the atomic level. Among the previously reported ERK1/2 inhibitors [36], tetrahydropyridine, indazole and tetrahydropyrazole pyridine compounds, such as MK8353 (SCH772984 analog) [37], BVD-523 [7] and GDC-0994 [9], provide the most suitable backbones for further research. Some of these compounds have shown efficacy in the preclinical stage of development [36, 38]. However, with the exception of FR148083 [27] and CC-90003 [26], most ERK1/2 inhibitors bind to their target via reversible and noncovalent interactions. Kinome analysis [39] has shown that many kinases have cysteine residues in and around the ATP-binding site, offering numerous opportunities for covalent reactions with compounds harboring an electrophilic Michael Acceptor in the required position. However, to date, only a few cysteine positions have been targeted by covalent inhibitors. Hence, our discovery of the covalent binding of ERK1/2 by Laxiflorin B is an exciting finding that highlights the possibility of further modification of Laxiflorin B to enhance its potential as an ERK1/2 inhibitor.
Due to the significance of the MEK-ERK axis in tumor development and progression, MEK inhibitors, such as trametinib, selumetinib and MEK162, have been widely studied in RAS- or RAF-driven cancers. As the unique downstream effector of MEK, ERK1/2 activation is critical for cancer progression, and its inhibitors, such as LY3124996 and SCH772984, have been extensively investigated in lung cancer, colorectal cancer and pancreatic cancer with KRAS mutations [15, 40]. However, inhibition of the MEK-ERK axis often results in compensatory AKT activation [37], which is associated with negative therapeutic outcomes. Therefore, we speculate that the therapeutic efficacy of MEK-ERK inhibition could be maximized by synergy with TKI or AKT inhibitors.
Regarding to the role of AREG and EREG in our study, we demonstrated that Laxiflorin B binds irreversibly and covalently to its target site in ERK1/2 to suppress its activation, leading to downregulation of its downstream target genes, AREG and EREG. In turn, this inhibition attenuates not only the RAF-MEK-ERK axis, but also AKT compensation via inhibition of the positive feedback loop. As our knowledge, EGFR and RAS are the two major types of constitutively active mutations identified in lung cancer, and both are upstream kinases of the ERK-involved MAPK pathway. Either EGFR or RAS can trigger ERK-involved MAPK pathway activation either cooperatively or individually. Intriguingly, we found NSCLC cells harboring constitutively active EGFR are more sensitive than those with constitutively active KRAS, suggesting that inhibition of the positive feedback loop of the AREG/EREG-EGFR axis plays a key role in Laxiflorin B-induced tumor suppression. Under this scenario, we indicated LB is more efficacious in tumor cells with constitutively active EGFR, not only in NSCLC, but also in breast cancer, head and neck cancer, bladder cancer, colon cancer, etc.
Phosphorylated-ERK1/2 plays a vital role in cancer progression [35]. Mechanistically, phosphorylated-ERK1/2 interacts with Importin7 (Imp7) to facilitate its nuclear translocation, which can be blocked by the myr-EPE peptide [41]. Nuclear ERK1/2 then binds directly to specific DNA elements to influence genes expression in immune cells [42], pluripotent embryonic stem cells [43], and cancer cells [44, 45]. Compared with other ERK inhibitors analyzed in this study (Figs. 7g and S11f), Laxiflorin B strongly repressed ERK1/2 phosphorylation, which might sequentially result in inhibition of AKT compensation due to AREG and EREG suppression. Thus, compared with noncovalent ERK1/2 inhibitors, the primary inhibitory effects of Laxiflorin B are enhanced by the secondary feedback inhibition. However, whether phospho-ERK1/2 is associated with ARGE and EREG expression and nuclear translocation of ERKi-bound ERK1/2 remain to be clarified. Our data suggest that phospho-ERK1/2 levels and ERK1/2 downstream gene expression, such as ARGE and EREG, should be evaluated in combination with phospho-RSK status when evaluating ERKi efficacy in the future.
Previous studies showed that expression of AREG and EREG, which encode secretory proteins, is regulated by FOSL1 [46] and ETS1 [47], respectively, which are transcription factor components of the MEK-ERK axis. High AREG or EREG expression is associated with a poor prognosis and lower survival rate in lung cancer patients, thus implicating these genes as targets for personalized cancer therapy [29, 48]. In our study, AREG, EREG and AKT compensation was repressed by Laxiflorin B treatment due to ERK1/2 inhibition. Therefore, it can be speculated that AREG or EREG levels in blood or body fluids represent a valuable marker that directly reflects the efficacies of Laxiflorin B and other ERK inhibitors for use in future clinical applications. However, EREG might be as a more accurate indicator because AREG can be adsorbed by extracellular matrix heparin sulfate proteoglycans in the extracellular space [49].
Laxiflorin B is a liposoluble molecule with low solubility in normal saline for peritoneal injection, which is a challenge that must be overcome for in vivo studies. To improve its water-solubility, alanine trifluoroacetate was used to generate the analog Laxiflorin B-Ala by esterification modification of the C-6 hydroxyl group of Laxiflorin B. Despite the slightly inferior inhibitory activity of Laxiflorin B-Ala in vitro, this modification facilitated pharmacodynamic evaluation of Laxiflorin B in vivo. After diffusing into the plasma, the C-6 position ester group of Laxiflorin B-Ala is hydrolyzed by an esterase, leading to Laxiflorin B release. However, since alanine trifluoroacetate accounts for 35% of the molecular weight of Laxiflorin B-Ala, the actual concentration of Laxiflorin B in our model was far lower than the administered dose of Laxiflorin B-Ala. In the xenograft model, we used three different concentrations of Laxiflorin B. At days 11 and 14 after Laxiflorin B treatment at 20 and 10 mg/kg, respectively, the weight of mice was slightly decreased (Supplementary Fig. S10d). At this point, we suspended the drug administration and continued to monitor the mice and tumor growth. Up to the study end-point (day 21), the body weight and all the side-effects were recovered. Furthermore, the tumor showed only a slight increase in size under the drug-free conditions, with no cytotoxicity detected in the organs (Supplementary Fig. S10e). Therefore, the dose-dependent effects of the inhibitor were not observed at the end-point of the study.
Laxiflorin B was the first covalent-natural compound shown to exhibit ERK-targeting and dramatic anticancer activities. Although previous studies have shown that Laxiflorin A, an analog of Laxiflorin B, has little antitumor activity [17], our clarification of the molecular mechanism has redefined the value of Laxiflorin A for this purpose. First, since Laxiflorin A binds to Cys-178/161 on ERK1, removal of the A-ring activity of Laxiflorin B might increase its effective binding to ERK1/2. Therefore, the drug sensitivity of tumor cells could be promoted by combined treatment with Laxiflorin A and B. Moreover, as a molecular probe of ERK, Laxiflorin A might offer great potential for the design of compounds as molecular probes of ERK for use in research or clinical applications. The synergistic activity of multiple components of herbs is a longstanding focus of research into their clinical application [50]. This phenomenon, which is consistent with the compound concept of traditional Chinese medicine, first prompted us to propose the existence of non-inhibitory competition (NIC) between Laxiflorin A and B. This type of interaction between natural small molecules might greatly improve the efficacy of active ingredients in natural substances.
In this study, we developed a well-defined procedure for the synthesis and modification of Laxiflorin B and validated its activity as an ERK1/2 inhibitor both in vitro and in vivo. With its immense potential for modification, we propose that Laxiflorin B represents an important compound that can be manipulated to improve specificity and affinity. As a novel ERK inhibitor for suppressing MEK-ERK-dependent malignancies, Laxiflorin B offers the potential for use alone or in combination with TKI or other inhibitors in both antitumor therapy and addressing drug resistance. Our finding also provides a reference for further investigation of the anticancer effects of compounds derived from herbs.
Supplementary information
Acknowledgements
The authors would like to thank Dr. Jessica Tamanini of ETediting for editing the manuscript prior to submission and the Instrumental Analysis Center of Shenzhen University for the support. This work was supported by the Natural Science Foundation of Guangdong Province (No. 2021A1515011046, 2021A1515010996), the Guangdong Provincial Science and Technology Program (No. 2017B030301016), the Regional Joint Fund of Guangdong Province (Grant No. 2019B1515120080), the Shenzhen Municipal Government of China (No. JCYJ20210324093408024), the Shenzhen Key Medical Discipline Construction Fund (No. SZXK060).
Author contributions
Conceived and designed the experiments: DZ, LZZ and CYC. Chemical compound synthesis: JRH and MY. Conducted most of the experiments and analyzed the data: MZ, CYC, JRH, CLC, DMP, TTZ, HY and TX. Construction of plasmids and lentivirus packaging: MZ, CYC. and CLC. Animal studies: CYC and FY. Performed the LC–MS/MS and proteomic analysis: QQH, ZW and YDZ. Performed the computer simulation of Laxiflorin B and ERK1/2 crystal structures: JZ. Planning and discussion of the project: DZ and LZZ. Supervised the entire project: YCC, DZ and LZZ. Wrote the manuscript, designed the layout of figures and tables: CYC, DZ, ZGL, and JZ.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Cheng-Yao Chiang, Min Zhang, Junrong Huang, Juan Zeng
Contributor Information
Lizhi Zhu, Email: lzzhu86@pku.edu.cn.
Duo Zheng, Email: dzheng@szu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01164-w.
References
- 1.Lavoie H, Gagnon J, Therrien M. ERK signalling: a master regulator of cell behaviour, life and fate. Nat Rev Mol Cell Biol. 2020;21:607–32.. doi: 10.1038/s41580-020-0255-7. [DOI] [PubMed] [Google Scholar]
- 2.Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13:928–42. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 3.Arcila ME, Drilon A, Sylvester BE, Lovly CM, Borsu L, Reva B, et al. MAP2K1 (MEK1) mutations define a distinct subset of lung adenocarcinoma associated with smoking. Clin Cancer Res. 2015;21:1935–43. doi: 10.1158/1078-0432.CCR-14-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degirmenci U, Wang M, Hu J. Targeting aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells. 2020;9:198. doi: 10.3390/cells9010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenan L, Andreev A, Cohen O, Pantel S, Kamburov A, Cacchiarelli D, et al. Phenotypic characterization of a comprehensive set of MAPK1/ERK2 missense mutants. Cell Rep. 2016;17:1171–83.. doi: 10.1016/j.celrep.2016.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roskoski R., Jr Targeting ERK1/2 protein-serine/threonine kinases in human cancers. Pharmacol Res. 2019;142:151–68.. doi: 10.1016/j.phrs.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Germann UA, Furey BF, Markland W, Hoover RR, Aronov AM, Roix JJ, et al. Targeting the MAPK signaling pathway in cancer: promising preclinical activity with the novel selective ERK1/2 inhibitor BVD-523 (Ulixertinib) Mol Cancer Ther. 2017;16:2351–63.. doi: 10.1158/1535-7163.MCT-17-0456. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan RJ, Infante JR, Janku F, Wong DJL, Sosman JA, Keedy V, et al. First-in-class ERK1/2 inhibitor ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: results of a phase I dose-escalation and expansion study. Cancer Discov. 2018;8:184–95.. doi: 10.1158/2159-8290.CD-17-1119. [DOI] [PubMed] [Google Scholar]
- 9.Blake JF, Burkard M, Chan J, Chen H, Chou KJ, Diaz D, et al. Discovery of (S)-1-(1-(4-Chloro-3-fluorophenyl)-2-hydroxyethyl)-4-(2-((1-methyl-1H-pyrazol-5-y l)amino)pyrimidin-4-yl)pyridin-2(1H)-one (GDC-0994), an extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor in early clinical development. J Med Chem. 2016;59:5650–60. doi: 10.1021/acs.jmedchem.6b00389. [DOI] [PubMed] [Google Scholar]
- 10.Sunaga N, Kaira K, Imai H, Shimizu K, Nakano T, Shames DS, et al. Oncogenic KRAS-induced epiregulin overexpression contributes to aggressive phenotype and is a promising therapeutic target in non-small-cell lung cancer. Oncogene. 2013;32:4034–42. doi: 10.1038/onc.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhagwat SV, McMillen WT, Cai S, Zhao B, Whitesell M, Shen W, et al. ERK inhibitor LY3214996 targets ERK pathway-driven cancers: a therapeutic approach toward precision medicine. Mol Cancer Ther. 2020;19:325–36.. doi: 10.1158/1535-7163.MCT-19-0183. [DOI] [PubMed] [Google Scholar]
- 12.Varga A, Soria JC, Hollebecque A, LoRusso P, Bendell J, Huang SA, et al. A first-in-human phase I study to evaluate the ERK1/2 inhibitor GDC-0994 in patients with advanced solid tumors. Clin Cancer Res. 2020;26:1229–36.. doi: 10.1158/1078-0432.CCR-19-2574. [DOI] [PubMed] [Google Scholar]
- 13.Kirouac DC, Schaefer G, Chan J, Merchant M, Orr C, Huang SA, et al. Clinical responses to ERK inhibition in BRAF(V600E)-mutant colorectal cancer predicted using a computational model. NPJ Syst Biol Appl. 2017;3:14. doi: 10.1038/s41540-017-0016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moschos SJ, Sullivan RJ, Hwu WJ, Ramanathan RK, Adjei AA, Fong PC, et al. Development of MK-8353, an orally administered ERK1/2 inhibitor, in patients with advanced solid tumors. JCI Insight. 2018;3:e92352. doi: 10.1172/jci.insight.92352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 2013;3:742–50. doi: 10.1158/2159-8290.CD-13-0070. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Wang WG, Sun HD, Pu JX. Diterpenoids from Isodon species: an update. Nat Prod Rep. 2017;34:1090–140.. doi: 10.1039/C7NP00027H. [DOI] [PubMed] [Google Scholar]
- 17.Wang WG, Du X, Li XN, Wu HY, Liu X, Shang SZ, et al. New bicyclo[3.1.0]hexane unit ent-kaurane diterpene and its seco-derivative from Isodon eriocalyx var. laxiflora. Org Lett. 2012;14:302–5. doi: 10.1021/ol203061z. [DOI] [PubMed] [Google Scholar]
- 18.Sun HD, Lin ZW, Niu FD, Shen PQ, Pan LT, Lin LZ, et al. Diterpenoids from Isodon eriocalyx var. laxiflora. Phytochemistry. 1995;38:1451–5. doi: 10.1016/0031-9422(94)00815-B. [DOI] [PubMed] [Google Scholar]
- 19.van Zundert GCP, Rodrigues JPGLM, Trellet M, Schmitz C, Kastritis PL, Karaca E, et al. The HADDOCK2.2 web server: user-friendly integrative modeling of biomolecular complexes. J Mol Biol. 2016;428:720–5. doi: 10.1016/j.jmb.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Rao S, Gurbani D, Du G, Everley RA, Browne CM, Chaikuad A, et al. Leveraging compound promiscuity to identify targetable cysteines within the kinome. Cell Chem Biol. 2019;26:818–29.e9. doi: 10.1016/j.chembiol.2019.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Case DA, Ben-Shalom IY, Brozell SR, Cerutti DS, Cheatham TEI, Cruzeiro VWD, et al. AMBER 2018, San Francisco: University of California; 2018.
- 22.Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE, Simmerling C. FF14SB: improving the accuracy of protein side chain and backbone parameters from FF99SB. J Chem Theory Comput. 2015;11:3696–713. doi: 10.1021/acs.jctc.5b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of a general amber force field. J Comput Chem. 2004;25:1157–74. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–35. doi: 10.1063/1.445869. [DOI] [Google Scholar]
- 25.Qiu D, Shenkin PS, Hollinger FP, Still WC. The GB/SA continuum model for solvation. a fast analytical method for the calculation of approximate born radii. J Phys Chem A. 1997;101:3005–14.. doi: 10.1021/jp961992r. [DOI] [Google Scholar]
- 26.Aronchik I, Dai Y, Labenski M, Barnes C, Jones T, Qiao L, et al. Efficacy of a covalent ERK1/2 inhibitor, CC-90003, in KRAS-mutant cancer models reveals novel mechanisms of response and resistance. Mol Cancer Res. 2019;17:642–54.. doi: 10.1158/1541-7786.MCR-17-0554. [DOI] [PubMed] [Google Scholar]
- 27.Ohori M, Kinoshita T, Yoshimura S, Warizaya M, Nakajima H, Miyake H. Role of a cysteine residue in the active site of ERK and the MAPKK family. Biochem Biophys Res Commun. 2007;353:633–7. doi: 10.1016/j.bbrc.2006.12.083. [DOI] [PubMed] [Google Scholar]
- 28.Niu XM, Li SH, Li ML, Zhao QS, Mei SX, Na Z, et al. Cytotoxic ent-kaurane diterpenoids from Isodon eriocalyx var. laxiflora. Planta Med. 2002;68:528–33. doi: 10.1055/s-2002-32551. [DOI] [PubMed] [Google Scholar]
- 29.Elangovan IM, Vaz M, Tamatam CR, Potteti HR, Reddy NM, Reddy SP. FOSL1 promotes Kras-induced lung cancer through amphiregulin and cell survival gene regulation. Am J Respir Cell Mol Biol. 2018;58:625–35.. doi: 10.1165/rcmb.2017-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lechtenberg BC, Mace PD, Sessions EH, Williamson R, Stalder R, Wallez Y, et al. Structure-guided strategy for the development of potent bivalent ERK inhibitors. ACS Med Chem Lett. 2017;8:726–31.. doi: 10.1021/acsmedchemlett.7b00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garai Á, Zeke A, Gógl G, Törő I, Fördős F, Blankenburg H, et al. Specificity of linear motifs that bind to a common mitogen-activated protein kinase docking groove. Sci Signal. 2012;5:ra74. doi: 10.1126/scisignal.2003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee P, Botello-Smith WM, Zhang H, Qian L, Alsamarah A, Kent D, et al. Can relative binding free energy predict selectivity of reversible covalent inhibitors? J Am Chem Soc. 2017;139:17945–52.. doi: 10.1021/jacs.7b08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward RA, Colclough N, Challinor M, Debreczeni JE, Eckersley K, Fairley G, et al. Structure-guided design of highly selective and potent covalent inhibitors of ERK1/2. J Med Chem. 2015;58:4790–801. doi: 10.1021/acs.jmedchem.5b00466. [DOI] [PubMed] [Google Scholar]
- 34.Ohori M, Kinoshita T, Okubo M, Sato K, Yamazaki A, Arakawa H, et al. Identification of a selective ERK inhibitor and structural determination of the inhibitor-ERK2 complex. Biochem Biophys Res Commun. 2005;336:357–63. doi: 10.1016/j.bbrc.2005.08.082. [DOI] [PubMed] [Google Scholar]
- 35.Zhao S, Qiu ZX, Zhang L, Li WM. Prognostic values of ERK1/2 and p-ERK1/2 expressions for poor survival in non-small cell lung cancer. Tumour Biol. 2015;36:4143–50. doi: 10.1007/s13277-015-3048-4. [DOI] [PubMed] [Google Scholar]
- 36.Blake JF, Gaudino JJ, De Meese J, Mohr P, Chicarelli M, Tian H, et al. Discovery of 5,6,7,8-tetrahydropyrido[3,4-d]pyrimidine inhibitors of Erk2. Bioorg Med Chem Lett. 2014;24:2635–9. doi: 10.1016/j.bmcl.2014.04.068. [DOI] [PubMed] [Google Scholar]
- 37.Chiang CY, Pan CC, Chang HY, Lai MD, Tzai TS, Tsai YS, et al. SH3BGRL3 protein as a potential prognostic biomarker for urothelial carcinoma: a novel binding partner of epidermal growth factor receptor. Clin Cancer Res. 2015;21:5601–11. doi: 10.1158/1078-0432.CCR-14-3308. [DOI] [PubMed] [Google Scholar]
- 38.Deng Y, Shipps GW, Jr., Cooper A, English JM, Annis DA, Carr D, et al. Discovery of novel, dual mechanism ERK inhibitors by affinity selection screening of an inactive kinase. J Med Chem. 2014;57:8817–26. doi: 10.1021/jm500847m. [DOI] [PubMed] [Google Scholar]
- 39.Leproult E, Barluenga S, Moras D, Wurtz JM, Winssinger N. Cysteine mapping in conformationally distinct kinase nucleotide binding sites: application to the design of selective covalent inhibitors. J Med Chem. 2011;54:1347–55. doi: 10.1021/jm101396q. [DOI] [PubMed] [Google Scholar]
- 40.Kohler J, Zhao Y, Li J, Gokhale PC, Tiv HL, Knott AR, et al. ERK inhibitor LY3214996-based treatment strategies for RAS-driven lung cancer. Mol Cancer Ther. 2021;20:641–54.. doi: 10.1158/1535-7163.MCT-20-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plotnikov A, Flores K, Maik-Rachline G, Zehorai E, Kapri-Pardes E, Berti DA, et al. The nuclear translocation of ERK1/2 as an anticancer target. Nat Commun. 2015;6:6685. doi: 10.1038/ncomms7685. [DOI] [PubMed] [Google Scholar]
- 42.Hu S, Xie Z, Onishi A, Yu X, Jiang L, Lin J, et al. Profiling the human protein-DNA interactome reveals ERK2 as a transcriptional repressor of interferon signaling. Cell. 2009;139:610–22. doi: 10.1016/j.cell.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tee WW, Shen SS, Oksuz O, Narendra V, Reinberg D. Erk1/2 activity promotes chromatin features and RNAPII phosphorylation at developmental promoters in mouse ESCs. Cell. 2014;156:678–90. doi: 10.1016/j.cell.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madak-Erdogan Z, Lupien M, Stossi F, Brown M, Katzenellenbogen BS. Genomic collaboration of estrogen receptor alpha and extracellular signal-regulated kinase 2 in regulating gene and proliferation programs. Mol Cell Biol. 2011;31:226–36. doi: 10.1128/MCB.00821-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Cheng HS, Chng WJ, Tergaonkar V. Activation of mutant TERT promoter by RAS-ERK signaling is a key step in malignant progression of BRAF-mutant human melanomas. Proc Natl Acad Sci USA. 2016;113:14402–7. doi: 10.1073/pnas.1611106113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee MH, Yanagawa J, Tran L, Walser TC, Bisht B, Fung E, et al. FRA1 contributes to MEK-ERK pathway-dependent PD-L1 upregulation by KRAS mutation in premalignant human bronchial epithelial cells. Am J Transl Res. 2020;12:409–27.. [PMC free article] [PubMed] [Google Scholar]
- 47.Cho MC, Choi HS, Lee S, Kim BY, Jung M, Park SN, et al. Epiregulin expression by Ets-1 and ERK signaling pathway in Ki-ras-transformed cells. Biochem Biophys Res Commun. 2008;377:832–7. doi: 10.1016/j.bbrc.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 48.Sunaga N, Kaira K. Epiregulin as a therapeutic target in non-small-cell lung cancer. Lung Cancer. 2015;6:91–8. doi: 10.2147/LCTT.S60427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh B, Coffey RJ. Trafficking of epidermal growth factor receptor ligands in polarized epithelial cells. Annu Rev Physiol. 2014;76:275–300. doi: 10.1146/annurev-physiol-021113-170406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Zhang Z, Li S, Ye X, Li X, He K. Synergy effects of herb extracts: pharmacokinetics and pharmacodynamic basis. Fitoterapia. 2014;92:133–47. doi: 10.1016/j.fitote.2013.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.