Abstract
Sustained attention, the ability to focus on an activity or stimulus over time, is significantly impaired in many psychiatric disorders, and there remains a major unmet need in treating impaired attention. Continuous performance tests (CPTs) were developed to measure sustained attention in humans, non-human primates, rats, and mice, and similar neural circuits are engaged across species during CPT performance, supporting their use in translational studies to identify novel therapeutics. Here, we identified electrophysiological correlates of attentional performance in a touchscreen-based rodent CPT (rCPT) in the locus coeruleus (LC) and prelimbic cortex (PrL), two inter-connected regions that are implicated in attentional processes. We used viral labeling and molecular techniques to demonstrate that neural activity is recruited in LC-PrL projections during the rCPT, and that this recruitment increases with cognitive demand. We implanted male mice with depth electrodes within the LC and PrL for local field potential (LFP) recordings during rCPT training, and identified an increase in PrL delta and theta power, and an increase in LC delta power during correct responses in the rCPT. We also found that the LC leads the PrL in theta frequencies during correct responses while the PrL leads the LC in gamma frequencies during incorrect responses. These findings may represent translational biomarkers that can be used to screen novel therapeutics for drug discovery in attention.
Subject terms: Neuroscience, Cognitive neuroscience
Introduction
Attention deficits are common in patients diagnosed with, as well as those at high genetic risk for a range of neuropsychiatric disorders. This includes children [1, 2], and adults [3] with attention-deficit hyperactivity disorder (ADHD), patients in remission from major depressive disorder [4, 5], as well as adults diagnosed with schizophrenia [6–8] and children at risk for schizophrenia [9]. Attention function in individuals with these disorders is commonly measured with continuous performance tests (CPTs) [10]. To successfully perform CPTs, subjects must focus their attention on an auditory [11] or visual stimulus. If one stimulus exemplar is presented (the “target” or S+), the subject must initiate a “go” response (for example, pressing a lever [10]). If a second stimulus exemplar is presented (the “non-target”), subjects must withhold responding. Subjects can therefore make errors of omission (failing to respond to a target), or errors of commission (responding to a non-target), which may differentiate underlying deficits in motivation or cognitive control [12, 13]. CPTs are highly effective tools for assessing attention deficits in patient populations, as CPT performance is a much better predictor of clinical ADHD diagnosis than parent reports of ADHD symptoms [14]. Despite widespread use of CPTs as attention assays, the neural mechanisms that govern performance are not well-understood.
Investigating the neural correlates of behavior during CPTs has been impeded by several barriers. First, multiple versions of CPTs exist [15–19], which complicates attempts to identify brain activity patterns that might generalize across experimental settings. Second, it is inherently difficult to obtain brain activity data in humans that is both spatially and temporally resolved without using invasive techniques. To overcome these barriers, a standardized touchscreen-based rodent version of the CPT (rCPT) was developed using task parameters that influence sustained attention in human CPTs [20–22]. This rCPT provides the opportunity to study how brain function contributes to performance in a task with high translational potential; indeed, rodent models of developmental disruption with schizophrenia-relevant phenotypes are consistently impaired in this rCPT [23, 24]. Identifying biomarkers in this task would therefore be invaluable for advancing diagnostic criteria and novel treatment strategies for the myriad disorders that feature sustained attention deficits.
The anterior cingulate cortex (ACC) is active during attention tasks in humans, particularly under conditions of high attentional load (i.e., when distractors are present [25–28]). In concert with these findings, ACC activity is diminished during CPT performance in individuals diagnosed with ADHD [3, 29], and schizophrenia [30, 31], suggesting that the ACC is a neuroanatomical locus for attentional processing. Recent studies suggest that the primate ACC may be most anatomically and functionally homologous with the area commonly referred to as the medial prefrontal cortex (mPFC) in the rodent [32, 33], which typically includes both the prelimbic cortex (PrL) and the infralimbic cortex. The PrL shows functional and anatomical similarity to the human dorsal ACC (dACC), and these regions in both the mouse and human play critical roles in attentional processes [28, 34]. In the rodent, neurons in the PrL increase their firing rates prior to correct trials in sustained attention tasks [35–37], indicating that the PrL’s role in attention function is phylogenetically conserved across species. The rodent PrL is also implicated in rCPT performance [37, 38], but the neurobiological mechanisms by which this brain region controls behavior in the rCPT is, to our knowledge, unexplored.
To assess the relationship between PrL function and rCPT performance with circuit-level detail, we investigated recruitment of neural activity in projections to the PrL from the locus coeruleus (LC), the mediodorsal thalamus (MD) and the ventral hippocampus (vHC). The LC is a brainstem nucleus that is implicated in attention; neurons in this nucleus synthesize and release norepinephrine (NE), and respond strongly to unexpected salient sensory stimuli [38]. The MD is the major thalamic input to the PrL [39, 40], and functional inactivation of this nucleus impairs flexible attention [41, 42]. While the vHC has not typically been associated with attention, a potential contribution via its connection with the prefrontal cortex was recently suggested [43]. We found that neurons in the LC with direct axonal input to the PrL (but not neurons in the MD or vHC) are recruited during the rCPT, and that this recruitment increases with cognitive demand. To further query activity in the LC-PrL circuit during rCPT performance, we simultaneously recorded local field potentials (LFPs) in the LC and PrL and identified an increase in PrL delta and theta power, as well as LC delta power following correct responses in well-trained mice. Moreover, LC neuronal activity leads PrL activity during correct responses in delta frequencies while PrL activity leads the LC during incorrect responses in gamma frequencies.
Materials and methods
Abbreviated methods are provided here—full details provided in the Supplementary.
Animals
Eight to ten week old male C57BL/6J mice (Strain#: 000664, The Jackson Laboratory) were group-housed (4/cage) until electrode implantation and then double-housed. Procedures were approved by the Johns Hopkins Animal Care and Use Committee.
Food restriction protocol
Food was restricted to 2.5 g chow/mouse/day, and mice were maintained at 85–90% of predicted free-feeding weight.
Surgical procedures
For immunohistochemistry experiments, holes were drilled with a 0.9 mm burr above the PrL (A/P: ±1.7 mm, M/L: +0.3 mm), and a retrograde virus expressing tdTomato (AAVrg-CAG-tdTomato, Addgene catalog # 59462-AAVrg) was injected (4 nl/s, 600 nl/hemisphere). For electrophysiology experiments, holes were drilled with a 0.9 mm burr above the PrL (A/P: ±1.7 mm, M/L: +0.3 mm), LC (A/P −5.4 mm, M/L: +0.9 mm), and lambda. Two stereotrodes (Cat #8245-M, Pinnacle Technologies) attached to a head-mount, with two wires soldered to bone screws were implanted unilaterally in PrL (A/P: +1.7 mm, M/L: +0.3 mm, D/V: −1.7) and LC (A/P:−5.4, M/L: +0.9, D/V: −3.5) (Supplementary Fig. 3).
Behavioral training
The rCPT training protocol was based on a previously described protocol [44]. Briefly, mice were trained in Bussey-Saksida mouse touchscreen chambers (Lafayette Instruments). Training stage details are provided in the Supplementary.
Behavioral scoring
Performance scoring parameters were similar to those described by [20] and [44]. Briefly, to assess attention performance during Stage 3, we calculated the discrimination index “d’”, which is a measure of sensitivity bias (perceptual discriminability between S+ and S−) and “c”, which is a measure of response bias (willingness to make responses).
Immunohistochemistry for c-Fos
Coronal sections were incubated in 1:1000 anti-c-Fos antibody (SySy; cat # 226003), 1:1000 goat anti-rabbit AlexaFluor 555 (Sigma), and 1:5000 DAPI (Sigma). Images were visualized at 40x.
In vivo electrophysiology and analysis
LFPs were recorded with a Pinnacle Technology recording system: (1) After mice reached performance criteria (≥60 hits/session) during Stage 2; (2) during the first Stage 3 session (Stage 3-early); and (3) after mice reached performance criteria during Stage 3 (Stage 3-late). LFPs were sampled at 2 kHz, and multitaper spectral analysis was used for power density estimation and phase coherence (http://chronux.org) [45]. For phase-amplitude coupling, values for frequency pairs were extracted via Morlet wavelet convolution. Phase and amplitude values were binned and mean amplitude values were normalized by dividing each bin value by the summed value over all bins. A modulation index value was derived by calculating the Kullback-Leibler distance between the observed phase-amplitude distribution and a uniform (null) phase-amplitude distribution [46]. For Granger causality analysis, we performed multiple lagged autoregressions on LFP traces with the model order for each set of autoregressions determined by the Bayes’ information criteria. Values for directionality were normalized into a lead index value.
Results
LC-PrL circuit is recruited during rCPT performance
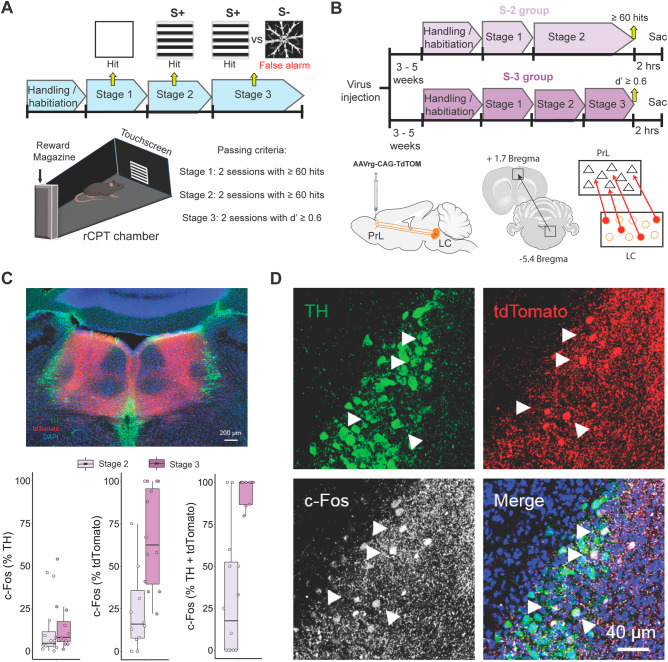
The PrL is necessary for rCPT performance, but how PrL circuits control distinct aspects of behavior, and which circuits provide neuromodulatory control over the PrL to regulate this behavior are not known. To better understand PrL-associated circuits involved in rCPT behavior, we asked which afferent projections to the PrL are selectively activated during task-related behavior. To answer this question, we used a behavioral circuit-tracing approach that allowed us to map recruitment of neurons projecting to the PrL across two stages of rCPT training (Stage 2 vs. Stage 3) with different cognitive demand requirements (Fig. 1A, B). We chose several candidate regions that have direct projections to the PrL, including the ventral hippocampus (vHC), mediodorsal thalamus (MD), and the locus coeruleus (LC), and have been implicated in attention-related behavior. We injected AAVrg-CAG-tdTomato into the PrL to label these afferent projections. Following viral labeling, mice were trained until they achieved asymptotic performance on Stage 2 of the rCPT (Supplementary Fig. 1). Half of the mice were further trained on Stage 3 (Stage 3 group), while the other half were continuously run on Stage 2 (Stage 2 group). Once a mouse in the Stage 3 group reached performance criteria, that mouse and a yoked mouse from the Stage 2 group were killed 2 h following a behavioral session. Brains were then collected for c-Fos immunohistochemistry and imaging. Double labeling for c-Fos and tdTomato was quantified to determine the proportion of neurons projecting to the PrL from the chosen regions that were activated during different stages of rCPT training (Fig. 1C, D). Within these brain regions, the LC contained the highest percentage of c-Fos+/tdTomato+ neurons across both groups (Stage 2: MD = 11.3% ± 11.8%; vHC = 3.17% ± 5.5%; LC = 28.2% ± 31.4%; Stage 3: MD = 14.9% ± 13.8%; vHC = 2.38% ± 2.07%; LC = 66.4% ± 29.2%; means ± standard deviation) (Fig. 1C and Supplementary Fig. 2). Moreover, more PrL-projecting LC neurons were recruited during Stage 3, when cognitive demand is higher, compared to yoked Stage 2 controls (t(22) = −3.0855, p = 0.005; Fig. 1C, D). This effect was not observed in either the MD (t(7) = −0.42438, p = 0.684), or the vHC (t(4) = 0.23309, p = 0.8271) (Supplementary Fig. 2).
Fig. 1. rCPT training recruits PrL-projecting LC neurons.
A Timeline of rCPT training (top). Arrows indicate the type of stimuli presented across training stages and the response when interacting with the stimulus. Mice are moved to the next training stage when they reach performance criteria (bottom right) in two consecutive sessions. Schematic of the touchscreen chambers (bottom left) depicting a stimulus presentation during the rCPT at the center of the touchscreen located in front of the chamber and the reward magazine in the back. B Timeline of c-Fos experiments (top) and schematics of viral injections designed to label PrL-projecting LC neurons (bottom). C Low magnification confocal image of an LC coronal slice immunostained against TH (green), TdTomato (red), c-Fos (white), and DAPI (Blue) (top), and boxplots summarizing the quantification c-Fos in TH+ (left), tdTomato+ (middle) and TH+/tdTomato+ (right) neurons in the LC. D High magnification confocal images of TH, tdTomato and c-Fos expression in LC neurons. Arrows show examples of ACC-projecting (tdTomato+) and NE (TH+) neurons in the LC that co-express c-Fos following the last session of rCPT training.
Cognitive demand and proficiency during rCPT training increase power of low-frequency bands within PrL and LC LFPs
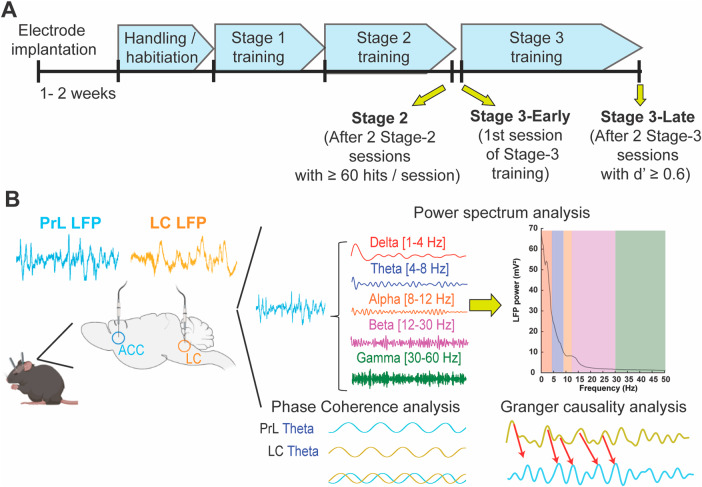
To better understand the role of LC-PrL circuitry in sustained attention during rCPT performance, we recorded simultaneous local field potentials (LFPs) within the PrL and LC at three timepoints: Stage 2, Stage 3-early, corresponding to the first Stage 3 session when the S− is introduced and performance is close to chance, and Stage 3-late when mice have reached the performance criterion of two consecutive sessions with a d’ above 0.6 (Fig. 2). We chose these three timepoints for comparison because they allow us to interrogate the LC-PrL circuitry across the switch from stimulus detection (Stage 2) to stimulus discrimination (Stage 3-early) and the switch from chance performance (Stage 3-early) to significant and consistent stimulus discrimination (Stage 3-late). At the Stage 2 time point, mice have demonstrated proficiency in detecting stimuli presented on the touchscreen and responding appropriately with a nose poke (Supplementary Fig. 4A). In Stage 3-early, which is the first Stage 3 session following advancement from Stage 2, mice are required to not only detect stimuli on the touchscreen, but to discriminate between the S+ and S− and respond accordingly. Performance, measured as d’, is lower in Stage 3-early compared to Stage 3-late, which is the last Stage 3 session, during which overall performance reached the established criteria. As expected, mice in the Stage 3-late group had better task performance than mice in the Stage 3-early group, as reflected by a significantly higher mean dʹ score (Stage 3-early d’ = −0.0485 ± 0.0706 N = 11; Stage 3-late d’ = 0.9395 ± 0.0767 N = 10 t(19) = −9.4951, p < 0.001) (Supplementary Fig. 4A, B). Despite better performance, mice in the Stage 3-late group did not tend to change their overall response strategy (as reflected in their mean c score), preferring to maintain a fairly conservative response bias across both Stage 3-early and Stage 3-late sessions (Stage 3-early c score’ = 1.1095 ± 0.0803 N = 11; Stage 3-late c score = 1.0843 ± 0.0902 N = 10 t(19) = 0.20951, p = 0.8363) (Supplementary Fig. 4C). Latency to the reward trough also did not differ between Stage 3-early and Stage 3-late sessions. However, there was a significant difference in the response latency between hits and false alarms, with mice responding significantly faster during false alarms than hits (F(1,38) = 8.364, p = 0.0063, main effect of response type for session × response type mixed-factorial ANOVA) (Supplementary Fig. 4D). These data show that mice improve their ability to discriminate between the S+ and the S− over time without changing their response bias while false alarms are associated with an impulsive motor pattern.
Fig. 2. LFP recording sessions within rCPT training stages.
A Timeline of rCPT training showing the three recording sessions: Stage 2, Stage 3-Early, and Stage 3-Late. B Schematic of depth electrode placements within the PrL and the LC (left) and summary of LFP data analysis (right).
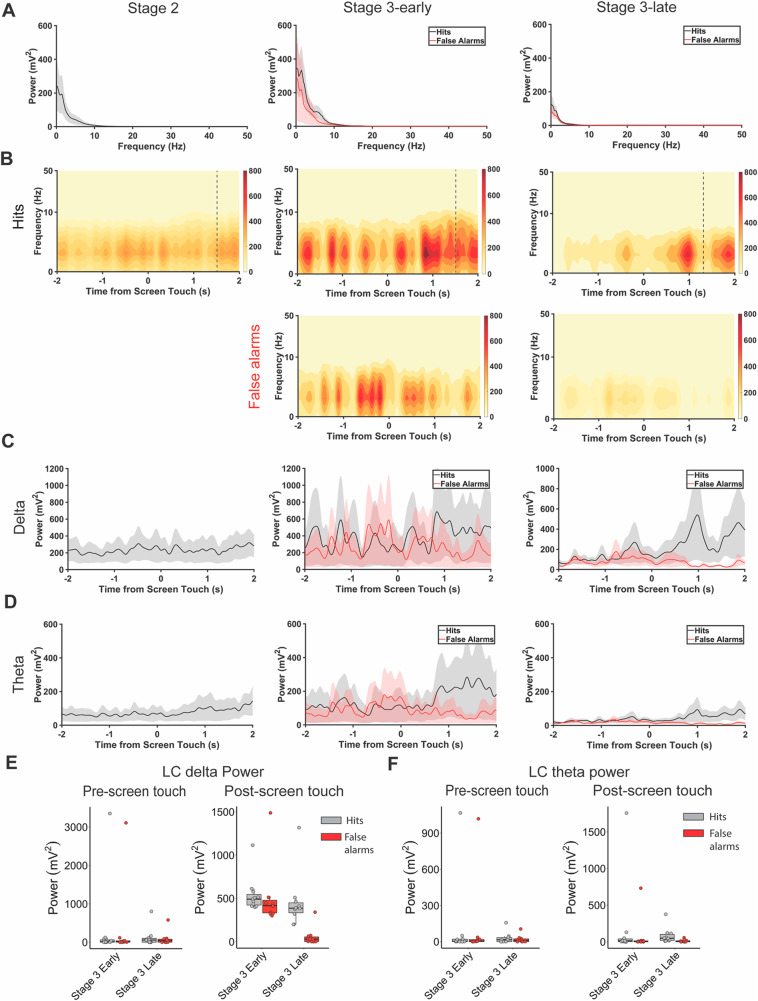
To understand the contributions of LC-PrL circuitry to rCPT learning and performance, we analyzed PrL and LC LFPs in the 4-s time window surrounding mouse responses (screen touches) to the S+ (hits) and S− (false alarms) to identify electrophysiological correlates of performance across recording sessions. We further split this time window into two distinct timepoints centered around the screen nosepoke: pre-screen touch (from −2 to 0 s), and post-screen touch (from 0 to 2 s). We first analyzed amplitude (power) within the PrL LFP during these timepoints, and found differences in lower-frequency bands during hits and false alarms (Fig. 3A). Specifically, we found no significant difference in either delta (1–4 Hz), or theta (4–12 Hz), power (p > 0.05 for session × response type mixed-factorial ANOVA) during pre-screen touch, but found that both delta (F(1,38) = 4.54, p = 0.0396) and theta (F(1,38) = 6.115, p = 0.018) power were significantly higher during post-screen touch hits vs. false alarms in both Stage 3-early and Stage 3-late sessions (main effect of response type for session × response type mixed-factorial ANOVA) (Fig. 3B–F). When analyzing the same frequency bands from the LC LFP, a different pattern emerged; namely, post-screen touch, but not pre-screen touch, theta and delta power differed between hits and false alarms for Stage 3-late sessions, but not Stage 3-early sessions (F(1,38) = 5.107, p = 0.0297, session × response type interaction) (Fig. 4). Importantly, the onset of the changes in PrL and LC LFP power during hit responses precede reward retrieval (Stage 3-early reward retrieval = 1.6 s onset PrL LFP changes = 0.7 s, onset LC LFP changes = 0.8 s; Stage 3-late reward retrieval = 1.2 s, onset PrL LFP changes = 0.9 s, onset LC LFP changes = 0.9 s). These data suggest a disparity between the PrL and LC with regard to task engagement during rCPT learning, with the PrL differentiating between hits and false alarms (the S+ and S−) regardless of task proficiency, and the LC differentiating between the two response types only when the task is well-learned.
Fig. 3. Correct responses (hits) during Stage 3 of rCPT training increase delta and theta power within the PrL.
A Power spectral density of PrL electrode during hits (black) and false alarms (red) in Stage 2 (left), Stage 3-early (middle), and Stage 3-late (right) recording sessions. B Spectrograms of PrL electrode surrounding hits (top) and false alarms (bottom) in Stage 2 (left), Stage 3-early (middle), and Stage 3-late (right) recording sessions. Dotted lines indicate reward retrieval. PrL delta (C) and theta (D) power across time surrounding hits and false alarms in Stage 2 (left), Stage 3-early (middle), and Stage 3-late (right) recording sessions. E Boxplots summarizing changes in PrL delta power before (left) and after (right) screen touch during hits and false alarms. F Boxplots summarizing changes in PrL theta power before (left) and after (right) screen touch during hits and false alarms.
Fig. 4. Correct responses during Stage 3-late of rCPT training increase delta power within the LC.
A Power spectral density of LC electrode during hits (black) and false alarms (red) in Stage 2 (left), Stage 3-early (middle), and Stage 3-late (right) recording sessions. B Spectrograms of LC electrode surrounding hits (top) and false alarms (bottom) in Stage 2 (left), Stage 3-early (middle), and Stage 3-late (right) recording sessions. Dotted lines indicate reward retrieval. LC delta (C) and theta (D) power across time surrounding hits and false alarms in Stage 2 (left), Stage 3-early (middle), and Stage 3-late (right) recording sessions. E Boxplots summarizing changes in LC delta power before (left) and after (right) screen touch during hits and false alarms. F Boxplots summarizing changes in LC theta power before (left) and after (right) screen touch during hits and false alarms.
To further assess relationships between activity in the PrL and behavior during CPT learning, we analyzed the degree to which the phase of delta and theta oscillations modulates the amplitude of gamma oscillations during the entire 4 s pre- and post-screen touch period. Phase-amplitude coupling between oscillations in different frequency bands has been linked to numerous cognitive functions, including attention [47–49]. We found a selective effect of response type (hits vs. false alarms) on delta-slow gamma (30–55 Hz) phase-amplitude coupling in the PrL LFP (F(1,38) = 4.451, p = 0.0415, main effect of response type for session × response type mixed-factorial ANOVA) that was not present for coupling between other frequency bands (delta-fast gamma (65–120 Hz), theta-slow gamma, or theta-fast gamma) (Supplementary Fig. 5). Delta-slow gamma coupling and delta power therefore increase in tandem during hits, regardless of which session (Stage 3-early or Stage 3-late) the mouse is performing.
The LC and PrL dynamically interact during rCPT training and proficient task performance
To investigate how the LC and PrL interact during rCPT performance, we assessed the degree to which LFPs in the two brain regions phase-locked with one another during the 4 s pre- and post-screen touch period across different stages. Phase coherence in the delta frequency band was significantly higher during hits vs. false alarms during both Stage 3-early and Stage 3-late sessions (F(1,38) = 7.77, p = 0.0083, main effect of response type for session × response type mixed-factorial ANOVA) (Fig. 5A, B). Although theta phase coherence did not significantly differ between response types, it did significantly decrease from Stage 3-early to Stage 3-late sessions (F(1,38) = 8.836, p = 0.0051, main effect of session for session × response type mixed-factorial ANOVA) (Fig. 5A, B), indicating that distinct pieces of information (the mouse’s response vs. the session the mouse is performing) can be decoded from frequency-specific patterns of synchrony between the PrL and LC.
Fig. 5. LC leads PrL in theta and beta frequencies during hits in Stage 3-late.
A Coherence analysis of LC and PrL LFPs across Stage 2 (left), Stage 3-early (middle), and Stage 3-late (right) recording sessions. Stage 3-late false alarms exhibit lower coherence within low-frequency bands (0–8 Hz) than Stage-3 hits. B Boxplots summarizing coherence across LC and PrL LFPs within delta (0–4 Hz; left) and theta (4–8 Hz; right) bands during hits (gray) and false alarms (red). C Granger causality index across recording sessions. Lead index is normalized where values higher than 0.5 (dotted line) indicate LC leading PrL, while values below 0.5 indicate PrL leading LC. Only Stage 3-late shows directionality where LC leads PrL in theta/beta frequencies while PrL leads LC in gamma frequencies during false alarms. D Boxplot summarizing lead index across recording sessions.
Since the LC-PrL circuit is bi-directional with neurons in the LC sending axons to the PrL, and the LC additionally receiving direct input from PrL neurons, we investigated whether the LC conveys information to the PrL, or vice versa, during distinct rCPT training stages. To infer directionality, we used Granger causality to determine how the LFP in one brain area predicted future changes in the LFP in the other brain area. We then normalized Granger causality values into a “lead index” variable, which allowed us to determine which brain region led the other within defined frequency bands (lead index values > 0.5 indicate that the LC LFP predicts the PrL LFP, and lead index values < 0.5 indicate that the PrL LFP predicts the LC LFP). During Stage 2, there was no clear pattern of directionality across delta, theta, slow gamma (30–55 Hz), or fast gamma (65–120 Hz) frequency bands (all lead index values not significantly different from 0.5, F(3,40) = 0.088, p = 0.966, one-way ANOVA across frequency bands with lead index as the dependent variable) (Fig. 5C, D). A similar pattern emerged during Stage 3-early sessions for both hits and false alarms (p > 0.05 for frequency band × response type mixed-factorial ANOVA) (Fig. 5C, D). During Stage 3-late sessions, however, the LC LFP led the PrL LFP in lower frequency bands (delta and theta) during hits (Fig. 5C, D). During false alarms, the LC LFP also led the PrL LFP in these frequency bands, but the PrL LFP additionally led the LC LFP in the slow gamma frequency band (F(3,72) = 19.712, p < 0.001, main effect of frequency band, and F(3,72) = 4.645, p = 0.033, frequency band × response type interaction for frequency band × response type mixed-factorial ANOVA) (Fig. 5C, D), suggesting that communication from the LC to the PrL is a hallmark of proficient task performance generally, but that the PrL additionally communicates back to the LC during errors.
Discussion
The LC plays a major role in general arousal and vigilance [50–52]; for review [53]. Phasic activation of LC neurons is associated with both sleep-to-wake transitions [54, 55], and orienting the response to salient or unexpected sensory stimuli [56] for review [57]. NE-producing neurons in the rodent LC send efferents to many cortical areas, including the PrL [58], and arousal-related patterns of neuronal activity in the PrL are linked with phasic LC activity [59, 60]. The “adaptive gain theory” of LC function posits that enhanced attention is, in part, a direct result of the arousal-inducing properties of phasic activity in the LC [61, 62]. Supporting this idea, lesioning LC-NE efferents to the frontal cortex impairs attentional performance in rodents [63, 64] and non-human primates [65]; for review [66], and catecholaminergic signaling in the PrL regulates behavior in attention tasks [67, 68], including the rCPT [24, 69, 70]. Our results further support the notion that communication between the LC and the PrL underlies successful stimulus discrimination and discrimination-guided behavior in mice. Specifically, viral labeling and immunohistochemical analysis showed that LC neurons that target the PrL are selectively activated after mice learn to asymptotically perform the touchscreen-guided rCPT. We investigated recruitment of attention-related immediate early gene expression in two other regions, the MD and vHC, which have dense inputs to the PrL [39, 40, 71, 72]. The MD is also strongly associated with attentional processing. While the vHC has not typically been associated with attention processing, recent data has suggested a potential contribution via its connection with the prefrontal cortex [43]. In contrast to the LC, we found no evidence that PrL inputs from these regions are activated during rCPT performance. That LC-PrL projection neurons, but not MD-PrL or vHC-PrL projection neurons, were recruited during the rCPT suggests that these pathways differentially contribute to functional aspects of attention in rodents, and support the theory that LC-mediated NE release is important for this behavior.
To investigate roles of the PrL and LC in the rCPT, we simultaneously recorded LFPs in these brain areas during rCPT performance. We found that activity in both regions distinguishes between correct and incorrect responses (e.g., hits and false alarms). These patterns of activity, however, are only present in the LC when the rCPT is well-learned, suggesting that the LC encodes the attention-related task rule. Specifically, amplitude in the delta and theta frequency bands is increased during hits (directly after the mouse pokes the screen), supporting a role for the LC in the exploitation of correct responses. In the rCPT, the mouse receives sensory feedback directly after a hit (specifically, the reward port light illuminates and a short tone is played), but not after a false alarm. The LC LFP may therefore reflect the association of this feedback with the learned task rule. This result is perhaps surprising given that both increased activity in the LC [73], and increases in pupil diameter [74, 75] are correlated with uncertainty during decision-making [76]. Both optogenetic [77], and chemogenetic [78], stimulation of LC neurons, however, accelerates learning a new rule (exploitation), rather than accelerated disengagement from an old role (exploration), indicating that the LC promotes cognitive flexibility by facilitating adaptation to new behavioral policies. The LC may promote both exploration and exploitation via tonic and phasic firing of LC neurons, respectively [61, 79, 80]; if this is the case, then activity in the LC that leads to phasic spiking may also underlie increases in low-frequency oscillations in the LC LFP during well-learned attention-guided behavior. A limitation of this study is that LFPs were only recorded during the first rCPT Stage 3 session (Stage 3-early), and the final rCPT Stage 3 session (Stage 3-late), which precludes analyzing how brain-behavior relationships shift during the entire learning process. Understanding how delta and theta activity in the LC LFP develop as a function of learning will be informative for understanding the relationship between the LC and exploration-exploitation during this task.
In contrast to activity in the LC, delta and theta power in the PrL LFP distinguishes between hits and false alarms even when mice have not yet learned the task rule. Indeed, response time differences in this study demonstrate a dissociation between visual discrimination and behavioral choice, indicating that mice discriminate between the target and non-target well in advance of learning to respond differently to the two [81]. Increased PrL power tracks this visual discrimination across orthogonal learning states, consistent with a role for the PrL in attention-related sensory processing [82, 83] and decision making during both periods of certainty and uncertainty [84, 85]. Concurrently with increased delta power, phase-amplitude coupling between delta and slow-gamma oscillations is also increased during hits, regardless of session. Phase-amplitude coupling in the PrL is linked to visual cue detection [48], reaction time [49], and stimulus orientation [86] during attentional tasks, signaling a role for the PrL in attention-guided sensory discrimination. The dissociation between LFP activity in the LC and PrL in this study suggests that these two brain areas process distinct aspects of experience during correct and incorrect choices, and argues against the possibility that motor behavior or reward consumption (the temporal onset of power differences between hits and false alarms is highly aligned to screen touch, occurring before the mouse reaches the reward port) is driving this dissociation, as would be expected if both brain areas responded similarly during Stage 3-early and Stage 3-late sessions.
LC-NE neurons make synaptic contact with [87–90], and also receive synaptic contact from the PrL [91, 92], suggesting that these two brain areas may functionally communicate during the rCPT. To test this, we analyzed phase coherence (the degree to which phases within a defined frequency band are aligned on successive cycles) between LFPs in the LC and PrL during Stage 3-early and Stage 3-late sessions. Coherence in the delta frequency band was highest during hits, regardless of session type, and coherence in the theta frequency band was highest during Stage 3-late sessions, regardless of response, signaling a dissociation between the type of information that is conveyed by these two oscillations. To uncover directionally-specific (i.e., from LC-to-PrL, or vice versa) patterns of communication between the two brain areas, we used Granger causality, and found that LC-to-PrL directionality in the delta and theta frequency bands was highest during Stage 3-late sessions. This directionality did not reach statistical significance during either Stage 2 or Stage 3-early sessions, indicating that phase coherence during Stage 3-early is a product of bi-directional transmission between the LC and PrL. LC-to-PrL low-frequency directionality during Stage 3-late sessions was observed during both hits and false alarms, implicating this pattern of directionality in attention-based discrimination performance (i.e., in overtrained animals), rather than attention-based discrimination learning. Directionality from the PrL-to-LC, however, did discriminate between hits and false alarms—specifically, PrL-to-LC directionality in the slow gamma frequency band was higher during false alarms, suggesting that the PrL conveys an error signal to the LC during higher periods of certainty in attention tasks. This finding represents a potential behavioral correlate to data in anesthetized rats showing that gamma power in the PrL precedes low-frequency activity in the LC [93], and provides experimental data to support a recently proposed computational model where PrL inputs to the LC encode prediction errors [94].
Follow up work using molecular genetic tools that allow us to bi-directionally manipulate the LC-PrL circuit will allow us to causally test whether recruitment of activity in direct projections between these brain regions impacts the identified electrophysiological biomarkers of attention behavior. Further investigation of the relationship between the LC and PrL in the rCPT will serve at least two purposes. First, regulation of these electrophysiological biomarkers may represent “second order” mechanisms of action for FDA-approved treatments for attention deficits, providing an opportunity to increase understanding of how these compounds work [95]. Second, these biomarkers may help us identify novel therapeutics. If these biomarkers are causal regulators of performance, then they can be used to screen and validate therapeutic strategies. In summary, our findings reinforce the well-known roles of the LC and PrL in attention, and further demonstrate multiplexed relationships between specific patterns of oscillatory synchrony between the two brain regions and different stages of learning in a translationally-relevant attentional discrimination task. That communication between the LC and PrL is routed through distinct frequency bands at different timepoints is perhaps unsurprising in light of the complex relationships between multi-unit activity in these two areas, even when animals are anesthetized [96, 97]. The results described here could be compared with neural responses during human CPTs to identify a translational benchmark for circuit function in patients with attentional deficits. This may be useful because attention deficits related to LC-PrL function are supported across multiple complex brain disorders, including major depressive disorder [98], post-traumatic stress disorder [99], schizophrenia [100]. Hence, establishing biomarkers of function in this circuit during attentional tasks is of significant value.
Supplementary information
Acknowledgements
We thank members of the Martinowich and Carr laboratories for helpful comments and suggestions. We also thank Aimee Ormond and Deveren Manley for assistance with animal care.
Author contributions
Conceptualization: HLH, GVC, and KM. Methodology: HLH, JMM, JMB, SSA, ACD, and YL. Validation: JMB and SO. Formal analysis: HLH and SSA. Investigation: HLH, SSA, JMB, JMM, and SO. Data curation: HLH, SA, and JMB. Writing—original draft: HLH, SSA, JMB, GVC, and KM. Writing—review and editing: HLH, SSA, JMB, GVC, and KM. Visualization: HLH, SSA, and JMB. Supervision: HLH, JMB, and KM. Project administration: JMB, KM, and GVC. Funding acquisition: GVC and KM.
Funding
This work was supported by internal funding from the Lieber Institute for Brain Development, and the National Institute of Mental Health (R01MH105592 to KM; R56MH126233 to GVC and KM).
Competing interests
KM is the Social Media Editor for Neuropsychopharmacology. GVC is a scientific advisor for LongTermGevity, Inc. and owns stock options in the company. LongTermGevity, Inc. was not involved in the funding, design, or execution of these studies. No other authors have financial relationships with commercial interests, and the authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Henry L. Hallock, Suhaas S. Adiraju.
These authors jointly supervised this work: Gregory V. Carr, Keri Martinowich.
Contributor Information
Gregory V. Carr, Email: greg.carr@libd.org
Keri Martinowich, Email: keri.martinowich@libd.org.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01692-3.
References
- 1.Hooks K, Milich R, Pugzles Lorch E. Sustained and selective attention in boys with attention deficit hyperactivity disorder. J Clin Child Psychol. 1994;23:69–77. doi: 10.1207/s15374424jccp2301_9. [DOI] [Google Scholar]
- 2.Brodeur DA, Pond M. The development of selective attention in children with attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2001;29:229–39. doi: 10.1023/A:1010381731658. [DOI] [PubMed] [Google Scholar]
- 3.Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60:1071–80. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disord. 2005;89:125–35. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 5.van der Meere J, Börger N, van Os T. Sustained attention in major unipolar depression. Percept Mot Skills. 2007;104:1350–4. doi: 10.2466/PMS.104.3.1350-1354. [DOI] [PubMed] [Google Scholar]
- 6.Neuchterlein KH, Dawson ME, Ventura J, Miklowitz D, Konishi G. Information-processing anomalies in the early course of schizophrenia and bipolar disorder. Schizophr Res. 1991;5:195–6. doi: 10.1016/0920-9964(91)90069-4. [DOI] [PubMed] [Google Scholar]
- 7.Hain C, Maier W, Klingler T, Franke P. Positive/negative symptomatology and experimental measures of attention in schizophrenic patients. Psychopathology. 1993;26:62–68. doi: 10.1159/000284801. [DOI] [PubMed] [Google Scholar]
- 8.Young JW, Bismark AW, Sun Y, Zhang W, McIlwain M, Grootendorst I, et al. Neurophysiological characterization of attentional performance dysfunction in schizophrenia patients in a reverse-translated task. Neuropsychopharmacology. 2017;42:1338–48. doi: 10.1038/npp.2016.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutschmann J, Cornblatt B, Erlenmeyer-Kimling L. Sustained attention in children at risk for schizophrenia. Report on a continuous performance test. Arch Gen Psychiatry. 1977;34:571–5. doi: 10.1001/archpsyc.1977.01770170081007. [DOI] [PubMed] [Google Scholar]
- 10.Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Beck LH. A continuous performance test of brain damage. J Consult Psychol. 1956;20:343–50. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- 11.Sanford JA, Turner A. The integrated visual and auditory continuous performance test. Interpretive manual. Richmond, VA: BrainTrain; 2004.
- 12.Halperin JM, Wolf LE, Pascualvaca DM, Newcorn JH, Healey JM, O’Brien JD, et al. Differential assessment of attention and impulsivity in children. J Am Acad Child Adolesc Psychiatry. 1988;27:326–9. doi: 10.1097/00004583-198805000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Halperin JM, Sharma V, Greenblatt E, Schwartz ST. Assessment of the continuous performance test: reliability and validity in a nonreferred sample. Psychol Assess. 1991;3:603–8. doi: 10.1037/1040-3590.3.4.603. [DOI] [Google Scholar]
- 14.Berger I, Slobodin O, Cassuto H. Usefulness and validity of continuous performance tests in the diagnosis of attention-deficit hyperactivity disorder children. Arch Clin Neuropsychol. 2017;32:81–93. [DOI] [PubMed]
- 15.Gordon M, Mettelman BB. The assessment of attention: I. Standardization and reliability of a behavior-based measure. J Clin Psychol. 1988;44:682–90. doi: 10.1002/1097-4679(198809)44:5<682::AID-JCLP2270440504>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Earle-Boyer EA, Serper MR, Davidson M, Harvey PD. Continuous performance tests in schizophrenic patients: stimulus and medication effects on performance. Psychiatry Res. 1991;37:47–56. doi: 10.1016/0165-1781(91)90105-X. [DOI] [PubMed] [Google Scholar]
- 17.Fitzpatrick PA, Klorman R, Brumaghim JT, Borgstedt AD. Effects of sustained-release and standard preparations of methylphenidate on attention deficit disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:226–34. doi: 10.1097/00004583-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Sanford JA, Turner A. Manual for the integrated visual and auditory continuous performance test. Richmond, VA: BrainTrain; 1995.
- 19.Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS ONE. 2009;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim CH, Hvoslef-Eide M, Nilsson SRO, Johnson MR, Herbert BR, Robbins TW, et al. The continuous performance test (rCPT) for mice: a novel operant touchscreen test of attentional function. Psychopharmacology. 2015;232:3947–66. doi: 10.1007/s00213-015-4081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KAL, Nithianantharajah J, et al. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–203. doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parasuraman R. Memory load and event rate control sensitivity decrements in sustained attention. Science. 1979;205:924–7. doi: 10.1126/science.472714. [DOI] [PubMed] [Google Scholar]
- 23.Mar AC, Nilsson SRO, Gamallo-Lana B, Lei M, Dourado T, Alsiö J, et al. MAM-E17 rat model impairments on a novel continuous performance task: effects of potential cognitive enhancing drugs. Psychopharmacology. 2017;234:2837–57. doi: 10.1007/s00213-017-4679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson SRO, Heath CJ, Takillah S, Didienne S, Fejgin K, Nielsen V, et al. Continuous performance test impairment in a 22q11.2 microdeletion mouse model: improvement by amphetamine. Transl Psychiatry. 2018;8:247. doi: 10.1038/s41398-018-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 26.Fallgatter AJ, Bartsch AJ, Herrmann MJ. Electrophysiological measurements of anterior cingulate function. J Neural Transm. 2002;109:977–88. doi: 10.1007/s007020200080. [DOI] [PubMed] [Google Scholar]
- 27.Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 28.Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex. 2005;15:229–37. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- 29.Fallgatter AJ, Ehlis A-C, Seifert J, Strik WK, Scheuerpflug P, Zillessen KE, et al. Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clin Neurophysiol. 2004;115:973–81. doi: 10.1016/j.clinph.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 30.Salgado-Pineda P, Junqué C, Vendrell P, Baeza I, Bargalló N, Falcón C, et al. Decreased cerebral activation during CPT performance: structural and functional deficits in schizophrenic patients. Neuroimage. 2004;21:840–7. doi: 10.1016/j.neuroimage.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Honey GD, Pomarol-Clotet E, Corlett PR, Honey RAE, McKenna PJ, Bullmore ET, et al. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laubach M, Amarante LM, Swanson K, White SR. What, if anything, is rodent prefrontal cortex? ENeuro. 2018;5:ENEURO.0315-18.2018. doi: 10.1523/ENEURO.0315-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Heukelum S, Mars RB, Guthrie M, Buitelaar JK, Beckmann CF, Tiesinga PHE, et al. Where is cingulate cortex? A cross-species view. Trends Neurosci. 2020;43:285–99. doi: 10.1016/j.tins.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA. 2002;99:523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Totah NKB, Kim YB, Homayoun H, Moghaddam B. Anterior cingulate neurons represent errors and preparatory attention within the same behavioral sequence. J Neurosci. 2009;29:6418–26. doi: 10.1523/JNEUROSCI.1142-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu D, Deng H, Xiao X, Zuo Y, Sun J, Wang Z. Persistent neuronal activity in anterior cingulate cortex correlates with sustained attention in rats regardless of sensory modality. Sci Rep. 2017;7:43101. doi: 10.1038/srep43101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H, Ährlund-Richter S, Wang X, Deisseroth K, Carlén M. Prefrontal parvalbumin neurons in control of attention. Cell. 2016;164:208–18. doi: 10.1016/j.cell.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sara SJ. Locus coeruleus reports changes in environmental contingencies. Behav Brain Sci. 2016;39:e223. doi: 10.1017/S0140525X15001946. [DOI] [PubMed] [Google Scholar]
- 39.Barbas H, Henion TH, Dermon CR. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1991;313:65–94. doi: 10.1002/cne.903130106. [DOI] [PubMed] [Google Scholar]
- 40.Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol. 1985;242:535–60. doi: 10.1002/cne.902420406. [DOI] [PubMed] [Google Scholar]
- 41.Chudasama Y, Muir JL. Visual attention in the rat: a role for the prelimbic cortex and thalamic nuclei? Behav Neurosci. 2001;115:417–28. doi: 10.1037/0735-7044.115.2.417. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, Halassa MM. Thalamic amplification of cortical connectivity sustains attentional control. Nature. 2017;545:219–23. doi: 10.1038/nature22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGarrity S, Mason R, Fone KC, Pezze M, Bast T. Hippocampal neural disinhibition causes attentional and memory deficits. Cereb Cortex. 2017;27:4447–62. doi: 10.1093/cercor/bhw247. [DOI] [PubMed] [Google Scholar]
- 44.DeBrosse AC, Li Y, Wiseman R, Ross R, Garrison S, Hallock HL, et al. Stimulus degradation impairs performance in a rodent continuous performance test. BioRxiv. 2021 doi: 10.1101/2021.08.31.458320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarvis MR, Mitra PP. Sampling properties of the spectrum and coherency of sequences of action potentials. Neural Comput. 2001;13:717–49. doi: 10.1162/089976601300014312. [DOI] [PubMed] [Google Scholar]
- 46.Tort ABL, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104:1195–210. doi: 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szczepanski SM, Crone NE, Kuperman RA, Auguste KI, Parvizi J, Knight RT. Dynamic changes in phase-amplitude coupling facilitate spatial attention control in fronto-parietal cortex. PLoS Biol. 2014;12:e1001936. doi: 10.1371/journal.pbio.1001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howe WM, Gritton HJ, Lusk NA, Roberts EA, Hetrick VL, Berke JD, et al. Acetylcholine release in prefrontal cortex promotes gamma oscillations and theta-gamma coupling during cue detection. J Neurosci. 2017;37:3215–30. doi: 10.1523/JNEUROSCI.2737-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chacko RV, Kim B, Jung SW, Daitch AL, Roland JL, Metcalf NV, et al. Distinct phase-amplitude couplings distinguish cognitive processes in human attention. Neuroimage. 2018;175:111–21. doi: 10.1016/j.neuroimage.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA. 1980;77:3033–7. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 52.Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–23. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 54.Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–8. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- 55.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grant SJ, Aston-Jones G, Redmond DE. Responses of primate locus coeruleus neurons to simple and complex sensory stimuli. Brain Res Bull. 1988;21:401–10. doi: 10.1016/0361-9230(88)90152-9. [DOI] [PubMed] [Google Scholar]
- 57.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/S0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 58.Chandler DJ, Gao W-J, Waterhouse BD. Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc Natl Acad Sci USA. 2014;111:6816–21. doi: 10.1073/pnas.1320827111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB, et al. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci. 2010;30:14543–51. doi: 10.1523/JNEUROSCI.3037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joshi S, Gold JI. Context-dependent relationships between locus coeruleus firing patterns and coordinated neural activity in the anterior cingulate cortex. ELife. 2022;11:e63490. doi: 10.7554/eLife.63490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 62.Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–41. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 63.McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newman LA, Darling J, McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology. 2008;200:39–50. doi: 10.1007/s00213-008-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnsten AF, Goldman-Rakic PS. Catecholamines and cognitive decline in aged nonhuman primates. Ann N Y Acad Sci. 1985;444:218–34. doi: 10.1111/j.1749-6632.1985.tb37592.x. [DOI] [PubMed] [Google Scholar]
- 66.Robbins TW. Arousal systems and attentional processes. Biol Psychol. 1997;45:57–71. doi: 10.1016/S0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- 67.Mair RD, Zhang Y, Bailey KR, Toupin MM, Mair RG. Effects of clonidine in the locus coeruleus on prefrontal- and hippocampal-dependent measures of attention and memory in the rat. Psychopharmacology. 2005;181:280–8. doi: 10.1007/s00213-005-2263-x. [DOI] [PubMed] [Google Scholar]
- 68.Ramos BP, Arnsten AFT. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharm Ther. 2007;113:523–36. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding Z, Brown JW, Rueter LE, Mohler EG. Profiling attention and cognition enhancing drugs in a rat touchscreen-based continuous performance test. Psychopharmacology. 2018;235:1093–105. doi: 10.1007/s00213-017-4827-y. [DOI] [PubMed] [Google Scholar]
- 70.Caballero-Puntiverio M, Lerdrup LS, Arvastson L, Aznar S, Andreasen JT. ADHD medication and the inverted U-shaped curve: a pharmacological study in female mice performing the rodent continuous performance test (rCPT) Prog Neuropsychopharmacol Biol Psychiatry. 2020;99:109823. doi: 10.1016/j.pnpbp.2019.109823. [DOI] [PubMed] [Google Scholar]
- 71.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 72.Condé F, Maire-Lepoivre E, Audinat E, Crépel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–93. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- 73.Payzan-LeNestour E, Dunne S, Bossaerts P, O’Doherty JP. The neural representation of unexpected uncertainty during value-based decision making. Neuron. 2013;79:191–201. doi: 10.1016/j.neuron.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn Affect Behav Neurosci. 2010;10:252–69. doi: 10.3758/CABN.10.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jepma M, Nieuwenhuis S. Pupil diameter predicts changes in the exploration-exploitation trade-off: evidence for the adaptive gain theory. J Cogn Neurosci. 2011;23:1587–96. doi: 10.1162/jocn.2010.21548. [DOI] [PubMed] [Google Scholar]
- 76.Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–92. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 77.Glennon E, Carcea I, Martins ARO, Multani J, Shehu I, Svirsky MA, et al. Locus coeruleus activation accelerates perceptual learning. Brain Res. 2019;1709:39–49. doi: 10.1016/j.brainres.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cope ZA, Vazey EM, Floresco SB, Aston Jones GS. DREADD-mediated modulation of locus coeruleus inputs to mPFC improves strategy set-shifting. Neurobiol Learn Mem. 2019;161:1–11. doi: 10.1016/j.nlm.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 79.McClure SM, Gilzenrat MS, Cohen JD. An exploration-exploitation model based on norepinepherine and dopamine activity. In: Advances in Neural Information Processing Systems. Vancouver, BC, Canada: 2005.
- 80.Kane MJ, Gross GM, Chun CA, Smeekens BA, Meier ME, Silvia PJ, et al. For whom the mind wanders, and when, varies across laboratory and daily-life settings. Psychol Sci. 2017;28:1271–89. doi: 10.1177/0956797617706086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeBrosse AC, Wheeler AM, Barrow JC, Carr GV. Inhibition of catechol-O-methyltransferase does not alter effort-related choice behavior in a fixed ratio/concurrent chow task in male mice. Front Behav Neurosci. 2020;14:73. doi: 10.3389/fnbeh.2020.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J Cogn Neurosci. 2006;18:766–80. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- 83.Hickey C, Chelazzi L, Theeuwes J. Reward changes salience in human vision via the anterior cingulate. J Neurosci. 2010;30:11096–103. doi: 10.1523/JNEUROSCI.1026-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kennerley SW, Walton ME, Behrens TEJ, Buckley MJ, Rushworth MFS. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–7. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 85.Luttrell A, Stillman PE, Hasinski AE, Cunningham WA. Neural dissociations in attitude strength: distinct regions of cingulate cortex track ambivalence and certainty. J Exp Psychol Gen. 2016;145:419–33. doi: 10.1037/xge0000141. [DOI] [PubMed] [Google Scholar]
- 86.Mento G, Astle DE, Scerif G. Cross-frequency phase-amplitude coupling as a mechanism for temporal orienting of attention in childhood. J Cogn Neurosci. 2018;30:594–602. doi: 10.1162/jocn_a_01223. [DOI] [PubMed] [Google Scholar]
- 87.Jones BE, Halaris AE, McIlhany M, Moore RY. Ascending projections of the locus coeruleus in the rat. I. Axonal transport in central noradrenaline neurons. Brain Res. 1977;127:1–21. doi: 10.1016/0006-8993(77)90377-8. [DOI] [PubMed] [Google Scholar]
- 88.Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res. 1977;127:25–53. doi: 10.1016/0006-8993(77)90378-X. [DOI] [PubMed] [Google Scholar]
- 89.Foote SL, Morrison JH. Extrathalamic modulation of cortical function. Annu Rev Neurosci. 1987;10:67–95. doi: 10.1146/annurev.ne.10.030187.000435. [DOI] [PubMed] [Google Scholar]
- 90.Koga K, Yamada A, Song Q, Li X-H, Chen Q-Y, Liu R-H, et al. Ascending noradrenergic excitation from the locus coeruleus to the anterior cingulate cortex. Mol Brain. 2020;13:49. doi: 10.1186/s13041-020-00586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buchanan SL, Thompson RH, Maxwell BL, Powell DA. Efferent connections of the medial prefrontal cortex in the rabbit. Exp Brain Res. 1994;100:469–83. doi: 10.1007/BF02738406. [DOI] [PubMed] [Google Scholar]
- 92.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 93.Totah NK, Logothetis NK, Eschenko O. Synchronous spiking associated with prefrontal high γ oscillations evokes a 5-Hz rhythmic modulation of spiking in locus coeruleus. J Neurophysiol. 2021;125:1191–201. doi: 10.1152/jn.00677.2020. [DOI] [PubMed] [Google Scholar]
- 94.Sales AC, Friston KJ, Jones MW, Pickering AE, Moran RJ. Locus Coeruleus tracking of prediction errors optimises cognitive flexibility: an active inference model. PLoS Comput Biol. 2019;15:e1006267. doi: 10.1371/journal.pcbi.1006267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cavanagh JF, Gregg D, Light GA, Olguin SL, Sharp RF, Bismark AW, et al. Electrophysiological biomarkers of behavioral dimensions from cross-species paradigms. Transl Psychiatry. 2021;11:482. doi: 10.1038/s41398-021-01562-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Totah NK, Neves RM, Panzeri S, Logothetis NK, Eschenko O. The locus coeruleus is a complex and differentiated neuromodulatory system. Neuron. 2018;99:1055–68.e6. doi: 10.1016/j.neuron.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 97.Noei S, Zouridis IS, Logothetis NK, Panzeri S, Totah NK. Distinct ensembles in the noradrenergic locus coeruleus are associated with diverse cortical states. Proc Natl Acad Sci USA. 2022;119:e2116507119. doi: 10.1073/pnas.2116507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Del Cerro I, Martínez-Zalacaín I, Guinea-Izquierdo A, Gascón-Bayarri J, Viñas-Diez V, Urretavizcaya M, et al. Locus coeruleus connectivity alterations in late-life major depressive disorder during a visual oddball task. Neuroimage Clin. 2020;28:102482. doi: 10.1016/j.nicl.2020.102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naegeli C, Zeffiro T, Piccirelli M, Jaillard A, Weilenmann A, Hassanpour K, et al. Locus coeruleus activity mediates hyperresponsiveness in posttraumatic stress disorder. Biol Psychiatry. 2018;83:254–62. doi: 10.1016/j.biopsych.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 100.Craven RM, Priddle TH, Crow TJ, Esiri MM. The locus coeruleus in schizophrenia: a postmortem study of noradrenergic neurones. Neuropathol Appl Neurobiol. 2005;31:115–26. doi: 10.1111/j.1365-2990.2004.00597.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.