Abstract
Around the world, a variety of crops, including tomatoes, suffer serious economic losses due to the Rhizoctonia root-rot disease. Herein, Bacillus velezensis, Bacillus megaterium, and Herpaspirillum huttiense isolated from strawberry (Fragaria chiloensis var. ananassa) plants were pragmatic as plant growth promotors for battling the Rhizoctonia root rot disease and bringing about defense mechanisms as well as growth promotional strategies in tomato plants. These endophytic bacteria demonstrated potent antifungal activity against R. solani in vitro along in vivo. Data explained that the isolated endophytic bacteria could produce Indole acetic acid, Gibberellic acid GA, and siderophore as well as solubilize phosphate in the soil. The consortium of (Bacillus velezensis, Bacillus megaterium, and Herpaspirillum huttiense) increased the protection % against Rhizoctonia infection by (79.4%), followed by B. velezensis by (73.52%), H. huttiense by (70.5%), and B. megaterium by (67.64%), respectively. There was an increase in soluble proteins and carbohydrates in infected plants treated with a consortium of endophytic bacteria by 30.7% and 100.2% over untreated infected plants, respectively. Applying endophytic bacteria either alone or in combination lowered the level of malondialdehyde MDA and hydrogen peroxide H2O2 and improved the activities of antioxidant enzymes in both infected and uninfected plants. Also, bacterial endophytes have distinctive reactions regarding the number and concentrations of isozymes in both infected and uninfected plants. It could be recommended the commercial usage of a mixture of targeted bacterial endophyte strains as therapeutic nutrients against Rhizoctonia root-rot disease as well as plant growth inducer.
Subject terms: Microbiology, Plant sciences
Introduction
By 2025, there will be around 8 billion individuals on the planet, and by 2050, there will be 9 billion. To provide food for this fast-growing global population, agricultural productivity must rise1. Unfortunately, crop loss brought on by pathogen attacks, especially those by fungi2,3, is a danger to food security. According to Bramhanwade et al. 4, almost one-third of the world's annual crop declines due to plant infections. Phytopathogenic fungi reduce crop productivity by 20–40% per year5. Vegetable diseases that result in either total or partial crop loss are a problem for the protection of plants globally6. All vegetables have been affected by infections, particularly tomatoes, which yearly cause output losses of between 70 and 95 percent7. The tomato (Lycopersicon esculentum L. Solanum lycopersicon Mill), which accounts for 14% of global fruit and vegetable production, is the 2nd most significant and valuable Solanaceous vegetable crop after potatoes8–11. About 4 million hectares of arable land are cultivated to produce tomatoes worldwide, which produces 100 million tons annually valued at between 5 and 6 billion US$12. One of the most significant Solanaceous commodities in Egypt is the tomato, which is grown for both local consumption and exportation13. Notably, Egypt ranks among the top 10 tomato-producing countries in the world, producing 6.4 million tons of tomatoes annually on an estimated 181,000 acres8,14. Tomatoes are considered a rich source of nutrients, minerals, organic acids, vital amino acids, dietary fibers, and vitamins A and C15,16. Because tomato infections severely reduce crop yield, they are seen as being of major economic significance17,18. Numerous harmful diseases affect tomato production in both quantity as well as quality19. Under the danger posed by global warming and the prevalence of infections, refining crop yield and minimizing the employment of pesticides is an urgent need for the agricultural sector20. The tomato crop is susceptible to fungal, viral, nematode, and bacterial illnesses21. In Egypt, fungal diseases are among the most hazardous biotic stresses that seriously harm crops22–24. Fungal plant pathogens have negative impacts on both the quantity and quality of crops, but these effects can be reversed by nonpathogenic fungi that induce plant biochemical defense25. Amongst fungal pathogens, Rhizoctonia solani is the most destructive for tomato plants26. One of the worst fungi damaging tomato crops is Rhizoctonia solani (Khun). This plant pathogenic fungus affects many kinds of hosts and is widely distributed. It causes plant diseases such as collar rot, root rot, damping off, and wire stem27. Because spores can live for years, the conventional techniques of controlling disease, such as the application of fungicides in and rotation of crops, have not proven successful. It is critical to create more efficient management practices that ensure the preservation of the environment. In contrast to fungicides, biological control of plant diseases uses antagonist nonpathogenic microorganisms that can reduce the disease's potential dangers on a variety of crops28. According to recent studies, the application of natural agents is preferred as a safety method for managing Rhizoctonia root rot29. The physiological immunity known as "induced resistance" (IR) is a critical defense mechanism against plant fungal infections. IR is elicited by specific environmental stimuli. Pathogenesis-related (PR) proteins and increased phenolic chemicals led to the development of resistance23. It is known that endophytic microorganisms can colonize the intracellular areas of higher plant parts without causing visible harm to the plants where they live. They have often been found to be rich in bioactive substances30. Endophytes and host plants may cooperate mutualistically to the benefit of both parties' fitness31. Endophytic microorganisms may help their host plant survive and give protection by creating a variety of chemicals32. Endophytes have the capacity to offer direct chemical defense in plants by producing secondary compounds that hinder pests and dangerous microorganisms33,34. Various endophytes were reported to have higher biosynthetic skills because they live and reproduce inside healthy plant tissues and may have undergone gene recombination with the host35. Therefore, the main objects of the present work are to (1) Isolate and identify endophytic bacteria from Strawberry plants (2) Evaluate the antifungal activity of these endophytic bacteria against Rhizoctonia solani root rot of tomato in vitro and in vivo, (3) Evaluate photosynthetic pigments, metabolic indicators, protein, and phenolics compounds of tomato, and (4) Recognize the impacts of endophytic bacterial metabolites on oxidative enzymes in tomato plants under pot conditions.
Materials and methods
Chemicals and reagents
All chemicals were purchased from Sigma-Aldrich Chemical Co (St. Louis Missouri, 63103, USA). The media were gained from Difco (United Kingdom).
Isolation and purification of endophytes
The fresh and healthy strawberry plants were collected from Badr City, El-Behera Governorate, Egypt in January 2022. The plant collection and use were in accordance with all the relevant guidelines. All plants were immediately taken to the lab and prepared for future work. The strawberry plants have been washed with tap water to remove surface dust and then rinsed twice with distilled water. Next, the plant materials were immersed in 75% ethanol for 2 min, rinsed with 2% sodium hypochlorite (Na Cl O) for 3 min, and finally washed 3 times with sterile distilled water36. The plant parts were checked for any surface contamination, in which 100 µL water from the third rinsing was inoculated on three types of media (Reasoner’s 2A agar, PDA, and nutrient agar). The plates were incubated at 30 °C for 2 days to determine surface sterilization efficacy. The sterilized explants were cut with a sterile blade into 0.5 to 0.3 cm pieces and placed on nutrient agar media. The plates were incubated at 30 °C for 2 days. The isolated endophytic bacteria were imperiled for morphological and microscopic description.
Quantitative determination of plant growth-promoting substances in liquid culture
Bacterial Endophyte isolates were checked for their quantitative capabilities to produce plant growth-promoting substances. The efficacy of (IAA) production is done using the colorimetric technique37. Determination of total Gibberellins production was done using the colorimetric technique38. Siderophore production was detected by the CAS plate assay method39. The ability of isolates to solubilize phosphate was tested by streaking the isolates in the center of Pikovyskyaya’s agar medium plates containing a known amount of tri-calcium phosphate Ca3(PO4)2. The plates were incubated at 37 ºC for 72 h. Phosphate solubilization was detected by the appearance of a clear halo zone around the streak of endophytic strains40. The ability of isolates to solubilize potassium was tested according to Zahra41. The formation of hydrogen cyanide (HCN) by the endophytic bacterial isolates was assessed according to the methods of Frey et al. and Daigham et al.42,43.
Source of pathogen
The Pathogen Rhizoctonia solani was obtained from the Plant Pathology Institute, Agriculture Research Center, Giza, Egypt. The pathogenic fungus' inoculum was established following the method of Hibar et al.44.
Molecular identification of endophytic bacterial isolates
The endophytic bacterial isolates (L1–L2–S7) were cultured in a nutrient broth medium45 and incubated at 28ºC for 48 h. for DNA extraction. Patho-gene-spin DNA/RNA extraction kit afforded by Intron Biotechnology Company; Korea was applied. PCR was operated utilizing two universal primers namely 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTT ACGACTT-3′). The purified PCR products were reconfirmed using a size nucleotide marker (100 base pairs) by electrophoreses on 1% agarose gel. Purified PCR products were sequenced in the sense and antisense directions using 27F and 1492R primers with the incorporation of dideoxynucleosides (dd NTPs) in the reaction mixture46. Sequences were analyzed via the Basic Local Alignment Search Tool (BLAST) from (NCBI) website. Meg Align (DNA Star) software version 5.05 was utilized for the phylogenetic testing of sequences.
In vitro antagonistic activity of endophytic bacteria against R. solani by the dual culture assay
Antagonistic activity test of endophytic bacterial strains (L1–L2–S7) was conducted as illustrated by Rosa et al.47with minor modification. PDA media in Petri plates have been streaked individually by a fine line of the isolates (24 h. old) along one end of the plate and incubated at 30 °C for 24 h. Next, along the edge of the plate opposing the bacterial inoculum, a 5-mm-diameter mycelial plug of a 7-day-old culture of R. solani was applied. As a negative control, a PDA plate loaded with only a mycelial plug of R. solani at one side was employed. All treatments were employed in triplicates and then incubated for 5 days at 30 °C. Lastly, the fungal radial growth was estimated, and inhibition was calculated.
Antagonistic activity of the endophytic bacterial extract
The bacterial isolates under study (L1–L2–S7) were grown on the PDB broth and incubated on the shaker for 48 h. at 30 °C. After incubation, the samples were centrifuged at 20,000 rpm for 10 min. and the supernatant was filtered through a 0.22 µm microbiological filter. The resulting supernatant was kept in sterilized flasks. A disk of each pathogenic fungus Rhizoctonia solani was added to the surface of the extract. Incubation was done at 28 °C for 7 days, and the spread of fungal growth was observed on the surface of the extract.
Experimental design
Three weeks age tomato seedlings (Solanum lycopersicum L. var. 023) were achieved from Agriculture Research Centre, Giza, Egypt. Similar seedlings were planted into plastic pots (40 × 40 cm), encompassing a combination of sand and clay (1: 3 W/W), a total of 6 kg, in a plastic greenhouse. Pots stayed in the greenhouse at Day/night temperature (22/18 °C) and relative humidity (70–85%). After planting, the seedlings were normally irrigated and left for 7 days without treatment. The pots were set up with 6 replicates in a subsequent random order : (T1) Tomato seedlings planted in soil that was recently sterilized (Healthy control); (T2) seedlings planted in Rhizoctonia solani -inoculated, soil (Control infected); (T3) Healthy seedlings treated with B. velezensis; (T4) Healthy seedlings treated with B. megaterium; (T5) Healthy seedlings treated with H. huttiense; (T6) Healthy seedlings treated with a combination of (B. velezensis, B. megaterium, and H. huttiense ratio 1:1:1); (T7) Infected seedlings managed with B. velezensis; (T8) infected seedlings handled with B. megaterium; (T9) infected seedlings treated with H. huttiense, and (T10) Infected seedlings managed with a mixture of (B. velezensis, B. megaterium, and H. huttiense). Disease severity was recorded 15 days post-inoculation. After 60 days of inoculation, the biochemical indicators resistance was detected.
Disease symptoms and disease index
The disease indicators were recorded, and the following equation was used to determine the severity of the disease along with the protection percentage.
Protection % = A–B/A × 100%, where A = PDI in diseased control plants B = PDI in diseased-treated plants as reported by Hashem et al.48.
Biochemical defense indicators
Total soluble carbohydrate content in dry leaves was assessed utilizing the anthrone technique according to Irigoyen,49. Also, the content of total protein was measured in the dry leaves50. The phenol content of the dry leaves was also estimated51. Malondialdehyde (MDA) content in fresh leaf was assessed by the thiobarbituric acid (TBA) method conferring to Hu et al.52 with slight modification. Hydrogen peroxide (H2O2) levels were determined53.
Evaluation of antioxidant enzymes activity
Peroxidase (POD) activity was detected corresponding to Verduyn et al.54. The activity of polyphenol oxidase (PPO) and SOD were evaluated affording to methods of Matta and Dimond55 and Marklund and Marklund56, respectively.
Isozyme electrophoresis
Peroxidase (POD) and polyphenol oxidase (PPO) isozymes were detected corresponding to Trivedi et al.57, while polyphenol oxidase (PPO) isozymes were assessed as stated by Knegt & Bruinsma58. Detection of SOD isozymes in fresh leaves was done following the method of Beauchamp & Fridovich59.
Statistical analyses
The obtained results had been imperiled to one-way variance analysis (ANOVA). The significant variances between treatments were demonstrated by CoStat (CoHort, Monterey, CA, USA) utilizing the Least Significant Difference (LSD) test at p < 0.05. The results were provided as means of standard errors (n = 3).
Plant collection
The plant collection and use were in accordance with all the relevant guidelines.
Ethical approval
There are no experiments on people or animals in this study.
Results
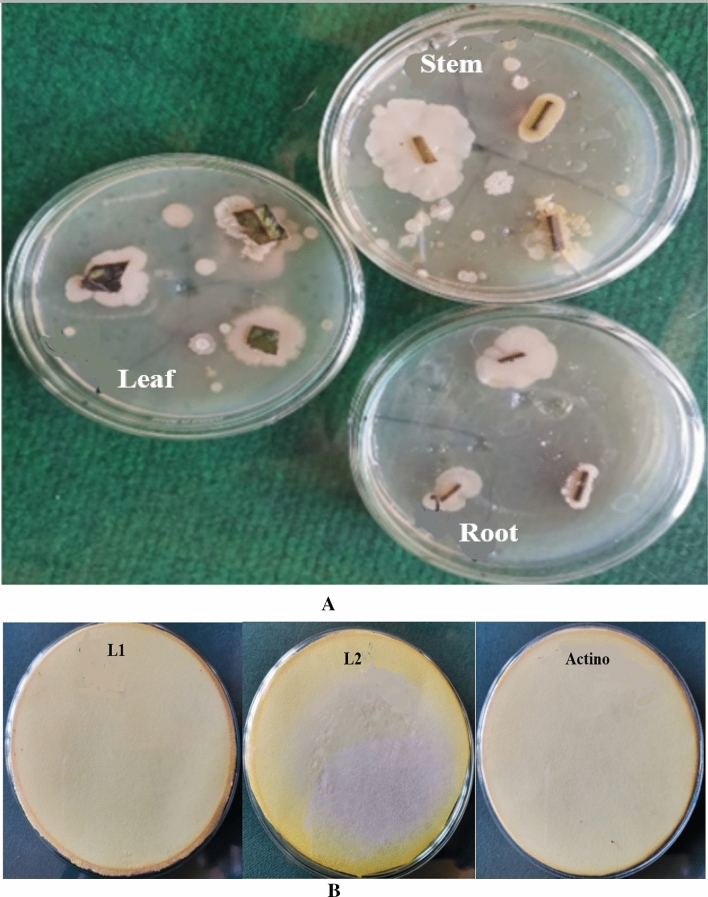
In the present investigation, a total of 31 endophytes were isolated and enumerated from leaves, stems, and roots of Strawberry plants Fig. 1A. All isolates were investigated according to promoting properties. The highest HCN production was recorded by the L1 isolate followed by S7 and then L2 isolate Fig. 1B. The results in Table 1 indicated that none of the isolates were capable of solubilizing potassium. All isolates could produce IAA, where L1 isolate showed a higher production rate (+++), followed by L2 (++) and S7 (++) isolates. The highest potassium solubilization was attained by L2 (+++), followed by S7 (++) and L1 (++) isolates. Moreover, all isolates showed proficiency in siderophores and GA production.
Figure 1.
(A) Isolation of endophytic bacteria from stem, leaves, and roots of strawberry plants. (B) HCN production by endophytic bacterial isolates.
Table 1.
Determination of plant growth-promoting substances of endophytic bacterial isolates.
| Endophytic isolates | K solubilization | IAA | Siderophores | P solubilization | GA | HCN |
|---|---|---|---|---|---|---|
| L1 | − | +++ | +++ | ++ | +++ | +++ |
| L2 | − | ++ | ++ | +++ | ++ | + |
| S7 | − | ++ | ++ | ++ | ++ | ++ |
Molecular identification of bacterial isolates
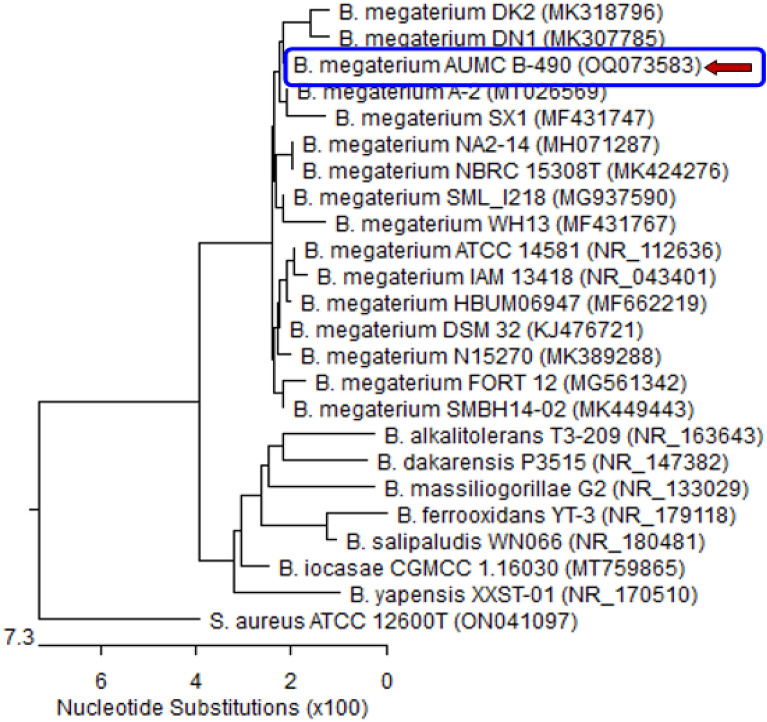
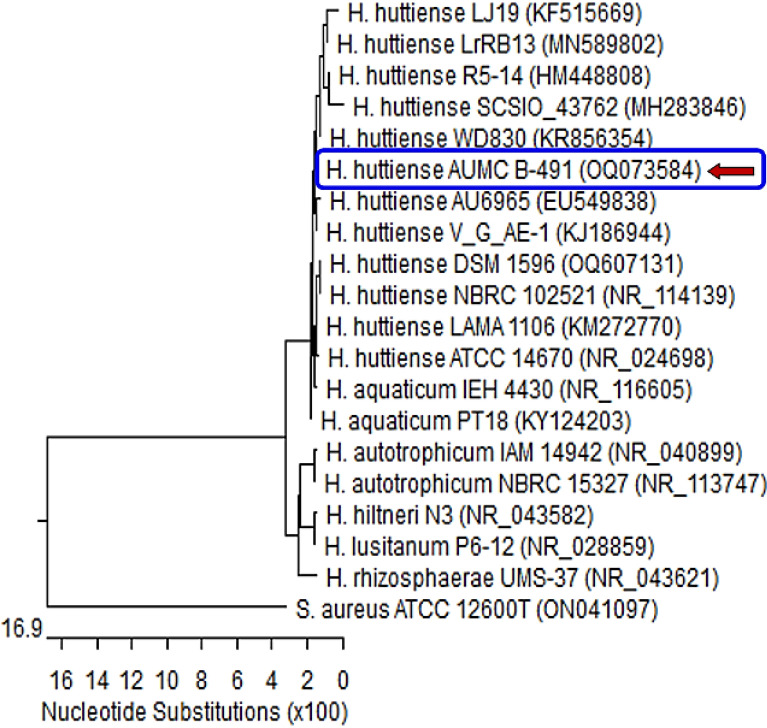
Based on a mega blast search of the NCBIs GenBank nucleotide database, the closest hit using the sequence of the L1 strain is Bacillus velezensis. Figure 2. B. velezensis L-1 showed 99.79–100% identity and 99–100% coverage with several strains of the same species with GenBank accession no. OQ073573. On the other hand, the Phylogenetic analysis based on the 16S rRNA gene of Bacillus megaterium isolate L2 (arrowed) aligned with sequences of closely related bacterial species. B. megaterium L2 showed 99.72–100% identity and 99–100% coverage with several strains of the same species including the type of material Bacillus megaterium ATCC14581 with GenBank accession no. OQ073583. Staphylococcus aureus represents an outgroup strain Fig. 3. Additionally, based on the 16S rRNA gene of Herpaspirillum huttiense isolate S7 (arrowed) aligned with sequences of closely related bacterial species. H. huttiense isolate S7 showed 99.50–99.86% identity and 93–100% coverage with several strains of the same species including the type of material H. huttiense ATCC14670 with GenBank accession no. OQ073584. Staphylococcus aureus represents an outgroup strain, Fig. 4.
Figure 2.
Phylogenetic tree of Bacillus velezensis isolate L-1 (arrowed) aligned with sequences of closely related bacterial species. B. velezensis L-1 displayed 99.79–100% identity and 99–100% reporting with various strains of the same species with GenBank accession no. OQ073573. B. = Bacillus and S = Staphylococcus. S. aureus represents an outgroup strain.
Figure 3.
Phylogenetic tree of Bacillus megaterium isolate L2 (arrowed) aligned with sequences of closely related bacterial species. B. megaterium L2 disclosed 99.72–100% identity and 99–100% reporting with various strains of the same species including the type of material Bacillus megaterium ATCC14581 with GenBank accession no. OQ073583. Staphylococcus aureus represents an outgroup strain.
Figure 4.
Phylogenetic tree of Herpaspirillum huttiense isolate S7 (arrowed) aligned with sequences of closely related bacterial species. H. huttiense isolate S7 exhibited 99.50–99.86% identity and 93–100% reporting with various strains of the same species including the type of material H. huttiense ATCC14670 with GenBank accession no. NR_024698. Staphylococcus aureus represents an outgroup strain.
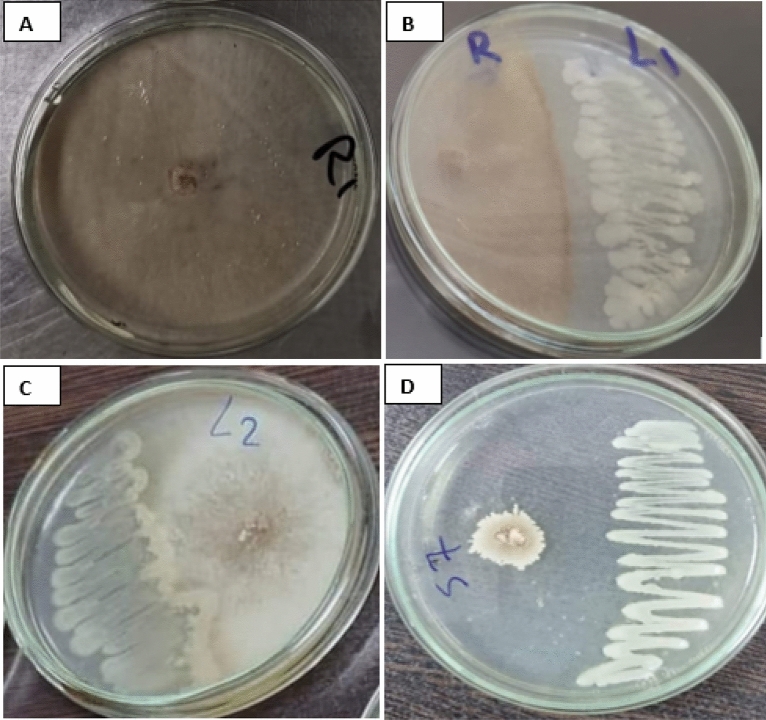
Antagonistic activity of endophytes
Varying degrees of mycelial growth inhibition of the phytopathogenic fungus R. solani using the dual culture assay were observed with antagonistic endophytic bacteria Bacillus velezensis L1, Bacillus megaterium L2 and Herpaspirillum huttiense S7. The results indicated that Herpaspirillum huttiense S7 had the maximum inhibitory effect on the mycelial growth of Rhizoctonia solani followed by Bacillus velezensis isolate L1 and then Bacillus megaterium isolate L2. The interactions between antagonistic endophytic bacteria and R. solani were shown in Fig. 5. Moreover, the extract of endophytic bacteria under study exerts variable degrees of R. solani mycelial growth inhibition, and the most effective was Bacillus velezensis isolate L1 followed by Herpaspirillum huttiense isolate S7 Table 2 and Fig. 6.
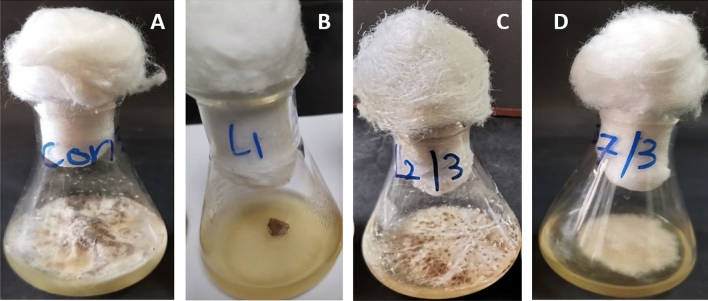
Figure 5.
Antagonistic action of endophytic strains versus of R. solani using the dual culture method; (A) R. solani (Control); (B) B. velezensis (L1) versus R. solani; (C) Bacillus megaterium (L2) versus R. solani; (D) Herpaspirillum huttiense (S7) versus R. solani.
Table 2.
Percentage of R. solani mycelial growth inhibition by endophytic bacterial strains.
| Endophytic isolates | Mycelial growth inhibition % of R Solani |
|---|---|
| B. velezensis (L1) | 100% |
| Bacillus megaterium (L2) | 48 |
| Herpaspirillum huttiense (S7) | 65% |
Figure 6.
Antagonistic activity of endophytic bacterial extract; (A) R. solani (Control); (B) extract of B. velezensis (L1); (C) extract of Bacillus megaterium (L2); (D) extract of Herpaspirillum huttiense (S7).
Disease symptoms and disease index
As shown in Table 3, the disease index reached 85%, due to R. solani infection. The results showed that R. solani infects the roots of the plant, causing yellowing, root rot, and eventually death. The results in Table 3 and Fig. 7 showed that both B. velezensis, H. huttiense, and B. megaterium filtrates alone were effective in reducing the severity of the Rhizoctonia root rot disease by 22.5%, 25%, and 27.5%, and increase protection by 73.52%, 70.5% and 67.64%, respectively. However, the combining treatment of B. velezensis, B. megaterium, and H. huttiense filtrates was even more effective, with a DI 17.5% and Protection 79.4%.
Table 3.
Effect of B. velezensis, H. huttiense, and B. megaterium filtrates on root rot disease of tomato plants caused by Rhizoctonia solani under pots conditions.
| Treatments | Disease symptoms Classes | DI (disease index) (%) | Protection (%) | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||
| Control infected | 0 | 0 | 1 | 4 | 5 | 85 | 0 |
| Infected treated with B. velezensis | 4 | 4 | 1 | 1 | 0 | 22.5 | 73.52 |
| Infected treated with B. megaterium | 4 | 3 | 2 | 0 | 1 | 27.5 | 67.64 |
| Infected treated with H. huttiense | 6 | 1 | 1 | 1 | 1 | 25 | 70.5 |
| Infected B. velezensis, B. megaterium and H. huttiense) | 7 | 0 | 2 | 1 | 0 | 17.5 | 79.4 |
Figure 7.
Effect of T1, T2, and T3 on protection of tomato plant against root rot caused by Rhizoctonia solani T1 = Infected treated with B. velezensis; T2 = Infected treated with B. megaterium; T3 = Infected treated with H. huttiense, Mix = Infected B. velezensis, B. megaterium and H. huttiense).
Biochemical defense indicators
Total soluble carbohydrates and protein
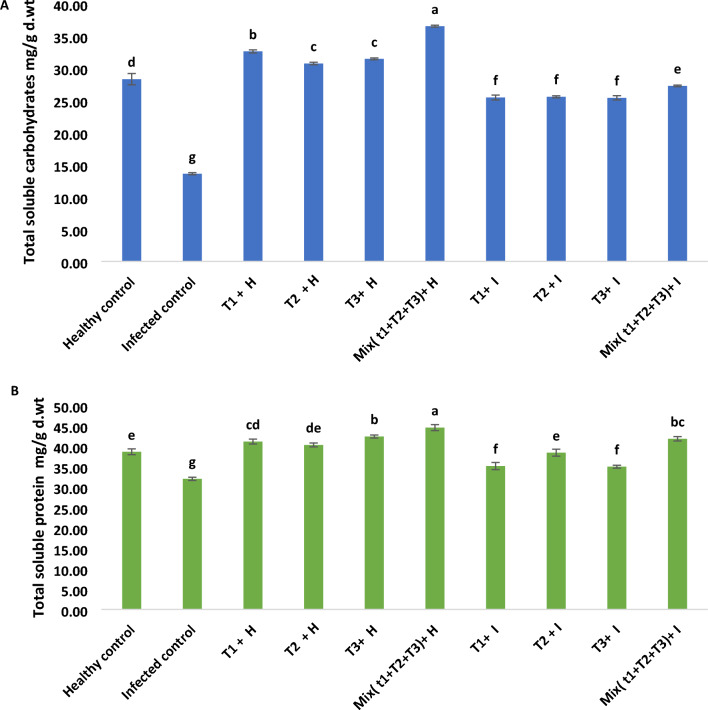
Results recorded in Fig. 8A declared that infected plants established significant declines in contents of total soluble carbohydrates by 51.9% in comparison with healthy control. Relating the consequence of tested treatments on healthy plants, it was found that all filtrates alone were effective in increasing total soluble carbohydrates by 15.24%, 8.59%, and 11.14%. However, the treatment with mixed filtrates from B. velezensis, B. megaterium, and H. huttiense was even more effective in increasing total soluble carbohydrates by 29.09%. Also, all filtrates alone were effective in increasing total soluble carbohydrates by 86.95%, 87.79%, and 86.65% in infected plants. However, the mixed treatment of all filtrates was even more effective in increasing total soluble carbohydrates by 100.2%. Additionally, results in Fig. 8 B illustrated a substantial decrease in the soluble protein content of R. solani-infected plants. Application of all filtrates individual or combination, resulted in, mostly, significant increases in total soluble protein in both healthy and diseased plants. About the effect of tested treatments on the diseased plants with R. solani. It was recovered that mixed treatment with (B. velezensis, B. megaterium, and H. huttiense) filtrates showed a highly substantial rise in the soluble protein contents by 30.07% related to B. megaterium 20.8%, B. velezensis 9.8% and H. huttiense 9.3% when being compared with untreated infected control.
Figure 8.
(A) Effect of B. velezensis, B. megaterium, and H. huttiense filtrates on of total soluble carbohydrates of healthy and infected tomato plants (B) Effect of B. velezensis, B. megaterium, and H. huttiense filtrates on of total soluble protein of healthy and infected tomato plants. T1: Healthy control, T2: Infected control, (T3) Healthy + B. velezensis; (T4) Healthy + B. megaterium; (T5) Healthy treated with H. huttiense,; (T6) Healthy + combination of (B. velezensis, B. megaterium and H. huttiense); (T7) Infected + B. velezensis; (T8) Infected + B. megaterium; (T9) Infected + H. huttiense,; and (T10) Infected + combination of (B. velezensis, B. megaterium, and H. huttiense). The results were represented as (mean ± SD, n = 3), letters authoritative to significant statical assessment.
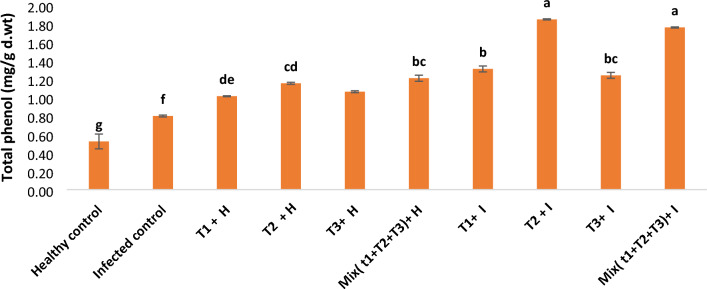
Phenol content
As shown from the results in Fig. 9, the phenol content increased by 52.77%, due to R. solani infection in comparison with healthy control. Application of all filtrates alone or in combination resulted in, mostly, a significant increase of phenol content in both healthy and infected plants. Regarding the effect of the tested treatment on the affected plants with R. solani, the B. megaterium treatment showed a significant rise in the content of phenol by 131.2% related to the mixed treatment of B. velezensis, B. megaterium, and H. huttiense by 120.3%, B. velezensis by 64% and H. huttiense 55.2%, when being compared with untreated infected control.
Figure 9.
Effect of B. velezensis, B. megaterium, and H. huttiense filtrates on phenol contents of healthy and infected tomato plants. T1: Healthy control, T2: Infected control, (T3) Healthy + B. velezensis; (T4) Healthy + B. megaterium; (T5) Healthy treated with H. huttiense,; (T6) Healthy + combination of (B. velezensis, B. megaterium, and H. huttiense); (T7) Infected + B. velezensis; (T8) Infected + B. megaterium; (T9) Infected + H. huttiense; and (T10) Infected + combination of (B. velezensis, B. megaterium, and H. huttiense). The results were represented as (mean ± SD, n = 3), letters authoritative to significant statical assessment.
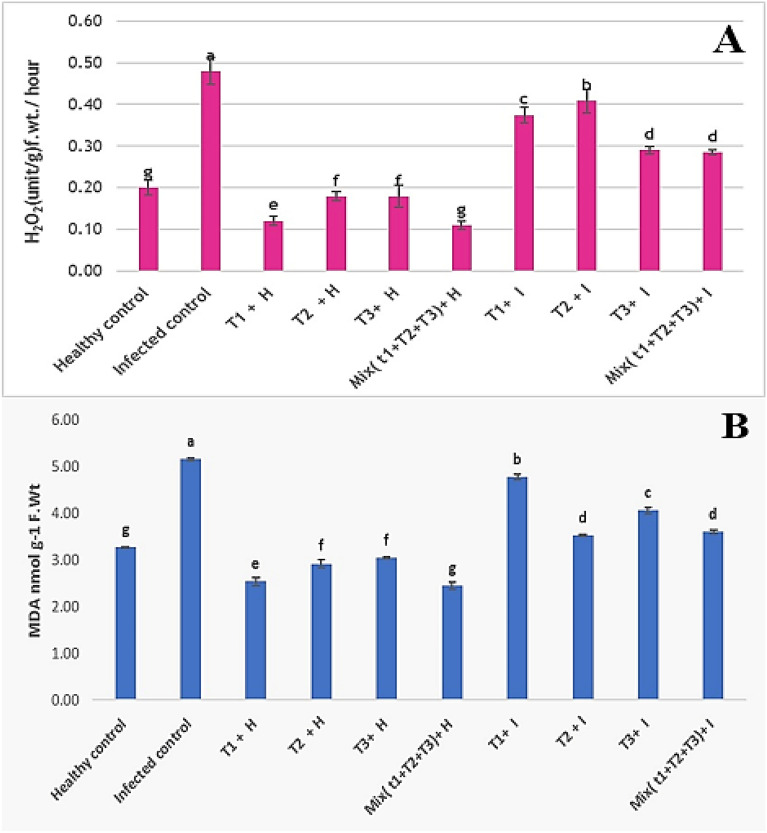
Oxidative stress (MDA and H2O2)
As shown in the results represented in Fig. 10A,B, R. solani infection accumulated the contents of MDA by 57.6% and H2O2 by 140%, compared to untreated healthy plants. Application of B. velezensis, B. megaterium, and H. huttiense individually or in combination, resulted in, mostly, a significant decrease in the contents of MDA by and H2O2. Also, B. megaterium exerts a significant decline in the MDA by 37.7% related to mixed treatment of B. velezensis, B. megaterium, and H. huttiense by 30.2%, H. huttiense by 21.3% and B. velezensis by 7.5%, on infected plants when being compared with untreated infected control. While treatment with a mix of B. velezensis, B. megaterium, and H. huttiense reduces H2O2 by 40.7% related to H. huttiense by 39.2%, B. velezensis by 22.1% and B. megaterium by 15%, when being compared with untreated infected control.
Figure 10.
Effect of B. velezensis, B. megaterium, and H. huttiense filtrates on (A) MDA and (B) H2O2 of healthy and infected tomato plants. T1: Healthy control, T2: Infected control, (T3) Healthy + B. velezensis; (T4) Healthy + B. megaterium; (T5) Healthy treated with H. huttiense, (T6) Healthy + combination of (B. velezensis, B. megaterium and H. huttiense); (T7) Infected + B. velezensis; (T8) Infected + B. megaterium; (T9) Infected + H. huttiense, and (T10) Infected + combination of (B. velezensis, B. megaterium and H. huttiense). The results were represented as (mean ± SD, n = 3), letters authoritative to significant statical assessment.
Antioxidant enzymes activity
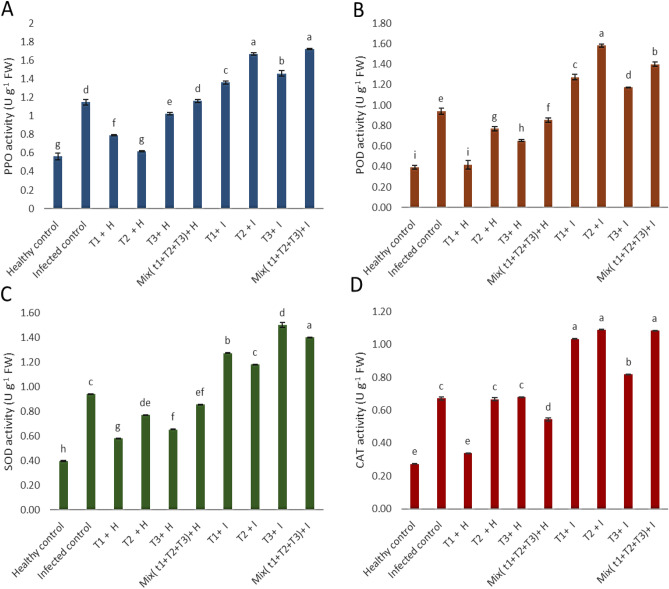
The activities of PPO, POD, SOD, and CAT highly increased in diseased plants linked to healthy control plants Fig. 11. Also, the application of B. velezensis, B. megaterium, and H. huttiense individually or in combination, resulted in, mostly, significant increase activities of antioxidant enzymes PPO, POD, SOD, and CAT, comparing to untreated healthy plants. Concerning the effect of tested treatments, on tomato plants infected with R. solani, the results revealed that the mix treatments of B. velezensis, B. megaterium, and H. huttiense filtrates show a highly significant increase in the PPO by 50.1% related to B. megaterium 45.5%, H. huttiense 27.1% and B. velezensis 18.5%. For POD, it was found that B. megaterium shows a highly significant increase of 67.81% followed by mixed treatment of B. velezensis, B. megaterium, and H. huttiense by 48.64%, B. velezensis by 35.2%, and H. huttiense 24.8%. Regarding the effect of tested treatments on tomato plants infected with R. solani, the results indicated that H. huttiense 27.3% shows a highly significant increase in the SOD by 59.6% related to mixing treatment of B. velezensis, B. megaterium, and H. huttiense by 48.94%, B. velezensis 35.4%, and B. megaterium 25.53%. For CAT, it was found that B. megaterium shows a highly significant increase of 62.16% followed by mixed treatment of B. velezensis, B. megaterium, and H. huttiense by 61.43%, B. velezensis by 53.92% and H. huttiense 21.71%, comparing to untreated infected plants.
Figure 11.
Effect of B. velezensis, B. megaterium, and H. huttiense filtrates on (A) PPO, (B) POD, (C) SOD and (D) CAT activities of healthy and infected tomato plants. T1: Healthy control, T2: Infected control, (T3) Healthy + B. velezensis; (T4) Healthy + B. megaterium; (T5) Healthy treated with H. huttiense, (T6) Healthy + combination of (B. velezensis, B. megaterium and H. huttiense); (T7) Infected + B. velezensis; (T8) Infected + B. megaterium; (T9) Infected + H. huttiense; and (T10) Infected + combination of (B. velezensis, B. megaterium and H. huttiense). The results were represented as (mean ± SD, n = 3), letters authoritative to significant statical assessment.
Superoxide dismutase (SOD) isozymes
Seven SOD isozymes were noticed on native PAGE in Fig. 12 and Table 4 at Rf 0.515, 0.566, 0.571, 0.729, 0.803, 0.853, and 0.903. Infected tomato plants with R. solani exhibited substantially overexpressed SOD, which was detected in three bands, two low and one dense at Rf 0.515 and 0.803 and 0.729 respectively. Under infection circumstances, the application of a mixture from B. velezensis, B. megaterium, and H. huttiense, H. huttiense, and B. velezensis treatments verified the similar 6 bands at the identical Rf. and came next the B. megaterium treatment which confirmed one moderate band at Rf 0.515 and one low band at Rf 0. 729.
Figure 12.
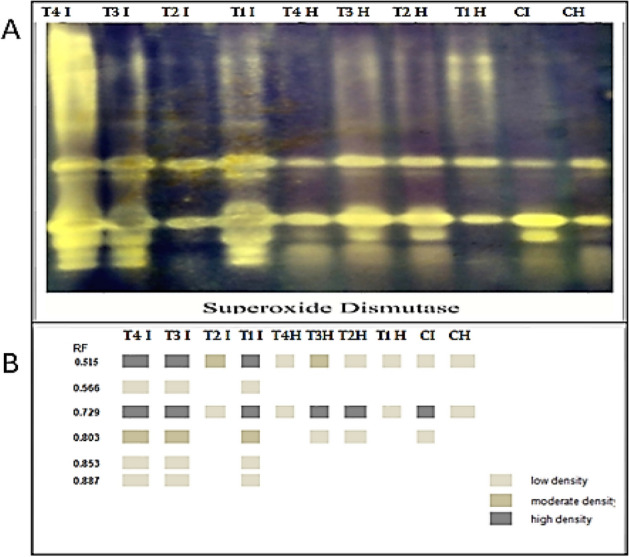
Consequence of R. solani infection, application of (B. velezensis, B. megaterium, and H. huttiense) and their impacts on tomato plants on (A) SOD isozyme and (B) Ideogram analysis of SOD isozyme of tomato plants.
Table 4.
Isomers of SOD enzymes (+/−) and their Retention factor (Rf) in response to R. solani, application of (B. velezensis, B. megaterium, and H. huttiense) and their interactions on tomato plants.
| RF | T4 I | T3 I | T2 I | T1 I | T4 H | T3 H | T2 H | T1 H | CI | CH |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.515 | +++ | +++ | ++ | +++ | + | ++ | + | + | + | + |
| 0.566 | + | + | − | + | − | − | − | − | − | − |
| 0.571 | − | − | − | − | − | − | − | − | − | − |
| 0.729 | +++ | +++ | + | +++ | + | +++ | +++ | + | +++ | + |
| 0.803 | ++ | ++ | − | ++ | − | + | + | − | + | − |
| 0.853 | + | + | − | + | − | − | − | − | − | − |
| 0.903 | + | + | − | + | − | − | − | − | − | − |
CH = Healthy Control, CI = Infected control, T1H = Healthy + B. velezensis; T2H = Healthy + B. megaterium; T3H = Healthy + H. huttiense, ; T4H = Healthy + combination of (B. velezensis, B. megaterium and H. huttiense), T1I = infected + B. velezensis; T2I = Infected + B. megaterium; T3I = Infected + H. huttiense, ;and T4I = Infected + combination of (B. velezensis, B. megaterium and H. huttiense).
Peroxidase (POD) isozymes
Data offered in Fig. 13 and Table 5 showed seven POD isozymes at Rf 0.146, 0.190, 0.381, 0.503, 0.741, 0.845 and 0.918. R. solani infected plants displayed substantially overexpressed POD that documented 6 bands. Regarding infected plants, application of a combination of B. velezensis, B. megaterium, and H. huttiense revealed substantially overexpressed POD that detailed 7 bands followed by B. megaterium documented 7 bands, H. huttiense gave 6 bands and came next B. velezensis treatments documented the same 6 bands.
Figure 13.
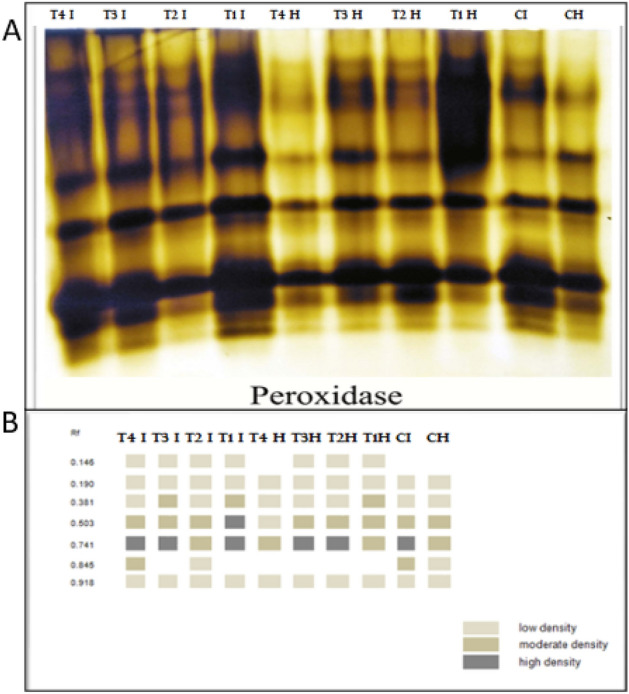
Consequences of R. solani infection, application of (B. velezensis, B. megaterium, and H. huttiense) and their impacts on tomato plants on (A) POD isozyme and (B) Ideogram analysis of POD isozyme of tomato plants.
Table 5.
Isomers of POD enzymes (+/−) and their Retention factor (Rf) in response to R. solani, application of (B. velezensis, B. megaterium, and H. huttiense) and their interactions on tomato plants.
| RF | T4 I | T3 I | T2 I | T1 I | T4 H | T3 H | T2 H | T1 H | CI | CH |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.146 | + | + | + | + | − | + | + | + | − | − |
| 0.190 | + | + | + | + | + | + | + | + | + | + |
| 0.381 | + | ++ | + | + | + | + | + | ++ | + | + |
| 0.503 | ++ | ++ | ++ | +++ | + | ++ | ++ | ++ | ++ | ++ |
| 0.741 | +++ | +++ | ++ | +++ | ++ | +++ | +++ | ++ | +++ | ++ |
| 0.845 | ++ | − | + | − | − | − | − | − | ++ | + |
| 0.918 | + | + | + | + | + | + | + | + | + | + |
CH = Healthy Control, CI = Infected control, T1H = Healthy + B. velezensis; T2H = Healthy + B. megaterium; T3H = Healthy + H. huttiense; T4H = Healthy + combination of (B. velezensis, B. megaterium and H. huttiense), T1I = infected + B. velezensis; T2I = Infected + B. megaterium; T3I = Infected + H. huttiense,; and T4I = Infected + combination of (B. velezensis, B. megaterium and H. huttiense).
Polyphenol oxidase (PPO)
Five PPO isozymes were visible on the native PAGE in Fig. 14 and Table 6. The infected plants revealed substantially overexpressed PPO 3 bands including 2 low bands at Rf 0.492 and 0.819, and a unique vastly dense band at Rf 0.732 related to healthy control. Additionally, under infection conditions, application B. velezensis revealed substantially overexpressed PPO that detailed two high dense and 3 low bands, followed by a combination of B. velezensis, B. megaterium, and H. huttiense, recorded the similar 5 bands at the identical Rf but the band at Rf 0.492 was moderate band, followed by H. huttiense recorded the same 5 bands two of them low at Rf 0.341and 0.819, while the other 1 moderate band was at Rf 0.49 and 1 high dense at Rf 0.732, and came next B. megaterium which recorded only two low bands at Rf 0.492 and 0.732.
Figure 14.
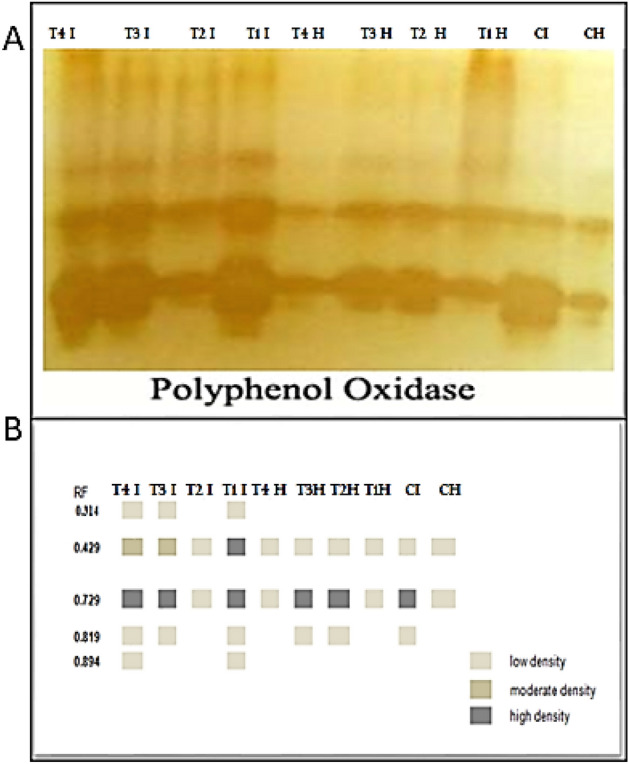
Consequences of R. solani infection, application of (B. velezensis, B. megaterium, and H. huttiense) and their impacts on tomato plants on (A) POD isozyme, and (B) Ideogram analysis of PPO isozyme of tomato plants.
Table 6.
Isomers of PPO enzymes (+/−) and their Retention factor (Rf) in response to R. solani, application of B. velezensis, B. megaterium, and H. huttiense) and their interactions on tomato plants.
| RF | T4 I | T3 I | T2 I | T1 I | T4 H | T3 H | T2 H | T1 H | CI | CH |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.341 | + | + | − | + | − | − | − | − | − | − |
| 0.492 | ++ | ++ | + | +++ | + | + | + | + | + | + |
| 0.732 | +++ | +++ | + | +++ | + | +++ | +++ | + | +++ | + |
| 0.819 | + | + | − | + | − | + | + | − | + | − |
| 0.894 | + | − | − | + | − | − | − | − | − | − |
CH = Healthy Control, CI = Infected control, T1H = Healthy + B. velezensis; T2H = Healthy + B. megaterium; T3H = Healthy + H. huttiense,; T4H = Healthy + combination of (B.velezensis, B. megaterium and H. huttiense), T1I = infected + B. velezensis; T2I = Infected + B. megaterium; T3I = Infected + H. huttiense,; and T4I = Infected + combination of (B.velezensis, B. megaterium and H. huttiense).
Discussion
Microorganisms are now used to control pathogens and pests to protect important plants60. The first and most important step in biological management is the identification and selection of active antagonistic microorganisms61. The goal of biological control is to sustain a balance in agrosystems in which the host suffers less damage in the presence of pathogens owing to the regulatory actions of non-pathogenic microorganisms that inhibit/antagonize plant pathogens. In the current work, endophytic bacteria Bacillus velezensis isolate L1, Bacillus megaterium isolate L2 and Herpaspirillum huttiense isolate S7 proved significant antifungal ability contrary to R. solani in Vitro. Our findings corresponded with those of Azevedo et al.62, who found that endophytic microorganisms have attracted attention for use in biological management and might be used to promote plant growth and prevent fungal infections. The isolated endophytic bacteria under study produce IAA, siderophores, HCN and solubilize phosphate in the soil. Indole acetic acid is a significant and powerful plant hormone, that controls cell growth and responds plants to light and gravity, as well as inducing plant growth and development63,64. Moreover, endophytic microorganisms produce bioactive compounds such as alkaloids, steroids, terpenoids, peptides, polyketides, flavonoids, quinols, and phenols. These compounds have a variety of agricultural, industrial, and therapeutic uses30,65,66. Endophytic bacteria have the ability to synthesize plant growth promotors, phosphate solubilization, mineral acquisition, and fixation of nitrogen67. Moreover, Shakirova68 indicated that IAA has been shown to play a significant fundamental function in cell division and root stimulation, which brings in improved plant development. HCN is a potent antifungal and has a significant role in the biocontrol of plant fungal pathogens43,69. Endophytes may have acted in various ways against plant pathogens, and this preliminary assessment suggests a potential ability to create metabolic substances that restrict pathogen growth or directly compete for nutrients and space, simulating conditions inside the plant that hosts them70,71. In addition, Schulz et al.72 proposed that endophytes are effective suppliers of secondary metabolites since they have an intimate connection to the host plants. Besides, Shukla et al.73 indicated that endophytes have been shown to produce a variety of bioactive compounds in a single plant or microbe, making them a promising source of drugs for the treatment of various diseases, as well as having conceivable uses in agriculture. The endophytes' production of bioactive compounds has been linked to the advancement of the host microbes, which might have adopted genetic codes from higher plants, thus allowing them to more easily adapt to their host plant and fulfill certain roles, such as protection from numerous sorts of pathogens74,75.
Similar to our findings, Devi et al.76 reported the usage of B. velezensis as a biocontrol agent has a wide antifungal spectrum and affords a sustainable alternative for hazardous chemicals. Besides, our results agreed with Hashem et al.48 who recorded the antifungal potential of B. megaterium against phytopathogenic R. solani. It's interesting to note that, except for a few isolates rarely reported in humans as a pathogen causing bacteremia in leukemic persons77,78, the majority of the Herbaspirillum genus were mostly isolated from soil, plants, or water. The isolation of the novel endophytic bacterium Herbaspirillum camelliae sp. from Camellia sinensis L. was also described by Liu et al.79. The study of Andreozzi et al.80 showed the beneficial endophyte Herpaspirillum huttiense RCA24 as a promising strain to improve rice plants in the greenhouse. Endophytic actinomycetes and bacteria are important contributors to the synthesis of bioactive substances. Moreover, Liarzi et al.81 reported that endophytic bacteria endure most of their lives inside plant tissues without causing any evident impairment to the host plant and improve plant tolerance to various abiotic and biotic stress, as well as plant durability against pests and insects.
The combination of endophytic bacteria under study increased the tomato's protection against R. solani disease by 79.4%, according to the results. B. velezensis has been shown to exert opposing effects on plant diseases by producing a variety of antimicrobial chemicals; siderophore bacillibactin82–84.
Besides, R. solani infectivity causes significant decreases in the contents of soluble carbohydrates and soluble proteins in our study. R. solani caused reduced photosynthetic rate and excessive respiration rate in infected plants resulting in lower concentrations of both soluble carbohydrates and protein85–87.
The results also showed that the application of (B. velezensis, B. megaterium, and H. huttiense) individually or in combinations had a substantial impact on the level of osmolytes (total soluble sugar and soluble protein), which may operate as a marker of resistance, in both healthy and diseased plants. The noticed increase in tomato plant soluble sugar and protein could be attributed to the ability of these endophytic bacteria to fix nitrogen, produce plant growth regulators; and solubilize phosphate, which enhances nutrient uptake from the root rhizosphere88,89.
The phenol content increased by 52.77%, due to Rhizoctonia solani infection in comparison with healthy control. These results are supported by many previous findings26,90. The role of phenolic compounds is fundamental in preventing the spread of root rot diseases by either making numerous metabolic products including those involved in host defense mechanisms, or by reducing the pathogen's toxicity and increasing host defense pathways91. Besides, phenolic chemicals were able to strengthen cellular membranes by restricting membrane flexibility, which lowers the ability of free radicals to cross membranes and causes membrane peroxidation92,93. Our results were supported by many previous findings48,94.
Herein, R. solani infection accumulated MDA by 57.6% and H2O2 by 140%, compared to untreated healthy plants. These results were supported by previous findings87,95. By boosting antioxidant molecules that eliminate ROS and protect cell membranes against oxidative stress, the application of endophytic bacteria reduced the generation of MDA and H2O296. One of the most important signs of stress resistance is avoidance and reduction of oxidative stress and capture of free radicals97.
Our findings confirmed that plants subjected to R. solani infection had considerably higher activity levels of antioxidant enzymes. The plant displayed various defense mechanisms against infection, boosting the action of antioxidant enzymes to maintain ROS levels in plant cells low. POD and other antioxidant enzymes assist in converting H2O2 to H2O98. Increasing the activity of antioxidant enzymes provides a key function in plant physiological immunity and defending cells from oxidation as a result of infection99. As part of the primary regulating mechanisms of metabolism within cells, several enzyme isoforms are essential for plant cellular defense versus biological stress100,101. The generation of these isozymes is thought to be essential for the cell's defense against oxidative damage102,103. In the leaf-soluble protein extracts of the tomato plant, active staining of antioxidants disclosed five PPO isozymes, seven SOD isozymes, and seven POD isozymes. The antioxidant enzyme levels in R. solani-infected plants handled with endophytic bacteria B. velezensis, B. megaterium, and H. huttiense were greater than in the control one since numerous unique bands were produced because of infection. The stressed tomato plants treated with B. velezensis, B. megaterium, and H. huttiense either in combination or alone displayed the highest bands of POD isozyme allied to another treatment. The outcomes demonstrate how treatments protect tomato plants from root rot disease in an ameliorative manner which is agreed with Hao et al.104. These findings are consistent with the findings of Rajendran et al.105, which reported increased antioxidant isozyme transcript abundances in plants. In comparison to untreated and infected control plants, the level of all SOD isozymes intensified when endophytic bacteria (B. velezensis, B. megaterium, and H. huttiense) were applied. The recent results were in the same link as prior investigations that claimed a range of proteins, including CAT and POD, may act as scavengers for these ROS106,107.
Conclusion
The present investigation used a promising approach that was focused on applying endophytic bacteria to promote systemic resistance in tomato plants against Rhizoctonia root-rot disease. Three endophytic bacteria; Bacillus velezensis OQ073573, Bacillus megaterium OQ073583, and Herpaspirillum huttiense OQ073584 isolated from strawberry plants boost the systemic resistance in tomato plants and reduce the severity of the Rhizoctonia root-rot disease. These endophytic bacteria demonstrated potent antifungal activity against R. solani in vitro along with in vivo. Pre-treated tomato plants with endophytic bacteria had significantly higher levels of total soluble proteins, total carbohydrates, and phenols. Interestingly, the harmful impact of Rhizoctonia root-rot disease on tomato plants was pointedly decreased and it can be clear from diminished MDA and H2O2 levels. In considering this, endophytic bacteria are promising isolates for application in agriculture, as an effective biological manager against Rhizoctonia root-rot, and for the induction of healthy tomato plants.
Author contributions
Conceptualization; A.Y.M., G.E.D., W.H.I. and M.S.A., Methodology; M.M.A., A.Y.M., G.E.D., W.H.I. and M.S.A., Data Analysis; A.Y.M., G.E.D., W.H.I. and M.S.A., Figures and tables preparation; A.Y.M., G.E.D., and M.S.A.; Writing original draft preparation; A.Y.M., G.E.D., and M.S.A., Writing review and editing A.Y.M., G.E.D., and M.S.A., Resources; A.Y.M., G.E.D., W.H.I. and M.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The datasets generated and/or analyzed during the current study are available in the Gene Bank database repository, under Accession No. OQ073573 for Bacillus velezensis, GenBank accession OQ073583 for Bacillus megaterium and GenBank Accession No. OQ073584 for Herpaspirillum huttiense.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amira Y. Mahfouz, Email: amira.mohamed@azhar.edu.eg
Ghadir E. Daigham, Email: ghadirdaigham@azhar.edu.eg
References
- 1.Singh Sekhon B. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014;7:31–53. doi: 10.2147/NSA.S39406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bebber DP, Gurr SJ. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genet. Biol. 2015;74:62–64. doi: 10.1016/j.fgb.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Khalil AMA, Hashem AH, Abdelaziz AM. Occurrence of toxigenic Penicillium polonicum in retail green table olives from the Saudi Arabia market. Biocatal Agric. Biotechnol. 2019;21:101314. doi: 10.1016/j.bcab.2019.101314. [DOI] [Google Scholar]
- 4.Bramhanwade K, Shende S, Bonde S, Gade A, Rai M. Fungicidal activity of Cu nanoparticles against Fusarium causing crop diseases. Environ. Chem. Lett. 2016;14:229–235. doi: 10.1007/s10311-015-0543-1. [DOI] [Google Scholar]
- 5.Srivastava S, et al. Unraveling aspects of bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Front. Plant Sci. 2016;7:1. doi: 10.3389/fpls.2016.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzola M, Freilich S. Prospects for biological soilborne disease control: Application of indigenous versus synthetic microbiomes. Phytopathology. 2017;107:256–263. doi: 10.1094/PHYTO-09-16-0330-RVW. [DOI] [PubMed] [Google Scholar]
- 7.Lukyanenko, A. N. Genetic Improvement of Tomato: Disease Resistance in Tomato. 14 (1991).
- 8.FAO. Summary of world food and agricultural statistics, 2003. FAO (2003) Agricultural statistics. Socio-Economic Statistics and Analysis Service.110 (2004).
- 9.Sahu DK, Khare CP, Singh HK, Thakur MP. (2013) Evaluation of newer fungicide for management of early blight of tomato in Chhattisgarh. The Bioscan. 2013;8:1255–1259. [Google Scholar]
- 10.Dhal A, Beura SK, Dash SK, Tripathy L. Fungicidal management of Early Blight disease in tomato. J. Mycopathol. Res. 2015;53:243–246. [Google Scholar]
- 11.Rahmatzai N, et al. In vitro and in vivo antifungal activity of botanical oils against Alternaria solani causing early blight of tomato. Int. J. Biosci. (IJB) 2017;10:91–99. doi: 10.12692/ijb/10.1.91-99. [DOI] [Google Scholar]
- 12.Costa, J. M. & Heuvelink, E. Introduction: The Tomato Crop and Industry. 1–20 Preprint at https://research.wur.nl/en/publications/introduction-the-tomato-crop-and-industry (2005).
- 13.Ashour AA. A protocol suggested for managing tomato early blight. Egypt. J. Phytopathol. 2009;37:9–20. [Google Scholar]
- 14.Selim ME. Effectiveness of trichoderma biotic applications in regulating the related defense genes affecting tomato early blight disease. J. Plant Pathol. Microbiol. 2015;6:1. doi: 10.4172/2157-7471.1000311. [DOI] [Google Scholar]
- 15.Borguini RG, da Silva Torres EAF. Tomatoes and tomato products as dietary sources of antioxidants. Food Rev. Int. 2009;25:313–325. doi: 10.1080/87559120903155859. [DOI] [Google Scholar]
- 16.Olaniyi JO, Akanbi WB, Adejumo TA, Akande OG. Growth, fruit yield and nutritional quality of tomato varieties. Afr. J. Food Sci. 2010;4:398–402. [Google Scholar]
- 17.LiNa W, et al. Effects of exogenous nitric oxide on growth and transcriptional expression of antioxidant enzyme mRNA in tomato seedlings under copper stress. Acta Horticulturae Sinica. 2010;37:47–52. [Google Scholar]
- 18.Attia MS, El-Wakil DA, Hashem AH, Abdelaziz AM. Antagonistic effect of plant growth-promoting fungi against fusarium wilt disease in tomato: in vitro and in vivo study. Appl. Biochem. Biotechnol. 2022;194:5100–5118. doi: 10.1007/s12010-022-03975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connell S, Rivard C, Peet MM, Harlow C, Louws F. High tunnel and field production of organic heirloom tomatoes: Yield, fruit quality, disease, and microclimate. HortScience. 2012;47:1283–1290. doi: 10.21273/HORTSCI.47.9.1283. [DOI] [Google Scholar]
- 20.Ramakrishna W, Yadav R, Li K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019;138:10–18. doi: 10.1016/j.apsoil.2019.02.019. [DOI] [Google Scholar]
- 21.Yashwant CK, Rao GM, Singh SK. Effect of different doses of fungicide (Thifluzamide) against early blight of tomato caused by Alternaria solani. Trends Biosci. 2017;10:1974–1976. [Google Scholar]
- 22.Aldinary AM, Morsy Abdelaziz A, Farrag AA, Attia MS. WITHDRAWN: Biocontrol of tomato Fusarium wilt disease by a new Moringa endophytic Aspergillus isolates. Mater. Today Proc. 2021 doi: 10.1016/J.MATPR.2021.03.423. [DOI] [Google Scholar]
- 23.Farrag A, Attia MS, Younis A, Abd Elaziz A. Potential impacts of elicitors to improve tomato plant disease resistance. Al Azhar Bull. Sci. 2017;9:311–321. [Google Scholar]
- 24.Abdelaziz AM, Kalaba MH, Hashem AH, Sharaf MH, Attia MS. Biostimulation of tomato growth and biocontrol of Fusarium wilt disease using certain endophytic fungi. Bot Stud. 2022;63:1. doi: 10.1186/s40529-022-00364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abd Alhakim A, Hashem A, Abdelaziz AM, Attia MS. Impact of plant growth promoting fungi on biochemical defense performance of Tomato under Fusarial infection. Egypt. J. Chem. 2022;65:1. [Google Scholar]
- 26.Heflish AA, Abdelkhalek A, Al-Askar AA, Behiry SI. Protective and curative effects of Trichoderma asperelloides Ta41 on tomato root rot caused by Rhizoctonia solani Rs33. Agronomy. 2021;11:1. doi: 10.3390/agronomy11061162. [DOI] [Google Scholar]
- 27.Yang G, Li C, Yang G, Li C. General description of Rhizoctonia species complex. Plant Pathol. 2012 doi: 10.5772/39026. [DOI] [Google Scholar]
- 28.Mohammed BL, Hussein RA, Toama FN. Biological control of Fusarium wilt in tomato by endophytic rhizobactria. Energy Procedia. 2019;157:171–179. doi: 10.1016/j.egypro.2018.11.178. [DOI] [Google Scholar]
- 29.Al-Hayani B, Ilhan H. Efficient cooperative image transmission in one-way multi-hop sensor network. Int. J. Electr. Eng. Educ. 2020;57:321–339. doi: 10.1177/0020720918816009. [DOI] [Google Scholar]
- 30.Molina G, Pimentel MR, Bertucci TCP, Pastore GM. Application of fungal endophytes in biotechnological processes. Chem. Eng. Trans. 2012;27:289–294. [Google Scholar]
- 31.Kogel KH, Franken P, Hückelhoven R. Endophyte or parasite—What decides? Curr. Opin. Plant. Biol. 2006;9:358–363. doi: 10.1016/j.pbi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Porras-Alfaro A, Bayman P. Hidden Fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011;49:291–315. doi: 10.1146/annurev-phyto-080508-081831. [DOI] [PubMed] [Google Scholar]
- 33.Arnold, A. E. Endophytic fungi: hidden components of tropical community ecology. (In: Carson W.P. Schnitzer S.A. Tropical Forest Community Ecology., 2008).
- 34.Rodriguez RJ, White JF, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytol. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Song YC, Liu JY, Ma YM, Tan RX. Anti-Helicobacter pylori substances from endophytic fungal cultures. World J. Microbiol. Biotechnol. 2005;21:553–558. doi: 10.1007/s11274-004-3273-2. [DOI] [Google Scholar]
- 36.Chen T, et al. Diversity and potential application of endophytic bacteria in ginger. Genet. Mol. Res. 2014;13:4918–4931. doi: 10.4238/2014.July.4.6. [DOI] [PubMed] [Google Scholar]
- 37.Leveau JHJ, Lindow SE. Utilization of the Plant Hormone Indole-3-Acetic Acid for Growth by Pseudomonas putida Strain 1290 †. Appl. Environ. Microbiol. 2005;71:2365–2371. doi: 10.1128/AEM.71.5.2365-2371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holbrook AA, Edge WJW, Bailey F. Spectrophotometric Method for Determination of Gibberellic Acid. 1961 doi: 10.1021/BA-1961-0028.CH018. [DOI] [Google Scholar]
- 39.Neilands JB. Iron absorption and transport in microorganisms. Annu. Rev. Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- 40.Rezzonico F, et al. Is the ability of biocontrol fluorescent pseudomonads to produce the antifungal metabolite 2,4-diacetylphloroglucinol really synonymous with higher plant protection? New Phytol. 2007;173:861–872. doi: 10.1111/j.1469-8137.2006.01955.x. [DOI] [PubMed] [Google Scholar]
- 41.Zahra, M. K. Studies on Silicate Bacteria. (Cairo Univ., 1969).
- 42.Frey-Klett P, et al. Ectomycorrhizal symbiosis affects functional diversity of rhizosphere fluorescent pseudomonads. New Phytol. 2005;165:317–328. doi: 10.1111/j.1469-8137.2004.01212.x. [DOI] [PubMed] [Google Scholar]
- 43.Daigham GE, Mahfouz AY, Abdelaziz AM, Nofel MM, Attia MS. Protective role of plant growth-promoting fungi Aspergillus chevalieri OP593083 and Aspergillus egyptiacus OP593080 as biocontrol approach against Alternaria leaf spot disease of Vicia faba plant. Biomass Convers. Biorefin. 2023;1:1–17. doi: 10.1007/S13399-023-04510-4/FIGURES/11. [DOI] [Google Scholar]
- 44.Hibar K, et al. Genetic diversity of fusarium oxysporum populations isolated from tomato plants in Tunisia. J. Phytopathol. 2007;155:136–142. doi: 10.1111/j.1439-0434.2007.01198.x. [DOI] [Google Scholar]
- 45.Zimbro, M. Jo. et al. Difco & BBL Manual: Manual of Microbiological Culture Media. (Becton, Dickinson and Company, 2009).
- 46.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics. PCR Protoc. 1990;1:315–322. doi: 10.1016/B978-0-12-372180-8.50042-1. [DOI] [Google Scholar]
- 47.Rosa LH, Almeida Vieira MDL, Santiago IF, Rosa CA. Endophytic fungi community associated with the dicotyledonous plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) in Antarctica. FEMS Microbiol. Ecol. 2010;73:178–189. doi: 10.1111/j.1574-6941.2010.00872.x. [DOI] [PubMed] [Google Scholar]
- 48.Hashem AH, et al. Bacillus megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants. J. Fungi (Basel) 2021;7:1. doi: 10.3390/jof7030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irigoyen JJ, Einerich DW, Sánchez-Díaz M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant. 1992;84:55–60. doi: 10.1111/j.1399-3054.1992.tb08764.x. [DOI] [Google Scholar]
- 50.Vernon, L. P. & Seely, G. R. The chlorophylls. 1296 (1966).
- 51.Diaz DH, Martin GC. Peach seed dormancy in relation to endogenous inhibitors and applied growth substances1. J. Am. Soc. Hortic. Sci. 1972;97:651–654. doi: 10.21273/JASHS.97.5.651. [DOI] [Google Scholar]
- 52.Hu Z, Richter H, Sparovek G, Schnug E. Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: A review. J. Plant. Nutr. 2004;27:183–220. doi: 10.1081/PLN-120027555. [DOI] [Google Scholar]
- 53.Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983;58:166–170. doi: 10.1111/j.1399-3054.1983.tb04162.x. [DOI] [Google Scholar]
- 54.Verduyn C, van Dijken JP, Scheffers WA. Colorimetric alcohol assays with alcohol oxidase. J. Microbiol. Methods. 1984;2:15–25. doi: 10.1016/0167-7012(84)90027-7. [DOI] [Google Scholar]
- 55.Matta A, Dimond A. Symptoms of Fusarium wilt in relation to quantity of fungus and enzyme activity in tomato stems. Phytopathology. 1963;53:574. [Google Scholar]
- 56.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 57.Trivedi P, Pandey A, Palni LMS. In vitro evaluation of antagonistic properties of Pseudomonas corrugata. Microbiol. Res. 2008;163:329–336. doi: 10.1016/j.micres.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Knegt E, Bruinsma J. A rapid, sensitive and accurate determination of indolyl-3-acetic acid. Phytochemistry. 1973;12:753–756. doi: 10.1016/0031-9422(73)80672-7. [DOI] [Google Scholar]
- 59.Beauchamp CO, Fridovich I. Isozymes of superoxide dismutase from wheat germ. Biochimica et Biophysica Acta (BBA) Prot. Struct. 1973;317:50–64. doi: 10.1016/0005-2795(73)90198-0. [DOI] [PubMed] [Google Scholar]
- 60.Talapatra K, Das AR, Saha A, Das P. In vitro antagonistic activity of a root endophytic fungus towards plant pathogenic fungi. J. Appl. Biol. Biotechnol. 2017 doi: 10.7324/JABB.2017.50210. [DOI] [Google Scholar]
- 61.Kamalannan A, et al. Biocontrol agents induce disease resistance in ‘Phyllanthus niruri’ Linn against damping-off disease caused by ‘Rhizoctonia solani’. Phytopathol. Mediterr. 2004;43:1. [Google Scholar]
- 62.Azevedo JL, Maccheroni W, Pereira JO, De Araújo WL. Endophytic microorganisms: A review on insect control and recent advances on tropical plants. Electron. J. Biotechnol. 2000;3:1. doi: 10.2225/vol3-issue1-fulltext-4. [DOI] [Google Scholar]
- 63.Gomes GLB, Scortecci KC. Auxin and its role in plant development: structure, signalling, regulation and response mechanisms. Plant Biol. (Stuttg) 2021;23:894–904. doi: 10.1111/plb.13303. [DOI] [PubMed] [Google Scholar]
- 64.Raheem A, Shaposhnikov A, Belimov AA, Dodd IC, Ali B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil. Sci. 2018;64:574–587. doi: 10.1080/03650340.2017.1362105. [DOI] [Google Scholar]
- 65.Zinniel DK, et al. Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl. Environ. Microbiol. 2002;68:2198–2208. doi: 10.1128/AEM.68.5.2198-2208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh M, Kumar A, Singh R, Pandey KD. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech. 2017;7:1. doi: 10.1007/s13205-017-0942-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glick BR. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica (Cairo) 2012;2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shakirova FM. Role of hormonal system in the manifestation of growth promoting and antistress action of salicylic acid. Salicyl. Acid Plant Hormone. 2007;1:69–89. doi: 10.1007/1-4020-5184-0_4/COVER. [DOI] [Google Scholar]
- 69.Rijavec T, Lapanje A. Hydrogen cyanide in the rhizosphere: Not suppressing plant pathogens, but rather regulating availability of phosphate. Front. Microbiol. 2016;7:216209. doi: 10.3389/fmicb.2016.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lacava, P. T., Sebastianes, F. L. S., & Azevedo, J. L. Fungos, uma introdução a biologia, bioquímica e biotecnologia. In Fungos endofíticos: Biodiversidades e aplicações biotecnológicas. (Revisada e ampliada. EDUCS, 2010).
- 71.Wenzel JB, et al. Isolamento E atividade antagonística de fungos Endofíticos de Soja (Glycine max (L.) Merrill) Sabios. 2012;7:86–96. [Google Scholar]
- 72.Schulz B, Boyle C, Draeger S, Römmert AK, Krohn K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002;106:996–1004. doi: 10.1017/S0953756202006342. [DOI] [Google Scholar]
- 73.Shukla S, et al. Endophytic microbes: A novel source for biologically/pharmacologically active secondary metabolites. Asian J. Pharmacol. Toxicol. 2014;2:01–16. [Google Scholar]
- 74.Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gouda S, Das G, Sen SK, Shin HS, Patra JK. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016;7:1. doi: 10.3389/fmicb.2016.01538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Devi S, et al. Depiction of secondary metabolites and antifungal activity of Bacillus velezensis DTU001. Synth. Syst. Biotechnol. 2019;4:142–149. doi: 10.1016/j.synbio.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ziga ED, Druley T, Burnham CAD. Herbaspirillum species bacteremia in a pediatric oncology patient. J. Clin. Microbiol. 2010;48:4320–4321. doi: 10.1128/JCM.01479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 79.Liu X, Zhou J, Tian J, Cheng W, Wang X. Herbaspirillum camelliae sp. nov., a novel endophytic bacterium isolated from Camellia sinensis L. Arch. Microbiol. 2020;202:1801–1807. doi: 10.1007/s00203-020-01892-1. [DOI] [PubMed] [Google Scholar]
- 80.Andreozzi A, et al. Efficient colonization of the endophytes Herbaspirillum huttiense RCA24 and Enterobacter cloacae RCA25 influences the physiological parameters of Oryza sativa L. cv. Baldo rice. Environ. Microbiol. 2019;21:3489–3504. doi: 10.1111/1462-2920.14688. [DOI] [PubMed] [Google Scholar]
- 81.Liarzi O, Bucki P, Miyara SB, Ezra D. Bioactive Volatiles from an Endophytic Daldinia cf. concentrica Isolate Affect the Viability of the Plant Parasitic Nematode Meloidogyne javanica. PLoS One. 2016;11:1. doi: 10.1371/journal.pone.0168437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao Y, et al. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-22782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen L, Heng J, Qin S, Bian K. A comprehensive understanding of the biocontrol potential of Bacillus velezensis LM2303 against Fusarium head blight. PLoS One. 2018;13:1. doi: 10.1371/journal.pone.0198560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen J, Mao S, Xu Z, Ding W. Various antibacterial mechanisms of biosynthesized copper oxide nanoparticles against soilborne Ralstonia solanacearum. RSC Adv. 2019;9:3788–3799. doi: 10.1039/C8RA09186B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akladious SA, Gomaa EZ, El-Mahdy OM. Efficiency of bacterial biosurfactant for biocontrol of Rhizoctonia solani (AG - 4) causing root rot in faba bean (Vicia faba) plants. Eur. J. Plant. Pathol. 2019;153:15–35. doi: 10.1007/s10658-018-01639-1. [DOI] [Google Scholar]
- 86.Singh S, et al. Seed biopriming with microbial inoculant triggers local and systemic defense responses against Rhizoctonia solani causing banded leaf and sheath blight in Maize (Zea mays L.) Int. J. Environ. Res. Public Health. 2020;17:1. doi: 10.3390/ijerph17041396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abdelaziz AM, et al. Efficient role of endophytic Aspergillus terreus in biocontrol of Rhizoctonia solani causing damping-off disease of Phaseolus vulgaris and Vicia faba. Microorganisms. 2023;11:1487. doi: 10.3390/microorganisms11061487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hossain, S. & Tofazzal, I. 27 Plant Endophytes in Growth. Biostimulants for Crop Production and Sustainable Agriculture (CABI, 2022). 10.1079/9781789248098.0000.
- 89.Yan Y, Xu W, Hu Y, Tian R, Wang Z. Bacillus velezensis YYC promotes tomato growth and induces resistance against bacterial wilt. Biol. Control. 2022;172:104977. doi: 10.1016/j.biocontrol.2022.104977. [DOI] [Google Scholar]
- 90.Behiry S, et al. Trichoderma pubescens Elicit Induced Systemic Resistance in Tomato Challenged by Rhizoctonia solani. J. Fungi (Basel) 2023;9:1. doi: 10.3390/jof9020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sahebi M, et al. Profiling secondary metabolites of plant defence mechanisms and oil palm in response to Ganoderma boninense attack. Int. Biodeterior. Biodegrad. 2017;122:151–164. doi: 10.1016/j.ibiod.2017.04.016. [DOI] [Google Scholar]
- 92.Papuc C, Goran GV, Predescu CN, Nicorescu V, Stefan G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017;16:1243–1268. doi: 10.1111/1541-4337.12298. [DOI] [PubMed] [Google Scholar]
- 93.Al-Surhanee AA, et al. The antifungal activity of Ag/CHI NPs against Rhizoctonia solani linked with tomato plant health. Plants (Basel) 2021;10:2283–2283. doi: 10.3390/plants10112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wekesa TB, Wafula EN, Kavesu N, Sangura RM. Taxonomical, functional, and cytopathological characterization of Bacillus spp. from Lake Magadi, Kenya, against Rhizoctonia solani Kühn in Phaseolus vulgaris L. J. Basic Microbiol. 2023;1:1. doi: 10.1002/JOBM.202300038. [DOI] [PubMed] [Google Scholar]
- 95.Kant R, Tyagi K, Ghosh S, Jha G. Host alternative NADH: Ubiquinone oxidoreductase serves as a susceptibility factor to promote pathogenesis of Rhizoctonia solani in Plants. Phytopathology. 2019;109:1741–1750. doi: 10.1094/PHYTO-02-19-0055-R. [DOI] [PubMed] [Google Scholar]
- 96.Sofy MR, Aboseidah AA, Heneidak SA, Ahmed HR. ACC deaminase containing endophytic bacteria ameliorate salt stress in Pisum sativum through reduced oxidative damage and induction of antioxidative defense systems. Environ. Sci. Pollut. Res. Int. 2021;28:40971–40991. doi: 10.1007/s11356-021-13585-3. [DOI] [PubMed] [Google Scholar]
- 97.McCord JM. The evolution of free radicals and oxidative stress. Am. J. Med. 2000;108:652–659. doi: 10.1016/S0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 98.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 99.Youssef SA, Tartoura KA, Abdelraouf GA. Evaluation of Trichoderma harzianum and Serratia proteamaculans effect on disease suppression, stimulation of ROS-scavenging enzymes and improving tomato growth infected by Rhizoctonia solani. Biol. Control. 2016;100:79–86. doi: 10.1016/j.biocontrol.2016.06.001. [DOI] [Google Scholar]
- 100.Demirevska-Kepova K, Simova-Stoilova L, Stoyanova Z, Hölzer R, Feller U. Biochemical changes in barley plants after excessive supply of copper and manganese. Environ. Exp. Bot. 2004;52:253–266. doi: 10.1016/j.envexpbot.2004.02.004. [DOI] [Google Scholar]
- 101.Berwal K, M. & Ram, C. Superoxide Dismutase: A Stable Biochemical Marker for Abiotic Stress Tolerance in Higher Plants. Abiotic Biotic Stress Plants. 2018;1:1. doi: 10.5772/INTECHOPEN.82079. [DOI] [Google Scholar]
- 102.Singh S, et al. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic. Biol. Med. 2013;56:89–101. doi: 10.1016/j.freeradbiomed.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abdelaziz AM, et al. Protective role of zinc oxide nanoparticles based hydrogel against wilt disease of pepper plant. Biocatal Agric Biotechnol. 2021;35:102083. doi: 10.1016/j.bcab.2021.102083. [DOI] [Google Scholar]
- 104.Hao S, et al. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae. 2021;7:1. doi: 10.3390/horticulturae7060132. [DOI] [Google Scholar]
- 105.Rajendran L, et al. Deciphering the Role of Growth-Promoting Bacterial Endophytes in Harmonizing Plant Health. 2023 doi: 10.1007/978-981-99-0030-5_11. [DOI] [Google Scholar]
- 106.Kiani T, et al. Control of stripe rust of wheat using indigenous endophytic bacteria at seedling and adult plant stage. Sci. Rep. 2021;11:1–14. doi: 10.1038/s41598-021-93939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahmad HM, et al. Plant growth-promoting Rhizobacteria eliminate the effect of drought stress in plants: A review. Front. Plant Sci. 2022;13:1. doi: 10.3389/fpls.2022.875774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the Gene Bank database repository, under Accession No. OQ073573 for Bacillus velezensis, GenBank accession OQ073583 for Bacillus megaterium and GenBank Accession No. OQ073584 for Herpaspirillum huttiense.