Abstract
The GRIN genes encoding N-methyl-D-aspartate receptor (NMDAR) subunits are remarkably intolerant to variation. Many pathogenic NMDAR variants result in their protein misfolding, inefficient assembly, reduced surface expression, and impaired function on neuronal membrane, causing neurological disorders including epilepsy and intellectual disability. Here, we investigated the proteostasis maintenance of NMDARs containing epilepsy-associated variations in the GluN2A subunit, including M705V and A727T. In the transfected HEK293T cells, we showed that the two variants were targeted to the proteasome for degradation and had reduced functional surface expression. We demonstrated that the application of BIX, a known small molecule activator of an HSP70 family chaperone BiP (binding immunoglobulin protein) in the endoplasmic reticulum (ER), dose-dependently enhanced the functional surface expression of the M705V and A727T variants in HEK293T cells. Moreover, BIX (10 μM) increased the surface protein levels of the M705V variant in human iPSC-derived neurons. We revealed that BIX promoted folding, inhibited degradation, and enhanced anterograde trafficking of the M705V variant by modest activation of the IRE1 pathway of the unfolded protein response. Our results suggest that adapting the ER proteostasis network restores the folding, trafficking, and function of pathogenic NMDAR variants, representing a potential treatment for neurological disorders resulting from NMDAR dysfunction.
Keywords: NMDA receptors, endoplasmic reticulum, unfolded protein response, proteostasis, epilepsy, channelopathy
Introduction
N-methyl-D-aspartate receptors (NMDARs) are excitatory neurotransmitter-gated ion channels in the mammalian central nervous system and play a key role in mediating synaptic plasticity and maintaining the excitation-inhibition balance within synapses [1, 2]. NMDARs are tetramers that are assembled from two obligatory GluN1 subunits and two GluN2 (A to D) subunits or two GluN3 (A and B) subunits. Each of these subunits shares a common domain architecture, including an extracellular amino-terminal domain (ATD), an extracellular ligand-binding domain (LBD), a transmembrane domain comprised of three transmembrane helices and one reentrant loop, and an intracellular carboxy-terminal domain (CTD) (Fig. 1a) (cartoons were built from the cryo-electron microscopy (cryo-EM) structure of the human GluN1GluN2A NMDA receptors. PDB: 7EOS) [3–5]. The most common subtype in the human brain is constructed from two GluN1 subunits and two GluN2A/2B subunits [6–8]. GluN1GluN2 channels are activated by the simultaneous binding of an agonist (glutamate or NMDA) in the GluN2 subunits and a co-agonist (glycine or D-serine) in the GluN1 subunits to the LBD. Numerous pathogenic variants in the GluN2A subunit-encoding gene, GRIN2A, have been identified in patients with a variety of neurological and neurodevelopmental disorders including epilepsies, autism spectrum disorder, and intellectual disabilities, with patients displaying phenotypes of cognitive dysfunction, dyspraxia, developmental delay, and speech difficulties [9, 10]. These GluN2A variants lead to impaired signaling at the plasma membrane [11]. Although recent advances in whole genome sequencing have identified an increasing number of pathogenic variants in various subunits of NMDARs, the precise mechanism of how these variants influence receptor surface delivery and function remains poorly understood [12].
Fig. 1. The alternations as a result of variants in GluN2A subunit on the expression and function of trafficking-deficient NMDARs.
a Upper panel, cartoon representation of tetrameric NMDARs, with the GluN1 subunits in light and dark gray and the GluN2A subunits in green and blue (PDB: 7EOS), each subunit consisting of an extracellular amino-terminal domain (ATD), an extracellular ligand-binding domain (LBD), a transmembrane domain comprised of three transmembrane helices and one reentrant loop (TMD), and an intracellular carboxy-terminal domain (CTD). The positions of the M705V and A727T are highlighted as spheres in LBD. Lower panel, linear representation of a GluN2A subunit. The LBD contains two segments of S1 and S2. b The effect of M705V and A727T on the total protein expression level of GluN2A and GluN1. β-Actin serves as the loading control of total protein lysates. c The effect of M705V and A727T on the surface protein expression level of GluN2A and GluN1 as determined by surface biotinylation assays. Na+/K+-ATPase serves as the loading control of membrane proteins. d Surface GluN2A staining was in green (column 1), and plasma membrane marker Na+/K+-ATPase staining was in red (column 2). Merge of these two signals with the nucleus stained in blue with DAPI was shown in column 3. Scale bar = 20 μm. The fluorescence intensity of the surface GluN2A was quantified from 30–50 cells per condition as shown on the right panel. e Automated patch-clamping was performed with the IonFlux Mercury 16 ensemble recording at a holding potential of −60 mV. Glutamate (10 mM) and glycine (100 µM) were applied simultaneously for 3 s, as indicated by the horizontal bar above the currents. The peak currents (Imax) were acquired and analyzed by the Fluxion Data Analyzer (n = 9–10 ensemble recording; each ensemble recording enclosed 20 cells). Each data point is reported as mean ± SEM. One-way ANOVA followed by a post-hoc Tukey test was used for statistical analysis. *P < 0.05; **P < 0.01; ***P < 0.001.
We focus on proteostasis maintenance of neurotransmitter-gated ion channels, including NMDARs [12, 13]. NMDAR subunits are folded and assembled into heterotetramers in the endoplasmic reticulum (ER), a cellular organelle for protein quality control with the assistance of molecular chaperones. The assembled receptors engage the trafficking machinery to exit the ER and travel through the Golgi apparatus en route to the plasma membrane. Reduced surface expression of variant NMDARs is a major molecular mechanism in the pathogenesis of related neurological diseases, such as epilepsy and intellectual disability [1, 9, 12]. Accumulating evidence indicates that the structural integrity of the LBD is essential for the surface trafficking of NMDARs [14–16]. Therefore, many disease-associated variations in the GluN2A subunit that are located in the LBD cause the reduced presence of the NMDARs on the plasma membrane and thus loss of their function resulting in epilepsy syndrome [9, 12]. For example, two missense variations, M705V and A727T in the S2 segment of the LBD of GluN2A subunit (Fig. 1a), which were identified in a cohort of patients with epilepsy, impair the delivery of the mature NMDARs to the plasma membrane [14, 17]. Presumably, trafficking-deficient variants in NMDAR subunits result in their protein misfolding and retention within the ER, which leads to their excessive degradation via the ER-associated degradation (ERAD) pathway [18]. Consequently, this can contribute to the loss-of-function phenotype displayed by variant NMDARs and corresponding disease phenotypes due to the inefficient processes of folding, assembly, and trafficking.
There is limited knowledge in the literature about how the proteostasis network regulates the folding, assembly, degradation, and trafficking of NMDARs [12]. The ER proteostasis network regulates the ER folding capacity to assure that newly synthesized proteins achieve their native multidimensional structures in the oxidative folding environments [19, 20]. Since membrane proteins need to fold in the ER, pharmacologic enhancement of ER proteostasis capacity to restore variant-containing NMDAR folding and assembly in the ER has the potential to improve the anterograde trafficking and function of NMDARs at the cell surface [21–23]. BiP (binding immunoglobulin protein, aka Grp78), an ER resident HSP70 family chaperone, plays a prominent role in the ER by controlling protein folding and preventing aggregation [24]. BiP is the master regulator of the unfolded protein response (UPR), which monitors the ER proteostasis status [25]. Activation of the UPR is a promising way to change the fate of pathogenic proteins that are associated with various protein misfolding diseases [23, 26, 27]. The UPR contains three integrated signaling arms, including ATF6 (activating transcription factor 6), IRE1 (inositol-requiring enzyme 1), and PERK (protein kinase R-like ER kinase) [25, 26]. In response to ER stress, these activated pathways result in both transcriptional and translational signaling cascades that function to regulate diverse aspects of cellular physiology including ER proteostasis. However, it remains unclear whether the UPR activation restores proteostasis of pathogenic, misfolded NMDAR proteins harboring clinical variants.
Here, we applied BIX (1-(3,4-dihydroxy-phenyl)-2-thiocyanate-ethanone), a potent BiP activator [28, 29], to investigate how regulating the ER proteostasis network influences the folding, trafficking, and function of NMDARs carrying M705V and A727T variants in the LBD of the GluN2A subunit.
Materials and methods
Plasmids, chemicals, and antibodies
The pcDNA3.1-GluN2A (catalog # OHu24642D, NM_000833, human) and the pcDNA3.1-GluN1 (catalog # OHu22255D, NM_007327, human) plasmids were purchased from GenScript Biotech (Piscataway, NJ, USA). The pcDNA3.1-BiP plasmid was provided by Dr. Tohru Mizushima (Kumamoto University, Kumamoto, Japan). N-terminal HA-tagged pCGN-ATF6N plasmid (catalog # 11974, human) came from Addgene (Watertown, MA, USA). The pcDNA3.1-XBP1s plasmid was a kind gift from Dr. Richard N. Sifers (Baylor College of Medicine, TX, USA) [30].
Thapsigargin (Tg; catalog # BML-PE180-0001), G418 (catalog # ALX-380-013) and cycloheximide (catalog # ALX-380-269) were obtained from Enzo Life Sciences (Farmingdale, NY, USA). (+)-MK801 maleate (catalog # HB0004) was purchased from Hellobio (Princeton, NJ, USA). Resazurin (catalog # 0219548101) and poly-L-lysine (catalog # 150177) were obtained from MP Biomedicals (Cleveland, Ohio, USA). SubAB, a bacterial toxin with protease activity that is highly selective for BiP and SubAA272B, a non-proteolytic negative control for SubAB, were prepared according to the published procedure [31]. MG132 (catalog # A2585) was obtained from ApexBio (Houston, TX, USA). Bafilomycin A1 (catalog # 11038) was purchased from Cayman Chemical (Ann Arbor, MI, USA). Dorsomorphin (catalog # A137399), Purmorphamine (catalog # A124253), CHIR-99021 (catalog # A133052) and SB431542 (catalog # A172016) were purchased from AMBEED (Chicago, IL, USA). ROCK inhibitor (catalog # 1254) was purchased from Tocris (Bristol, UK). GSK2606414 (catalog # HY-18072), ISRIB (catalog # HY-12495), Ceapin-A7 (catalog # HY-108434), PF429242 (catalog # HY-13447A), KIRA6 (catalog # HY-19708) and STF-083010 (catalog # HY-15845) were purchased from MedChemExpress (Monmouth Junction, NJ, USA). N-dodecyl-β-D-maltoside (DDM) (catalog # DDM5) was purchased from GoldBio (St. Louis, MO, USA). Protease inhibitor cocktail (catalog # 04693116001) was obtained from Roche (Basel, CH). BIX (BiP protein inducer X; catalog # SML1073), poly-D-lysine (catalog # P6407), laminin (catalog # L2020), cytosine arabinose (Ara C; catalog # C6645) and other chemicals were purchased from Sigma (St. Louis, MO, USA) unless stated otherwise.
The rabbit monoclonal anti-GluN2A (catalog # ab124913; 1:3000), mouse monoclonal anti-GluN2A (catalog # ab240884; 1:200), rabbit monoclonal anti-Na+/K+-ATPase antibody (catalog # ab76020; 1:3000), and rabbit monoclonal anti-GluN1 antibody (catalog # ab109182; 1:3000) were purchased from Abcam (Waltham, MA, USA). The mouse monoclonal anti-calreticulin (catalog # ADI-SPA-601; 1:3000), the rabbit polyclonal anti-calnexin (CANX) (catalog # ADI-SPA-860-F; 1:5000), and the rat monoclonal anti-GRP94 (clone 9G10) (catalog # ADI-SPA-850-F; 1:1000) antibodies were purchased from Enzo Life Sciences (Farmingdale, NY, USA). The mouse monoclonal anti-β-actin antibody (catalog # A1978; 1:10,000) was obtained from Sigma (St. Louis, MO, USA). The rabbit polyclonal anti-BiP antibody (catalog # AP50016; 1:5000) was obtained from Abgent (San Diego, CA, USA). The rabbit polyclonal anti-ATF6 antibody (catalog # 24169-1-AP; 1:3000) and rabbit polyclonal anti-calnexin antibody (catalog # 10427-2-AP; 1:200) were purchased from Proteintech (Rosemont, IL, USA). The rabbit monoclonal anti-XBP1s antibody (catalog # 12782S), the mouse monoclonal anti-CHOP antibody (catalog # 2895S; 1:1000), and the rabbit monoclonal anti-ubiquitin antibody (catalog # 43124S; 1:2000) were obtained from Cell Signaling (Danvers, MA, USA). The rabbit polyclonal anti-LC3B antibody (catalog # NB100-2220; 1:1500) was obtained from Novus Biologicals (Centennial, CO, USA). The rabbit polyclonal anti-PAX6 antibody (catalog # 901301; 1:200) was obtained from Biolegend (San Diego, CA, USA). The mouse monoclonal anti-Nestin antibody (catalog # MA1-110; 1:200), rat monoclonal anti-SOX2 antibody (catalog # 14-9811-82; 1:200), Alexa fluor 488 goat-anti-mouse antibody (catalog # A11029; 1:300), Alexa 488 goat anti-rabbit secondary antibody (catalog # A11034; 1:500), Alexa fluor 568 goat-anti-mouse antibody (catalog # A11031; 1:500), and Alexa 568-conjugated goat anti-rabbit secondary antibody (catalog # A11036; 1:500), and Alexa fluor 633 goat-anti-rat antibody (catalog # A21094; 1:300) were purchased from Invitrogen (Waltham, MA, USA).
The M705V or A727T mutation was introduced to the GluN2A subunit using QuikChange II site-directed mutagenesis Kit from Agilent (Santa Clara, CA, USA). DNA sequencing was used to confirm the cDNA sequences. The forward and reverse primers for GluN2A(M705V) are 5′-CCTTTCTGATTAAATTTGGTCACGTACTGATGCATGTAGGGATAG-3′ and 5′-CTATCCCTACATGCATCAGTACGTGACCAAATTTAATCAGAAAGG-3′; the forward and reverse primers for GluN2A(A727T) are 5′-GCATCGTAGATGAAAGTGTCCAGCTTCCCCGTT-3′ and 5′-AACGGGGAAGCTGGACACTTTCATCTACGATGC-3′.
Cell culture and transfection
HEK293T cells (catalog # CRL-3216) were obtained from ATCC (Manassas, VA, USA). Human A2780 cells (catalog # 93112519) were purchased from Sigma. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (ThermoFisher, catalog # SH3024301; Waltham, MA, USA) containing 10% heat-inactivated fetal bovine serum (ThermoFisher, catalog # SH3039603HI) and 1% penicillin streptomycin (Hyclone, catalog # sv30010; Logan, UT, USA) at 37 °C in 5% CO2. Cells were seeded in 10-cm dishes or 6-well plates and allowed to reach −70% confluency before transient transfection using TransIT-2020 (Mirus, catalog # MIR 5400; Brampton, ON, CA) according to the manufacturer’s instruction. MK801 (50 µM) and 2.5 mM MgCl2 were added to medium 4 h post transient transfection to avoid excitatory toxicity. Then, cells were harvested for further analysis after transfection for 24 h.
Stable cell lines for GluN1GluN2A, GluN1GluN2A(M705V) and GluN1GluN2A(A727T) were generated using the G418 selection method in the presence of MK801 and MgCl2 to block the receptors as they were expressed. Briefly, cells were transfected with GluN1 : GluN2A (1:1), GluN1 : GluN2A(M705V) (1:1) or GluN1 : GluN2A(A727T) (1:1) plasmids, selected in DMEM supplemented with 1.0 mg/mL G418 for 10 days, and then maintained in DMEM supplemented with 0.4 mg/mL G418. G418 resistant cells expressing NMDARs were verified by Western blot analysis and then used for experiments.
SDS-PAGE and Western blot analysis
Cells were harvested with cold Dulbecco’s phosphate-buffered saline (DPBS) (ThermoFisher, catalog # SH3002803) into a centrifuge tube and then lysed with lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, and 2 mM DDM supplemented with complete protease inhibitor cocktail). Cells were vortexed and ultrasonicated for 40 s three times. Lysates were cleared at 21,000 × g, 4 °C for 10 min. Protein concentrations were measured by Pierce MicroBCA kit (ThermoFisher, catalog # 23235). Cell lysates were loaded with 4× Laemmli buffer (Biorad, catalog # 1610747; Hercules, CA, USA) including β-mercaptoethanol (1:10, v/v; catalog # M3148) and subjected to 4%–20% SDS-PAGE gel. Protein ladder (Biorad, catalog # 1610395) was used to locate the molecular weight. Western blot analysis was carried out using appropriate antibodies with dilutions as listed above. Band intensity was quantified using ImageJ software. The β-actin and the Na+/K+-ATPase serve individually as a loading control of total protein and plasma membrane proteins respectively. The total protein was first normalized to the loading control and then the vehicle, DMSO, or WT control.
Biotinylation of cell surface proteins
HEK293T cells stably expressing NMDAR variants were plated in poly-L-lysine (0.1 mg/mL) pre-coated 10 cm dishes for surface biotinylation experiments according to published procedure [32]. In brief, cells were transfected with the corresponding GluN1GluN2A plasmids for 24 h, and then treated with BIX at 10 μM for another 24 h. The medium was supplemented with MK801 and MgCl2 to decrease the cell excitatory toxicity. Intact cells were rinsed gently twice with ice-cold DPBS and incubated with the membrane-impermeable biotinylation reagent Sulfo-NHS SS-Biotin (0.5 mg/mL; ThermoFisher, catalog # 21331) in DPBS containing 1 mM CaCl2 and 0.5 mM MgCl2 (DPBS-CM) for 30 min on ice to label surface membrane proteins. Glycine (50 mM) in ice-cold PBS-CM was added to plates for 5 min at 4 °C to quench the reaction. To block the sulfhydryl groups, 5 nM N-ethylmaleimide (NEM) in DPBS was added for 15 min at room temperature. Cells were scraped, transferred to a tube, and were solubilized at 4 °C overnight in homogenization buffer (Triton X-100, 1%; 50 mM Tris-HCl, 150 mM NaCl, and 5 mM EDTA; pH 7.5) supplemented with Roche protease inhibitor cocktail and 5 mM NEM. The supernatant containing the biotinylated surface proteins was obtained by centrifuging (16,000 × g, 10 min at 4 °C) the lysates. The concentration of the supernatant was measured using a microBCA assay. Biotinylated surface proteins were affinity-purified by incubating the obtained supernatant for 2 h at 4 °C with 40 μL of immobilized NeutrAvidin-conjugated agarose bead slurry (ThermoFisher, catalog # 29201). The beads were kept and washed with wash buffer (Triton X-100, 0.5%; 50 mM Tris-HCl; 150 mM NaCl, and 5 mM EDTA; pH 7.5) and the same wash buffer without Triton X-100, three times each. Surface proteins were eluted from beads with 70 μL of elution buffer (2× Laemmli sample buffer with 100 mM DTT and 6 M urea; pH 6.8) by vortex for 30 min at room temperature before being subjected to SDS-PAGE and Western blot analysis.
Confocal immunofluorescence
The labeling of surface NMDARs and confocal immunofluorescence microscopy analysis were performed according to published procedure [33]. Briefly, cells cultured on coverslips were fixed with 4% formaldehyde (ThermoFisher, catalog # 28908) in DPBS for 15 min at 4 °C, blocked with 10% goat serum for 30 min at room temperature, and incubated in 50 μL of 2% goat serum in DPBS containing mouse monoclonal anti-GluN2A antibody (1:200) and rabbit monoclonal anti-Na+/K+-ATPase, a plasma membrane marker (1:200) for overnight at 4 °C. The cells were incubated at room temperature with 50 μL of an Alexa 488-conjugated goat anti-mouse secondary antibody (1:500) and an Alexa 568-conjugated goat anti-rabbit secondary antibody (1:500) for 1 h. Then, cells were rinsed with DPBS two times and incubated with DAPI (1 μg/mL) for 5 min to stain the nucleus. The coverslips were then mounted and sealed. For confocal immunofluorescence microscopy, an Olympus IX-81 Fluoview FV3000 confocal laser scanning system was used. A 40× objective was used to collect images using FV31S-SW software. Quantification of the fluorescence intensity was achieved using the ImageJ software.
Cycloheximide-chase (CHX) assay
CHX assay was used to assess protein degradation and performed as previously mentioned [32]. Cycloheximide (100 µg/mL) was added to the culture medium to stop mRNA translation. Briefly, HEK293T cells expressing GluN1GluN2A(M705V) NMDARs were treated with DMSO or BIX for 24 h, and then chased for the respective time points and harvested. Cells were lysed for total protein and then subjected to SDS-PAGE.
Endoglycosidase H (endo H) enzyme digestion assay
Endo H assay was used to monitor the efficiency of protein trafficking from ER to Golgi. To remove asparaginyl-N-acetyl-D-glucosamine in the N-linked glycans incorporated on the GluN2A subunit in the ER, the Endo Hf enzyme (NEBiolab, catalog # P0703L; Ipswich, MA, USA) was used to digest the cell lysates at 37 °C for 24 h. The samples treated by the Peptide-N-Glycosidase F (PNGase F) enzyme (NEBiolab, catalog # P0704L) serve as a control for unglycosylated GluN2A subunits. Treated samples were then subjected to SDS-PAGE and Western blot analysis.
Automated patch-clamping recording with IonFlux Mercury 16 instrument
Whole-cell currents of NMDARs composed of wild type or variant GluN1GluN2A were recorded 24 h post-transfection of HEK293T cells. Automated patch-clamping was performed on the Ionflux Mercury 16 instrument (Fluxion Biosciences, CA, USA). The extracellular solution (ECS) contained the following: 145 mM NaCl, 4 mM KCl, 2 mM CaCl2 (2H2O), 10 mM glucose, 10 mM HEPES (pH 7.4 adjusted with NaOH). The intracellular solution (ICS) contained the following: 135 mM CsCl, 1 mM EGTA, 5 mM CsOH, 10 mM HEPES, 10 mM NaCl (pH 7.2 adjusted with CsOH). In brief, cells were grown to 70% confluence on 10-cm dishes and rinsed with DPBS. Then 3 mL of Accutase (StemCell Technologies, catalog # 07922; Vancouver, BC, CA) was added to the cells and incubated for 2 min at 37 °C until the cells were floating with minimal clumps under a microscope. Cells were pelleted via centrifugation for 1 min at 200 × g, supernatant was aspirated and cells were resuspended in serum-free medium 293 SFM II (ThermoFisher, catalog # 11686-029), supplemented with 25 mM HEPES (ThermoFisher, catalog # 15630-080) and 1% penicillin streptomycin. Cells were gently shaken at room temperature for 0.5–1 h before experiments. Mercury 16 plates were prepared according to manufacture’s procedure. Whole-cell NMDARs currents were recorded at a holding potential of −60 mV at the application of 10 mM glutamate and 100 µM glycine as indicated. The data were acquired and analyzed by Fluxion Data Analyzer.
Resazurin cell toxicity assay
Resazurin cell toxicity assay was performed colorimetrically as previously described [34]. HEK293T cells stably expressing GluN1GluN2A(M705V) NMDARs were seeded into a 96-well plate. The cells were separated into nine groups which were treated with DMSO or BIX (0.5, 1, 2.5, 5, 10, 20 or 40 μM) for 24 h, or thapsigargin (2 μM, 6 h). Resazurin stain (10 μL of 0.15 mg/mL in DPBS) was added to each well using a multichannel pipette and the plate was re-incubated at 37 °C for 2 h before plate reading. The fluorescence signal to proportion cell metabolism/viability was determined spectrophotometrically at excitation 530 nm using a reference wavelength of emission 590 nm with a microplate reader (Tecan-Spark, A-5082, Grödig, Austria).
Generation of GluN2A(M705V) knockin in human induced pluripotent stem cells (hiPSCs) and their differentiation into excitatory neurons
The knockin of the M705V GRIN2A variation (NM_001134407.3 (GRIN2A): c.2113A>G (p.Met705Val)) into apparently healthy female iPSCs (catalog # 802-30F) was generated using the CRISPR/Cas9 technique by Synthego Corporation (Redwood City, CA, USA). To generate these cells, ribonucleoproteins containing the Cas9 protein and synthetic chemically modified sgRNA produced at Synthego were electroporated into the cells along with a single-stranded oligodeoxynucleotide (ssODN) donor using Synthego’s optimized protocol. Editing efficiency is assessed upon recovery, 48 h post electroporation. Genomic DNA is extracted from a portion of the cells, PCR amplified and sequenced using Sanger sequencing. The resulting chromatograms are processed using Synthego Inference of CRISPR edits software (https://ice.synthego.com/). To create monoclonal cell populations, edited cell pools are seeded at 1 cell/well using a single cell printer into 96 or 384 well plates. All wells are imaged every 3 days to ensure expansion from a single-cell clone. Clonal populations are screened and identified using the PCR-Sanger-ICE genotyping strategy described above. The heterozygous knockin of the M705V GRIN2A variation was confirmed by genotyping using the following primers: forward: 5′-TGGACCACAGTCACTCTCCA-3′; reverse: 5′-TGTCATCCTGCCCTAATGCA-3′.
Excitatory neurons were generated from hiPSCs harboring the M705V GRIN2A variation. The generation of human neural progenitor cells (NPCs) was described as previous [35]. To briefly explain, hiPSCs were maintained on Geltrex (ThermoFisher, catalog # A1413201)-coated plates under feeder free conditions. HiPSCs at 80%–95% confluency were dissociated using Accutase and 3 × 106 cells were transferred into one well of an uncoated 6-well tissue culture plate and incubated at 37 °C, 5% CO2 on a shaker at 90 rpm. The neural induction of embryoid bodies (EBs) occurred within 24 h from iPSCs and their size increased during the following days in N2B27 medium (DMEM/F12, 1:100 N2 supplement (ThermoFisher, catalog # 17502001), 1:50 B27 supplement (ThermoFisher, catalog # A35828-01), 1:200 Glutamax, 150 µM ascorbic acid, and 1% penicillin streptomycin) supplemented with 1 µM dorsomorphin, 10 µM SB431542, 3 µM CHIR-99021, 0.5 µM purmorphamine, and 10 µM ROCK inhibitor. Neural induction was initiated by inhibiting BMP and TGFß signaling using dorsomorphin and SB431542 [36]. Simultaneously, CHIR-99021 and purmorphamine were added to stimulate the canonical WNT and SHH-pathways [37, 38]. EBs were cultivated under defined conditions to ensure uniform formation of EBs achieved. Neuroepithelial folds were clearly visible in all EBs after withdrawing dorsomorphin and SB431542 for a few days. Then, EBs were gently triturated and plated onto Geltrex-coated 6-well plates. After 3 – 4 days, attached EB fragments and outgrown cells were dissociated to single cells with Accutase and split for first few passages to make sure that the initially heterogeneous cell population became homogeneous after several passages. To confirm the efficiency and quality of the generated NPCs as well as the identity and maturation state of the NPCs, we evaluated the presence of NPCs biomarkers, including Nestin, SOX2, and PAX6.
For the generation of excitatory neurons, NPCs were cultured in N2B27 medium supplemented with 10 µM ROCK inhibitor, 10 ng/mL brain-derived neurotrophic factor (BDNF, Peprotech, catalog # 450-02; Cranbury, NJ, USA), and 10 ng/mL glial-derived neurotrophic factor (GDNF, Peprotech, catalog # 450-10). 2 µM Ara C was added into cultured medium at day 7 to specifically inhibit the proliferation of glial cells. After culturing for additional 10–14 days, differentiated neurons were dissociated using Accutase and plated onto poly-D-lysine (10 µg/mL) and laminin (10 µg/mL) co-coated coverslips in 24-well plates at 10,000 cells per well. Excitatory neurons were treated with DMSO or 10 µM BIX for 24 h and then subjected to immunofluorescence staining of surface GluN2A and plasma membrane marker Na+/K+-ATPase.
Quantification and statistical analysis
All values were presented as mean ± SEM. Data for statistical analysis were evaluated by using a two-tailed Student’s t test between two groups as appropriate, and the analysis of variance (ANOVA) followed by a post-hoc Tukey test was applied for comparisons in multiple groups. A P < 0.05 was defined as statistically significant. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
Results
Epilepsy-associated variants in the GluN2A subunits reduce the protein expression and function of NMDARs
To evaluate the protein levels of exogenously expressed NMDARs in HEK293T cells compared to those in the central nervous system, we transiently transfected HEK293T cells with different amounts of wild type (WT) GluN1 and GluN2A plasmids in a 6-well plate and compared GluN2A expression to endogenous GluN2A protein levels in the adult mouse cortex and hippocampus (Supplementary Fig. S1). Mouse cortex and hippocampus demonstrated high expression levels of GluN2A, which was consistent to previous reports [39–41]. We observed that transient transfection using 0.3 μg GluN1 and 0.3 μg GluN2A plasmids per well led to comparable GluN2A protein level as expressed in mouse hippocampus. Therefore, in the following experiments, we kept this transfection regime to express low levels of exogenous NMDARs in HEK293T cells to minimize potential disturbance to protein homeostasis, yet to achieve appreciable GluN2A protein levels that are physiologically relevant. Additionally, to lower the excitatory toxicity that associated with the expression of NMDA receptors, cells are routinely maintained in cell culture media supplemented with 50 µM of MK801 and 2.5 mM of MgCl2 to block the channels [42].
We determined the effect of the M705V and A727T variants, located in the S2 segment of the LBD of the GluN2A subunit (Fig. 1a), on the trafficking and function of NMDARs [43, 44]. Western blot analysis of cell lysates from the transfected HEK293T cells showed that total protein levels of GluN2A and GluN1 were significantly reduced for the M705V and A727T variations in GluN2A (Fig. 1b). Because NMDARs need to be transported to cell surface to function as ion channels, we determined whether variant NMDARs affected the cell surface expression. Surface biotinylation assay demonstrated that the M705V or A727T variations in GluN2A significantly decreased the surface protein levels of GluN2A and GluN1 in HEK293T cells (Fig. 1c). Since GluN1 and GluN2A need to assemble into the heterotetramers in the ER membrane prior to their ER exit and subsequent trafficking to the plasma membrane, it appears that the variations in the GluN2A subunits can also retain the GluN1 subunits in the ER and prevent the surface trafficking of GluN1, indicating a dominant negative effect of such variants. In addition, we used immunocytochemistry to visualize surface expression of GluN2A subunits. Cells were fixed with 4% formaldehyde without membrane permeabilization and incubated with an anti-GluN2A antibody with an epitope recognizing the GluN2A extracellular region. GluN2A surface staining merged well with the staining of a plasma membrane marker Na+/K+-ATPase (Fig. 1d). The fluorescence intensity of surface GluN2A was calculated for WT and each variant [45]. The immunocytochemistry analysis showed that M705V or A727T significantly reduced the GluN2A cell surface expression in comparison to WT (Fig. 1d), indicating that the variants have impaired efficiency to traffic to the plasma membrane.
Moreover, to determine whether GluN2A variants influence the function of NMDARs, we carried out whole-cell patch-clamping electrophysiological recording in HEK293T cells using the Fluxion automated patch clamping instrument. Application of co-agonists of 10 mM glutamate and 100 µM glycine opened the NMDAR channels and showed slowly decaying currents (Fig. 1e), consistent with the electrophysiological characteristics of the channels [1]. The peak agonist-induced current in the WT GluN1GluN2A group was 1083.9 ± 305.2 pA, whereas the peak current in NMDARs harboring GluN2A(M705V) or GluN2A(A727T) decreased to 576.9 ± 122.7 pA and 517.4 ± 156.5 pA, corresponding to 46.8% and 52.3% reduction, respectively (Fig. 1e), indicating the loss-of-function phenotype of these GluN2A variants. Previously, it was reported that the M705V and A727T variations reduced the potency of glutamate: EC50(WT) = 3.4 μM, EC50(M705V) = 5.7 μM, and EC50(A727T) = 5.1 μM according to the voltage-clamp recordings from Xenopus oocytes [14], consistent with the interpretation that mutating residues in the glutamate binding pocket led to deceased glutamate potency. Here, we applied glutamate at a high concentration (10 mM) to achieve saturating glutamate to obtain agonist-induced peak currents, which likely diminished the glutamate concentration-dependent current amplitudes of NMDARs. The mM glutamate concentrations were also used by other researchers to carry out electrophysiological recordings of NMDAR variants [46]. Nonetheless, a more detailed study on the electrophysiological properties of the M705V and A727T variants merits further investigation. Collectively, we demonstrated that the M705V and A7275T variations significantly reduced the steady-state protein levels and the surface expressions of GluN2A and peak current amplitudes of NMDARs.
Epilepsy-associated variants in the GluN2A subunits are retained in the ER and targeted to the proteasome for degradation
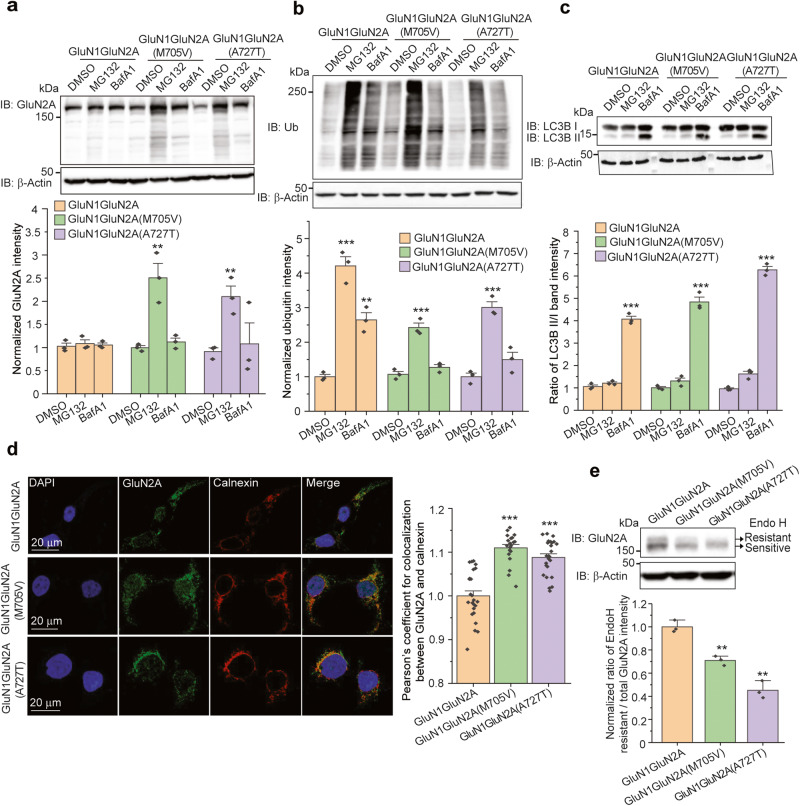
Ion channel mutations often cause reduced protein levels due to the degradation of misfolded proteins by the protein quality control machinery. Misfolded proteins can be targeted to the proteasome or the lysosome for their destruction. Therefore, we investigated the preferred degradation route of the GluN2A variants by inhibiting potential degradation pathways pharmacologically. Application of MG132 (10 μM, 6 h), a potent proteasome inhibitor, substantially increased both GluN2A variants of M705V and A727T in cells according to Western blot analysis without an apparent effect on the WT GluN2A (Fig. 2a). As expected, MG132 treatment substantially accumulated ubiquitinated proteome (Fig. 2b, cf. lane 2 to 1, lane 5 to 4, and lane 8 to 7), indicating the effective proteasome inhibition. In sharp contrast, application of bafilomycin A1 (Baf A1, 1 μM, 6 h), a vacuolar H+-ATPase inhibitor, did not change the total protein levels of GluN2A(M705V) and GluN2A(A727T) variants (Fig. 2a). As expected, bafilomycin A1 treatment substantially accumulated LC3B-II (isoform II of microtubule-associated protein 1 light chain 3 (LC3)) (Fig. 2c, cf. lane 3 to 1, lane 6 to 4, and lane 9 to 7), indicating the effective inhibition of the lysosomal degradation pathway [47]. Furthermore, to determine whether the M705V and A727T variants are retained in the ER, confocal microscopy was used to image GluN2A and calnexin, an ER marker. Clearly, both GluN2A variants colocalized with calnexin, and the ER colocalization for the variants was substantially greater than that for WT GluN2A (Fig. 2d), indicating that M705V and A727T variants aggravated the ER retention of GluN2A. The above results clearly demonstrated that these two GluN2A variants are retained in the ER and targeted to the proteasome for their degradation, presumably through the ERAD pathway.
Fig. 2. The GluN2A variants are retained in the ER and degraded by the proteasome.
a Inhibition of proteasomal degradation (MG132, 10 µM, 6 h) and lysosomal degradation (BafA1, 1 µM, 6 h) independently demonstrated that GluN2A subunits containing either M705V or A727T variants are mainly degraded by the proteasome pathway in HEK293T cells. β-Actin serves as the loading control of total protein lysates. b MG132 treatment (10 µM, 6 h) substantially accumulated ubiquitinated proteins. c BafA1 treatment (1 µM, 6 h) substantially accumulated the isoform II of LC3B. d The nucleus staining with DAPI was in blue (column 1), total GluN2A staining was in green (column 2), and ER marker calnexin staining was in red (column 3). Merge of the three channels was shown in column 4. Scale bar = 20 μm. The colocalization between GluN2A and calnexin was quantified using Pearson’s coefficient from 30–50 cells per condition as shown on the right panel. e The M705V and A727T variations reduced the Endo H-resistant post-ER glycoform of the GluN2A subunit in HEK293T cells. Quantification of the Endo H resistant/total GluN2A band intensities was shown on the bottom panel. Each data point is reported as mean ± SEM. One-way ANOVA followed by a post-hoc Tukey test was used for statistical analysis. **P < 0.01; ***P < 0.001.
The available high-resolution cryo-EM NMDAR structures [5] enabled us to predict the potential influence of the M705V and A727T variants on protein stability at the atomic level. Since methionine can form stabilizing interactions with adjacent aromatic residues [48] and M705 is surrounded by F682, Y704 and F708 in GluN2A (Supplementary Fig. S2a), the M705V variation potentially destabilizes this protein motif, leading to its misfolding and degradation. Since A727 is located in the hydrophobic pocket composed of V535, V537, F682, and I729 (Supplementary Fig. S2b), the A727T variation potentially compromises the hydrophobic interactions as well as introducing steric clashes, causing its excessive degradation.
To evaluate whether the destabilized M705V and A727T variants reduced the anterograde ER-to-Golgi trafficking of GluN2A proteins, we performed an endoglycosidase H (Endo H) enzyme digestion experiment to quantify the trafficking efficiency. Endo H-sensitive GluN2A subunits (Fig. 2e, bottom bands) are retained in the ER, either as nascent proteins or targeted for degradation, whereas Endo H-resistant GluN2A subunits (Fig. 2e, top bands) have exited the ER and traffic at least to the Golgi for further glycan modifications. The ER-to-Golgi trafficking efficiency was quantified by the ratio of Endo H resistant GluN2A/total GluN2A bands. Clearly, the M705V or A727T variations reduced the post-ER Endo H-resistant GluN2A subunit band compared to WT (Fig. 2e, cf. lanes 3 and 2 to lane 1) and decreased the ratio of the Endo H-resistant band/total GluN2A (Fig. 2e), indicating that both GluN2A variants reduced the trafficking efficiency of GluN2A. Taken together, GluN2A(M705V) and GluN2A(A727T) variants are retained in the ER and degraded by the proteasome. Therefore, their forward trafficking from the ER to the Golgi and onward to the plasma membrane was reduced.
BIX increases functional surface protein levels of loss-of-function GluN2A variants
We aim to correct the folding and surface trafficking of GluN2A variants to restore their function on the plasma membrane. Previously it has been shown that BIX has a neuro-protective effect against ER stress [29], and we used BIX to restore the function of pathogenic neuroreceptors [34]. Since BIX has the capacity to enhance the ER folding capacity [21], we determined the effect of BIX application on variant GluN2A protein levels in HEK293T cells stably expressing GluN1GluN2A(M705V) and GluN1GluN2A(A727T). Dose-response analysis showed that BIX (24 h treatment) increased total protein levels of GluN2A variants in a dose-dependent manner: the EC50 (half-maximal effective concentration) values of BIX were 5.28 μM for GluN2A(M705V) and 6.90 μM for GluN2A(A727T) (Fig. 3a). BIX increased GluN2A variant protein levels at a concentration above 2.5 μM and the effect of BIX plateaued at a concentration of 10 μM (Fig. 3a). Resazurin cell toxicity assay showed that a single dose application of BIX for 24 h did not significantly induce toxicity to cells at concentrations below 10 μM (Fig. 3b). Time-course experiments demonstrated that the increase of total GluN2A(M705V) or GluN2A(A727T) subunit protein levels by a single dose application of BIX (10 μM) was achieved as early as 6 h and maximized at 12–24 h after treatment (Fig. 3c). Thereby, we used the optimal treatment condition of BIX (10 μM, 24 h) for the following experiments. BIX treatment did not change the total protein levels of wild type GluN2A subunit in human A2780 cells that express endogenous NMDARs (Supplementary Fig. S3a) [49], indicating that BIX can achieve certain selectivity toward the destabilized ion channel variants. In addition, cycloheximide-chase experiments showed that BIX treatment only slightly slowed down the degradation of wild type GluN2A (Supplementary Fig. S3b).
Fig. 3. Effects of BIX on the expression and function of trafficking-deficient variants in GluN2A subunit of NMDARs.
a Dose-response analysis of BIX treatment (24 h) on the total protein levels of GluN2A subunits in HEK293T cells expressing GluN1GluN2A(M705V) or GluN1GluN2A(A727T). b The toxicity of BIX treatment (24 h) was quantified with a resazurin assay to determine cell viability under different concentrations of BIX application. Tg (2 µM, 6 h) was used as a positive control. Tg thapsigargin. c Time-course analysis of BIX (10 μM) treatment on the total protein levels of GluN2A subunits in HEK293T cells expressing GluN1GluN2A(M705V) or GluN1GluN2A(A727T). β-Actin serves as the total protein loading control. d BIX treatment (10 μM, 24 h) increases surface expression of variant NMDARs. Surface GluN2A staining was in green (column 1), and plasma membrane marker Na+/K+-ATPase staining was in red (column 2). Merge of these two signals with the nucleus stained in blue with DAPI was shown in column 3. Scale bar = 20 μm. The fluorescence intensity of the surface GluN2A was quantified from 30–50 cells per condition. e Surface biotinylation assays further demonstrated that BIX (10 µM, 24 h) enhances surface expression of variant NMDARs. Na+/K+-ATPase serves as the loading control of membrane protein. f BIX (10 μM, 24 h) restores function of variant NMDARs as ion channels, as shown by whole-cell patch-clamping recordings. Glutamate (10 mM) and glycine (100 µM) were applied simultaneously for 3 s, as indicated by the horizontal bar above the currents. The peak currents (Imax) were acquired and analyzed by the Fluxion Data Analyzer (n = 7–9 ensemble recording; each ensemble recording enclosed 20 cells). Student’s t test (for comparison of two groups) or one-way ANOVA followed by a post-hoc Tukey test (for comparison of three or more groups) was used for statistical analysis. Each data point is presented as mean ± SEM. *P < 0.05; **P < 0.01, ***P < 0.001.
Since NMDARs need to reach the plasma membrane to carry out their function, we performed experiments to determine how BIX treatment affected the surface levels of GluN2A variants. Immunofluorescence microscopy experiments demonstrated that BIX treatment substantially increased the surface staining of GluN2A(M705V) and GluN2A(A727T) subunits in HEK293T cells (Fig. 3d, column 1), which colocalized well with a plasma membrane marker Na+/K+-ATPase (Fig. 3d, column 3). Consistently, surface biotinylation assays revealed that BIX treatment significantly increased the surface protein levels of GluN2A(M705V) and GluN2A(A727T) as well (Fig. 3e).
To determine whether the enhanced surface trafficking of GluN2A variants led to enhanced function of NMDARs, we carried out whole-cell patch-clamping experiments to record the agonist-induced peak currents of NMDARs. BIX treatment (10 μM, 24 h) significantly increased the peak current amplitudes from 552.1 ± 169.9 pA to 951.8 ± 183.0 pA for the GluN1GluN2A(M705V) variant, and from 518.4 ± 125.0 pA to 839.0 ± 190.1 pA for the GluN1GluN2A(A727T) variant, corresponding to 72.3% and 61.8% increase, respectively (Fig. 3f). These results unambiguously demonstrated BIX’s capability to upregulate the surface trafficking and the function of NMDARs containing the GluN2A variants of M705V and A727T.
BIX treatment increases surface staining of the GluN2A(M705V) variant in hiPSC-derived neurons
To study NMDA receptor variants in neurons, we used CRISPR/Cas9 genome-editing technology to knockin M705V GRIN2A into apparently healthy hiPSCs, which were then differentiated into excitatory neurons. HiPSCs were subjected to neural induction to embryoid bodies (day 2–12) and then neural progenitor cells (NPCs) (day 13–21) (Fig. 4a). The efficient NPC generation was confirmed by immunostaining of NPC biomarkers, including Nestin, SOX2, and PAX6 (Fig. 4b). NPCs were further differentiated into excitatory neurons and matured for several weeks before performing immunostaining of surface GluN2A subunits. The surface GluN2A staining merged well with the staining of a plasma membrane marker, Na+/K+-ATPase (Fig. 4c, column 4). Moreover, BIX treatment (10 μM, 24 h) significantly increased the fluorescence intensities of the surface GluN2A staining (Fig. 4c, cf. row 2, column 2 to row 1, column 2), indicating that indeed BIX treatment enhanced the surface trafficking of the GluN2A(M705V) variant in hiPSC-derived excitatory neurons, the cell culture models under endogenously expressed conditions.
Fig. 4. Effects of BIX treatment on the surface expression of a trafficking-deficient GluN2A(M705V) variant in hiPSC-derived neurons.
a Schematic timeline for the induction of hiPSCs carrying M705V GRIN2A into neural progenitor cells (NPCs) and their further differentiation to excitatory neurons. Representative images from phase-contrast microscopy were shown for each stage. b Representative images from immunofluorescence staining of NPC markers, SOX2, Nestin, and PAX6, as well as DAPI, a nucleus marker. Scale bar, 10 μm. c Immunofluorescence labeling of surface GluN2A and a plasma membrane marker Na+/K+-ATPase. DAPI was used as a nucleus marker. Scale bar, 10 μm. The fluorescence intensity of the surface GluN2A was quantified from 50–60 cells per condition. Student’s t test was used for statistical analysis. Each data point is presented as mean ± SEM. ***P < 0.001.
BIX enhances variant NMDARs proteostasis by promoting their folding, assembly, and trafficking and reducing their ERAD
Thereafter, we focused on the GluN2A(M705V) variant to study the mechanism of action of BIX on restoring its surface trafficking and function. We determined whether BIX treatment enhanced folding and assembly and facilitated the anterograde trafficking of GluN2A(M705V). To quantify the relative folding extent of GluN2A(M705V), we used the n-dodecyl-β-d-maltoside (DDM) detergent solubility assay by measuring the ratio of its DDM-soluble / insoluble fractions in HEK293T cells. Application of BIX substantially increased this ratio for the variant GluN2A(M705V) subunits (Fig. 5a), which indicated that BIX redistributed variant GluN2A proteins from an aggregation-prone state to a pro-folding state. Furthermore, we used co-immunoprecipitation assay to quantify the interactions between GluN1 and GluN2A(M705V). BIX treatment significantly increased GluN2A(M705V) subunits that were pulled down by the GluN1 subunits (Fig. 5b, cf. lane 5 to 4), indicating that BIX treatment enhanced the interactions between GluN1 and GluN2A(M705V) and thus presumably promoted their assembly into heterotetrameric NMDARs.
Fig. 5. Effect of BIX treatment on the folding, assembly, trafficking, and degradation of variant NMDARs.
a Effect of BIX (10 μM, 24 h) on the folding of GluN2A subunits in HEK293T cells stably expressing GluN1GluN2A(M705V). The normalized ratio of the soluble to insoluble GluN2A was shown on the bottom panel. b Effect of BIX (10 μM, 24 h) on the interactions between GluN1 and GluN2A(M705V) subunits in HEK293T cells stably expressing GluN1GluN2A(M705V) according to co-immunoprecipitation assay. Quantification of the ratio of GluN2A/GluN1 band intensities post-immunoprecipitation (IP), as a measure of the interactions between GluN2A and GluN1, was shown on the bottom. c BIX (10 μM, 24 h) increases the Endo H-resistant post-ER glycoform of the GluN2A subunit in HEK293T cells stably expressing GluN1GluN2A(M705V). PNGase F, which cleaves all glycans in a glycoprotein, served as a non-glycosylated form of GluN2A. Quantification of the Endo H resistant/total GluN2A band intensities was shown on the bottom panel. d Effect of BIX (10 μM, 24 h) on the degradation of the GluN2A subunit in HEK293T cells stably expressing GluN1GluN2A(M705V) using cycloheximide (CHX)-chase analysis. Each data point was reported as mean ± SEM. Student’s t test (for comparison of two groups) or two-way ANOVA followed by a post-hoc Tukey test (for comparison of three or more groups) was used for statistical analysis. *P < 0.05; **P < 0.01.
To evaluate whether BIX enhanced the effective anterograde ER-to-Golgi trafficking of GluN2A(M705V) subunits, we performed an Endo H enzyme digestion assay. Endo H-sensitive GluN2A subunits (Fig. 5c, lanes 2 and 3, bottom bands) are retained in the ER, whereas Endo H-resistant GluN2A subunits (Fig. 5c, lanes 2 and 3, top bands) have exited the ER and traffic at least to the Golgi for further glycan modifications. PNGase F treatment, which cleaves all glycans in a glycoprotein, generated a non-glycosylated form of GluN2A (Fig. 5c, lane 4). The group of BIX treatment produced a stronger post-ER Endo H-resistant GluN2A subunit band (Fig. 5c, cf. lane 3 to 2) and consistently increased the ratio of the Endo H-resistant band/total GluN2A, indicating that BIX significantly enhanced the ER-to-Golgi trafficking efficiency of the variant GluN2A(M705V).
Next, we determined whether BIX influenced the degradation of GluN2A(M705V) variant. Cycloheximide, a potent protein synthesis inhibitor, was administered to the cell culture media for the indicated time. The cycloheximide-chase assay showed that BIX treatment significantly increased the normalized remaining GluN2A(M705V) protein levels at 2 h, 3 h, and 4 h after cycloheximide treatment (Fig. 5d), indicating that BIX attenuated the excessive degradation of variant GluN2A(M705V). An exponential decay fitting showed that BIX treatment increased the half-life of GluN2A(M705V) from 1.2 h to 2.7 h (Fig. 5d), indicating that BIX treatment reduced the degradation rate of this variant. Collectively, these data demonstrated that BIX enhanced the productive folding and assembly of the GluN2A(M705V) variant in the ER, inhibited its ERAD, and promoted its anterograde trafficking from the ER to the Golgi and onward to the plasma membrane. Consequently, BIX treatment restored the function of NMDARs containing this variant due to the increased surface expression.
BIX activates the IRE1 arm of UPR to increase the protein levels of variant GluN2A(M705V) subunits of NMDARs
Furthermore, since the ER proteostasis network plays a critical role in regulating the biogenesis of ion channels [12, 13], we determined the effect of BIX treatment on this network. We investigated how BIX treatment influenced major ER chaperones [50, 51], including BiP, GRP94 (glucose-regulated protein 94), calreticulin (CRT), and calnexin (CANX). Western blot analysis demonstrated that BIX treatment significantly increased the BiP protein level without an apparent effect on other selected chaperones in HEK293T cells expressing GluN1GluN2A(M705V) (Fig. 6a), consistent with the literature report that BIX is a potent BiP inducer [29].
Fig. 6. Effect of BIX on increasing the GluN2A variant total protein level is not dependent on BiP.
a Effect of BIX (10 μM, 24 h) on the ER proteostasis network components, including BiP, CRT, CANX, and GRP94, in HEK293T cells stably expressing GluN1GluN2A(M705V). CRT: calreticulin. CANX: calnexin. Quantifications of the normalized band intensities were shown on the lower panels. b Effect of BiP inhibition on BIX’s effect to increase the total expression levels of GluN2A and BiP in HEK293T cells stably expressing GluN1GluN2A(M705V) according to Western blot analysis. SubAB (0.5 μg/mL, 6 h), a potent BiP-specific protease, was applied to cell culture media to deplete BiP. SubAA272B served as a negative control of SubAB. Tg (thapsigargin) served as a positive control to increase the BiP protein level. c Effect of BiP overexpression on the total protein levels of GluN2A in HEK293T cells stably expressing GluN1GluN2A(M705V). Each data point is reported as mean ± SEM. Student’s t test (for comparison of two groups) or one-way ANOVA followed by a post-hoc Tukey test (for comparison of three or more groups) was used for statistical analysis. *P < 0.05; **P < 0.01; ***P < 0.001.
We next determined whether BIX’s effect on increasing GluN2A(M705V) protein level is dependent on BiP. SubAB is a bacterial toxin with protease activity that selectively cleaves BiP, and SubAA272B is a negative control SubAB mutant that lacks BiP-specific protease activity [31]. Western blot analysis confirmed that BiP was dramatically depleted with SubAB treatment (0.5 μg/mL, 6 h); however, such BiP depletion did not affect the GluN2A(M705V) protein levels (Fig. 6b. cf. lane 4 to 2). In addition, when BiP was depleted with SubAB, BIX treatment still significantly increased GluN2A(M705V) protein levels (Fig. 6b. cf. lane 5 to 4). Furthermore, we carried out the BiP depletion with SubAB post BIX treatment (10 µM, 24 h) and demonstrated that BiP depletion did not diminish the increased GluN2A(M705V) protein levels afforded by BIX treatment (Fig. 6b. cf. lane 5 to 3). In addition, we observed that BiP overexpression (up to 4.2-fold) did not change the GluN2A(M705V) subunit protein levels (Fig. 6c). Therefore, these results indicated that although BIX upregulated BiP protein levels, the BIX-mediated increase of GluN2A(M705V) protein levels did not principally rely on the BIX-upregulated BiP protein level. Other cellular signaling pathways must be involved in the regulation of the activity of BIX on GluN2A(M705V) subunits.
Since the UPR is a major cellular pathway to enhance the ER folding capacity to handle misfolded proteins, we sought to determine whether BIX utilized the UPR to correct the folding and trafficking of GluN2A(M705V). The UPR contains three signaling arms, namely IRE1, ATF6, and PERK, which are all ER integral membrane proteins. Activation of IRE1 and ATF6 is mainly cytoprotective by upregulating the expression of molecular chaperones to handle misfolded proteins, whereas activation of PERK often leads to apoptosis [25]. Upon activation, IRE1 oligomerizes, leading to the autophosphorylation of its kinase domain and the splicing of the mRNA of XBP1. Spliced XBP1 (XBP1s) is a potent transcription factor that promotes the ER folding capacity. Western blot analysis demonstrated that BIX treatment significantly increased the protein level of XBP1s (Fig. 7a), indicating the BIX modestly activated the IRE1 pathway. Upon activation, ATF6, a type-II membrane protein, is translocated form the ER to the Golgi and processed by S1P and S2P proteases to release the N-terminal cytoplasmic fragment of ATF6 (ATF6N), which acts as a transcription factor to enhance ER folding. Western blot analysis showed that BIX treatment increased the protein level of the cleaved ATF6N (Fig. 7a), as an indication of the activation of the ATF6 pathway. Upon activation, PERK phosphorylates eIF2α (eukaryotic translation initiation factor 2α) to reduce global translation, but eIF2α phosphorylation selectively induces the expression of ATF4 and its downstream target CHOP, a proapoptotic transcription factor. Western blot analysis showed that BIX treatment did not change the protein level of CHOP (Fig. 7a), indicating that the PERK arm was not activated. Thapsigargin (Tg), a well-established UPR activator, activated all three arms of the UPR, but did not increase the GluN2A(M705V) protein level in HEK293T cells (Fig. 7a). Thapsigargin activated CHOP and induced substantial ER stress, which was cytotoxic. Therefore, stress-independent activation of the UPR pathway is critical to correct GluN2A folding defects.
Fig. 7. BIX increases variant GluN2A subunits expression through pharmacological activation of the unfolded protein response (UPR).
a Effect of BIX (10 μM, 24 h) on the total protein levels of GluN2A and UPR-associated proteins, including ATF6N, XBP1s and CHOP, in HEK293T cells stably expressing GluN1GluN2A(M705V). Tg (thapsigargin) (1 μM, 6 h) serves as a positive control to activate the UPR. Effect of ATF6N (b) and XBP1s (c) overexpression on the total protein levels of GluN2A in HEK293T cells stably expressing GluN1GluN2A(M705V). d XPB-1s overexpression enhances surface protein levels of GluN2A in HEK293T cells stably expressing GluN1GluN2A(M705V). Na+/K+-ATPase serves as the loading control of surface protein. e Effect of specific IRE1 inhibitors, KIRA6 (1 µM, 24 h) or STF-083010 (30 µM, 24 h), on the BIX (10 µM, 24 h)-induced protein levels of GluN2A in HEK293T cells stably expressing GluN1GluN2A(M705V). Each data point is reported as mean ± SEM. Student’s t test (for comparison of two groups) or one-way ANOVA followed by a post-hoc Tukey test (for comparison of three or more groups) was used for statistical analysis. *P < 0.05; **P < 0.01; ***P < 0.001.
Since BIX treatment modestly activated the IRE1 and ATF6 arms of the UPR, we continued to determine the effect of genetic activation of ATF6 and IRE1 on the maturation of the GluN2A(M705V) subunits. Overexpression of active ATF6N up to 4.2-fold of the endogenous level did not increase the total protein expression levels of GluN2A(M705V) (Fig. 7b), suggesting that ATF6 activation did not contribute to the effect of BIX on increasing GluN2A(M705V) protein level. In contrast, Western blot analysis showed that the genetic activation of the IRE1 pathway by overexpressing XBP1s resulted in a substantial increase of the total protein level of GluN2A(M705V) subunits (Fig. 7c, cf. lanes 4 and 5 to lane 1). Moreover, it appeared that the modest overexpression of XBP1s at 2.15-fold to endogenous level, which is similar to the level that BIX induced, resulted in the most dramatic increase of GluN2A(M705V) protein (Fig. 7c, cf. lane 4 to 5). However, overexpressing XPB1s did not increase the wild type GluN2A protein levels in A2780 cells that express endogenous NMDARs (Supplementary Fig. S3c). In addition, surface biotinylation experiments showed that modest XBP1s overexpression substantially increased the surface protein level of the GluN2A(M705V) subunits (Fig. 7d).
To further determine whether the BIX’s effect depended on the activation of the IRE1 pathway, we co-applied specific IRE1 inhibitors, including KIRA6, an allosteric RNase inhibitor [52], and STF-083010, a RNase inhibitor without affecting the IRE1 kinase activity [53], with BIX treatment. Western blot analysis demonstrated that inhibiting the IRE1 pathway significantly reduced the GluN2A(M705V) protein levels that was induced by BIX treatment (Fig. 7e, cf. lane 4 to 2 for KIRA6 and cf. lane 6 to 2 for STF-083010), indicating that indeed the IRE1 activation contributed to the effect of BIX on enhancing GluN2A(M705V) proteostasis. In addition, to determine whether the ATF6 or PERK pathway played an important role in regulating BIX’s activity on increasing GluN2A(M705V) protein levels, we co-applied specific ATF6 inhibitors, including Ceapin-A7 [54] and PF429242 [55], or PERK inhibitors, including ISRIB [56] and GSK2606414 [57], with BIX treatment. Western blot analysis demonstrated that inhibiting the ATF6 or PERK pathway did not significantly change the GluN2A(M705V) protein levels that was induced by BIX treatment (Supplementary Fig. S4a, b), indicating that BIX’s effect on the GluN2A variant was not dependent on the activation of the ATF6 or PERK pathway. The differentiating effects of ATF6 and IRE1 on the GluN2A(M705V) variant protein levels could be due to the distinct downstream gene targets between ATF6 activation and IRE1 activation, which would adapt the ER proteostasis differently [58].
In summary, the above experiments clearly demonstrated that modest IRE1/XBP1s activation enhanced the surface trafficking of GluN2A(M705V). Therefore, through activating the IRE1/XBP1s pathway, BIX enhances the folding, trafficking, and function of the GluN2A(M705V) variant.
Discussion
Our data corroborate that BIX enhances surface trafficking of epilepsy-associated GluN2A variant subunits of NMDARs, including GluN2A(M705V) and GluN2A(A727T). Once they reach the plasma membrane, the function of the variant receptors is restored (Fig. 3f). Our results support the mechanism for BIX-mediated rescue of misfolding-prone GluN2A variant NMDARs proposed in Fig. 8. BIX treatment modestly activates the IRE1/XBP1s arm of the UPR pathway to remodel the ER proteostasis network, including the regulation of molecular chaperones [58], which leads to enhanced ER folding capacity. This operation further stabilizes the misfolding-prone GluN2A variant and enhances its productive protein folding. In the meantime, BIX treatment also inhibits ERAD of the variant. Enhancing protein folding and reducing ERAD would push more variant proteins to the NMDAR assembly process in the ER membrane. As a result, BIX treatment promotes the forward trafficking of the assembled variant-containing NMDARs from the ER to the Golgi and further onward to the plasma membrane. Consequently, BIX treatment corrects the function of the variant-containing NMDARs by enhancing their trafficking to the plasma membrane.
Fig. 8. Proposed mechanism of action of BIX effect on restoring proteostasis of NMDARs harboring GluN2A pathogenic variations.
BIX treatment adapts the ER proteostasis network by activating the IRE1/XBP1s arm of the UPR. Activated XBP1s acts as a transcription factor to upregulate genes associated with protein folding, assembly, trafficking, and degradation. Therefore, BIX treatment promotes productive folding of the GluN2A variants as well as attenuating their ERAD. Enhanced folding and reduced ERAD of variant GluN2A subunits result in an increased population of assembled tetrameric NMDARs on the ER membrane. As a result, BIX treatment promotes the forward trafficking of the assembled NMDARs from the ER to the Golgi and onward to the plasma membrane to perform their function as ion channels. Asterisk (*) represents mutations on the GluN2A subunits.
Increasing reports have investigated the effects of missense mutations on GluN2A protein function in various expression systems [59–61], a common outcome of which is that epilepsy-associated GluN2A variants have strikingly variable functional consequences on NMDARs [11]. The significance of NMDARs is well-demonstrated by the range of clinical phenotypes of patients with variants in the genes encoding GluN2A subunits. A standard approach for rescuing loss-of-function phenotypes is the usage of pharmacological modulators to enhance receptor function. The ability of ligand binding to control ion-channel gating has been shown to be a functional checkpoint for glutamate receptors, including NMDARs, to exit the ER [14, 15, 62]. Thereby, we used epilepsy-associated M705V and A727T variations, which are located in the LBD of the GluN2A subunits of NMDARs, to evaluate how BIX remodels the ER proteostasis network to rescue the misfolding-prone variants of NMDARs.
We first determined the protein expression level and functional characteristics of NMDARs containing M705V or A727T variants in the GluN2A subunits. The results from transfected HEK293T cells revealed that total and surface protein levels of GluN2A were significantly reduced in both M705V and A727T variants (Fig. 1b–d). The results coincided with the reduced peak currents recorded by whole-cell patch-clamp technique for GluN1GluN2A(M705V) and GluN1GluN2A(A727T) (Fig. 1e). Moreover, the variants in GluN2A subunits showed the increased degradation by the proteasome pathway with the application of MG132 compared to GluN2A WT (Fig. 2a). The results ascertain that NMDARs harboring M705V or A727T variants in the GluN2A subunits are prone to misfolding in the ER and inefficient trafficking to the cell membrane.
In the past, researchers attempted to validate BiP as a potentially potent therapeutic target for pharmacological manipulation of neuronal cell damage in the brain [63]. Previous reports revealed that treatment with BIX had anti-apoptotic and neuroprotective effects against neuronal cell damage in animal models [64–66], which implied that BIX may be developed as a promising candidate to attenuate ER stress for the treatment of ER stress-associated diseases. Our results showed that BIX treatment not only increased the total protein levels but also surface protein levels of GluN2A subunits containing M705V or A727T variant (Fig. 3a–e), indicating that BIX promotes forward trafficking and importantly surface expression of these variants. The time course study of single-dose BIX treatment showed the optimal rescue level was achieved at 12–24 h (Fig. 3c), which may be possibly due to the involvement of protein maturation, such as protein folding and trafficking. The ER-retained GluN2A(M705V) and GluN2A(A727T) variants were targeted to the proteasome for degradation (Fig. 2a–d). Adapting ER proteostasis can correct the misfolding of such variants (Fig. 5a) and enhance their assembly to tetrameric NMDARs (Fig. 5b). If such variants can pass the ER quality control and reach the plasma membrane in assembled NMDARs, presumably the rescued receptors on the plasma membrane would form functional channels, which was supported by the results that BIX treatment increased the electrophysiological currents in NMDARs containing these GluN2A variants (Fig. 3f). Furthermore, the increased currents induced by BIX treatment in NMDARs containing GluN2A variants were comparable to that recorded in GluN1GluN2A WT NMDARs, suggesting a clinical potential of this strategy. These results provided evidence that misfolding and trafficking and functional deficiency of NMDARs harboring GluN2A variants can be restored by BIX treatment.
We sought to investigate how BIX adapts the ER proteostasis network to correct GluN2A variant function. Among the major ER chaperones tested, BIX treatment did not significantly change the protein levels of calnexin, calreticulin, and GRP94, but increased BiP levels in HEK293T cells stably expressing GluN1GluN2A(M705V) (Fig. 6a). Interestingly, such BIX-upregulated BiP expression did not seem to affect BIX’s effect on the variant GluN2A subunits (Fig. 6b, c). Since the UPR is a major pathway to regulate ER proteostasis, we next determined the effect of BIX treatment on the activation of the UPR. BIX activated both the IRE1 and ATF6 arms of the UPR. However, IRE1 activation by XBP1s overexpression significantly increased the total protein level of GluN2A(M705V) subunits in HEK293T cells, whereas ATF6N overexpression did not (Fig. 7b, c). The differing effect between IRE1 and ATF6 activation on the GluN2A variants is likely due to the fact that they have different downstream targets. Our results also suggest that stress-independent activation of the IRE1/XBP1s pathway [21] is a promising strategy to correct the functional surface expression of pathogenic GluN2A variants. It merits further investigation to find out which downstream targets of the IRE1 activation play a key role in correcting the misfolding of GluN2A variants, especially in hiPSC-derived neurons that carry the pathogenic variants. In addition, we showed that the activation of the IRE1 pathway rather than the ATF6 or PERK pathway plays an important role in the BIX-induced rescue of misfolding-prone GluN2A variant receptors.
In conclusion, we demonstrated that BIX treatment enhances the productive folding and forward trafficking, and further restores the function of NMDARs containing pathogenic variant GluN2A subunits by modestly activating the IRE1 pathway. Importantly, BIX effectively restores the surface trafficking of a GluN2A variant in iPSC-derived neurons. This study provides a proof-of-concept case about remodeling ER proteostasis to rescue the loss-of-function GluN2A variant NMDARs associated with protein misfolding diseases. Such a folding-correction strategy has a dual role in the functional rescue of NMDARs: first, it will increase the number of the functional channels on the plasma membrane to enhance the post-synaptic activities; second, the increased surface receptors will be available to current known NMDAR-specific ligands, such as agonists and positive allosteric modulators, providing a potential synergistic action between two mechanistically distinct compounds.
Supplementary information
Acknowledgements
This work was supported by the National Institutes of Health (R01NS117176 to TWM, R01NS123524 to AES, and F30HD110088 to LYA) and the Brain Research Foundation (BRFSG-2021-08 to AES).
Author contributions
PPZ, TWM, and YJW designed research; PPZ, LYA, and YJW performed research; PPZ, TWM, and YJW analyzed data; JCP and AWP contributed reagents; PPZ, TWM and YJW wrote the original draft; PPZ, TMB, LYA, AES, JCP, AWP, TWM, and YJW reviewed and edited the manuscript. All authors discussed the study.
Competing interests
The authors declare no competing interests.
Contributor Information
Ting-wei Mu, Email: tingwei.mu@case.edu.
Ya-juan Wang, Email: yajuan.wang@case.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01172-w.
References
- 1.Hansen KB, Wollmuth LP, Bowie D, Furukawa H, Menniti FS, Sobolevsky AI, et al. Structure, function, and pharmacology of glutamate receptor ion channels. Pharmacol Rev. 2021;73:298–487. doi: 10.1124/pharmrev.120.000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yong XLH, Zhang L, Yang L, Chen X, Tan JZA, Yu X, et al. Regulation of NMDA receptor trafficking and gating by activity-dependent CaMKIIalpha phosphorylation of the GluN2A subunit. Cell Rep. 2021;36:109338. doi: 10.1016/j.celrep.2021.109338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karakas E, Furukawa H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science. 2014;344:992–7. doi: 10.1126/science.1251915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CH, Lü W, Michel JC, Goehring A, Du J, Song X, et al. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature. 2014;511:191–7. doi: 10.1038/nature13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Lv S, Stroebel D, Zhang J, Pan Y, Huang X, et al. Gating mechanism and a modulatory niche of human GluN1-GluN2A NMDA receptors. Neuron. 2021;109:2443–56.e2445. doi: 10.1016/j.neuron.2021.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Sanz-Clemente A, Nicoll RA, Roche KW. Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist. 2013;19:62–75. doi: 10.1177/1073858411435129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vieira M, Yong XLH, Roche KW, Anggono V. Regulation of NMDA glutamate receptor functions by the GluN2 subunits. J Neurochem. 2020;154:121–43. doi: 10.1111/jnc.14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroebel D, Casado M, Paoletti P. Triheteromeric NMDA receptors: from structure to synaptic physiology. Curr Opin Physiol. 2018;2:1–12. doi: 10.1016/j.cophys.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.XiangWei W, Jiang Y, Yuan H. De novo mutations and rare variants occurring in NMDA receptors. Curr Opin Physiol. 2018;2:27–35. doi: 10.1016/j.cophys.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers SJ, Yuan H, Kang JQ, Tan FCK, Traynelis SF, Low CM. Distinct roles of GRIN2A and GRIN2B variants in neurological conditions. F1000Res. 2019;8:1940. doi: 10.12688/f1000research.18949.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmasri M, Hunter DW, Winchester G, Bates EE, Aziz W, Van Der Does DM, et al. Common synaptic phenotypes arising from diverse mutations in the human NMDA receptor subunit GluN2A. Commun Biol. 2022;5:174. doi: 10.1038/s42003-022-03115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benske TM, Mu TW, Wang YJ. Protein quality control of N-methyl-D-aspartate receptors. Front Cell Neurosci. 2022;16:907560. doi: 10.3389/fncel.2022.907560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu YL, Wang YJ, Mu TW. Proteostasis maintenance of Cys-loop receptors. Adv Protein Chem Struct Biol. 2016;103:1–23. doi: 10.1016/bs.apcsb.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Swanger SA, Chen W, Wells G, Burger PB, Tankovic A, Bhattacharya S, et al. Mechanistic insight into NMDA receptor dysregulation by rare variants in the GluN2A and GluN2B agonist binding domains. Am J Hum Genet. 2016;99:1261–80. doi: 10.1016/j.ajhg.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.She K, Ferreira JS, Carvalho AL, Craig AM. Glutamate binding to the GluN2B subunit controls surface trafficking of N-methyl-D-aspartate (NMDA) receptors. J Biol Chem. 2012;287:27432–45. doi: 10.1074/jbc.M112.345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mah SJ, Cornell E, Mitchell NA, Fleck MW. Glutamate receptor trafficking: endoplasmic reticulum quality control involves ligand binding and receptor function. J Neurosci. 2005;25:2215–25. doi: 10.1523/JNEUROSCI.4573-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Addis L, Virdee JK, Vidler LR, Collier DA, Pal DK, Ursu D. Epilepsy-associated GRIN2A mutations reduce NMDA receptor trafficking and agonist potency - molecular profiling and functional rescue. Sci Rep. 2017;7:66. doi: 10.1038/s41598-017-00115-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Needham PG, Guerriero CJ, Brodsky JL. Chaperoning endoplasmic reticulum-associated degradation (ERAD) and protein conformational diseases. Cold Spring Harb Perspect Biol. 2019;11:a033928. doi: 10.1101/cshperspect.a033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams BM, Oster ME, Hebert DN. Protein quality control in the endoplasmic reticulum. Protein J. 2019;38:317–29. doi: 10.1007/s10930-019-09831-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Z, Brodsky JL. Protein quality control in the secretory pathway. J Cell Biol. 2019;218:3171–87. doi: 10.1083/jcb.201906047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grandjean JMD, Wiseman RL. Small molecule strategies to harness the unfolded protein response: where do we go from here? J Biol Chem. 2020;295:15692–711. doi: 10.1074/jbc.REV120.010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly JW. Pharmacologic approaches for adapting proteostasis in the secretory pathway to ameliorate protein conformational diseases. Cold Spring Harb Perspect Biol. 2020;12:a034108. doi: 10.1101/cshperspect.a034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marciniak SJ, Chambers JE, Ron D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat Rev Drug Discov. 2022;21:115–40. doi: 10.1038/s41573-021-00320-3. [DOI] [PubMed] [Google Scholar]
- 24.Otero JH, Lizák B, Hendershot LM. Life and death of a BiP substrate. Semin Cell Dev Biol. 2010;21:472–8. doi: 10.1016/j.semcdb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 26.Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21:421–38. doi: 10.1038/s41580-020-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiseman RL, Mesgarzadeh JS, Hendershot LM. Reshaping endoplasmic reticulum quality control through the unfolded protein response. Mol Cell. 2022;82:1477–91. doi: 10.1016/j.molcel.2022.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorbatyuk MS, Gorbatyuk OS. The molecular chaperone GRP78/BiP as a therapeutic target for neurodegenerative disorders: a mini review. J Genet Syndr Gene Ther. 2013;4:128. doi: 10.4172/2157-7412.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kudo T, Kanemoto S, Hara H, Morimoto N, Morihara T, Kimura R, et al. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15:364–75. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- 30.Termine DJ, Moremen KW, Sifers RN. The mammalian UPR boosts glycoprotein ERAD by suppressing the proteolytic downregulation of ER mannosidase I. J Cell Sci. 2009;122:976–84. doi: 10.1242/jcs.037291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, et al. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443:548–52. doi: 10.1038/nature05124. [DOI] [PubMed] [Google Scholar]
- 32.Wang M, Cotter E, Wang YJ, Fu X, Whittsette AL, Lynch JW, et al. Pharmacological activation of ATF6 remodels the proteostasis network to rescue pathogenic GABA(A) receptors. Cell Biosci. 2022;12:48. doi: 10.1186/s13578-022-00783-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di XJ, Han DY, Wang YJ, Chance MR, Mu TW. SAHA enhances proteostasis of epilepsy-associated alpha1(A322D)beta2gamma2 GABA(A) receptors. Chem Biol. 2013;20:1456–68. doi: 10.1016/j.chembiol.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu YL, Han DY, Wang YJ, Di XJ, Yu HB, Mu TW. Remodeling the endoplasmic reticulum proteostasis network restores proteostasis of pathogenic GABAA receptors. PLoS One. 2018;13:e0207948. doi: 10.1371/journal.pone.0207948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinhardt P, Glatza M, Hemmer K, Tsytsyura Y, Thiel CS, Höing S, et al. Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PLoS One. 2013;8:e59252. doi: 10.1371/journal.pone.0059252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim DS, Lee JS, Leem JW, Huh YJ, Kim JY, Kim HS, et al. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev Rep. 2010;6:270–81. doi: 10.1007/s12015-010-9138-1. [DOI] [PubMed] [Google Scholar]
- 37.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–80. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sineva GS, Pospelov VA. Inhibition of GSK3beta enhances both adhesive and signalling activities of beta-catenin in mouse embryonic stem cells. Biol Cell. 2010;102:549–60. doi: 10.1042/BC20100016. [DOI] [PubMed] [Google Scholar]
- 39.Huntley GW, Vickers JC, Morrison JH. Cellular and synaptic localization of NMDA and non-NMDA receptor subunits in neocortex: organizational features related to cortical circuitry, function and disease. Trends Neurosci. 1994;17:536–43. doi: 10.1016/0166-2236(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 40.Meador-Woodruff JH, Healy DJ. Glutamate receptor expression in schizophrenic brain. Brain Res Brain Res Rev. 2000;31:288–94. doi: 10.1016/S0165-0173(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 41.Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–3. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- 42.Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci USA. 1986;83:7104–8. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemke JR, Lal D, Reinthaler EM, Steiner I, Nothnagel M, Alber M, et al. Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nat Genet. 2013;45:1067–72. doi: 10.1038/ng.2728. [DOI] [PubMed] [Google Scholar]
- 44.Liu XR, Xu XX, Lin SM, Fan CY, Ye TT, Tang B, et al. GRIN2A variants associated with idiopathic generalized epilepsies. Front Mol Neurosci. 2021;14:720984. doi: 10.3389/fnmol.2021.720984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Tang W, Bhatia NK, Xu Y, Paudyal N, Liu D, et al. A de novo GRIN1 variant associated with myoclonus and developmental delay: from molecular mechanism to rescue pharmacology. Front Genet. 2021;12:694312. doi: 10.3389/fgene.2021.694312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang P, Mizushima N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods. 2015;75:13–18. doi: 10.1016/j.ymeth.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 48.Valley CC, Cembran A, Perlmutter JD, Lewis AK, Labello NP, Gao J, et al. The methionine-aromatic motif plays a unique role in stabilizing protein structure. J Biol Chem. 2012;287:34979–91. doi: 10.1074/jbc.M112.374504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.North WG, Liu F, Tian R, Abbasi H, Akerman B. NMDA receptors are expressed in human ovarian cancer tissues and human ovarian cancer cell lines. Clin Pharmacol. 2015;7:111–7. doi: 10.2147/CPAA.S90367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohli E, Causse S, Baverel V, Dubrez L, Borges-Bonan N, Demidov O, et al. Endoplasmic reticulum chaperones in viral infection: therapeutic perspectives. Microbiol Mol Biol Rev. 2021;85:e0003521. doi: 10.1128/MMBR.00035-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–51. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghosh R, Wang L, Wang ES, Perera BG, Igbaria A, Morita S, et al. Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell. 2014;158:534–48. doi: 10.1016/j.cell.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–4. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallagher CM, Garri C, Cain EL, Ang KK, Wilson CG, Chen S, et al. Ceapins are a new class of unfolded protein response inhibitors, selectively targeting the ATF6α branch. Elife. 2016;5:e11878. doi: 10.7554/eLife.11878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hawkins JL, Robbins MD, Warren LC, Xia D, Petras SF, Valentine JJ, et al. Pharmacologic inhibition of site 1 protease activity inhibits sterol regulatory element-binding protein processing and reduces lipogenic enzyme gene expression and lipid synthesis in cultured cells and experimental animals. J Pharmacol Exp Ther. 2008;326:801–8. doi: 10.1124/jpet.108.139626. [DOI] [PubMed] [Google Scholar]
- 56.Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife. 2013;2:e00498. doi: 10.7554/eLife.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem. 2012;55:7193–207. [DOI] [PubMed]
- 58.Shoulders MD, Ryno LM, Genereux JC, Moresco JJ, Tu PG, Wu C, et al. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep. 2013;3:1279–92. doi: 10.1016/j.celrep.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan H, Hansen KB, Zhang J, Pierson TM, Markello TC, Fajardo KV, et al. Functional analysis of a de novo GRIN2A missense mutation associated with early-onset epileptic encephalopathy. Nat Commun. 2014;5:3251. doi: 10.1038/ncomms4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sibarov DA, Bruneau N, Antonov SM, Szepetowski P, Burnashev N, Giniatullin R. Functional properties of human NMDA receptors associated with epilepsy-related mutations of GluN2A subunit. Front Cell Neurosci. 2017;11:155. doi: 10.3389/fncel.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen W, Tankovic A, Burger PB, Kusumoto H, Traynelis SF, Yuan H. Functional evaluation of a de novo GRIN2A mutation identified in a patient with profound global developmental delay and refractory epilepsy. Mol Pharmacol. 2017;91:317–30. doi: 10.1124/mol.116.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gill MB, Vivithanaporn P, Swanson GT. Glutamate binding and conformational flexibility of ligand-binding domains are critical early determinants of efficient kainate receptor biogenesis. J Biol Chem. 2009;284:14503–12. doi: 10.1074/jbc.M900510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawaguchi Y, Hagiwara D, Tsumura T, Miyata T, Kobayashi T, Sugiyama M, et al. Knockdown of endoplasmic reticulum chaperone BiP leads to the death of parvocellular AVP/CRH neurons in mice. J Neuroendocrinol. 2023;35:e13223. doi: 10.1111/jne.13223. [DOI] [PubMed] [Google Scholar]
- 64.Nakanishi T, Shimazawa M, Sugitani S, Kudo T, Imai S, Inokuchi Y, et al. Role of endoplasmic reticulum stress in light-induced photoreceptor degeneration in mice. J Neurochem. 2013;125:111–24. doi: 10.1111/jnc.12116. [DOI] [PubMed] [Google Scholar]
- 65.Oida Y, Hamanaka J, Hyakkoku K, Shimazawa M, Kudo T, Imaizumi K, et al. Post-treatment of a BiP inducer prevents cell death after middle cerebral artery occlusion in mice. Neurosci Lett. 2010;484:43–46. doi: 10.1016/j.neulet.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 66.Inokuchi Y, Nakajima Y, Shimazawa M, Kurita T, Kubo M, Saito A, et al. Effect of an inducer of BiP, a molecular chaperone, on endoplasmic reticulum (ER) stress-induced retinal cell death. Invest Ophthalmol Vis Sci. 2009;50:334–44. doi: 10.1167/iovs.08-2123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.