Abstract
People living with diabetes have many medical devices available to assist with disease management. A critical aspect that must be considered is how systems for continuous glucose monitoring and insulin pumps communicate with each other and how the data generated by these devices can be downloaded, integrated, presented and used. Not only is interoperability associated with practical challenges, but also devices must adhere to all aspects of regulatory and legal frameworks. Key issues around interoperability in terms of data ownership, privacy and the limitations of interoperability include where the responsibility/liability for device and data interoperability lies and the need for standard data-sharing protocols to allow the seamless integration of data from different sources. There is a need for standardised protocols for the open and transparent handling of data and secure integration of data into electronic health records. Here, we discuss the current status of interoperability in medical devices and data used in diabetes therapy, as well as regulatory and legal issues surrounding both device and data interoperability, focusing on Europe (including the UK) and the USA. We also discuss a potential future landscape in which a clear and transparent framework for interoperability and data handling also fulfils the needs of people living with diabetes and healthcare professionals.
Graphical Abstract
Supplementary Information
The online version contains a slideset of the figures for download available at 10.1007/s00125-023-06049-5.
Keywords: Big data, Diabetes therapy, Glucose monitoring, Insulin delivery systems, Interoperability, Medical devices, Review
Introduction
Many different medical devices are available to support people living with diabetes (PLWD) with diabetes management. However, to get the most benefit from these devices it is important that they communicate effectively with each other (device interoperability) and that the data generated are seamlessly integrated as well as viewed across platforms (data interoperability) (Figs 1 and 2). Data are invaluable for PLWD and their healthcare professionals (HCPs); however, issues related to data generation, transfer, storage, access and use must be considered. Several factors currently limit the full potential of the data generated by these medical products. Some are hurdles regarding costs and regulatory policies; however, many are related to data sharing. One major question in this respect is related to data ownership, which might look different in different countries. HCPs and, to a greater extent, device users are end-users and the data generated are crucial to population research and large-scale real-world evidence (RWE) studies.
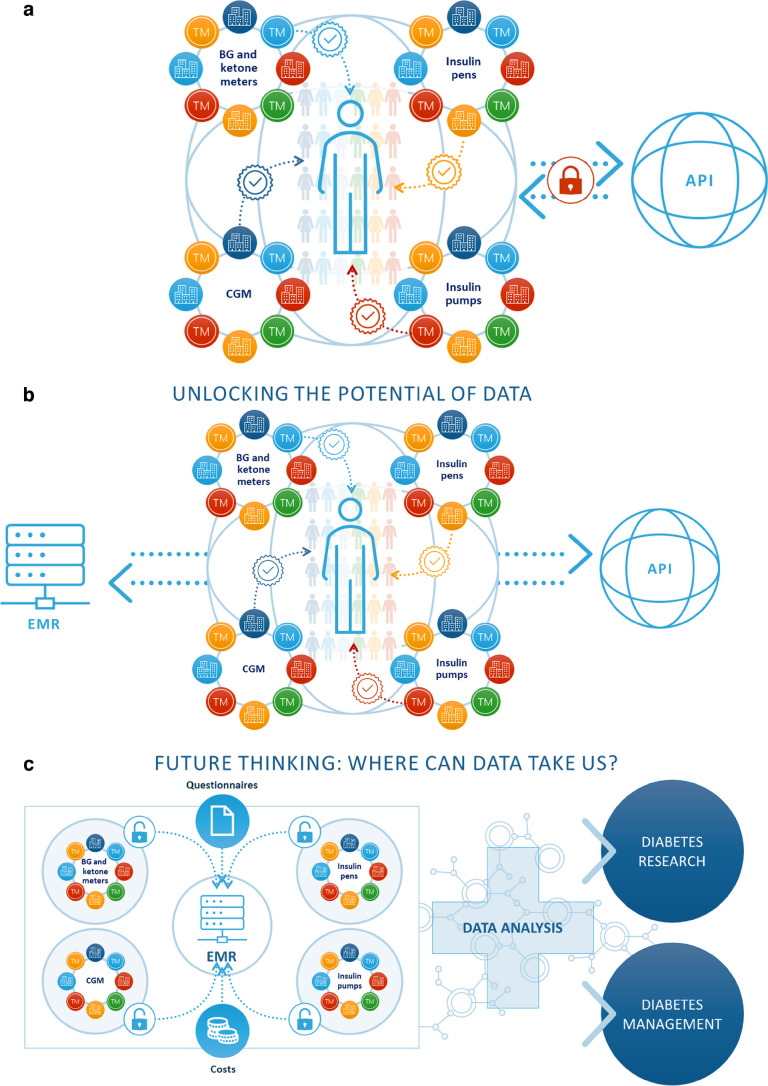
Fig. 1.
Device and data interoperability and the way ahead. Regarding the availability of medical devices for diabetes therapy, across most developed markets there are currently at least ten different continuous glucose monitor (CGM) systems available, at least 15 different insulin pumps, at least ten connected insulin pens or cap systems (also known as ‘smart pens’ [29, 30] and at least 150 systems for the measurement of (capillary) blood glucose (BG) and ketone body levels. Systems for the continuous monitoring of ketones will also enter the market soon [31]. (a) In this scenario, an API, which would allow the integration of data from different devices into a site’s/organisation’s EMR system, is not available, represented by the red padlock. As different brands are not always compatible with each other or with diabetes management software, fully personalised treatment is hampered. (b) Unlocking the potential of data. In this scenario, an open API, illustrated by removal of the red padlock, creates opportunities to use data from different devices for additional purposes, including data sharing and data documentation. (c) Future thinking: where can data take us? In this scenario the potential of data is unlocked, that is, the red padlocks are removed and there is free data flow between the devices and the EMR. These data also enable the costs of different treatment procedures to be calculated. Additional information from patient questionnaires and HCPs can be transferred to the EMR. The combined information allows advanced data analysis and further diabetes research and optimisation of diabetes management. Different devices (‘TM’) and companies (images of buildings) are represented in each group (BG and ketone meters, CGM, insulin pens and insulin pumps) in different-coloured circles. The PLWD is illustrated in the centre of the figures and individual patient choices are illustrated by the different-coloured ticks. TM, trademark. Images (a), (b) and (c) are provided courtesy of Novo Nordisk. These images were initially presented by Adolfsson P. at ATTD 2022, Barcelona [32]. Used with permission. This figure is available as part of a downloadable slideset
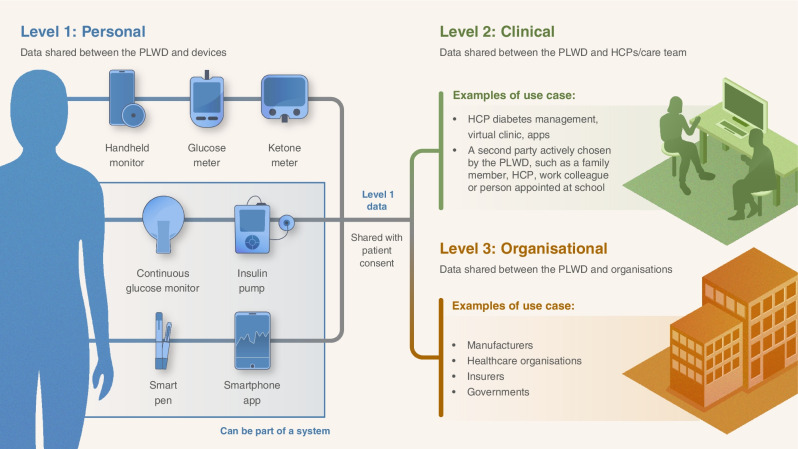
Fig. 2.
Present diabetes management and the handling of data from devices and access to data/device ecosystems can be broadly divided into three levels that require different degrees of data interoperability. At level 1, data are shared between the PLWD and the devices. At level 2, the data are shared between the PLWD and their HCPs/care team. At level 3, the data are shared between the PLWD and organisations (manufacturers, healthcare organisations, insurers and governments). Image designed by Science Graphic Design (UK). Used with permission. This figure is available as part of a downloadable slideset
A key objective in enabling data interoperability is to have a standard application programming interface (API), which would allow the integration of data from different devices into a site’s/organisation’s electronic medical record (EMR) system, with a single point of entry for the system [1] (Fig. 1). An open API enables software developers to handle data from multiple sources, for example for use in virtual diabetes clinics (VDCs). However, a VDC could require multiple data platforms and multiple sign-ins, which would be time-consuming and hinder efficient workflows. Consequently, a simplified process enabling the use and integration of data transfer from different products into digital platforms would reduce the burden for both PLWD and their HCPs, with automated or semi-automated data transfer making the process smoother [1]. The seamless transmission and interpretation of data could also be made more accessible through agreement on a minimum dataset and standardisation of interoperability between medical devices, device-specific software, EMR systems, apps and HCP interface portals [2]. Open access to data, while complying with the General Data Protection Regulation (GDPR), which came into effect in the EU in 2018, would also support data analysis, data interpretation and display of data in a clear and instructive manner [3]. Ongoing initiatives such as the integration of Continuous Glucose Monitoring Data into the Electronic Health Record (iCoDE) project are currently focusing on providing a data standard and workflow framework for integrating continuous glucose monitoring (CGM) data into EMRs to enable these data to be readily accessed and used in routine clinical care [4]. iCoDE is based on the US Health Insurance Portability and Accountability Act of 1996. A European version of iCoDE would need to be compliant with the GDPR. In addition to use by HCPs, manufacturers also use device-specific data for proprietary data management systems and quality control, as required by post-marketing surveillance requirements set out in the 2017 EU Medical Device Regulation (MDR) [5], wherein the manufacturer is responsible for the information presented by the proprietary data management system (not for third-party interpretation).
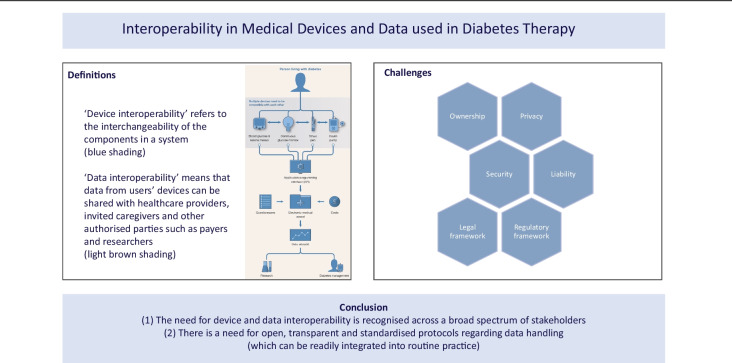
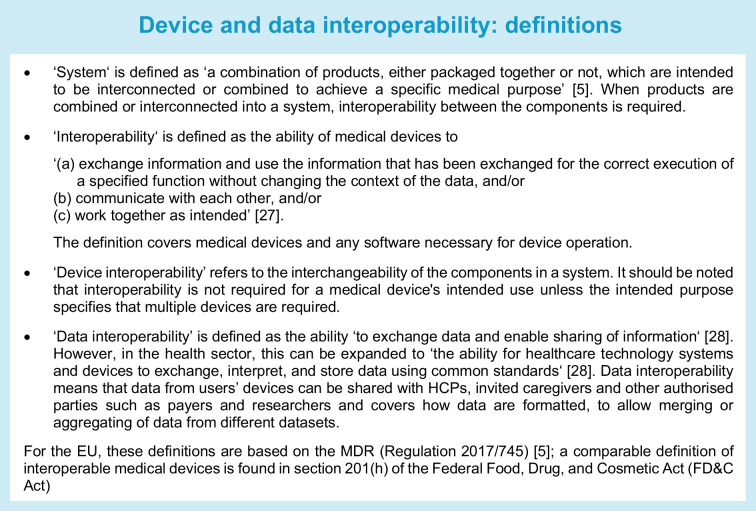
This article provides an overview of critical issues around interoperability in medical devices and data used in diabetes therapy, including data ownership, privacy, security, interoperability limitations and the influence of interoperability on the device marketplace. It also discusses the main challenges that must be addressed for device and data interoperability to generate a seamless, secure process that complies with local regulatory and legal requirements in Europe (including the UK) and the USA. For definitions related to device and data operability, see the textbox (‘Device and data interoperability: definitions’). This article is not intended to be a systematic (scientific) review or a consensus paper about interoperability. In a sense, it is more an academic commentary, mainly from an EU/UK/US perspective. We are fully aware of the fact that interoperability is a complex topic that undergoes rapid changes. One limitation of our approach is that we are a group of academics with a focus on the clinical point of view; consideration from other stakeholders is also needed.
Limitations of interoperability
Device interoperability is particularly vulnerable to limitations such as cybersecurity, quality and liability in terms of a system’s intended purpose. While the intended purpose of CGM systems is glucose monitoring and that of insulin pumps is insulin delivery, the intended use of automated insulin delivery (AID) systems is diabetes management as driven by an AID algorithm, the accuracy of insulin delivery and input by the CGM system. In the USA, the US Food and Drug Administration (FDA) tends to regulate end-to-end systems but differences in sensor accuracy, algorithms or insulin delivery, in particular, may impact clinical outcomes. On a similar note, the efficacy and safety of a system’s performance is based on the engineering specifications of the individual components and not on the performance in general of the system.
From the user perspective, using a combination of different products means that there may be some gaps in customer care that arise from interoperability considerations, as well as restricted or piecemeal clinical and technical support for HCPs. Additionally, the use of different products means that there is the potential for discrepancies within a model of open data handling with different devices from different manufacturers. For example, a user may have indicated to the manufacturer of a given CGM system that a third party may use their data. However, the user may not have granted permission for the pump manufacturer to use their data or may initially grant permission to both manufacturers and subsequently withdraw permission from one manufacturer but not the other. In this situation, it is necessary to consider how the CGM information may be passed from one manufacturer to the other. Another potential legal concern around combined products is that, in the eventuality of a malfunction, who is liable and how is this determined?
Given the limited computational power of AID systems, the checking of data integrity, imputation of missing data and reconciliation of outliers in data by these systems are limited. Hence, the coordination of such activities by various devices becomes another challenge for the interoperability of medical devices. Potential limitations from the regulatory perspective concerning AID system approval include that some markets have defined diabetes-specific operability standards but have not yet created the approval process for the system in its intended use. At the same time, new AID technologies are bringing new concepts regarding a ‘hands-off’ approach to glucose control. Therefore, it is likely that at least some of the challenges for users and HCPs in terms of coping with the volume of data and the provision of therapy recommendations by HCPs will be overcome by design.
Regulatory and legal frameworks
Overview
There are substantial differences across jurisdictions concerning the regulatory and legal frameworks for medical products and devices. The regulation of medical devices in the USA (where the FDA is the single national agency responsible for device approval) and the EU (where different notified bodies are designated and contracted for device review and approval) differ considerably in terms of requirements and organisational structure. There are differences across jurisdictions in terms of how data/RWE can be used. Companies may be subject to specific laws depending on where the company headquarters are located, where the diabetes technology devices are manufactured and where the servers for data storage are located. For example, the legal frameworks for data protection differ between Europe and the USA [6, 7].
Regulatory frameworks in the EU/UK/USA
The EU does not have an interoperable diabetes device pathway comparable to that in the USA. Instead, in the EU, the manufacturer specifies the intended purpose, technical specifications, indications and limits of use for its product supported by clinical evidence, which are detailed in the instructions/information for users and the technical documentation. Within the EU, the European Medicines Agency (EMA) is responsible for the regulation of some medicines but not for the regulation of medical devices; instead, the competent authorities are the regulators of medical devices and accredit notified bodies in Europe to assess manufacturers [8].
Since May 2017, a new regulatory framework for medical devices (MDR) has been in place in the EU [5], which represents a significant change in how they are regulated [9]. The implementation of the MDR commenced in May 2021 and, from May 2024, all medical devices placed on the market will have to conform to the MDR. Medical devices are evaluated and those that conform to the EU MDR are allowed to enter the market by the independent notified bodies. The MDR requires the manufacturer to provide technical documentation and perform clinical evaluation. Traditionally, medical devices, but not necessarily diabetes-related products, have reached the market sooner in the EU than in the USA. However, implementation of the MDR may reduce the differences in data requirements and marketing approval times between the EU and the USA. In particular, the MDR has new, stricter classification rules for AID systems (e.g. Rule 22, ‘Active therapeutic devices with an integrated or incorporated diagnostic function that significantly determines the patient management’) with a higher risk classification (class III) and is more complex than the modular US approach. In addition, unlike the USA, there are no standard technical specifications for any of the different devices within AID systems. Furthermore, no clear direction or guidance exists on how notified bodies should practically apply the definition of AID systems and the higher risk classification through their reviews and issuance of certificates.
Combining products from different manufacturers
The manufacturer of a system component defines its interoperability with other components. In the area of diabetes, for example, this results in the availability of AID system components intended to be combined only with other specified system components (e.g. from the same manufacturer), as well as the availability of system components intended to be used with a wider range of components (e.g. from different manufacturers). In instances where components from different manufacturers are combined, interoperability must be explicitly demonstrated (i.e. similar to the FDA’s interoperability provision). Key considerations for combined products include establishing who is responsible for the combined product in terms of liability and warranty and how to demonstrate the safety and efficacy of combined devices in accordance with the MDR. With regard to AID systems, other components might be added for continuous monitoring of physical activity or ketone bodies.
Liability in Europe
According to the EU Product Liability Directive, the manufacturer of a device is liable for the device and should ensure that it is working according to the product’s specifications, as formalised in the CE (Conformité Européenne) mark and the instructions for use (IFU) (per EU Council Directive 85/374/EEC of 1985 on the approximation of the laws, regulations and administrative provisions of the Member States concerning liability for defective products [10]. The labelling or IFU is defined in the MDR as the ‘information provided by the manufacturer to inform the user of a device’s intended purpose and proper use, and of any precautions to be taken’, and the manufacturer is defined as the ‘natural or legal person who manufactures or fully refurbishes a device or has a device designed, manufactured or fully refurbished, and markets that device under its name or trademark’ [5]. However, in terms of liability, the PLWD who uses the device also has a degree of responsibility in that they are expected to use the device according to the IFU provided by the manufacturer. Therefore, these instructions need to be clear, transparent and understandable. Some manufacturers provide specific warnings to intended users in case of misuse or modifications of a device, such as ‘modifying the devices can cause serious injury, interfere with your ability to operate the device and void your warranty’ [11, 12].
The EU’s position on interoperability should also be considered in the context of digital health, wherein the EU acknowledges the need for digital tools to support PLWD-centred care. However, many discussions about digital health and future directions are also ongoing within the EU, and the use of any digital tools should also consider the current data privacy protection guidelines (GDPR) and the corresponding data privacy provisions of the UK Data Protection Act 2018, which is the UK’s version of the GDPR [13]. Any exchange of information from PLWD will require a European EMR to be established, which in turn will require data interoperability specifications to be met, and only when data can be assessed in a standardised manner can the data generated by diabetes technology systems be successfully integrated into EMRs. However, a key component of the GDPR is the ‘right to be forgotten’ [14], which raises the questions of what happens to an individual’s data if that individual wishes to stop using a given AID system and whether this information can be appropriately deleted. Data are supposed to be stored on servers located in the EU only. From our point of view, all data generated in the context of this therapy are owned by the individual PLWD and not the manufacturer of a given device. PLWD should therefore have access to the data stored by the manufacturer and the opportunity to have these data deleted.
In Europe, several legislative initiatives have been designed to both ease and regulate access to data; these include the Artificial Intelligence Act [15], the Data Act [16] and the creation of the European Health Data Space [17]. The European Health Data Space, specifically, once formally adopted, will provide a health-specific ecosystem comprising rules, common standards and infrastructures. It will also include a governance framework that aims to empower individuals through increased digital access to and control of their electronic personal health data. This approach can work at both a national level and an EU-wide level and will support the free movement of data. Moreover, it will also foster a genuine single market for EMR systems, relevant medical devices and high-risk artificial intelligence systems. Finally, the availability of information in all EU languages, in accordance with EU regulations, will enable data searching by all individuals across the EU, providing a consistent, trustworthy and efficient set-up for using health data for research, innovation, policymaking and regulatory activities.
Regulatory framework on interoperability in the USA
The FDA has been highly supportive of the development of diabetes technology devices, beginning with detailed guidance introduced in 2012 [18]. This guidance covered CGM systems, primary endpoints that can be used to determine safety and effectiveness, indications for use, and clinical study progression and design. Moreover, the 2012 guidance explicitly notes that the intention is to apply the ‘least burdensome’ approach to investigating and developing AID systems and making them available to PLWD. The FDA has also encouraged interoperability among available insulin pumps, CGM systems and other diabetes devices, with the specification in 2018 of an integrated CGM (iCGM) regulatory pathway for CGM systems [19]. In 2019 the FDA authorised the first interoperable insulin pump, also known as an alternate controller-enabled (ACE) insulin pump, which would allow PLWD to customise treatment through their diabetes management devices. This designation meant that an ACE insulin pump could be used with different components that make up diabetes therapy systems [20]. In 2023 the FDA approved the first interoperable automated glycaemic controller app [21]. Connected diabetes devices allow PLWD to tailor their diabetes management according to their preferences for devices.
Additionally, the FDA is also able to grant clearance for different device enhancements and new products, which is designed to ease the burden on clinical practices and allow for greater PLWD-centred care and self-management. Such features can also help PLWD to make better decisions about how and when to treat their diabetes, reduce some of the challenges associated with glucose management and reduce the burden of living with diabetes.
Cybersecurity
In 2022, the Institute of Electrical and Electronics Engineers (IEEE) Standards Association in the USA completed IEEE 2621, a standard for wireless diabetes device security assurance. This is the first and only medical device cybersecurity standard that comprehensively evaluates performance claims by manufacturers. It is focused on wireless diabetes devices and supports interoperability. This standard includes a certification programme built with industry consensus to ensure that IEEE-certified products continuously demonstrate conformance to the standard [22]. The standard was officially recognised by the FDA in 2022 [23]. No such standards exist in the EU.
Data ownership, privacy and security
Even though HCPs often use the data generated by medical devices and, while these are invaluable in terms of generating RWE, it is important to remember that the data are owned by the PLWD, and it is the PLWD who ultimately determines the extent to which the data are shared and can be used. That is, it is the user who consents to whether the data can be used for personal health management, research, product development or marketing. In some cases, and from a legal perspective, some may argue that questions from companies regarding data use are not straightforward, nor are the potential usages of data asked about separately. Some AID devices send data directly via a Subscriber Identification Module (SIM) card without the PLWD doing anything to the upload device; there is one AID system on the market that requires the user to be notified. To ensure essential understanding, data use questions should explicitly state the purpose and potential area of data usage. Furthermore, PLWD should be allowed to respond 'yes' or 'no' to each question. Currently, companies may ask for data for research purposes; however, no ethical approval is needed for such data use.
For data emanating from single or combined devices, security, privacy and data protection are of fundamental importance, particularly with the trend among manufacturers to use the data in RWE studies. The use of RWE is gaining increasing recognition in both clinical and political decision-making (by policymakers, regulators and payers), given that RWE studies often involve large datasets and include data from all people who have consented for their data to be used, which usually represents a more diverse cohort than those who meet inclusion criteria for clinical trials [24]. The advantage of RWE studies is that PLWD who are ineligible for clinical trials because of the inclusion/exclusion criteria (age, diagnosis of dementia, HbA1c cut-off values, prone to hypoglycaemia, presence of comorbidities) are included in such datasets. Such studies therefore provide valuable insight into the effectiveness of devices in a real-world environment. However, a key question regarding RWE relates to whether device users know how their data will be used. Users are likely to indicate a willingness to donate their data for research purposes, but it should be noted that the consent should be accompanied by information on data usage. Ethics committee approvals for large datasets that ensure privacy and anonymity are not commonly required for such studies.
Other concerns regarding data privacy relate to the potential use of data by entities such as insurance companies or courts of law. For example, if data from individuals were accessible to insurance companies, would these companies be able to modify coverage? Additionally, could users refuse to share their data with HCPs if CGM data are identifiable? What would be the implications of such a refusal to share data?
Impact of device interoperability on the device marketplace
Consumer and market forces influence interoperability; for example, a key incentive for component interoperability is the user’s ability to select specific components based on merit or reviews/opinions expressed online or on social media. Similar to other industries, the user may choose between different systems, supporting the need for different components to be available. While AID systems are considered medical devices first and foremost, they are also devices that the user has to interact with frequently during the day. In this way, AID systems may also be considered consumer devices. Therefore, providing users with a choice of components that best suits their lifestyles and wishes may help with ongoing engagement and use of the system to achieve therapeutic goals. This choice can be considered analogous to assembling a desktop computer with a choice of components compared with buying a fully assembled desktop. PLWD may want to choose a pump, an algorithm and a CGM based on their preferences, or a fully assembled system. It should be noted that neither option has been shown to be better than the other.
Interoperability between the different components needed to establish an AID system is a key hurdle for individuals who build their own systems. Such open-source AID systems are not addressed in detail here; however, their existence is in part due to the requirement for interoperability from PLWD. In the last few years a number of articles have been published that support some of the fundamental considerations presented here [25, 26].
Summary and conclusions
Transparent guidelines/standards developed using a stepwise approach should help ensure that innovations can be approved while also accounting for data ownership, safety of the devices used, and data safety and privacy. An open and transparent standard for data handling remains to be established, with the solution needing to fulfil the requirements of the reality of routine clinical practice. Only when data can be assessed in a standardised manner can the data generated by AID systems be integrated into EMRs. There is a need for an international committee on worldwide interoperability regulations; this role could be performed by an initiative of the WHO/IDF.
Through the combined efforts of many stakeholders (including legislative, regulatory and clinical stakeholders and medical device manufacturers), considerable progress has been made in formulating practical solutions for data and device interoperability in medical devices and data used in diabetes therapy. However, there is a need for professional diabetes associations and those directly involved in the care of PLWD to improve their understanding of the requirement to consent to analysis of their data. Furthermore, it is also important to make PLWD aware of how their data may be used with different degrees of anonymity and security, for example by hospitals, insurance companies and law enforcement agencies.
Finally, given the rapid pace of development of technology used in the management of PLWD, a regular update on data and device interoperability appears advisable.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- ACE
Alternate controller-enabled
- AID
Automated insulin delivery
- API
Application programming interface
- CGM
Continuous glucose monitoring
- EMR
Electronic medical record
- EMA
European Medicines Agency
- FDA
US Food and Drug Administration
- GDPR
General Data Protection Regulation
- HCP
Healthcare professional
- iCGM
Integrated continuous glucose monitoring
- iCoDE
Integration of Continuous Glucose Monitoring Data into the Electronic Health Record
- IEEE
Institute of Electrical and Electronics Engineers
- IFU
Instructions for use
- MDR
Medical Device Regulation
- PLWD
People living with diabetes
- RWE
Real-world evidence
- VDC
Virtual diabetes clinic
Acknowledgements
The authors would like to thank O. Cohen and S. de Portu (Medtronic) for their administrative support.
Funding
Open access funding provided by Örebro University. Medtronic commissioned and funded medical writing support. J. Smith-Palmer (Covalence Research, UK) produced the first draft of this article. The authors received no honoraria.
Authors’ relationships and activities
JJ has been a lecturer/member of the scientific advisory boards at the following companies: Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Medtronic, Nordic InfuCare, NovoNordisk and Sanofi. PA has received speaker’s honoraria from Dexcom, Eli Lilly, Insulet, NovoNordisk, Sanofi and Tandem. He has received consulting fees from and/or held advisory positions with Dexcom, Eli Lilly, Medtronic, NovoNordisk and Roche. PC has received personal fees from Medtronic, Abbott, Dexcom, Lilly, Sanofi, NovoNordisk, Glooko, DreaMed and MannKind. AF is the Executive Chairman of Kinexum, which advises multiple health product companies, including multiple insulin manufacturers. Relevant device companies advised include Abbott, Biolinq, CMC Magnetics, Diabeloop, Hagar, Know Labs, Modular Medical, SFC Fluidics and Surf Bio. He was formerly the Group Leader of the Division of Metabolism and Endocrine Drug Products at the US Food and Drug Administration. DCK is a consultant for Better Therapeutics, EOFlow, Integrity, Lifecare, Nevro, Novo Nordisk, Rockley Photonics and Sanofi. JKM is a member of the advisory boards of Abbott Diabetes Care, Becton Dickinson, Boehringer Ingelheim, Eli Lilly, Embecta, Medtronic, NovoNordisk, Roche Diabetes Care, Sanofi-Aventis and Viatris and has received speaker honoraria from A. Menarini Diagnostics, Abbott Diabetes Care, AstraZeneca, Boehringer Ingelheim, Dexcom, Eli Lilly, Medtrust, MSD, NovoNordisk, Roche Diabetes Care, Sanofi, Servier and Ypsomed. She is a shareholder of decide Clinical Software GmbH and elyte diagnostics, where she also serves as Chief Medical Officer. KD served on the advisory boards of Novo Nordisk, Pfizer and Sanofi. KD has also received honoraria for participation in the speaker’s bureaus of Abbott, Eli Lilly, Medtronic, NovoNordisk and Pfizer. NO has participated on the advisory boards of Medtronic, Roche Diabetes Care and Dexcom. He has received speaker fees from Dexcom and research funding from Medtronic Diabetes, Roche Diabetes Care and Dexcom. JLS has conducted clinical trials for Eli Lilly, Insulet, Medtronic and Provention Bio and has received in-kind support for research studies from Dexcom and Medtronic. She has consulted for and/or received speaker honoraria from Eli Lilly, Lexicon, Insulet, Medtronic and Zealand. She has been a member of the advisory boards of Bigfoot Biomedical, Cecelia Health, Eli Lilly, Insulet, StartUp Health’s T1D Moonshot, the T1D Fund and Vertex. JŠ has received speaker honoraria from and consulted for Abbott, Dexcom, Eli Lilly, Medtronic, NovoNordisk and Roche. LH holds shares in the Profil Institute for Metabolic Research, Neuss, Germany. LH is also a consultant for embecta, Lifecare and Roche Diabetes Care.
Contribution statement
All authors were responsible for drafting the article and reviewing it critically for important intellectual content. All authors approved the version to be published.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xu NY, Nguyen KT, DuBord AY, et al. The launch of the iCoDE Standard project. J Diabetes Sci Technol. 2022;16(4):887–895. doi: 10.1177/19322968221093662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espinoza J, Xu NY, Nguyen KT, Klonoff DC. The need for data standards and implementation policies to integrate CGM data into the electronic health record. J Diabetes Sci Technol. 2023;17(2):495–502. doi: 10.1177/19322968211058148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeung AM, Huang J, Klonoff DC, et al. iCoDE June 22, 2022 steering committee meeting summary report. J Diabetes Sci Technol. 2022;16(6):1575–1576. doi: 10.1177/19322968221119146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Union (2017) Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Available from https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32017R0745&from=IT#d1e1058-1-1 Accessed 3 Jan 2023
- 6.Vlahou A, Hallinan D, Apweiler R, et al. Data sharing under the General Data Protection Regulation: time to harmonize law and research ethics? Hypertension. 2021;77(4):1029–1035. doi: 10.1161/HYPERTENSIONAHA.120.16340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tom E, Keane PA, Blazes M, et al. Protecting data privacy in the age of AI-enabled ophthalmology. Transl Vis Sci Technol. 2020;9(2):36. doi: 10.1167/tvst.9.2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Commission (2023) National competent authorities. Available from https://health.ec.europa.eu/medical-devices-sector/new-regulations/contacts_en. Accessed 8 Mar 2023
- 9.MedTech Europe (2020) Impact of changes under the new EU Medical Devices Regulation (EU) 2017/745 to international registrations. Available from https://www.medtecheurope.org/wp-content/uploads/2020/05/20200526_Impact_Changes_Int_Reg_MedicalDevices_MDR.pdf. Accessed 13 Sep 2022
- 10.European Union (1985) Council Directive 85/374/EEC of 25 July 1985 on the approximation of the laws, regulations and administrative provisions of the Member States concerning liability for defective products. Available from https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A31985L0374. Accessed 13 Sep 2022
- 11.Sherr JL, Heinemann L, Fleming GA, et al. Automated insulin delivery: benefits, challenges, and recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association. Diabetologia. 2023;66(1):3–22. doi: 10.1007/s00125-022-05744-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Commission (2022) Directive of the European Parliament and of the Council on liability for defective products. Available from https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52022PC0495. Accessed 15 Oct 2023
- 13.UK Data Protection Act (2018) Available from https://www.legislation.gov.uk/ukpga/2018/12/contents/enacted. Assessed 15 Oct 2023
- 14.European Union (2016) Regulation (EU) 2016/679 of the European Parliament and of the council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation). Available from https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32016R0679&from=EN#d1e2606-1-1. Accessed 8 Mar 2023
- 15.European Union (2021) Proposal for a regulation of the European Parliament and of the Council laying down harmonised rules on artificial intelligence (Artificial Intelligence Act) and amending certain Union legislative acts. Available from https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52021PC0206. Accessed 8 Mar 2023
- 16.European Commission (2022) Data Act: Proposal for a Regulation on harmonised rules on fair access to and use of data. Available from https://digital-strategy.ec.europa.eu/en/library/data-act-proposal-regulation-harmonised-rules-fair-access-and-use-data. Accessed 8 Mar 2023
- 17.European Commission (2023) European Health Data Space. Available from https://health.ec.europa.eu/ehealth-digital-health-and-care/european-health-data-space_en#more-information. Accessed 8 Mar 2023
- 18.US Food and Drug Administration (2012) Guidance for Industry and Food and Drug Administration Staff. The content of investigational drug exemption (IDE) and premarket approval applications for artificial pancreas systems. Available from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/content-investigational-device-exemption-ide-and-premarket-approval-pma-applications-artificial. Accessed 25 Aug 2022
- 19.US Food and Drug Administration (2018) FDA news release: FDA authorizes first fully interoperable continuous glucose monitoring system, streamlines review pathway for similar devices. Available from https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-fully-interoperable-continuous-glucose-monitoring-system-streamlines-review. Accessed 6 Jan 2023
- 20.US Food and Drug Administration (2019) Food and Drug Administration News Release. FDA authorizes first interoperable insulin pump intended to allow patients to customize treatment through their individual diabetes management devices. Available from https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-interoperable-insulin-pump-intended-allow-patients-customize-treatment-through. Accessed 6 Mar 2023
- 21.Tidepool. Tidepool announces FDA clearance of Tidepool Loop. Available from https://www.businesswire.com/news/home/20230124006085/en/Tidepool-Announces-FDA-Clearance-of-Tidepool-Loop. Accessed 16 Oct 2023
- 22.Institute of Electrical and Electronics Engineers (2023) Medical devices cybersecurity – IEEE 2621 series of standards. Available from https://standards.ieee.org/products-programs/icap/programs/ieee-2621-standards/. Accessed 6 Mar 2023
- 23.US Food and Drug Administration (2022) Recognized consensus standards: medical devices. IEEE Std 2621.2-2022 Standard for Wireless Diabetes Device Security: information security requirements for connected diabetes solutions. Available from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfStandards/detail.cfm?standard__identification_no=43895. Accessed 6 Mar 2023
- 24.Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763–1774. doi: 10.1007/s12325-018-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braune K, Lal RA, Petruzelkova L, et al. Open-source automated insulin delivery: international consensus statement and practical guidance for health-care professionals. Lancet Diabetes Endocrinol. 2022;10(1):58–74. doi: 10.1016/S2213-8587(21)00267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimentstein K, Sosenko JM, Goodman KW. Do-it-yourself diabetes management: perspectives of a patient, a physician, and an ethicist. Clin Diabetes. 2022;40(1):70–74. doi: 10.2337/cd20-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medical Device Regulation (2023) MDR – Article 2 – definitions: interoperability. Available from https://www.medical-device-regulation.eu/2019/07/10/mdr-article-2-definitions/. Accessed 17 Oct 2023
- 28.Healthcare IT skills (2018) What is interoperability in healthcare IT? Available from https://healthcareitskills.com/what-is-interoperability-in-healthcare-it/#:~:text=Interoperability%20is%20the%20ability%20for,that%20is%20useful%20to%20clinicians. Accessed 3 Jan 2023
- 29.Klonoff DC, Kerr D. Smart pens will improve insulin therapy. J Diabetes Sci Technol. 2018;12(3):551–553. doi: 10.1177/1932296818759845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinemann L, Jendle J. Language matters: connected pens, smart pens, connected smart pens, or just digital pens? J Diabetes Sci Technol. 2023 doi: 10.1177/19322968221148508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen KT, Xu NY, Zhang JY, et al. Continuous ketone monitoring consensus report 2021. J Diabetes Sci Technol. 2022;16(3):689–715. doi: 10.1177/19322968211042656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adolfsson P (2022) Smart futures: creating a digital ecosystem for diabetes. A digital dawn for diabetes: how data can transform patient care (Industry Symposium oral presentation, supported by Novo Nordisk). 15th International Conference on Advanced Technologies & Treatments for Diabetes, 27–30 April, Barcelona, Spain. 2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.