Abstract
Genomic DNA fingerprint analysis was performed on 39 Staphylococcus aureus and 28 Enterococcus faecalis endophthalmitis isolates collected from multiple clinical centers. Among 21 S. aureus genomic DNA fingerprint patterns identified, five clonotypes were recovered from multiple unrelated patients and accounted for 58.9% (23 of 39) of the isolates analyzed. Compared with strains having unique genomic DNA fingerprint patterns, the S. aureus clonotypes occurring more than once were more likely to result in visual acuities of 20/200 or worse (P = 0.036 [χ2 test]). In contrast to the S. aureus isolates, the E. faecalis endophthalmitis isolates were a clonally diverse population, enriched for the expression of a known toxin, cytolysin, which is plasmid encoded.
Infectious endophthalmitis is a sight-threatening clinical crisis that occurs as a complication of ocular surgery (postoperative endophthalmitis) or penetrating ocular injury (posttraumatic endophthalmitis). The severity of vision loss in endophthalmitis is related to the pathogenic potential of the infecting organism (7, 19, 25–29, 33). Coagulase-negative staphylococci are generally associated with final visual acuities of 20/40 or better, whereas in endophthalmitis caused by more virulent organisms such as Staphylococcus aureus, Enterococcus faecalis, or Bacillus cereus, visual outcomes ranging from 20/100 to enucleation occur in approximately 50 to 90% of cases (1, 5, 7). Despite a general association between visual outcome and the infectious agent, relatively little is known about the species-specific factors that account for the characteristic severity of each disease.
We previously focused on determining the role of secreted bacterial toxins in the pathogenesis of endophthalmitis caused by S. aureus and E. faecalis by using well-characterized laboratory strains in animal models of disease. These studies showed that cytolytic E. faecalis not only causes more fulminant disease but also renders the infection unresponsive to therapeutic intervention (17, 18). The production of most secreted and cell surface proteins in S. aureus is coordinately controlled by chromosomal regulatory loci termed accessory gene regulator (agr) and staphylococcal accessory regulator (sar) (6, 16, 20). Mutant strains of S. aureus with insertional mutations in the sar and agr loci are attenuated in virulence in experimental endophthalmitis compared with parental strains (3, 4). Since sar and agr affect the expression of 12 or more unrelated genes (6, 20), the staphylococcal toxin(s) that contributes most significantly to the severity of disease has not yet been identified.
To further analyze the bacterial factors that contribute to the pathogenesis of endophthalmitis, we performed a genomic DNA fingerprint analysis on 39 S. aureus and 28 E. faecalis strains isolated from the vitreous or aqueous humor of endophthalmitis patients treated at multiple clinical centers. The purpose of this investigation was to assess whether common traits that may be related to ocular colonization and/or the severity of disease outcome exist among isolates of a particular species.
The S. aureus and E. faecalis isolates analyzed in this study were collected from patients with endophthalmitis between 1984 and 1995 at Cullen Eye Institute, Houston, Tex. (CE), Dean A. McGee Eye Institute, Oklahoma City, Okla. (DM), University of Pittsburgh School of Medicine, Pittsburgh, Pa. (UP), King Fahd Hospital, Al Hasa, Saudi Arabia (KF) (a kind gift from LouAnn Bartholomew), and Bascom Palmer Eye Institute, Miami, Fla. (BP). S. aureus strains were collected from DM (7 isolates), UP (7 isolates), and BP (25 isolates), while E. faecalis strains were collected from CE (10 isolates), DM (3 isolates), UP (4 isolates), KF (2 isolates), and BP (9 isolates). Twenty-nine additional S. aureus clinical isolates of extraocular origin were a kind gift from Mark Huycke, Veterans Administration Medical Center, Oklahoma City, Okla. Twenty-one S. aureus keratitis isolates were obtained from the Alcon Microbiology Culture Collection (Fort Worth, Tex.).
Pulsed-field gel electrophoretic analysis of endophthalmitis isolates.
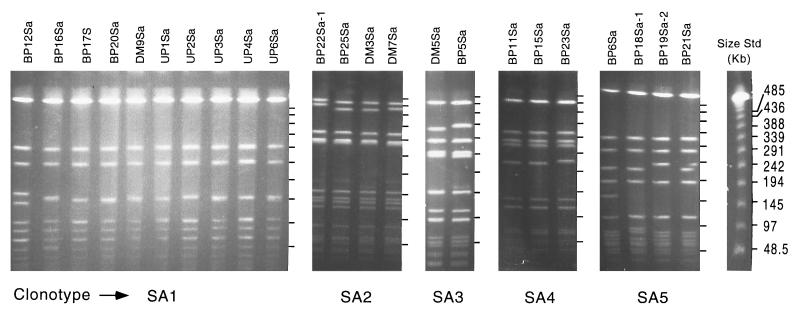
Bacterial genomic DNA was prepared as previously described (24), except that lysostaphin (50 μg/ml) was added to the lysis solution for the preparation of S. aureus chromosomal DNA. Isolates with similar banding patterns and no more than three band differences were considered clonally related (32). Isolates with banding patterns similar to clonally related strains but with no more than four band differences were considered subtypes of the clonal group. Once isolates were recognized as having identical or similar banding patterns, a second gel containing all isolates from the same group was run to verify clonal relationships. Twenty-one distinct fingerprint patterns were identified among the S. aureus isolates. Of these, five clonotypes were present more than once and accounted for 58.9% (23 of 39) of the total number of isolates. The clonotype represented most frequently was designated SA1 and accounted for 25.6% (10 of 39) of the isolates tested (Fig. 1). Isolates in this group were derived from each of the clinical centers from which S. aureus isolates were obtained (DM, UP, and BP). Clonotypes SA2 (n = 4) and SA3 (n = 2) were also derived from multiple clinical centers (DM and BP). All isolates comprising clonotypes SA4 (n = 3) and SA5 (n = 4) were derived from the same clinical center (BP) (Fig. 1). The remaining 16 isolates (41%) were present only once (data not shown) and were derived from all three clinical centers. To ensure that the general clonality observed among the S. aureus endophthalmitis isolates was not attributable to a methicillin-resistant S. aureus (MRSA) genotype (21), strains comprising each of the five S. aureus clonotypes were analyzed for the presence of the mecA antibiotic resistance determinant (8). Briefly, bacteria from a 0.5-ml suspension of bacterial cells in phosphate-buffered saline were lysed by boiling in a sealed tube for 10 min, followed by centrifugation (10,000 × g for 1 min) to remove cell debris. PCR was performed on cell lysates with previously published mecA-specific primers (8). Only clonotype SA4 (three isolates), was found to be mecA positive; all other clonotypes were mecA negative.
FIG. 1.
PFGE of SmaI-digested chromosomal DNA of endophthalmitis-derived S. aureus clinical isolates collected from three clinical centers, BP, DM, and UP. Separate panels show clonally related isolates. Where present, subtypes are designated with the suffix 1 or 2. Molecular size standards are the New England Biolabs lambda ladder.
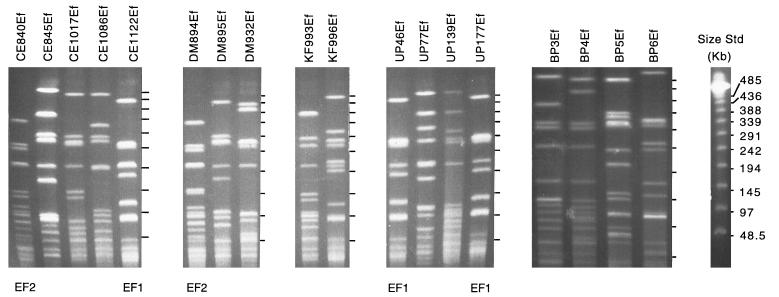
In contrast to the S. aureus isolates, substantial clonal diversity was observed among the E. faecalis isolates. Of the 28 isolates collected from five clinical centers (CE, DM, UP, KF, and BP), 25 unique genomic DNA fingerprints were identified. Two E. faecalis clonotypes, EF1 (n = 3) and EF2 (n = 2), occurred more than once (Fig. 2). EF1 isolates were derived from either UP (two isolates) or CE (one isolate), while EF2 isolates were derived from CE and DM. The remaining 23 E. faecalis endophthalmitis isolates had unique genomic DNA fingerprints.
FIG. 2.
PFGE of SmaI-digested chromosomal DNA of selected endophthalmitis-derived E. faecalis clinical isolates collected from five clinical centers, BP, DM, UP, KF, and CE. Clonally related strains are identified below the panels. Molecular size standards are the New England Biolabs lambda ladder.
Comparison of S. aureus endophthalmitis clonotypes with S. aureus isolated from various sources.
Since it was determined that the general clonality observed among the endophthalmitis-derived S. aureus isolates was not due to an MRSA genotype, it was considered that the clonotypes identified might represent species subsets uniquely associated with ocular infection. To test this hypothesis, we examined chromosomal DNA fingerprints for 21 S. aureus keratitis isolates and 29 S. aureus strains isolated from extraocular infections, such as soft-tissue, catheter-associated, and surgical-wound infections. The frequency of occurrence of clonotypes SA1 to SA5 within these populations of isolates is shown in Table 1. Clonotypes SA1, SA3, SA4, and SA5 were found among the S. aureus keratitis isolates and accounted for 47.6% (10 of 21) of the total number of isolates analyzed; the remaining isolates in this group all showed unique genomic DNA fingerprints. Interestingly, as in the case of endophthalmitis-derived clonotypes SA1, SA2 and SA3, keratitis-derived clonotypes SA1, SA3, SA4, and SA5 were collected from geographically diverse clinical centers (6a). All five endophthalmitis-derived S. aureus clonotypes occurred at least once among the extraocular-infection isolates. One new clonotype consisting of three strains (SA6), which was not represented among either the endophthalmitis- or keratitis-derived isolates, was identified among the soft-tissue-wound isolates. The frequency of occurrence of clonotype SA1 among the extraocular-infection isolates was approximately 2.5-fold lower than that observed for the endophthalmitis isolates (10% versus 25.6%); however, the difference was not statistically significant (P = 0.136 [Fisher’s exact test]). When analyzed in combination, the frequency of occurrence of clonotypes SA1 to SA5 was not significantly different between the groups (P = 0.340 [χ2 test]). These results indicate that although clonotypes SA1 to SA5 are isolated frequently from ocular infections, these isolates are not unique to this site of infection.
TABLE 1.
Frequency of S. aureus clonotypes SA1 to SA5 among isolates from various sources
| Clonotype | No. (%) of S. aureus isolates from:
|
||
|---|---|---|---|
| Endophthalmitis (n = 39) | Keratitis (n = 21) | Soft-tissue wounds (n = 29) | |
| SA1 | 10 (25.6) | 2 (9.5) | 3 (10.3) |
| SA2 | 4 (10.2) | 0 | 4 (13.7) |
| SA3 | 2 (5.1) | 2 (9.5) | 2 (3.4) |
| SA4 | 3 (7.6) | 3 (19.0) | 1 (3.4) |
| SA5 | 4 (10.2) | 3 (9.5) | 2 (6.8) |
| SA6 | 3 (10.32) | ||
| Total (clonal) | 23 (58) | 10 (48) | 15 (52) |
| Other isolates (nonclonal) | 16 (41) | 11 (52) | 14 (48) |
Frequency of cytolysin expression among E. faecalis endophthalmitis isolates.
The frequency of the cytolytic genotype among the E. faecalis isolates was determined by performing PCR on bacterial cell lysates with primers specific for cylA, the proteolytic activator gene of the E. faecalis cytolysin operon. The following oligonucleotide primers were selected from published sequences: 5′ AAT GGA TAA TAT TTC AGA ATT TGA AGT 3′ (cylA1) and 5′ TTC CCA CGA AAA TTT TAT AAA CCC 3′ (cylA2) (9). Briefly, a suspension of each isolate was prepared by removing bacterial colonies from an overnight plate culture with a moistened sterile swab and resuspending them in 1 ml of sterile 10 mM Tris, pH 7.5. Bacteria from 0.5 ml of the suspension were lysed with a Mini-Beadbeater (Biospec Products, Bartlesville, Okla.) according to the manufacturers instructions. One hundred fifty microliters of the lysate was removed to a clean tube and centrifuged (10,000 × g for 1 min) to remove cell debris. PCR was performed in 10-μl reaction mixtures containing 1 μl of cell lysate, 1 μl of 3 mM MgSO4, 1 μl of cylA1, 1 μl of cylA2 (10 μM each in sterile H2O), 1 μl of diluted Taq polymerase (diluted 1:12.5), 1 μl of 2 mM deoxynucleotide triphosphates, and 4 μl of H2O in a Rapidcycler PCR machine (Idaho Technologies, Idaho Falls, Idaho). Following an initial hold step (94°C for 30 s), the PCR mixtures were cycled 30 times as follows: denaturation, 94°C for 0 s; annealing, 50°C for 0 s; and elongation, 72° for 35 s. An additional hold step of 72°C for 2 min was included at the end of the 30 cycles. The cytolytic phenotype was confirmed by observing zones of hemolysis on brain heart infusion agar plates containing 5% rabbit blood incubated for 2 days at 37°C. E. faecalis FA2-2 (pAM714) and plasmid-free E. faecalis FA2-2 were used as positive and negative controls, respectively, for the detection of both cytolytic phenotype and genotype (14). Of the 28 E. faecalis endophthalmitis isolates collected for this study, 13 (46.4%) possessed the cylA gene, and all cylA-positive strains were phenotypically positive for cytolysin expression as indicated by zones of hemolysis on brain heart infusion agar. This represents an enrichment for the cytolytic phenotype among endophthalmitis isolates compared with its occurrence among isolates from the gastrointestinal tracts of healthy subjects (0 to 17%; P < 0.028 [χ2 test]) (12, 15). All isolates comprising both EF1 and EF2 clonotypes were cytolytic.
Relationship between clonality of endophthalmitis isolates and final visual outcome.
In the present study, a number of S. aureus clonotypes which are known to have caused endophthalmitis in multiple, apparently unrelated cases were identified. Therefore, it was of interest to determine whether a correlation between strains of S. aureus and disease outcome exists. The severity-of-outcome measure used in this study was final best-corrected visual acuity achieved following treatment for endophthalmitis and was ascertained following a retrospective review of patient records. The range of final visual acuities observed was in agreement with that found in a previous series for S. aureus endophthalmitis: 20/40 or better, 30.7%; 20/100 or better, 48.7%; 5/200 or better, 64% (7). The relationship between final best-corrected visual acuity and the clonality of the endophthalmitis-derived isolates was analyzed by Pearson’s chi square (χ2) test. Due to the wide range of visual acuities recorded in patient charts, only two levels of severity were analyzed: better than 20/200 and 20/200 or worse. Clonotypes were analyzed either individually (when adequate numbers of isolates made up the group) or in combination. All isolates that occurred more than once were designated “clonal” and all those occurring only once were designated “nonclonal.” Table 2 shows the distribution of isolates comprising each clonotype for each level of severity. Visual acuities of 20/200 or worse were found in 70%, 50%, 50%, 66%, and 75% of cases infected with clonotypes SA1, SA2, SA3, SA4, and SA5, respectively, compared with 31% of the nonclonal isolates (for SA1 versus nonclonal isolates, P was 0.053 [χ2 test]). When clonal isolates were combined (SA1 to SA5; n = 23) and compared for severity of outcome with isolates occurring only once (nonclonal isolates; n = 16), it was found that a statistically significant relationship existed between clonality and visual outcomes of 20/200 or worse (P = 0.036 [χ2 test]). These results suggest that clonotypes SA1 to SA5 not only possess traits that enhance ocular colonization, thereby favoring their occurrence at this site, but also possess traits that contribute to poor visual outcomes following intraocular infection.
TABLE 2.
Correlation between severity of outcome and S. aureus endophthalmitis clonotype
| Clonotype (n) | Severity of outcome (no. [%] of patients)
|
Pb | |
|---|---|---|---|
| 20/200 or worsea | Better than 20/200a | ||
| SA1 (10) | 7 (70) | 3 (30) | 0.054 |
| SA2 (4) | 2 (50) | 2 (50) | ND |
| SA3 (2) | 1 (50) | 1 (50) | ND |
| SA4 (3) | 2 (66) | 1 (33) | ND |
| SA5 (4) | 3 (75) | 1 (25) | ND |
| Total (clonal) (23) | 15 (65) | 8 (35) \ | 0.036 |
| Other isolates (nonclonal) (16) | 5 (31) | 11 (69) / | |
Final best-corrected visual acuity.
By Pearson’s chi square test. ND, not done (the numbers of isolates in these groups fall below the threshold for Pearson’s chi square test).
Clinical data were available for 20 of the 28 E. faecalis isolates. Of these, 15 (75%) had outcomes of 20/200 or worse and 5 (25%) had outcomes better than 20/200, confirming observations of poor visual outcome associated with most cases of enterococcal endophthalmitis (7). Since most of the E. faecalis endophthalmitis cases were associated with poor outcomes, no enrichment in the cytolytic phenotype was observed among the severe outcome group. Specifically, of isolates associated with severe outcome, eight (53.3%) were cytolytic and seven were noncytolytic (46.6%). In the better-than-20/200 outcome group, two were cytolytic and three were noncytolytic (P = 0.605 [χ2 test]). For clonotype EF1 (n = 3), two isolates were associated with visual outcomes of 20/200 or worse. No clinical information was available for the third EF1 isolate. For clonotype EF2 (n = 2), one was associated with a visual outcome of 20/200 or worse and the other with a visual outcome of better than 20/200. Therefore, no correlation between clonotype and severity of visual outcome was observed in this study. Interestingly, two of the E. faecalis isolates (CE200Ef and CD695Ef) were collected 4 years apart from the same patient presenting with separate episodes of an infected filtering bleb. The two isolates were shown not only to be distinct by pulsed-field gel electrophoresis (PFGE) (data not shown) but also to be cytolytic in one case and noncytolytic in the other. On both occasions, final best-corrected visual acuities of better than 20/200 were achieved. This finding suggests that in this particular case, factors unrelated to the infectious agent may have been important in determining the outcome of the endophthalmitis.
Two studies have analyzed genomic DNA fingerprint patterns of bacterial endophthalmitis clinical isolates (2, 31). In these cases, Staphylococcus epidermidis was either the predominant or the only species examined. In one study, unique fingerprints were found for all S. epidermidis isolates (11 isolates) analyzed. The second study compared genomic DNA fingerprints of 105 S. epidermidis strains isolated from endophthalmitis patients at several clinical centers in the United States. With the exception of three strains that were isolated from two patients each, unique banding patterns were observed for all isolates from any given clinical center. This contrasts with the substantial degree of clonality observed in the present study for S. aureus endophthalmitis isolates. In both S. aureus and S. epidermidis endophthalmitis, a likely source for the infecting organism is the periocular skin, eyelid margins, or nares (22, 31). With PFGE used to identify clonal relationships between strains, it was recently shown that nasal colonization patterns by S. aureus and S. epidermidis differ (10, 11). In the case of S. aureus, the same strain was observed to persistently colonize the host for periods of at least 2 years, while predominant S. epidermidis strains colonizing the nares were observed to change frequently, with the same organism persisting for less than 5 months. These data suggest that S. aureus exists stably at the nasal mucosal surface under environmental selection pressure that favors the persistence of particular strains, perhaps due to highly efficient adherence or clearance avoidance mechanisms. This selection for particular strain types may be reflected in the incidence of S. aureus isolates that cause endophthalmitis. Since nasal and ocular mucosal surfaces are continuous through the nasolacrimal duct, the same colonization mechanisms may be important for the establishment of ocular infection and nasal colonization. Recent studies have described cell surface proteins that mediate the binding of S. aureus to nasal mucin (30). It would be of interest to determine whether clonotypes SA1 to SA5 express similar mucosal surface binding proteins. Clonotypes SA1 to SA5 were also observed to occur multiple times among the keratitis-derived isolates, supporting the suggestion that these clonotypes possess colonization traits that enhance their abilities to establish ocular infection. However, the finding that SA1 to SA5 also occur among nonocular soft-tissue-infection isolates indicates that SA1 to SA5 clonotype strains possess traits favoring colonization of extramucosal sites as well.
When the relationship between clonality and final visual outcome was analyzed, S. aureus clonotypes SA1 to SA5 were found to be significantly associated with final best-corrected visual acuities of 20/200 or worse. This finding suggests that these strains possess not only characteristics that enhance ocular colonization but also traits that lead to loss of organ function. Because toxin production by S. aureus was previously shown to be related to the severity of endophthalmitis in animal models (3, 4), current studies are assessing the profiles of toxins expressed by clonotypes SA1 to SA5 to determine whether a particular toxin(s) is selectively expressed by these strains.
Substantial clonal diversity was observed among E. faecalis strains, suggesting that no particular E. faecalis genomic DNA fingerprint type is more likely than another to cause ocular infection. However, we observed an enrichment for cytolysin expression among the E. faecalis isolates over the raw incidence of its occurrence among isolates derived from the gastrointestinal tracts of healthy volunteers, as previously reported (12, 15). This observation is consistent with an enrichment in the cytolysin genotype among E. faecalis clinical isolates from other anatomical sites (12, 13, 15). Cytolysin is a toxin capable of lysing both eukaryotic and prokaryotic cells and is most commonly encoded by highly transmissible pheromone-responsive plasmids (15). Cytolysin has been shown previously to be cytotoxic for mouse macrophages and polymorphonuclear leukocytes (23). The enrichment for the cytolytic phenotype among the endophthalmitis-derived E. faecalis isolates may therefore be due to the ability of cytolytic strains to resist host clearance mechanisms.
S. aureus and E. faecalis are opportunistic pathogens that reside at preferred commensal colonization sites in or on the human host without ill effect. When either of these species is introduced into the eye, as a consequence of a surgical or traumatic wound, an infection that invariably threatens vision can result. The substantial clonality observed among the endophthalmitis-derived S. aureus isolates may relate to the fact that this species colonizes sites in close proximity to the eye (eyelid margins and nares), while E. faecalis rarely does so. Among the S. aureus isolates residing close to the eye, certain subsets possessing colonization traits (e.g., binding proteins and clearance resistance mechanisms) that position them well for introduction through surgical or traumatic wounds to the eye may exist. Since E. faecalis rarely colonizes ocular structures and adjacent surfaces, introduction of these organisms is more likely the result of seeding from contaminated material and is therefore not dependent on specialized, chromosomally encoded colonization mechanisms. However, possession of variable traits, such as a plasmid-encoded cytolysin, may provide a colonization advantage for E. faecalis.
Acknowledgments
We gratefully acknowledge Gail Cupp of Alcon Laboratories for providing the keratitis strains analyzed in this study.
This work was supported by Public Health Service grants EY10867 (to M.C.B.), EY08289 (to M.S.G.), EY06813 (to M.C.C.), and EY00357 (to J.C.) and by Research to Prevent Blindness (RPB), Inc. J.C. is the recipient of a career development award from RPB, Inc.
REFERENCES
- 1.Affeldt J C, Flynn H W, Forster R K, Mandelbaum S, Clarkson J G, Jarus G D. Microbial endophthalmitis resulting from ocular trauma. Ophthalmology. 1987;94:407–413. doi: 10.1016/s0161-6420(87)33447-5. [DOI] [PubMed] [Google Scholar]
- 2.Bannerman T L, Rhoden D L, McAllister S K, Miller J M, Wilson L A. The source of coagulase negative staphylococci in the endophthalmitis vitrectomy study. Arch Ophthalmol. 1997;115:357–361. doi: 10.1001/archopht.1997.01100150359008. [DOI] [PubMed] [Google Scholar]
- 3.Booth M C, Atkuri R V, Nanda S K, Iandolo J J, Gilmore M S. Accessory gene regulator controls Staphylococcus aureus virulence in endophthalmitis. Investig Ophthalmol Vis Sci. 1995;36:1828–1836. [PubMed] [Google Scholar]
- 4.Booth M C, Cheung A L, Hatter K L, Jett B D, Callegan M C, Gilmore M S. Staphylococcal accessory regulator (sar) contributes to Staphylococcus aureus virulence in endophthalmitis only in conjunction with agr. Infect Immun. 1997;65:1550–1556. doi: 10.1128/iai.65.4.1550-1556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinton G S, Topping T M, Hyndiuk R A, Aaberg T M, Reeser F H, Abrams G W. Posttraumatic endophthalmitis. Arch Ophthalmol. 1984;102:547–550. doi: 10.1001/archopht.1984.01040030425016. [DOI] [PubMed] [Google Scholar]
- 6.Cheung A L, Koomey J M, Butler C A, Projan S J, Fischetti V A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Cupp, G. (Alcon Microbiology). Personal communication.
- 7.Endophthalmitis Vitrectomy Study Group. Microbiologic factors and visual outcome in the endophthalmitis vitrectomy study. Am J Ophthalmol. 1996;122:830–846. doi: 10.1016/s0002-9394(14)70380-0. [DOI] [PubMed] [Google Scholar]
- 8.Geha D J, Uhl J R, Gustaferro C A, Persing D H. Multiplex PCR for identification of methicillin-resistant staphylococci in the clinical laboratory. J Clin Microbiol. 1994;32:1768–1772. doi: 10.1128/jcm.32.7.1768-1772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilmore M S, Segarra R A, Booth M C. An HlyB-type function is required for expression of the Enterococcus faecalis hemolysin/bacteriocin. Infect Immun. 1990;58:3914–3923. doi: 10.1128/iai.58.12.3914-3923.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu L, Umeda A, Amako K. Typing of Staphylococcus epidermidis colonizing in human nares by pulsed-field gel electrophoresis. Microbiol Immunol. 1995;39:315–319. doi: 10.1111/j.1348-0421.1995.tb02207.x. [DOI] [PubMed] [Google Scholar]
- 11.Hu L, Umeda A, Kondo S, Amako K. Typing of Staphylococcus aureus colonising human nasal carriers by pulsed-field gel electrophoresis. J Med Microbiol. 1995;42:127–132. doi: 10.1099/00222615-42-2-127. [DOI] [PubMed] [Google Scholar]
- 12.Huycke M M, Gilmore M S. Frequency of aggregation substance and cytolysin genes among enterococcal endocarditis isolates. Plasmid. 1995;34:152–156. doi: 10.1006/plas.1995.9992. [DOI] [PubMed] [Google Scholar]
- 13.Huycke M M, Speigel C A, Gilmore M S. Bacteremia caused by hemolytic high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1991;35:1626–1634. doi: 10.1128/aac.35.8.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ike Y, Clewell D B, Segarra R A, Gilmore M S. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: Tn917 insertional mutagenesis and cloning. J Bacteriol. 1990;172:155–163. doi: 10.1128/jb.172.1.155-163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ike Y, Hashimoto H, Clewell D B. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol. 1987;25:1524–1528. doi: 10.1128/jcm.25.8.1524-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janzon L, Arvidson S. The role of the δ-lysin gene (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J. 1990;9:1391–1399. doi: 10.1002/j.1460-2075.1990.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jett B D, Jensen H G, Atkuri R V, Gilmore M S. Evaluation of therapeutic measures for treating endophthalmitis caused by isogenic toxin-producing and toxin-nonproducing Enterococcus faecalis. Investig Ophthalmol Vis Sci. 1995;36:9–15. [PubMed] [Google Scholar]
- 18.Jett B D, Jensen H G, Nordquist R E, Gilmore M S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992;60:2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kattan H M, Flynn H W, Pflugfelder S C, Robertson C, Forster R K. Nosocomial endophthalmitis survey. Current incidence of infection after intraocular surgery. Ophthalmology. 1991;98:227–238. [PubMed] [Google Scholar]
- 20.Kornblum J, Kreiswirth B N, Projan S J, Ross H, Novick R P. Agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, Skurry S A, editors. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 373–402. [Google Scholar]
- 21.Kreiswirth B, Kornblum J, Arbeit R D, Eisner W, Maslow J N, McGeer A, Low D E, Novick R P. Evidence for a clonal origin of methicillin resistance in Staphylococcus aureus. Science. 1993;259:227–230. doi: 10.1126/science.8093647. [DOI] [PubMed] [Google Scholar]
- 22.Locatcher-Khorazo D, Sullivan N, Gutierrez E. Staphylococcus aureus isolated from normal and infected eyes. Arch Ophthalmol. 1967;77:370–377. doi: 10.1001/archopht.1967.00980020372015. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki S, Ohno A, Kobayashi I, Uji T, Yamaguchi K, Goto S. Cytotoxic effect of hemolytic culture supernatant from Enterococcus faecalis on mouse polymorphonuclear neutrophils and macrophages. Microbiol Immunol. 1993;37:265–270. doi: 10.1111/j.1348-0421.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- 24.Murray B E, Singh K V, Heath J D, Sharma B R, Weinstock G M. Comparison of genomic DNAs of different enterococcal isolates using restriction fragments with infrequent recognition sites. J Clin Microbiol. 1990;28:2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen J C, Flynn H W, Forster R K, Culbertson W W. Results in the treatment of post-operative endophthalmitis. Ophthalmology. 1983;90:692–699. doi: 10.1016/s0161-6420(83)34511-5. [DOI] [PubMed] [Google Scholar]
- 26.Philips W B, Tasman W S. Postoperative endophthalmitis in association with diabetes mellitus. Ophthalmology. 1994;101:508–518. doi: 10.1016/s0161-6420(13)31268-8. [DOI] [PubMed] [Google Scholar]
- 27.Puliafito C A, Baker A S, Haaf J, Foster S. Infectious endophthalmitis: review of 36 cases. Ophthalmology. 1982;89:921–929. [PubMed] [Google Scholar]
- 28.Rowsey J J, Newsom D L, Sexton D J, Harms W K. Endophthalmitis: current approaches. Ophthalmology. 1982;89:1055–1066. doi: 10.1016/s0161-6420(82)34691-6. [DOI] [PubMed] [Google Scholar]
- 29.Shrader S K, Band J D, Lauter C B, Murphy P. The clinical spectrum of endophthalmitis: incidence, predisposing factors and features influencing outcome. J Infect Dis. 1990;162:115–120. doi: 10.1093/infdis/162.1.115. [DOI] [PubMed] [Google Scholar]
- 30.Shuter J, Hatcher V B, Lowy F D. Staphylococcus aureus binding to human nasal mucin. Infect Immun. 1996;64:310–318. doi: 10.1128/iai.64.1.310-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speaker M G, Milch F A, Shah M K, Eisner W, Krieswirth B N. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98:639–649. doi: 10.1016/s0161-6420(91)32239-5. [DOI] [PubMed] [Google Scholar]
- 32.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber D J, Hoffman K L, Thoft R A, Baker A S. Endophthalmitis following intraocular lens implantation: report of 30 cases and review of the literature. Rev Infect Dis. 1986;8:12–20. doi: 10.1093/clinids/8.1.12. [DOI] [PubMed] [Google Scholar]