Abstract
Bone defects are primarily the result of high-energy trauma, pathological fractures, bone tumor resection, or infection debridement. The treatment of bone defects remains a huge clinical challenge. The current treatment options for bone defects include bone traction, autologous/allogeneic bone transplantation, gene therapy, and bone tissue engineering amongst others. With recent developments in the field, composite scaffolds prepared using tissue engineering techniques to repair bone defects are used more often. Among the various composite scaffolds, hydrogel exhibits the advantages of good biocompatibility, high water content, and degradability. Its three-dimensional structure is similar to that of the extracellular matrix, and as such it is possible to load stem cells, growth factors, metal ions, and small molecule drugs upon these scaffolds. Therefore, the hydrogel-loaded drug system has great potential in bone defect repair. This review summarizes the various natural and synthetic materials used in the preparation of hydrogels, in addition to the latest research status of hydrogel-loaded drug systems.
Keywords: Bone defects, Bone tissue engineering, Hydrogel, Drug release
1. Introduction
Bone defects caused by trauma, bone tumors or infection are common and serious clinical presentations [1]. Without external intervention, it is very difficult for bone tissue to self-repair and regenerate. In the past few decades, bone transplantation has remained the “gold standard” for the treatment of bone defects [2]. Bone transplantation procedure types can be divided into autologous and allogeneic. The source of material for bone transplant is limited, and transplantation can result in donor bone tissue defects, and infection risks, and thus there is a limit to the use of bone transplantation in the clinic [3,4]. Bone tissue engineering scaffolds can not only be used as carriers to transport various substances to bone defects, but they also provide good adhesive support and an environment for cellular survival due to their spatial structure and physicochemical properties. Therefore, designing and preparing novel biological scaffolds is an important avenue for bone tissue engineering research.
Hydrogels are three-dimensional network polymers that can be shaped into distinct shapes and sizes [5]. Moreover, they further exhibit high-density hydrophilic groups, which are capable of retaining a large amount of water or biological fluid [6]. In addition, hydrogels are similar to natural extracellular matrices and can provide a platform for cells to transport nutrition, proliferation and differentiation, and minimize damage to biological tissues [7]. Recently developed natural and synthetic hydrogels exhibit great variety and those produced with gelatin, chitosan, alginate, hyaluronic acid, and polyethylene glycol. Each hydrogel type has its advantages and disadvantages. It is necessary to fully understand the characteristics of each hydrogel to better select the best carrier according to the specific requirement of the case.
In addition to utilizing the inherent characteristics of hydrogels for bone repair, bone regeneration can be promoted by loading with polypeptides, growth factors, metal ions or small molecule drugs. Studies have identified that a variety of polypeptides are beneficial and contribute to the promotion of cell osteogenic differentiation, the activation of bone healing, and the subsequent repairing of bone defects. The study of metal ions provides a novel avenue for the development of bone tissue engineering. For example, magnesium participates in bone tissue formation, accelerating bone defect repair by promoting osteogenic differentiation and bone mineralization; Manganese promotes the formation of chondroitin sulfate and is beneficial to the synthesis of extracellular matrix and cartilage. Furthermore, small molecule drugs have good stability and are economical. Combining small molecule drugs with tissue engineering composite scaffolds can further improve bone repair efficiency. In this review, we summarized the basic properties of natural and synthetic materials commonly used to prepare hydrogels in recent research. In addition, we summarized the latest progress in the bioactive molecules loaded on hydrogel drug delivery systems.
2. Various hydrogels for bone tissue engineering
2.1. Natural polymer materials
2.1.1. Gelatin
Gelatin (Gel) is a natural polymer derived from the hydrolysis of collagen. Due to its low price, excellent biocompatibility, low biodegradability and immunogenicity, it has been widely used in biomedicine [8]. In addition, Gel also has strong cell adhesion and migration capabilities and is considered one of the preferred proteins for tissue engineering [9,10]. Gel is soluble in warm water>40 °C. After cooling, they form hydrogels due to the existence of stable hydrogen bonds between amino and carbonyl groups [11]. However, a rapid degradation rate and low mechanical strength of Gel in physiological conditions limit their wider application. Considering the rich side chain groups of Gel, they can be modified by coupling with various substances to synthesize composite materials and subsequently overcome their shortcomings [12,13]. For example, cross-linked gelatin prepared by the coupling of enzymatically crosslinked hydroxyphenyl propionic acid (GHPA) was revealed to significantly induce the growth and differentiation of mesenchymal stem cell endothelial cells and was also reported to reduce host macrophage activation [12]. Bhushan et al. prepared gelatin chitosan cerium oxide nanoparticle composite scaffolds (CG-CNPs) using a freeze-drying method. The CG CNPs scaffold with the bulk porosity of 75–85 % and the pore size of 100–200 μm was beneficial for promoting the diffusion of nutrients and gases. Due to the increased cross-linking between CG and CNPs, the degradation rate of this scaffold was lower than that of CG scaffold. In addition, compared to CG (137.84 kPa), CG CNPs had a higher compressive modulus (178.25 kPa) [14]. The scaffold not only exhibited superior physical, chemical, mechanical, and biological properties, but also significantly inhibited the growth of gram-positive/negative bacteria. Moreover, the CG-CNP2 scaffolds are highly biocompatible and therefore can used to repair bone defects [14]. In addition, cross-linking agents have been commonly employed to improve the performance of Gel. Skopinska Wisniewska et al. confirmed that EDC-NHS, a classic crosslinking agent, could significantly improve the mechanical properties and thermal stability of Gel. Compared to EDC-NHS, dialdehyde starch had a better effect; it not only increased the strength, stiffness, and thermal stability of Gel, but also increased its swelling capacity [15].
2.1.2. Collagen
Collagen (Col) is a rich functional protein in mammals, accounting for about 30 % of the total protein weight of the human body. Types I, II, and III Col are the most common, with Type I accounting for 90 % of the total collagen, mainly distributed in skin, tendons, ligaments, bone, and cartilage [16,17]. Col has many advantageous properties, including high biocompatibility, low antigenicity, and biodegradability; Col has a wide range of potential sources and can be easily integrated with other biological materials [18,19]. Collagen, as a component of cartilage tissue, is therefore an ideal material for repairing human cartilage tissue due to its inherent biocompatibility. In addition, the three-dimensional skeleton of Col possesses functional groups coupled with a variety of factors or molecules, so collagen-based hydrogels have been used as scaffold materials or carriers to treat bone and cartilage defects. For example, the recombinant human collagen (rhCol) hydrogel scaffold loaded with basic fibroblast growth factor (bFGF) exhibits not only good biological properties, but also improves the utilization rate of bFGF and promotes the repair of rat skull defects by continuously releasing bFGF [20]. The application of Col hydrogel in bone tissue engineering has been limited due to its insufficient mechanical strength and fast rate of degradation in physiological conditions. Researchers have developed composite scaffold materials by combining Col with other biomaterials to improve the performance of Gol hydrogel and promote bone defect repair. For example, chitin hydroxyapatite collagen composite scaffolds (CHCS) have been reported to not only exhibit increased mechanical strength but have also been shown to accelerate the repair of bone defects by promoting collagen and new bone formation [21]. In addition, there are several other common materials, including chitosan [22], alginate [23], and PLGA [24], all of which can improve the mechanical strength and stability of collagen hydrogel.
2.1.3. Hyaluronic acid
Hyaluronic acid (HA) is a linear anionic polysaccharide that exists widely in the extracellular matrix of connective tissue, its content varies significantly in content amongst different species and tissues. HA is the most abundant compound in synovial fluid in the joint cavity and possesses both viscoelastic and rheological properties. HA has high viscoelasticity, high biocompatibility, and high biodegradability, and plays a key role in cartilage formation and differentiation. Compared to Col, HA can be extracted from a wider array of sources and has lower immunogenicity [25,26]. HA, as an ECM molecule, plays an important role in immune regulation and secretion, providing a natural microenvironment for stem cells. When stem cells are present in an HA matrix, ensuing synergistic effects promote the tissue repair processes [27]. Given the above characteristics, HA is utilized frequently to treat bone/cartilage defects, osteoarthritis, and rheumatoid arthritis [28,29]. However, the sub-optimal mechanical properties of HA result in difficulties in maintaining its structural stability during implantation, and its high degradability after implantation can also lead to unsatisfactory therapeutic effects. At present, increasing numbers of studies report the addition of hydroxyapatite and other materials to HA hydrogel to improve the therapeutic effect [30].
2.1.4. Alginate
Alginate (Alg) is a natural linear polysaccharide derived from the cell wall of brown algae. Under the action of several distinct divalent cations, including Mn2+, Co2+and Cu2+, it is rapidly transformed into hydrogel by associated coupling reactions, each different cation exerts diverse effects upon the properties and structure of alginate gel [31]. As a natural polymer, alginate has a high level of biocompatibility, low immunogenicity and biodegradability, and a wide range of sources. Therefore, alginate is an ideal material for bone repair tissue engineering; it has received widespread attention in recent years [32,33]. Similar to other hydrogels, Alg possesses weak mechanical properties. Among the inorganic compounds used to reinforce hydrogels, bioglass and calcium phosphate are the most studied. For example, Jin et al. prepared a porous hydroxyapatite (HAP)/chitosan alginate composite scaffold. As the content of HAP was increased, the porosity of the scaffold decreased, and in vivo experiments in mice revealed that the composite scaffold significantly promoted bone formation [34].
2.1.5. Chitosan
Chitosan (CS) is primarily produced during the deacetylation process of chitin obtained from the exoskeleton of crustaceans. It is composed of glucosamine and N-acetylglucosamine and is a natural linear polysaccharide [35]. CS has components similar to natural extracellular matrices, providing the microenvironment required for cell growth. Moreover, CS can promote the proliferation and differentiation of osteoblasts [36]. CS can further be combined with other chemical polymers to prepare biological scaffolds for the treatment of bone defects. Additionally, CS has inhibitory effects upon a wide range of bacteria and fungi through direct binding to negatively charged bacteria at the surface, binding to bacterial DNA, and metal ion chelation [37]. CS has been widely used in the field of bone tissue engineering due to its biocompatibility, biodegradability, and other biological characteristics required by bone tissue biomaterials. The positive charge characteristics of chitosan help to control drug release, mucosal adhesion, in situ gel generation, transfection, osmotic enhancement and efflux pump inhibition [38]. The above features enable a CS hydrogel stent to be targeted at the defect site to achieve optimal therapeutic effect. As such, a CS hydrogel scaffold can act as a drug carrier system, transporting antibiotics, hormones, growth factors, statins and various genes to the injury center; a characteristic with an important role in bone tissue engineering [39]. In a recent study, Weng et al. prepared a chitosan/silk fibroin hydrogel mixed with MgFe double hydroxide nanosheets loaded with platelet-derived growth factor (PDGF). This system not only enhanced the mechanical properties of the hydrogel, but also resulted in the release of PDGF and bone morphogenetic protein 2 (BMP-2) in turn promoting the regeneration of vascular cells and bone [40].
2.1.6. Silk fibroin
Silk fibroin (SF) is a natural protein polymer derived from the fibrin of silk, accounting for about 70 %–75 % of the total mass of silk fibers [41]. SF is rich in 18 amino acids, with combined alanine, glycine, and serine contents reaching 85 %. Because sericin in silk can cause allergic reactions, before preparing SF tissue engineering scaffolds, it is necessary to first remove the immunogenic sericin [42]. Using the difference in the solubility of silk fibroin and sericin in an aqueous solution system, the sericin is dissolved in the solution system and the silk fibroin is separated by centrifugation, filtration, dialysis, and other methods. Biocompatibility refers to the various biological, physical, and chemical reactions that occur after the interaction between biomaterials and living organisms. Biocompatibility includes two parts: biological reactions and material reactions, among which biological reactions include blood reactions, immune reactions, and tissue reactions; Material reactions mainly manifest in changes in the physical and chemical properties of materials. SF has a high degree of biocompatibility. Unlike collagen, hyaluronic acid, and other polymers mentioned above, SF has excellent mechanical strength and physicochemical properties [43], making it one of the ideal biomaterials for bone tissue engineering. Similar to other polymer materials, hydrogels can be prepared by chemical modification and the crosslinking of SF fibers. SF however has the distinct advantage of utilizing β- Folding to make silk fibroin gel so the use of toxic chemicals can be avoided [44], while β-fold hydrogen bonds in crystals determine the rigidity and strength of the proteins. Inspired by the unique role of glue molecules in the mechanical strength and fracture resistance of strong bones, Bai et al. prepared a novel method using the spontaneous co-assembly of tannic acid (TA), SF, and hydroxyapatite (HA); the SF@TA @HA inorganic-organic hybrid hydrogel not only significantly improved hydrogel performance but also promoted bone defect regeneration in the early stage [45].
2.2. Synthetic polymer materials
2.2.1. Polyethylene glycol
Polyethylene glycol (PEG) is a water-soluble synthetic polymer. PEG and its derivatives can be manipulated to form hydrogels using physical, ionic, chemical or covalent interactions [46]. PEG is still widely used in the field of tissue engineering due to its multifunctional adjustability, mechanical strength, biocompatibility, and biodegradability [47]. In addition, PEG is easily crosslinked by various functional groups (such as acrylate, mercaptan, etc.) to promote hydrogel formation [48]. PEG is an inert polymer with low biological activity. Therefore, biologically active molecules can be functionalized and added to PEG hydrogel to simulate a natural ECM, improve cell vitality, and promote osteogenesis and/or cartilage generation [49]. In 1999, Elisseeff et al. prepared PEG hydrogel for the first time and reported that transdermal photopolymerization could be used for minimally invasive subcutaneous implantation of hydrogels and chondrocytes for cartilage regeneration in vivo [50].
2.2.2. Polyvinyl alcohol
Polyvinyl alcohol (PVA) is a water-soluble polymer. Because it is biologically similar to cartilage, it possesses good biocompatibility, a high elastic modulus, a high-water content, and excellent biological tribological properties. As such, PVA has been widely utilized to prepare hydrogel materials for the repairing of cartilage defects [51]. In addition, PVA can also be used to prepare composite materials with a variety of polymers, nanomaterials, metal ions, and biomolecules [52]. Based on the above advantages and characteristics, PVA has been revealed to exhibit broad application prospects in bone tissue engineering. For example, PVA and CS can be combined to prepare a new double network hydrogel through physical crosslinking in a KOH/urea dissolution system. This hydrogel has strong tensile strength, elongation at break and high compressive strength, and was shown to significantly accelerate the regeneration of rabbit bone defects [53]. Although 3D-printed porous titanium (pTi) is beneficial to bone growth, its microporous structure can cause bacterial proliferation. Qiao et al. prepared a compatible implant system by combining sodium tetraborate (Na2B4O7), PVA, silver nanoparticles (AgNPs), and tetraethyl orthosilicate (TEOS) with pTi, which could not only promote the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells but also inhibited bacterial growth. This scaffold could thus be an ideal material for treating infectious bone defects [54].
2.2.3. Others
Other noteworthy synthetic polymers are used to prepare hydrogels. For example, polylactic acid glycolic acid copolymer (PLGA) is a biodegradable synthetic polymer material with good biocompatibility and no biological toxicity; it has been widely used in the fields of biomedicine and tissue engineering [55]. Our previous research identified that PLGA hydrogel provided a suitable environment for rat-derived bone marrow stromal stem cells (rBMSCs). In addition, PLGA hydrogel containing LINC00473 modified rBMSCs could significantly promote bone repair and reconstruction of the femoral head necrosis area [56]. Poly (N-isopropyl acrylamide) (PNiPAAm), polyamide amine (PAMAM), and others have also been utilized in the development of hydrogel scaffolds for bone and cartilage tissue engineering [57,58].
3. Types of hydrogel-loaded drugs
Due to the recent developments in bone tissue engineering technology and the refinement and controllability of drug delivery systems, research regarding hydrogel drug delivery for bone defects has become a hot spot. Next, we introduce the substances commonly loaded onto hydrogel drug delivery systems, including polypeptides, metal ions and a variety of osteoinductive small molecules.
3.1. Peptides
3.1.1. Bone morphogenetic protein
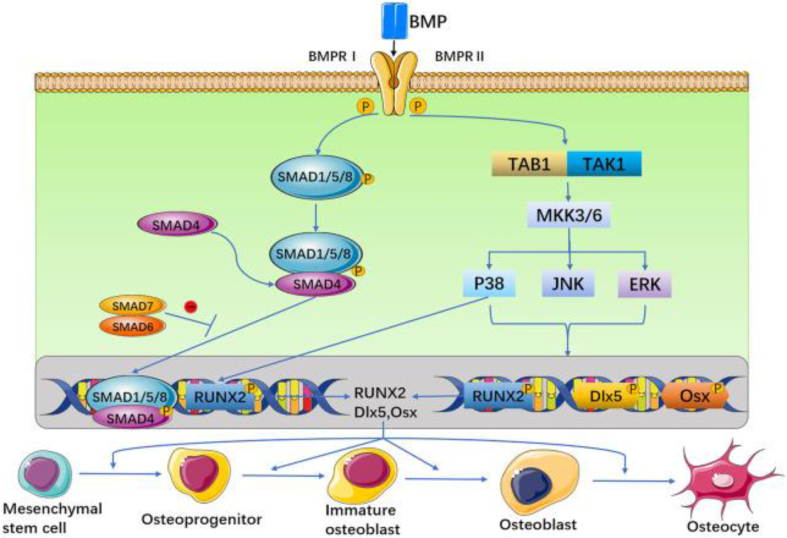
The bone morphogenetic protein (BMP) family consists of a large group of glycoproteins that do not contain collagen. It is one of the important bone development signals and belongs to an important member of the TGF-b family. Among its various subtypes, BMP-2,4,6,7, and 9 possess bone formation-promoting effects [59], and BMP-2 and 7 have been approved by FDA for clinical use in the treatment of bone defects. It is reported that after BMP-2 is knocked out, with increasing age mice are prone to spontaneous fractures and long-term nonunion after fractures, although the development of long bones in embryonic mice is not affected [60]. Muscle-derived stem cells (MDSCs) expressing BMP4 can ameliorate critical size skull defects in immunocompetent mice [61]. Compared with WT mice, mice with BMP-6 mutations exhibit delayed mineralization of the sternal skeleton in embryos during the third trimester of pregnancy, but this effect can be functionally compensated for by BMP-2 [62]. BMP-9 is a newly discovered member of the BMP family; it facilitates bone progenitor cell differentiation into osteoblastic progenitor cells and osteoblasts [63]. BMP-3 is a subtype of bone morphogenetic protein that inversely regulates bone formation, regulating bone mass by inhibiting BMP-2-mediated differentiation of bone progenitor cells into osteoblasts [64]. Overall, BMP signaling pathways are conducted in bone in both a Smad dependent and non-Smad pathway dependent manner [65] (Fig. 1). In Smad dependent signaling, phosphorylated R-Smad (Smad1, 5, or 8) binds to Smad4 to activate Runx2 expression, thereby regulating osteoblast gene expression. The non-Smad dependent pathway initiates transcription through the MKK-p38MAPK induced MKK-ERK1/2 signal cascade reaction [66,67].
Fig. 1.
The BMP signal is transduced through BMPRI and BMPRII receptors. These two receptors are combined into a functional complex, to initiate further signaling pathways. On the one hand, activated BMP type I receptor phosphorylates Smad-dependent signaling pathways. On the other hand, BMP receptors activate non-Smad-dependent signaling pathways, that is, activate p38 MAPK, JNK, and ERK signaling pathways. Then, BMP can stimulate the expression of three osteogenic main transcription factors Runx2, D1x5 and Osx. Reproduced from Ref. [65] with permission.
A large number of experimental studies have revealed that a hydrogel scaffold loaded with BMP has great potential for the treatment of bone defects, and BMP-2 is the most widely studied and applied at present. To overcome high treatment costs and complication rates caused by the routine requirement to apply BMP in excess of physiological doses for bone repair, Shekaran et al. utilized the triple helix α2β1 integrin specific peptide (GFOGER) that was functionalized to employ BMP-2 and loaded with biodegradable PEG hydrogel. This construct could continuously release BMP-2 in low doses in vivo and enhance bone formation, a function related to the matrix metalloproteinase (MMP) modified therein being conducive to cell adhesion and proliferation [68]. Like BMP-2, BMP-7 is considered by some to be the most effective growth factor for inducing bone morphogenesis [69]. CS has bone conductivity but lacks bone inductivity. Therefore, CS scaffolds loaded with growth factors can be used as potential substitutes. Researchers have identified that the biocompatibility of CS particles contributes to improving the loading efficiency of BMP-7, in such constructs the BMP-7 is released in a controlled manner, promoting the attachment, proliferation, and differentiation of osteoblasts at bone defect sites [70]. In the latest study, Song et al. prepared a PLGA scaffold whose surface was modified with GFOGER peptide and BMP-9. In vivo studies 12 weeks after implantation revealed that new bone formed and merged with surrounding bone tissue, and furthermore, bone-related factors were significantly upregulated [71].
3.1.2. Osteogenic growth polypeptide
Osteogenic growth polypeptide (OGP) was first isolated from a culture medium conditioned by newly formed rat bones produced by mechanical bone marrow ablation in vivo [72,73]. It is mainly composed of 14 amino acid residues, and OGP is homologous with the C terminal amino acid sequence (89–102) of histone H4 (HH4) [72,73]. When bone marrow ablation occurs, the expression of HH4 is upregulated due to increased cell cycle activity, increasing the serum concentration of OGP [74]. OGP exists in various forms in the serum, and each form of osteogenic growth factor plays an important role in regulation and protection [74]. OGP enhances the mitotic activity and differentiation of osteoblasts through the protein MAP kinase signal cascade and the RhoA-ROCK cell pathway, respectively [74]. Combining OGP as a functional bioconjugate with natural or synthetic polymers has more recently become widely used in bone tissue engineering. For example, Liu et al. prepared a poly (l-lactic acid) (PLLA) nanofiber scaffold using poly (dopamine) (PDA) coating to immobilize OGP; this preparation significantly enhanced the osteogenic differentiation and calcium mineralization of hMSCs in vitro, and also promoted bone regeneration in rat skull defect sites [75]. In a recent study, researchers developed two self-assembled supramolecular hydrogels loaded with OGP. This hydrogel not only promoted cell proliferation, but also promoted bone defect healing by upregulating the expression of osteogenic factors. Its mediated osteogenic differentiation is primarily the result of the modulation of inflammatory pathways and folate pathways [76].
3.1.3. Vascular endothelial growth factor
Vascular Endothelial Growth Factor (VEGF) is a heparin binding polypeptide that was first isolated from bovine pituitary follicular cells in 1989 [77]. As a specific mitogen of vascular endothelial cells, VEGF can specifically stimulate the proliferation of vascular endothelial cells and induce angiogenesis. There are six isoforms of VEGF, and different isoforms play distinct but dominant roles in the promoting of angiogenesis and bone remodeling. A large number of studies have revealed that VEGF promotes the production of vascular endothelial cells, and that angiogenesis is an important basis for cartilage ossification, enabling the transformation of cartilage into bone tissue [78]. In addition, VEGF not only promotes cartilage ossification, but also modulates intramembrane osteogenesis [79,80]. Although experiments have confirmed that VEGF can promote the angiogenesis and osteogenic differentiation of large segmental bone defects [81], considering the short half-life of exogenous VEGF monomers [82] especially concerning the lack of stent and blood vessels at the defect site, the application of VEGF is limited. The gradual increase in popularity of bone tissue engineering has made up for the deficiency of exogenous VEGF. Kanczler et al. prepared biodegradable scaffolds that encapsulate human bone marrow mesenchymal stem cells and VEGF before transplanting them into mouse femoral bone defects for 4 weeks. It was revealed that VEGF-encapsulated scaffolds resulted in more significant bone regeneration compared to scaffolds alone and those implanted with HBMSC [83]. García et al. prepared PEG hydrogel functionalized by αvβ3 integrin targeting peptide (RGD) and loading these with VEGF increased vascularization compared with the RGD hydrogel without VEGF [84]. Similarly, a gelatin water gel system functionalized with angiogenic vascular endothelial growth factor (VEGF) mimic peptide KLTWQELYQLKYKGI (KLT) and bone anabolic peptide parathyroid hormone (PTH) 1–34 also potentiated a significant effect in promoting bone regeneration in rat skull defect sites [85].
3.1.4. Parathyroid hormone
Parathyroid hormone (PTH) is primarily secreted by the main parathyroid cells, the N-terminal 1–34 fragments of PTH are its biologically active site. PTH modulates bone and cartilage metabolism by maintaining a balance of calcium and phosphorus concentrations in the blood. PTH is a “double-edged sword” in bone metabolism. As such, continuous administration of PTH promotes the maturation and differentiation of osteoclasts, while intermittent injection of PTH (iPTH) promotes the differentiation of preosteoblasts and increases the activity of osteoblasts [86,87]. Recombinant human parathyroid hormone (1-34) (rhPTH 1–34), trade name Teriparatide was the first bone anabolic drug approved by the FDA and was initially used to treat osteoporosis [88]. Experiments have revealed that delayed, short-term administration of PTH can promote the healing of bone defect allografts by increasing bone formation at the junction of the graft and bone defect [89]. As mentioned above, hydrogels loaded with PTH (1-34) and KLT help promote bone regeneration [85]. In the latest research, an environment responsive hydrogel coated with a mesoporous bioactive glass (MBG) scaffold releases PTH by a continuous and pulsatile dual mode, promoting the proliferation, osteogenic differentiation and proliferation of BMSCs, the migration of human umbilical vein endothelial cells (HUVEC). and endothelial angiogenesis [90].
3.1.5. Calcitonin gene related peptide
Calcitonin gene related peptide (CGRP) is a peptide chain composed of 37 amino acids, which is divided into α- and β- CGRP. There are two forms of CGRP. Research has confirmed that CGRP receptors can be identified on the surface of 4 cell types directly related to bone metabolism, including osteoblasts, osteoclasts, bone marrow stromal cells, and bone marrow macrophages [91]. CGRP can promote the proliferation and differentiation of osteoblasts, and thereby the formation of bone tissue. The specific mechanism of CGRP may involve multiple aspects. After analyzing the effects of five neuropeptides including CGRP on the viability of osteoblasts, Ma et al. identified that CGRP increased alkaline phosphatase (ALP) activity in a dose-dependent manner, and significantly promoted the gap junctional intercellular communication (GJIC) of osteoblasts. Therefore, the authors speculated that CGRP promoted the proliferation and differentiation of osteoblasts by enhancing GJIC [92]. In another study, CGRP significantly stimulated the proliferation of MG-63 cells, while increasing the expression of BMP-2, ALP, osteocalcin, and ColIa1. The mechanism by which CGRP operated in this study might be through the activation of intracellular signal transduction through the cyclic adenosine monophosphate (cAMP) pathway, the increasing BMP-2 expression, and through the participation of CGRP in the induction of osteogenic differentiation [93]. In addition to promoting the proliferation and differentiation of osteoblasts, CGRP also plays a role in osteogenesis by promoting the apoptosis of osteoclasts [94]. In addition, CGRP can further promote the differentiation and proliferation of mesenchymal stem cells towards osteogenesis, thereby promoting bone regeneration [91,95]. The above studies indicate that CGRP can be applied as an ideal loading factor for biomaterials in bone tissue engineering. Implantation of CGRP loaded gelatin microspheres into rabbit femoral bone defect sites for 3 months was revealed to effectively promote bone formation, and the best effect was achieved when loaded with 1 μ M CGRP [96].
3.1.6. Substance P
Substance P (SP) is a member of the mammalian tachykinin family [97]. It is primarily distributed in the central and peripheral nervous systems. SP mainly functions through interactions with receptors, which can be divided into NK-1, NK-2, and NK-3. NK-1 is known as an SP receptor due to its high-affinity binding to SP [98]. There are NK-1 receptors in bone marrow stromal cells. Low concentrations of SP promote the expression of ALP and osteocalcin, and the activity of ALP is upregulated by SP. High concentrations of SP can promote the mineralization of bone marrow stromal cells [99]. Moreover, SP has been observed to improve the bone formation efficacy of advanced osteoblasts via the activation of NK-1 receptors [100]. In addition, SP has been revealed to regulate osteoclasts by activating downstream signal transduction through NK-1 receptors. Studies have shown that bone marrow macrophages (BMMs) express NK-1 receptors, and that SP promotes RANKL induced osteoclast and bone resorption activity by activating the NK-KB signaling pathway of bone marrow macrophages [99]. Some studies have reported that gelatin microspheres containing SP promote bone repair in osteoporotic bone defects in a dose dependent manner [96]. Finally, electrospun nanofibrous polycaprolactone (PCL)-polydopamine (PDA) - hydroxyapatite (HA) scaffolds combined with SP were reported to enhance bone tissue regeneration through stem cell mobilization [101].
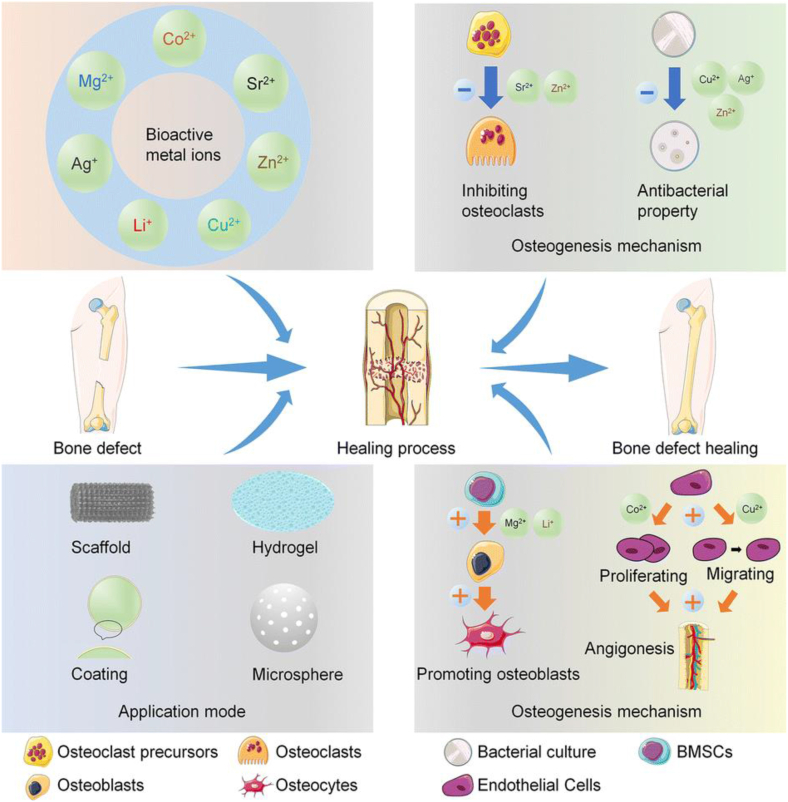
3.2. Metal nanoparticles
3.2.1. Mg2+
Metal ions play an important role in bone tissue engineering, and many studies have explored the mechanism of metal ions promoting bone growth and repair [102] (Fig. 2). Magnesium is involved in physiological processes such as bone tissue formation and bone metabolism. Mg2+ has been shown to promote cell osteogenic differentiation and bone mineralization and is thus beneficial for the repair of bone defects. Moreover, Mg2+induces osteogenic reactions in bone marrow by activating the classic Wnt signaling pathway, thereby promoting the differentiation of HBMSCs into osteogenic differentiation lineages [103]. Distinct Mg2+ concentrations have been shown to induce opposing effects on human osteoclasts. As such, when the applied concentration was lower than 15 mmol/L, Mg2+ promoted the proliferation and differentiation of osteoclasts. Meanwhile, when the applied concentration was higher than 15 mmol/L, Mg2+ induced the proliferation and differentiation of osteoclasts in a concentration-dependent manner [104]. Furthermore, a large number of studies have demonstrated that loading Mg2+ into hydrogels can effectively promote the repair of bone defects. Chen et al. mixed MgO nanoparticles with a water-soluble phosphocreatine functionalized chitosan (CSMP) aqueous solution to prepare CSMP MgO injectable hydrogel. This hydrogel not only avoided the mechanical performance shortcomings of simple CS hydrogel, but also exhibited excellent efficacy to promote bone formation in rat skull bone defect sites [105]. In order to accurately control the release of Mg2+to ensure therapeutic concentration of Mg2+in the microenvironment, Yuan et al. designed biodegradable microspheres (PMg) that were co-encapsulated with PLGA microspheres and MgO/MgCO3. PMg microspheres were revealed to control the release of Mg2+by adjusting the ratio of MgO/MgCO3 to achieve optimally efficient bone defect regeneration [106]. Although the current results demonstrate that Mg2+has a broad application prospect within bone defect repair composites, caution must be taken due rapid degradation potential and low concentrations of Mg2+promoting osteoclast proliferation. These issues should be addressed by further study.
Fig. 2.
Loading mode and mechanism of metal ions in the treatment of bone defects. Reproduced from Ref. [102] with permission.
3.2.2. Sr2+
Sr2+can promote the proliferation and differentiation of osteoblasts, inhibit the activity of osteoclasts, and promote vascular regeneration in bone defect sites. As such, Ding et al. prepared a composite scaffold combining chitosan/aldehyde dextran hydrogel (CDH) and strontium nano hydroxyapatite (Sr nHA) nanoparticles. Compared with CDH, Sr-nHA not only significantly improved the physical properties of CDH, but also significantly enhanced osteoblast proliferation and osteogenic differentiation. In addition, Sr2nHA/CDH induced macrophages to polarize to the M1 phenotype, which is beneficial for reducing inflammatory responses in the microenvironment [107]. Ionic crosslinking agents Ca2+, Ba2+, and Sr2+ can be used to prepare sodium alginate nanohydroxyapatite collagen (Alg nHA Coll) microspheres. Compared to other metal ions, Sr2+ crosslinked microspheres resulted in reduced swelling, enhanced stability, and mechanical strength, and appropriately induced various signal transduction pathways in human osteoblasts, leading to osteogenic activity and dynamic growth [108].
3.2.3. Cu2+
Copper (Cu2+) has been revealed to promote the differentiation of BMSCs into osteoblasts in addition to its antibacterial effects [109]. Cu2+ has good biocompatibility and antibacterial activity, and can inhibit osteoclast differentiation, therefore it is used to treat infected bone defects. Lu et al. added Cu nanoparticles to a mixture of carboxymethyl chitosan (CMC) and alginate (Alg); the study observed that Cu2+ was gradually released and cross-linked into the mixture. Further, CMC/Alg/Cu scaffolds have been prepared by freeze-drying. A series of studies have reported that this particular scaffold enhances the adhesion of osteoblastic precursor cells by upregulating the expression of adhesion related genes and bone related genes and that its application results in the promotion of the osteogenic differentiation and the inhibition of bacterial infection [110].
3.2.4. Zn2+
A PLGA/β-Tricalcium phosphate(β-TCP) stent has been reported to possess good bone conductivity, but it has shortcomings including but not limited to poor mechanical properties, low bone induction ability, and a high infection rate after implantation. Therefore, Li et al. added 1 wt% zinc to the stent for continuous release of Zn2+ over 16 weeks. Compared to PLGA/β-TCP stents, the new stents exhibited higher osteogenic and anti-inflammatory capabilities, which was reported to be mainly a result of modulation of Wnt/β- Catenin, P38 MAPK and NF-kB signaling pathways [111].
3.2.5. Others
In addition, metal ions such as Mn2+ [112], Ca2+ [33], and Co2+ [113] have further been applied in bone tissue engineering and have exhibited ideal properties for promoting bone defect repair. However, issues such as fine-tuning the proportion of metal ions combined with materials, cytotoxicity, and osteogenic/angiogenic mechanisms still need to be addressed by further experimentation.
3.3. Osteoinductive small molecule
Osteoinductive small molecule drugs can be divided into two groups: natural small molecules and synthetic small molecules. Natural small molecules are primarily extracted from plants or animals, while synthetic small molecules are commonly obtained by chemical synthesis after designing imitation structures of natural small molecules by pharmacological mechanisms. Quercetin can directly act upon osteoclasts through interaction with estrogen receptors (ER) to induce apoptosis of mature osteoclasts, effectively inhibiting bone resorption, and by activating BMP receptors by inducing the expression of NF-kB and the production of reactive oxygen species to promote osteoblast differentiation and inhibit osteoclasis [114]. Naringin is a quercetin analog that can increase the level of BMP in osteoblasts, promote the proliferation of BMSCs, and increase the expression level of bone formation related genes [115]. Moreover, Naringin inhibits RANK mediated NF- κ B and ERK signal transduction to inhibit osteoclast formation and bone resorption [116]. Another flavonoid drug, hesperidin, can inhibit bone loss in ovariectomized mice [117]. Pyrroloquinoline quinine (PQQ) has also been revealed to promote bone metabolism by inhibiting osteoclast differentiation in RANKL mediated BMM in a dose dependent manner resulting in the promotion of bone formation [118]. Emodin has also been reported to promote the proliferation and differentiation of osteoblasts, primarily a result of the activation of the bone morphogenetic protein receptor Smad (BMPR Smad) signaling axis and p38 mitogen-activated protein kinase (p38 MAPK) pathway [119]. Bone regeneration involves multiple signaling pathways, including Wnt and TGF- β/BMP, MAPK, amongst others [120] Artificially synthesized small molecules play a role in bone metabolism by regulating related signaling pathways. The Wnt signaling pathway is crucial in osteoblast differentiation and regulates the expression of osteogenic genes by inhibiting the formation of the Axin/GSK-3/APC complex. Therefore, targeting GSK-3 is a key strategy in bone repair. For example, oral administration of an active GSK-3 inhibitor (AZD2858) resulted in a dose-dependent increase in bone trabecular mass in rats because AZD2858 has a bone building effect in the trabecular region [121]. Similarly, Genistein, Daidzein, Formononetin, and 2-Acetyldibenzothiophen were all reported to regulate the BMP-2 pathway and promote the expression of the BMP-2 gene in MG-63 cells [122], thereby increasing osteoblast differentiation and bone formation.
4. Conclusion and perspectives
This review summarizes the recent progress in research regarding hydrogel scaffold materials in the field of bone tissue engineering, including synthetic materials for hydrogels (Table 1), and a series of studies investigating hydrogel scaffolds as a drug delivery system in the repair of bone and cartilage defects. As a relatively novel polymer material, hydrogel is now favored in the biomedical field due to its high biocompatibility, high tissue moisture content, degradability and other aforementioned characteristics. Hydrogel is therefore becoming more widely used in the research of bone and cartilage defect repair. However, natural synthetic materials such as pure gelatin and alginate have drawbacks including insufficient mechanical strength and rapid degradation, limiting their application in bone tissue engineering. Therefore, synthetic polymers have been employed to effectively improve the shortcomings of natural synthetic materials, but this avenue is associated with risks of increased immunogenicity, which needs to be addressed in the future. Among the reviewed hydrogels, some functional scaffolds have high manufacturing costs and are associated with difficulties in achieving large-scale production. In addition, at present, a large number of studies are reliant on laboratory animal models, and regular bone defect models cannot fully reflect the complex bone defects of patients in the clinic. Therefore, further clinical experiments are required to verify the repairing effect of hydrogel scaffolds.
Table 1.
Summary of research progress of hydrogel materials involved in this paper.
| Polymer materials | Source | Composite hydrogel scaffold | Results | References |
|---|---|---|---|---|
| Gelatin | Natural | GHPA | The polymer can significantly induce the growth and differentiation of mesenchymal stem cell endothelial cells, and was also reported to reduce host macrophage activation | [12] |
| Gelatin | Natural | CG-CNPs | The scaffold not only exhibited superior physical, chemical, mechanical, and biological properties, but also significantly inhibited the growth of gram positive/negative bacteria, and the CG-CNP2 scaffolds are highly biocompatible, so it can be used to repair bone defects. | [14] |
| Collagen | Natural | rhCol | The scaffold not only good biological properties, but also improves the utilization rate of bFGF and promotes the repair of rat skull defects by continuously releasing bFGF | [20] |
| Collagen | Natural | CHCS | The scaffold not only exhibit increased mechanical strength, but have also been shown to accelerate the repair of bone defects by promoting collagen and new bone formation | [21] |
| Hyaluronic acid | Natural | HAP + HA1-ALG | HAP enhanced mechanical strength of composite hydrogel scaffold and the hydrogel system loaded with exosomes can significantly enhance bone regeneration. | [30] |
| Alginate | Natural | HAP/CS-ALG | As the content of HAP was increased, the porosity of the scaffold decreased, and in vivo experiments in mice revealed that the composite scaffold significantly promoted bone formation | [34] |
| Chitosan | Natural | MgFe-LDH nanosheet + chitosan/silk fibroin | This system not only enhanced the mechanical properties of the hydrogel, but also resulted in the release of PDGF and bone morphogenetic protein 2 (BMP-2) in turn promoting the regeneration of vascular cells and bone | [40] |
| Silk fibroin | Natural | SF@TA@HA2 | The inorganic organic hybrid hydrogel not only significantly improved hydrogel performance, but also promoted bone defect regeneration in the early stage | [45] |
| Polyethylene glycol | Synthetic | PEG | Biologically active molecules can be functionalized and added to PEG hydrogel to simulate a natural ECM, improve cell vitality, and promote osteogenesis and/or cartilage generation | [49] |
| Polyvinyl alcohol | Synthetic | PVA + CS | This hydrogel has strong tensile strength, elongation at break and high compressive strength, and was shown to significantly accelerate the regeneration of rabbit bone defects | [53] |
| Polyvinyl alcohol | Synthetic | Na2B4O7+ PVA + AgNPs, +TEOS + pTi | This scaffold could not only promote the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells, but also inhibited bacterial growth. | [54] |
| polylactic acid glycolic acid copolymer | Synthetic | PLGA | PLGA hydrogel provided a suitable environment for rat derived bone marrow stromal stem cells (rBMSCs). In addition, PLGA hydrogel containing LINC00473 modified rBMSCs could significantly promote bone repair and reconstruction of femoral head necrosis area. | [56] |
As we described above, hydrogel systems can achieve bone tissue engineering regeneration and repair by promoting the osteogenic differentiation of mesenchymal stem cells and/or osteoblasts, by inhibiting oxidative stress and the inflammatory polarization of macrophages (M1 type in the bone defect microenvironment), and by promoting angiogenesis and other mechanisms. However, when hydrogels are loaded with exogenous cells or biological factors, there is a risk of immune response, and the hydrogel implantation itself may cause an inflammatory reaction, both of which could affect the efficacy of the hydrogel scaffold in treating bone defects. In addition, although drug delivery systems based on hydrogels have achieved positive results in in-vivo and in-vitro experiments, the determination of the optimal concentration of each loaded drug and the method by which to maintain concentrations of some ions (e. g. Mg2+) needs to be refined.
Although the hydrogel drug delivery system faces challenges in utilization for repairing bone defects, with the development of various bioengineering technologies and broad scientific research study, the field has the potential to optimize the preparation of ideal functional hydrogels. The hydrogel drug delivery system exhibits broad applicational prospects in bone tissue engineering and could achieve successful clinical translation through further preclinical experimentation.
Author contributions
T. L. contributed to the conception and design of the review. W.S., Y.J. contributed to searching for information or preparing figures. W.S., Y.J., T.W. and T.L. contributed to drafting and revising of manuscript. All authors contributed to the article and approved the final manuscript.
Funding
National Natural Science Foundation of China; Grant number: 82272489, 82203588; Qingdao Traditional Chinese Medicine Science and Technology Project; Grant number: 2021-zyym28. Science and technology Development Project of Shandong Geriatric Society; Grant number: LKJGG2021W082.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Data availability statement
Not applicable.
Declaration of competing interest
No conflict of interest exits in the submission of this manuscript, and all the authors listed have approved the manuscript that is enclosed.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Zhang Y., Yu T., Peng L., Sun Q., Wei Y., Han B. Advancements in hydrogel-based drug sustained release systems for bone tissue engineering. Front Pharmacol. 2020;11:622. doi: 10.3389/fphar.2020.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keating J.F., Simpson A.H., Robinson C.M. The management of fractures with bone loss. J Bone Joint Surg Br. 2005;87:142–150. doi: 10.1302/0301-620x.87b2.15874. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y., Wang L., Zhao X., Hou S., Guo B., Ma P.X. Self-healing supramolecular bioelastomers with shape memory property as a multifunctional platform for biomedical applications via modular assembly. Biomaterials. 2016;104:18–31. doi: 10.1016/j.biomaterials.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Hsu R.S., Chen P.Y., Fang J.H., Chen Y.Y., Chang C.W., Lu Y.J., Hu S.H. Adaptable microporous hydrogels of propagating NGF-gradient by injectable building blocks for accelerated axonal outgrowth. Adv Sci. 2019;6 doi: 10.1002/advs.201900520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 6.Jose G., Shalumon K.T., Chen J.P. Natural polymers based hydrogels for cell culture applications. Curr Med Chem. 2020;27:2734–2776. doi: 10.2174/0929867326666190903113004. [DOI] [PubMed] [Google Scholar]
- 7.Wei M., Hsu Y.I., Asoh T.A., Sung M.H., Uyama H. Injectable poly(gamma-glutamic acid)-based biodegradable hydrogels with tunable gelation rate and mechanical strength. J Mater Chem B. 2021;9:3584–3594. doi: 10.1039/d1tb00412c. [DOI] [PubMed] [Google Scholar]
- 8.Su K., Wang C. Recent advances in the use of gelatin in biomedical research. Biotechnol Lett. 2015;37:2139–2145. doi: 10.1007/s10529-015-1907-0. [DOI] [PubMed] [Google Scholar]
- 9.Bello A.B., Kim D., Kim D., Park H., Lee S.H. Engineering and functionalization of gelatin biomaterials: from cell culture to medical applications. Tissue Eng Part B. 2020;26:164–180. doi: 10.1089/ten.TEB.2019.0256. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Guillen M.C., Gimenez B., Lopez-Caballero M.E., Montero M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocolloids. 2011;25:1813–1827. doi: 10.1016/j.foodhyd.2011.02.007. [DOI] [Google Scholar]
- 11.Guo L., Liang Z., Yang L., Du W., Yu T., Tang H., Li C., Qiu H. The role of natural polymers in bone tissue engineering. J Contr Release. 2021;338:571–582. doi: 10.1016/j.jconrel.2021.08.055. [DOI] [PubMed] [Google Scholar]
- 12.Lee S.H., Lee Y., Chun Y.W., Crowder S.W., Young P.P., Park K.D., Sung H.J. In situ crosslinkable gelatin hydrogels for vasculogenic induction and delivery of mesenchymal stem cells. Adv Funct Mater. 2014;24:6771–6781. doi: 10.1002/adfm.201401110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yue K., Trujillo-de Santiago G., Alvarez M.M., Tamayol A., Annabi N., Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhushan S., Singh S., Maiti T.K., Das A., Barui A., Chaudhari L.R., Joshi M.G., Dutt D. Cerium oxide nanoparticles disseminated chitosan gelatin scaffold for bone tissue engineering applications. Int J Biol Macromol. 2023;236 doi: 10.1016/j.ijbiomac.2023.123813. [DOI] [PubMed] [Google Scholar]
- 15.Skopinska-Wisniewska J., Tuszynska M., Olewnik-Kruszkowska E. Comparative study of gelatin hydrogels modified by various cross-linking agents. Materials. 2021;14 doi: 10.3390/ma14020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deshmukh S.N., Dive A.M., Moharil R., Munde P. Enigmatic insight into collagen. J Oral Maxillofac Pathol. 2016;20:276–283. doi: 10.4103/0973-029X.185932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abazari M.F., Soleimanifar F., Amini Faskhodi M., Mansour R.N., Amini Mahabadi J., Sadeghi S., Hassannia H., Saburi E., Enderami S.E., Khani M.M., et al. Improved osteogenic differentiation of human induced pluripotent stem cells cultured on polyvinylidene fluoride/collagen/platelet-rich plasma composite nanofibers. J Cell Physiol. 2020;235:1155–1164. doi: 10.1002/jcp.29029. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira A.M., Gentile P., Chiono V., Ciardelli G. Collagen for bone tissue regeneration. Acta Biomater. 2012;8:3191–3200. doi: 10.1016/j.actbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Antoine E.E., Vlachos P.P., Rylander M.N. Tunable collagen I hydrogels for engineered physiological tissue micro-environments. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Y., Hu Z., Chen J., Zhang Z., Liu Q., Li J., Yang J., Ma Z., Zhao J., Hu J., et al. Injectable TG-linked recombinant human collagen hydrogel loaded with bFGF for rat cranial defect repair. Int J Biol Macromol. 2023;236 doi: 10.1016/j.ijbiomac.2023.123864. [DOI] [PubMed] [Google Scholar]
- 21.Xing F., Chi Z., Yang R., Xu D., Cui J., Huang Y., Zhou C., Liu C. Chitin-hydroxyapatite-collagen composite scaffolds for bone regeneration. Int J Biol Macromol. 2021;184:170–180. doi: 10.1016/j.ijbiomac.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Chacon E.L., Bertolo M.R.V., de Guzzi Plepis A.M., da Conceicao Amaro Martins V., Dos Santos G.R., Pinto C.A.L., Pelegrine A.A., Teixeira M.L., Buchaim D.V., Nazari F.M., et al. Collagen-chitosan-hydroxyapatite composite scaffolds for bone repair in ovariectomized rats. Sci Rep. 2023;13:28. doi: 10.1038/s41598-022-24424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng L., Jiang X., Chen X., Fan H., Zhang X. Evaluation of novel in situ synthesized nano-hydroxyapatite/collagen/alginate hydrogels for osteochondral tissue engineering. Biomed Mater. 2014;9 doi: 10.1088/1748-6041/9/6/065004. [DOI] [PubMed] [Google Scholar]
- 24.Quinlan E., Lopez-Noriega A., Thompson E., Kelly H.M., Cryan S.A., O'Brien F.J. Development of collagen-hydroxyapatite scaffolds incorporating PLGA and alginate microparticles for the controlled delivery of rhBMP-2 for bone tissue engineering. J Contr Release. 2015;198:71–79. doi: 10.1016/j.jconrel.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y., Guo W., Hu L., Yi X., Tang F. Application of hydrogels as sustained-release drug carriers in bone defect repair. Polymers. 2022;14 doi: 10.3390/polym14224906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu D., Wang H., Trinh P., Heilshorn S.C., Yang F. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials. 2017;127:132–140. doi: 10.1016/j.biomaterials.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal G., Agiwal S., Srivastava A. Hyaluronic acid containing scaffolds ameliorate stem cell function for tissue repair and regeneration. Int J Biol Macromol. 2020;165:388–401. doi: 10.1016/j.ijbiomac.2020.09.107. [DOI] [PubMed] [Google Scholar]
- 28.Fakhari A., Berkland C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013;9:7081–7092. doi: 10.1016/j.actbio.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dicker K.T., Gurski L.A., Pradhan-Bhatt S., Witt R.L., Farach-Carson M.C., Jia X. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater. 2014;10:1558–1570. doi: 10.1016/j.actbio.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang S., Zhu B., Yin P., Zhao L., Wang Y., Fu Z., Dang R., Xu J., Zhang J., Wen N. Integration of human umbilical cord mesenchymal stem cells-derived exosomes with hydroxyapatite-embedded hyaluronic acid-alginate hydrogel for bone regeneration. ACS Biomater Sci Eng. 2020;6:1590–1602. doi: 10.1021/acsbiomaterials.9b01363. [DOI] [PubMed] [Google Scholar]
- 31.Agulhon P., Robitzer M., Habas J.P., Quignard F. Influence of both cation and alginate nature on the rheological behavior of transition metal alginate gels. Carbohydr Polym. 2014;112:525–531. doi: 10.1016/j.carbpol.2014.05.097. [DOI] [PubMed] [Google Scholar]
- 32.Ho S.S., Vollmer N.L., Refaat M.I., Jeon O., Alsberg E., Lee M.A., Leach J.K. Bone morphogenetic protein-2 promotes human mesenchymal stem cell survival and resultant bone formation when entrapped in photocrosslinked alginate hydrogels. Adv Healthcare Mater. 2016;5:2501–2509. doi: 10.1002/adhm.201600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H.S., Jang J., Oh J.S., Lee E.J., Han C.M., Shin U.S. Injectable remodeling hydrogels derived from alendronate-tethered alginate calcium complex for enhanced osteogenesis. Carbohydr Polym. 2023;303 doi: 10.1016/j.carbpol.2022.120473. [DOI] [PubMed] [Google Scholar]
- 34.Jin H.H., Kim D.H., Kim T.W., Shin K.K., Jung J.S., Park H.C., Yoon S.Y. In vivo evaluation of porous hydroxyapatite/chitosan-alginate composite scaffolds for bone tissue engineering. Int J Biol Macromol. 2012;51:1079–1085. doi: 10.1016/j.ijbiomac.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Kyzas G.Z., Bikiaris D.N. Recent modifications of chitosan for adsorption applications: a critical and systematic review. Mar Drugs. 2015;13:312–337. doi: 10.3390/md13010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zang S., Zhu L., Luo K., Mu R., Chen F., Wei X., Yan X., Han B., Shi X., Wang Q., et al. Chitosan composite scaffold combined with bone marrow-derived mesenchymal stem cells for bone regeneration: in vitro and in vivo evaluation. Oncotarget. 2017;8:110890–110903. doi: 10.18632/oncotarget.22917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raafat D., von Bargen K., Haas A., Sahl H.G. Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol. 2008;74:3764–3773. doi: 10.1128/AEM.00453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernkop-Schnurch A., Dunnhaupt S. Chitosan-based drug delivery systems. Eur J Pharm Biopharm. 2012;81:463–469. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Bharathi R., Ganesh S.S., Harini G., Vatsala K., Anushikaa R., Aravind S., Abinaya S., Selvamurugan N. Chitosan-based scaffolds as drug delivery systems in bone tissue engineering. Int J Biol Macromol. 2022;222:132–153. doi: 10.1016/j.ijbiomac.2022.09.058. [DOI] [PubMed] [Google Scholar]
- 40.Lv Z., Hu T., Bian Y., Wang G., Wu Z., Li H., Liu X., Yang S., Tan C., Liang R., et al. A MgFe-ldh nanosheet-incorporated smart thermo-responsive hydrogel with controllable growth factor releasing capability for bone regeneration. Adv Mater. 2023;35 doi: 10.1002/adma.202206545. [DOI] [PubMed] [Google Scholar]
- 41.Rockwood D.N., Preda R.C., Yucel T., Wang X., Lovett M.L., Kaplan D.L. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6:1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melke J., Midha S., Ghosh S., Ito K., Hofmann S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016;31:1–16. doi: 10.1016/j.actbio.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Kim U.J., Park J., Li C., Jin H.J., Valluzzi R., Kaplan D.L. Structure and properties of silk hydrogels. Biomacromolecules. 2004;5:786–792. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- 44.Lu Q., Huang Y., Li M., Zuo B., Lu S., Wang J., Zhu H., Kaplan D.L. Silk fibroin electrogelation mechanisms. Acta Biomater. 2011;7:2394–2400. doi: 10.1016/j.actbio.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai S.M., Zhang X.L., Lv X.L., Zhang M.Y., Huang X.W., Shi Y., Lu C.H., Song J.B., Yang H.H. Bioinspired mineral-organic bone adhesives for stable fracture fixation and accelerated bone regeneration. Adv Funct Mater. 2020;30 doi: 10.1002/adfm.201908381. ARTN 1908381. [DOI] [Google Scholar]
- 46.Lin C.C., Anseth K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res (N Y) 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engebretson B., Sikavitsas V.I. Long-term in vivo effect of PEG bone tissue engineering scaffolds. J Long Term Eff Med Implants. 2012;22:211–218. doi: 10.1615/jlongtermeffmedimplants.2013006244. [DOI] [PubMed] [Google Scholar]
- 48.Tran H.D.N., Park K.D., Ching Y.C., Huynh C., Nguyen D.H. A comprehensive review on polymeric hydrogel and its composite: matrices of choice for bone and cartilage tissue engineering. J Ind Eng Chem. 2020;89:58–82. doi: 10.1016/j.jiec.2020.06.017. [DOI] [Google Scholar]
- 49.Nguyen M.K., Jeon O., Dang P.N., Huynh C.T., Varghai D., Riazi H., McMillan A., Herberg S., Alsberg E. RNA interfering molecule delivery from in situ forming biodegradable hydrogels for enhancement of bone formation in rat calvarial bone defects. Acta Biomater. 2018;75:105–114. doi: 10.1016/j.actbio.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elisseeff J., Anseth K., Sims D., McIntosh W., Randolph M., Yaremchuk M., Langer R. Transdermal photopolymerization of poly(ethylene oxide)-based injectable hydrogels for tissue-engineered cartilage. Plast Reconstr Surg. 1999;104:1014–1022. doi: 10.1097/00006534-199909040-00017. [DOI] [PubMed] [Google Scholar]
- 51.Maiolo A.S., Amado M.N., Gonzalez J.S., Alvarez V.A. Development and characterization of Poly (vinyl alcohol) based hydrogels for potential use as an articular cartilage replacement. Mater Sci Eng C. 2012;32:1490–1495. doi: 10.1016/j.msec.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 52.Kumar A., Sood A., Han S.S. Poly (vinyl alcohol)-alginate as potential matrix for various applications: a focused review. Carbohydr Polym. 2022;277 doi: 10.1016/j.carbpol.2021.118881. [DOI] [PubMed] [Google Scholar]
- 53.Bi S., Wang P., Hu S., Li S., Pang J., Zhou Z., Sun G., Huang L., Cheng X., Xing S., et al. Construction of physical-crosslink chitosan/PVA double-network hydrogel with surface mineralization for bone repair. Carbohydr Polym. 2019;224 doi: 10.1016/j.carbpol.2019.115176. [DOI] [PubMed] [Google Scholar]
- 54.Qiao S., Wu D., Li Z., Zhu Y., Zhan F., Lai H., Gu Y. The combination of multi-functional ingredients-loaded hydrogels and three-dimensional printed porous titanium alloys for infective bone defect treatment. J Tissue Eng. 2020;11 doi: 10.1177/2041731420965797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoop R. Smart biomaterials for tissue engineering of cartilage. Injury. 2008;39(Suppl 1):S77–S87. doi: 10.1016/j.injury.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 56.Xu Y., Jiang Y., Wang Y., Jia B., Gao S., Yu H., Zhang H., Lv C., Li H., Li T. LINC00473-modified bone marrow mesenchymal stem cells incorporated thermosensitive PLGA hydrogel transplantation for steroid-induced osteonecrosis of femoral head: a detailed mechanistic study and validity evaluation. Bioeng Transl Med. 2022;7 doi: 10.1002/btm2.10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren Z., Wang Y., Ma S., Duan S., Yang X., Gao P., Zhang X., Cai Q. Effective bone regeneration using thermosensitive poly(N-Isopropylacrylamide) grafted gelatin as injectable carrier for bone mesenchymal stem cells. ACS Appl Mater Interfaces. 2015;7:19006–19015. doi: 10.1021/acsami.5b02821. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y., Zhao Q., Zhang H., Yang S., Jia X. A novel poly(amido amine)-dendrimer-based hydrogel as a mimic for the extracellular matrix. Adv Mater. 2014;26:4163–4167. doi: 10.1002/adma.201400323. [DOI] [PubMed] [Google Scholar]
- 59.Luu H.H., Song W.X., Luo X., Manning D., Luo J., Deng Z.L., Sharff K.A., Montag A.G., Haydon R.C., He T.C. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 60.Tsuji K., Bandyopadhyay A., Harfe B.D., Cox K., Kakar S., Gerstenfeld L., Einhorn T., Tabin C.J., Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 61.Shen H.C., Peng H., Usas A., Gearhart B., Cummins J., Fu F.H., Huard J. Ex vivo gene therapy-induced endochondral bone formation: comparison of muscle-derived stem cells and different subpopulations of primary muscle-derived cells. Bone. 2004;34:982–992. doi: 10.1016/j.bone.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 62.Solloway M.J., Dudley A.T., Bikoff E.K., Lyons K.M., Hogan B.L., Robertson E.J. Mice lacking Bmp6 function. Dev Genet. 1998;22:321–339. doi: 10.1002/(SICI)1520-6408(1998)22:4<321::AID-DVG3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 63.Bharadwaz A., Jayasuriya A.C. Osteogenic differentiation cues of the bone morphogenetic protein-9 (BMP-9) and its recent advances in bone tissue regeneration. Mater Sci Eng C. 2021;120 doi: 10.1016/j.msec.2020.111748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daluiski A., Engstrand T., Bahamonde M.E., Gamer L.W., Agius E., Stevenson S.L., Cox K., Rosen V., Lyons K.M. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet. 2001;27:84–88. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 65.Zhu L., Liu Y., Wang A., Zhu Z., Li Y., Zhu C., Che Z., Liu T., Liu H., Huang L. Application of BMP in bone tissue engineering. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.810880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee K.S., Hong S.H., Bae S.C. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene. 2002;21:7156–7163. doi: 10.1038/sj.onc.1205937. [DOI] [PubMed] [Google Scholar]
- 67.Franceschi R.T., Xiao G. Regulation of the osteoblast-specific transcription factor, Runx2: responsiveness to multiple signal transduction pathways. J Cell Biochem. 2003;88:446–454. doi: 10.1002/jcb.10369. [DOI] [PubMed] [Google Scholar]
- 68.Shekaran A., Garcia J.R., Clark A.Y., Kavanaugh T.E., Lin A.S., Guldberg R.E., Garcia A.J. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials. 2014;35:5453–5461. doi: 10.1016/j.biomaterials.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bessa P.C., Casal M., Reis R.L. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 70.Mantripragada V.P., Jayasuriya A.C. Injectable chitosan microparticles incorporating bone morphogenetic protein-7 for bone tissue regeneration. J Biomed Mater Res. 2014;102:4276–4289. doi: 10.1002/jbm.a.35100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song X., Li X., Wang F., Wang L., Lv L., Xie Q., Zhang X., Shao X. Bioinspired protein/peptide loaded 3D printed PLGA scaffold promotes bone regeneration. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.832727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gazit D., Shteyer A., Bab I. Further characterization of osteogenic-cell growth promoting activity derived from healing bone marrow. Connect Tissue Res. 1989;23:153–161. doi: 10.3109/03008208909002415. [DOI] [PubMed] [Google Scholar]
- 73.Bab I., Gazit D., Muhlrad A., Shteyer A. Regenerating bone marrow produces a potent growth-promoting activity to osteogenic cells. Endocrinology. 1988;123:345–352. doi: 10.1210/endo-123-1-345. [DOI] [PubMed] [Google Scholar]
- 74.Policastro G.M., Becker M.L. Osteogenic growth peptide and its use as a bio-conjugate in regenerative medicine applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:449–464. doi: 10.1002/wnan.1376. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y., Xu C., Gu Y., Shen X., Zhang Y., Li B., Chen L. Polydopamine-modified poly(l-lactic acid) nanofiber scaffolds immobilized with an osteogenic growth peptide for bone tissue regeneration. RSC Adv. 2019;9:11722–11736. doi: 10.1039/c8ra08828d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Y., Xing Y., Wang M., Huang Y., Xu H., Su Y., Zhao Y., Shang Y. Supramolecular hydrogel based on an osteogenic growth peptide promotes bone defect repair. ACS Omega. 2022;7:11395–11404. doi: 10.1021/acsomega.2c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferrara N., Henzel W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 78.Augustin G., Antabak A., Davila S. The periosteum. Part 1: anatomy, histology and molecular biology. Injury. 2007;38:1115–1130. doi: 10.1016/j.injury.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y.Q., Luk J.M., Chu A.C., Ikeda K., Man K., Kaneda K., Fan S.T. TNP-470 blockage of VEGF synthesis is dependent on MAPK/COX-2 signaling pathway in PDGF-BB-activated hepatic stellate cells. Biochem Biophys Res Commun. 2006;341:239–244. doi: 10.1016/j.bbrc.2005.12.175. [DOI] [PubMed] [Google Scholar]
- 80.Tsiridis E., Giannoudis P.V. Transcriptomics and proteomics: advancing the understanding of genetic basis of fracture healing. Injury. 2006;37(Suppl 1):S13–S19. doi: 10.1016/j.injury.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 81.Geiger F., Bertram H., Berger I., Lorenz H., Wall O., Eckhardt C., Simank H.G., Richter W. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J Bone Miner Res. 2005;20:2028–2035. doi: 10.1359/JBMR.050701. [DOI] [PubMed] [Google Scholar]
- 82.Spector J.A., Mehrara B.J., Luchs J.S., Greenwald J.A., Fagenholz P.J., Saadeh P.B., Steinbrech D.S., Longaker M.T. Expression of adenovirally delivered gene products in healing osseous tissues. Ann Plast Surg. 2000;44:522–528. doi: 10.1097/00000637-200044050-00011. [DOI] [PubMed] [Google Scholar]
- 83.Kanczler J.M., Ginty P.J., Barry J.J., Clarke N.M., Howdle S.M., Shakesheff K.M., Oreffo R.O. The effect of mesenchymal populations and vascular endothelial growth factor delivered from biodegradable polymer scaffolds on bone formation. Biomaterials. 2008;29:1892–1900. doi: 10.1016/j.biomaterials.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 84.Garcia J.R., Clark A.Y., Garcia A.J. Integrin-specific hydrogels functionalized with VEGF for vascularization and bone regeneration of critical-size bone defects. J Biomed Mater Res. 2016;104:889–900. doi: 10.1002/jbm.a.35626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang G., Yuan N., Li N., Wei Q., Qian Y., Zhang J., Qin M., Wang Y., Dong S. Vascular endothelial growth factor mimetic peptide and parathyroid hormone (1-34) delivered via a blue-light-curable hydrogel synergistically accelerate bone regeneration. ACS Appl Mater Interfaces. 2022;14:35319–35332. doi: 10.1021/acsami.2c06159. [DOI] [PubMed] [Google Scholar]
- 86.Ma Y.L., Cain R.L., Halladay D.L., Yang X., Zeng Q., Miles R.R., Chandrasekhar S., Martin T.J., Onyia J.E. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142:4047–4054. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- 87.Rowshan H.H., Parham M.A., Baur D.A., McEntee R.D., Cauley E., Carriere D.T., Wood J.C., Demsar W.J., Pizarro J.M. Effect of intermittent systemic administration of recombinant parathyroid hormone (1-34) on mandibular fracture healing in rats. J Oral Maxillofac Surg. 2010;68:260–267. doi: 10.1016/j.joms.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 88.Baron R., Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab. 2012;97:311–325. doi: 10.1210/jc.2011-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takahata M., Schwarz E.M., Chen T., O'Keefe R.J., Awad H.A. Delayed short-course treatment with teriparatide (PTH(1-34)) improves femoral allograft healing by enhancing intramembranous bone formation at the graft-host junction. J Bone Miner Res. 2012;27:26–37. doi: 10.1002/jbmr.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu S., Han Z., Hao J.N., Zhang D., Li X., Cao Y., Huang J., Li Y. Engineering of a NIR-activable hydrogel-coated mesoporous bioactive glass scaffold with dual-mode parathyroid hormone derivative release property for angiogenesis and bone regeneration. Bioact Mater. 2023;26:1–13. doi: 10.1016/j.bioactmat.2023.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang L., Shi X., Zhao R., Halloran B.P., Clark D.J., Jacobs C.R., Kingery W.S. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-kappaB activation, osteoclastogenesis and bone resorption. Bone. 2010;46:1369–1379. doi: 10.1016/j.bone.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma W., Zhang X., Shi S., Zhang Y. Neuropeptides stimulate human osteoblast activity and promote gap junctional intercellular communication. Neuropeptides. 2013;47:179–186. doi: 10.1016/j.npep.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 93.Tian G., Zhang G., Tan Y.H. Calcitonin gene-related peptide stimulates BMP-2 expression and the differentiation of human osteoblast-like cells in vitro. Acta Pharmacol Sin. 2013;34:1467–1474. doi: 10.1038/aps.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suzuki A., Uemura T., Nakamura H. [Control of bone remodeling by nervous system. Neural involvement in fracture healing and bone regeneration] Clin Calcium. 2010;20:1820–1827. [PubMed] [Google Scholar]
- 95.Fang Z., Yang Q., Xiong W., Li G.H., Liao H., Xiao J., Li F. Effect of CGRP-adenoviral vector transduction on the osteoblastic differentiation of rat adipose-derived stem cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen J., Liu W., Zhao J., Sun C., Chen J., Hu K., Zhang L., Ding Y. Gelatin microspheres containing calcitonin gene-related peptide or substance P repair bone defects in osteoporotic rabbits. Biotechnol Lett. 2017;39:465–472. doi: 10.1007/s10529-016-2263-4. [DOI] [PubMed] [Google Scholar]
- 97.Lotz M., Vaughan J.H., Carson D.A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- 98.Mutolo D., Bongianni F., Cinelli E., Pantaleo T. Role of neurokinin receptors and ionic mechanisms within the respiratory network of the lamprey. Neuroscience. 2010;169:1136–1149. doi: 10.1016/j.neuroscience.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 99.Wang L., Zhao R., Shi X., Wei T., Halloran B.P., Clark D.J., Jacobs C.R., Kingery W.S. Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone. 2009;45:309–320. doi: 10.1016/j.bone.2009.04.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goto T., Nakao K., Gunjigake K.K., Kido M.A., Kobayashi S., Tanaka T. Substance P stimulates late-stage rat osteoblastic bone formation through neurokinin-1 receptors. Neuropeptides. 2007;41:25–31. doi: 10.1016/j.npep.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 101.Lee J.S., Jin Y., Park H.J., Yang K., Lee M.S., Yang H.S., Cho S.W. In situ bone tissue engineering with an endogenous stem cell mobilizer and osteoinductive nanofibrous polymeric scaffolds. Biotechnol J. 2017;12 doi: 10.1002/biot.201700062. [DOI] [PubMed] [Google Scholar]
- 102.Li S., Cui Y., Liu H., Tian Y., Wang G., Fan Y., Wang J., Wu D., Wang Y. Application of bioactive metal ions in the treatment of bone defects. J Mater Chem B. 2022;10:9369–9388. doi: 10.1039/d2tb01684b. [DOI] [PubMed] [Google Scholar]
- 103.Hung C.C., Chaya A., Liu K., Verdelis K., Sfeir C. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway. Acta Biomater. 2019;98:246–255. doi: 10.1016/j.actbio.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 104.Wu L., Luthringer B.J., Feyerabend F., Schilling A.F., Willumeit R. Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater. 2014;10:2843–2854. doi: 10.1016/j.actbio.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 105.Chen Y., Sheng W., Lin J., Fang C., Deng J., Zhang P., Zhou M., Liu P., Weng J., Yu F., et al. Magnesium oxide nanoparticle coordinated phosphate-functionalized chitosan injectable hydrogel for osteogenesis and angiogenesis in bone regeneration. ACS Appl Mater Interfaces. 2022;14:7592–7608. doi: 10.1021/acsami.1c21260. [DOI] [PubMed] [Google Scholar]
- 106.Yuan Z., Wei P., Huang Y., Zhang W., Chen F., Zhang X., Mao J., Chen D., Cai Q., Yang X. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomater. 2019;85:294–309. doi: 10.1016/j.actbio.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 107.Ding X., Li X., Li C., Qi M., Zhang Z., Sun X., Wang L., Zhou Y. Chitosan/dextran hydrogel constructs containing strontium-doped hydroxyapatite with enhanced osteogenic potential in rat cranium. ACS Biomater Sci Eng. 2019;5:4574–4586. doi: 10.1021/acsbiomaterials.9b00584. [DOI] [PubMed] [Google Scholar]
- 108.Hassani A., Avci C.B., Kerdar S.N., Amini H., Amini M., Ahmadi M., Sakai S., Bagca B.G., Ozates N.P., Rahbarghazi R., et al. Interaction of alginate with nano-hydroxyapatite-collagen using strontium provides suitable osteogenic platform. J Nanobiotechnol. 2022;20:310. doi: 10.1186/s12951-022-01511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ali A., Ershad M., Vyas V.K., Hira S.K., Manna P.P., Singh B.N., Yadav S., Srivastava P., Singh S.P., Pyare R. Studies on effect of CuO addition on mechanical properties and in vitro cytocompatibility in 1393 bioactive glass scaffold. Mater Sci Eng C. 2018;93:341–355. doi: 10.1016/j.msec.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 110.Lu Y., Li L., Zhu Y., Wang X., Li M., Lin Z., Hu X., Zhang Y., Yin Q., Xia H., et al. Multifunctional copper-containing carboxymethyl chitosan/alginate scaffolds for eradicating clinical bacterial infection and promoting bone formation. ACS Appl Mater Interfaces. 2018;10:127–138. doi: 10.1021/acsami.7b13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li C., Sun F., Tian J., Li J., Sun H., Zhang Y., Guo S., Lin Y., Sun X., Zhao Y. Continuously released Zn(2+) in 3D-printed PLGA/beta-TCP/Zn scaffolds for bone defect repair by improving osteoinductive and anti-inflammatory properties. Bioact Mater. 2023;24:361–375. doi: 10.1016/j.bioactmat.2022.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu L., Xu S., Xiang T.Y., Chen L.W., Zhong W.X., Zhu L., Liu H., Wu L., Li W.D., Wang Y.T., et al. A novel peptide hydrogel of metal ion clusters for accelerating bone defect regeneration. J Contr Release. 2023;353:738–751. doi: 10.1016/j.jconrel.2022.12.031. [DOI] [PubMed] [Google Scholar]
- 113.Perez R.A., Kim J.H., Buitrago J.O., Wall I.B., Kim H.W. Novel therapeutic core-shell hydrogel scaffolds with sequential delivery of cobalt and bone morphogenetic protein-2 for synergistic bone regeneration. Acta Biomater. 2015;23:295–308. doi: 10.1016/j.actbio.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 114.Wattel A., Kamel S., Mentaverri R., Lorget F., Prouillet C., Petit J.P., Fardelonne P., Brazier M. Potent inhibitory effect of naturally occurring flavonoids quercetin and kaempferol on in vitro osteoclastic bone resorption. Biochem Pharmacol. 2003;65:35–42. doi: 10.1016/s0006-2952(02)01445-4. [DOI] [PubMed] [Google Scholar]
- 115.Saito A., Suzuki Y., Ogata S., Ohtsuki C., Tanihara M. Prolonged ectopic calcification induced by BMP-2-derived synthetic peptide. J Biomed Mater Res. 2004;70:115–121. doi: 10.1002/jbm.a.30071. [DOI] [PubMed] [Google Scholar]
- 116.Ang E.S., Yang X., Chen H., Liu Q., Zheng M.H., Xu J. Naringin abrogates osteoclastogenesis and bone resorption via the inhibition of RANKL-induced NF-kappaB and ERK activation. FEBS Lett. 2011;585:2755–2762. doi: 10.1016/j.febslet.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 117.Chiba H., Uehara M., Wu J., Wang X., Masuyama R., Suzuki K., Kanazawa K., Ishimi Y. Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. J Nutr. 2003;133:1892–1897. doi: 10.1093/jn/133.6.1892. [DOI] [PubMed] [Google Scholar]
- 118.Kong L., Yang C., Yu L., Smith W., Zhu S., Zhu J., Zhu Q. Pyrroloquinoline quinine inhibits RANKL-mediated expression of NFATc1 in part via suppression of c-Fos in mouse bone marrow cells and inhibits wear particle-induced osteolysis in mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen X., Zhang S., Chen X., Hu Y., Wu J., Chen S., Chang J., Wang G., Gao Y. Emodin promotes the osteogenesis of MC3T3-E1 cells via BMP-9/Smad pathway and exerts a preventive effect in ovariectomized rats. Acta Biochim Biophys Sin. 2017;49:867–878. doi: 10.1093/abbs/gmx087. [DOI] [PubMed] [Google Scholar]
- 120.Rahman M.S., Akhtar N., Jamil H.M., Banik R.S., Asaduzzaman S.M. TGF-beta/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3 doi: 10.1038/boneres.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marsell R., Sisask G., Nilsson Y., Sundgren-Andersson A.K., Andersson U., Larsson S., Nilsson O., Ljunggren O., Jonsson K.B. GSK-3 inhibition by an orally active small molecule increases bone mass in rats. Bone. 2012;50:619–627. doi: 10.1016/j.bone.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 122.Li X., Yang J., He X., Yang Z., Ding Y., Zhao P., Liu Z., Shao H., Li Z., Zhang Y., et al. Identification of upregulators of BMP2 expression via high-throughput screening of a synthetic and natural compound library. J Biomol Screen. 2009;14:1251–1256. doi: 10.1177/1087057109346446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.