Abstract
Background/Aims
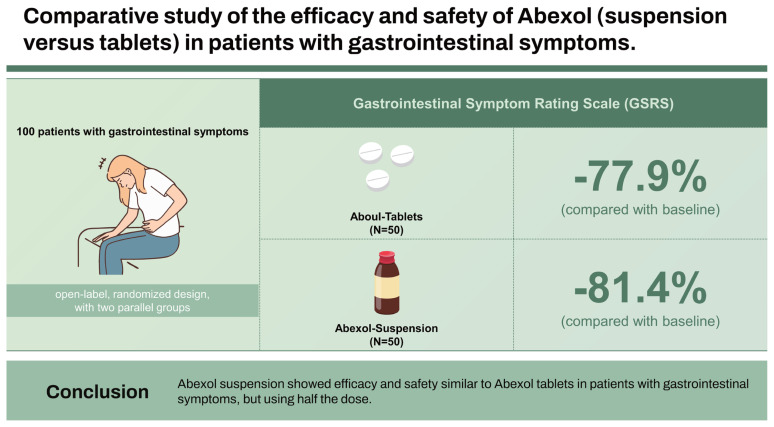
Abexol is a mixture of primary aliphatic alcohols purified from beeswax (Apis mellifera), that produces anti-inflammatory, antioxidant and gastroprotective effects, as well as it is safe and well tolerated. To investigate and compare the efficacy and safety of Abexol (suspension versus tablets) in patients with gastrointestinal symptoms.
Methods
Monocentric study, open-label, randomized design, with two parallel groups receiving Abexol tablets (150 mg/d) or Abexol suspension (75 mg/d) for 8 weeks. Primary efficacy variable (significant improvement in the total score of Gastrointestinal Symptom Rating Scale [GSRS]). Significant reduction in the intensity of the gastrointestinal-symptoms and the reduction in the consumption of antacids are considered secondary efficacy variable. Short form-36 (SF-36) quality of life questionnaire was evaluated as collateral variable. Data were analyzed as per intention to treat.
Results
A significantly decrease in the overall score of the survey was observed with respect to the baseline level (p < 0.001) of 81.4% in the Abexol suspension group and 77.9% in the Abexol tablets group. At the end of the trial, most gastrointestinal-symptoms disappeared or reduced significantly. The frequency of consumption of neutralizing antacids was low. The significantly improvement in the perception of the state of health obtained in the Abexol is in correspondence with the improvement achieved in some of the components evaluate in the SF-36 questionnaire. Both treatments were safe and well tolerated.
Conclusions
Abexol suspension showed efficacy and safety similar to Abexol tablets in patients with gastrointestinal symptoms, but using half the dose.
Keywords: Abexol suspension, D-002, Beeswax, Gastroprotective effects, Gastrointestinal symptoms
Graphical abstract
INTRODUCTION
Gastrointestinal diseases (which include diseases of the upper and lower gastrointestinal tract, liver and pancreas) as a whole are a major health problem worldwide, constituting the third leading cause of death, if cancers of the gastrointestinal system are included [1–3].
Acid peptic diseases are a group of disorders of the gastrointestinal tract that involve damage to the gastric mucosa owing to the action of pepsin and hydrochloric acid secretion that usually occur in the stomach and proximal duodenum. These diseases affect approximately 10% of the world population and constitute 10% of the causes of hospital admissions [1].
Some of the manifestations of acid peptic diseases are gastroesophageal reflux, gastritis, gastric, duodenal and esophageal ulcers. Ulcers are lesions produced by the loss of continuity of the mucosa of the gastrointestinal tract whose most common symptoms include urgent epigastric pain associated with other symptoms such as belching, nausea, vomiting, heartburn, upper digestive bleeding [4].
For its part, the acute or chronic gastritis consists of an inflammation of the stomach lining, which also causes discomfort or epigastric pain, nausea, vomiting, a feeling of fullness, bleeding and heartburn [5]. Gastroesophageal reflux occurs when gastroduodenal contents reflux into the esophagus, injuring the mucosa to varying degrees. The typical symptoms are heartburn and regurgitation [6].
Control of “external” factors (Helicobacter pylori infection, consumption of non-steroidal anti-inflammatory drugs, consumption of ethanol, stress) is a first step in the management of acid peptic diseases and must be maintain as part of a healthy lifestyle to prevent recurrences. When this is not enough, it is necessary to use pharmacological therapy with the aim of relieving symptoms, healing existing ulcers and preventing recurrences and complications. Among the medications that can be uses for this purpose, proton pump inhibitors (PPIs) and histaminergic type 2 receptor antagonists (H2RAs) represent the first-line treatment of acid-peptic disease. Cytoprotectors such as sucralfate, colloidal bismuth succitrate, shovstakosky balm, as well as over-the-counter antacids are also used, and prokinetic agents such as metoclopramide, domperidone, cinitapride, among others, have been uses to manage gastroesophageal reflux [7].
On the other hand, based on the involvement of oxidative stress in mucosal damage [7], it’s logical to expect that substances with antioxidant effects may have a gastroprotective effect. In line with this, the effects of the active ingredient of Abexol have been evaluate in different models of gastrointestinal mucosal damage and in clinical trials in subjects with symptoms of gastrointestinal discomfort, typical of acid- peptic disease.
Abexol (D-002), a mixture of higher primary aliphatic alcohols (tetracosanol, hexacosanol, octacosanol, triacontanol, dotriacontanol y tetratriacontanol) obtained from beeswax (Apis mellifera) [8], is a nutritional supplement that produces anti-inflammatory, antioxidant and gastroprotective effects in experimental models [9–19] and in clinical studies [20–29], as well as safe and very well tolerated.
Experimental toxicology studies have shown the safety of short and long-term treatment with the substance, finding no toxicity related to its use, even when doses 1,000 times higher than the recommended therapeutic dose are used. On the other hand, it does not present mutagenic or geno toxic potential in the different in vitro and in vivo tests carried out, nor does it show evidence of fetal or reproductive toxicity [30–33].
Clinical trials on patients with symptoms of acid peptic disease, demonstrating its efficacy, safety and tolerability [24–28].
Clinical evidence suggests greater bioavailability and immediacy of the pharmacological action of drugs in liquid forms than in solid forms, so it is logical to expect that Abexol (suspension) shows similar or superior efficacy, safety and tolerability to Abexol (tablets) in patients with gastrointestinal symptoms, eliminating the difficulty reported by some patients to swallow the tablets.
The aim of the study was to investigate and compare the efficacy and safety of Abexol (suspension versus tablets) for 8 weeks in patients with gastrointestinal symptoms.
METHODS
The study was conduct according to the principles reflected in the Helsinki Declaration [34], as well as the recommendations of the World Health Organization and the Cuban regulations on Good Clinical Practices. The study protocol was approved by the Ministry of Public Health and by the Ethics Committee in Clinical Research of the National Gastroenterology Institute (IRB-2021-01), and was register in the Cuban Public Registry of Clinical Trials (RPCEC-00000372).
Study design
The study had a monocentric, open-label, randomized design, with two parallel groups that received Abexol 150 mg/d tablets (3 tablets of 50 mg) or Abexol 75 mg/d suspension (3 teaspoons of 5 mL, 5 mg/mL), 3 times a day (30 minutes before breakfast, lunch and dinner) for 8 weeks.
Patients with symptoms of gastric discomfort typical of acid peptic diseases were study. The sample was composed of 100 subjects, 50 in each treatment group.
This study evaluated the effects of two finished forms with the same active ingredient, the tablets and the suspension.
However, the doses used are not the same, the daily dose in the tablet treatment group was 150 mg/d while the patients treated with the suspension received 75 mg/d, without there being a group with the same dose. This makes comparison between groups difficult and prevents establishing the superiority of the suspension, with the same tablet dose; however, it has the value of comparing the dosage unit indicated in each case, 1 tablet versus 1 teaspoon, and the effects in each case. Subsequent studies should be designed to establish the dose-effect relationship of the suspension and to establish the comparison between equal doses of both finished forms.
Batches used for suspension and tablet formulation are different. The batches used to manufacture these finished forms fulfill the quality specifications approved for this active ingredient.
All patients in the Abexol tablets group received tablets from the same batch and all patients in the Abexol suspension group received suspension from the same batch.
The study consisted of six consultations: recruitment, inclusion, and four follow-up consultations at 2, 4, 6 and 8 weeks of treatment.
Recruitment/inclusion criteria
The sample was selected from patients who attended the Outpatient Clinic of the National Institute of Gastroenterology for presenting gastrointestinal symptoms that required medical attention, and agreed to participate in the study, after signing their informed consent.
Patients of both sexes, aged between 20 and 70 years, who reported some symptom of gastrointestinal discomfort, typical of acid-peptic diseases such as epigastralgia (abdominal pain located in the epigastric region), heartburn, nausea, flatulence, regurgitation, belching, abdominal distension, early satiety, vomiting, anorexia and feeling of incomplete emptying.
Exclusion criteria
Patients with confirmatory endoscopy of gastric, duodenal or esophageal ulcer; organic lesions of the upper digestive tract that require specific treatment; benign and malignant digestive neoplasms; alarm symptoms (digestive bleeding, anemia, significant loss of body weight, progressive dysphagia, odynophagia, persistent vomiting, lymphadenopathy, palpation of an abdominal mass), active liver or kidney disease. Other non-digestive neoplasms, disorders of the thyroid gland, irritable bowel syndrome, pancreatic conditions (acute or chronic pancreatitis), intrahepatic and extrahepatic bile duct affections, ischemic changes, glucose > 7 mmol/L, aspartate amine transferase (AST) and alanine amine transferase (ALT) > 55 IU, creatinine > 130 μmol/L, diastolic arterial hypertension (> 105 mmHg), patients with any other special condition that, in the opinion of the doctor, put their health and life at risk during the study.
The consumption of PPIs, H2RAs, mucoprotectors, drugs and/or supplements with antioxidant, prokinetic, antiemetic and antiflatulant action other than the study medication was not allow.
Primary efficacy endpoints
The significantly improvement in the total score of the Gastrointestinal Symptom Rating Scale (GSRS) survey was evaluated [35]. Treatments considered effective if the improvement obtained at the end of the study was significantly different from the baseline level.
This is a validated questionnaire, to discriminate digestive symptoms, which consists of 15 questions that are answer using a scale ranging from 0–3 points: from not presenting symptoms (0) to suffering them in the most frequent and intense way (3). The overall score ranges from 0–45 points, the lower the better the patient’s status in terms of gastrointestinal-symptoms [35].
Secondary efficacy endpoints
The significantly reduction in the intensity of the gastrointestinal-symptoms was evaluated as a secondary efficacy variable, while the reduction in the consumption of antacids with neutralizing action was allowed.
Collateral variable
The effect on quality of life was evaluate using the Short form-36 (SF-36) questionnaire [36]. Questionnaire is made up of 36 questions (items) that assess both positive and negative states of health. The questionnaire covers eight scales, which represent the health concepts most frequently used in the main health questionnaires, as well as the aspects most related to the disease and treatment. The 36 items of the instrument cover the following scales: physical function, physical role, body pain, general health, vitality, social function, emotional role, and mental health. Additionally, the SF-36 includes a transition item that asks about the change in the general state of health with respect to the previous evaluation. This variable was evaluated at the beginning and at 8 weeks of treatment.
Safety and tolerability
Data from a physical examination (bodyweight, pulse rate and arterial pressure), laboratory indicators and requests for adverse events (AE) were included for safety and tolerability analysis. All undesirable events that newly appeared to patients during the trials, disregarding the cause, considered as AE. In accordance with their intensity, AE were classified as mild, moderate or severe [37].
Laboratory analyses
For laboratory analysis, venous blood samples obtained under fasting conditions for not less than 12 hours nor more than 16 hours. The hematological variables (hemoglobin, hematocrit, red blood cell count, white blood cell count, and platelet count) were determined automatically in a hematological complex. Blood biochemistry variables (AST, ALT, glucose, creatinine) were determine by enzymatic methods using reagent kits (Roche, Basel, Switzerland). The determinations made in automatized equipment located in the Clinical Laboratory of the National Gastroenterology Institute.
Statistical analysis
Data analysis performed according to the intention-to-treat method, including all randomized patients, regardless of compliance with the treatments studied, and data imputation using the carryover method.
Variables with categorical values were present in contingency tables with absolute values, proportions, and percentages for each category, using the Pearson χ2 test with Yates correction. In the case of quantitative variables, the Student’s test was applied, if the data follow a normal distribution, and the Levene’s test to evaluate the homogeneity of the variances in the mean contrasts.
All the statistical tests were two-tailed and the level of significance established a priori for all the statistical tests that were used was α = 0.05.
Data management and statistical analysis were carried out in the Department of Data Management and Processing of National Clinical Trials Coordinators Centre.
RESULTS
Baseline characteristics
In this study, 123 patients were recruit, of whom 100 were included in the active treatment phase. Twenty-three patients were not included due to the following reasons: consumption of illegal drugs (n = 2), anemia (n = 1), chronic liver disease (n = 1), diagnosed ulcer (n = 15), esophagitis (n = 2), older than 70 years (n = 2).
Table 1 shows the main baseline characteristics of the study population. Abexol suspension group and Abexol tablets group were statistically homogeneous in all the comparisons made. During the studied there were three dropouts: one due to incorporating ranitidine from the Abexol suspension group, and two due to incorporating Omeprazole: one from the Abexol suspension group and one from the Abexol tablets group, the rest of the included patients completed the trial.
Table 1.
Baseline characteristic of study populations
| Variable | Abexol-tablets (n = 50) | Abexol-suspension (n = 50) | Total (n = 100) |
|---|---|---|---|
| Age (yr) | 51.6 ± 14.6 | 51.7 ± 13.5 | 51.6 ± 14.0 |
| Body mass index (kg/m2) | 25.7 ± 5.3 | 25.6 ± 4.6 | 25.7 ± 4.9 |
| Sex | |||
| Female | 38 (76.0) | 32 (64.0) | 70 (70.0) |
| Male | 12 (24.0) | 18 (36.0) | 30 (30.0) |
| Personal history | |||
| Arterial hypertension | 19 (38.0) | 20 (40.0) | 39 (39.0) |
| Overweight (25–30 kg/m2) | 13 (26.0) | 16 (32.0) | 29 (29.0) |
| Obesity (≥ 30 kg/m2) | 11 (22.0) | 8 (16.0) | 19 (19.0) |
| Smoking | 12 (24.0) | 5 (10.0) | 17 (17.0) |
| Diabetes mellitus | 3 (6.0) | 2 (4.0) | 5 (5.0) |
| Hypercholesterolemia | 4 (8.0) | 1 (2.0) | 5 (5.0) |
| Coronary disease | 1 (2.0) | 1 (2.0) | 2 (2.0) |
| Family history | |||
| Gastrointestinal cancer | 7 (14.0) | 10 (20.0) | 17 (17.0) |
| CM | |||
| Patients consuming CM | 28 (56.0) | 30 (60.0) | 58 (58.0) |
| IACE | 13 (26.0) | 13 (26.0) | 26 (26.0) |
| Diuretics | 12 (24.0) | 10 (20.0) | 22 (22.0) |
| β-blockers | 5 (10.0) | 5 (10.0) | 10 (10.0) |
| Calcium antagonists | 4 (8.0) | 2 (4.0) | 6 (6.0) |
| Antiplatelets drugs | 2 (4.0) | 3 (6.0) | 5 (5.0) |
| Antiallergic | 1 (2.0) | 4 (8.0) | 5 (5.0) |
| Lipid lowering drugs | 4 (8.0) | 1 (2.0) | 5 (5.0) |
| Oral hypoglycemic drugs | 1 (2.0) | 2 (4.0) | 3 (3.0) |
| Antiasthmatic | 1 (2.0) | 1 (2.0) | 2 (2.0) |
| Antidepressants | 2 (4.0) | 0 (0.0) | 2 (2.0) |
Values are presented as mean ± standard deviation or number (%).
CM, concomitant medications; IACE, inhibitors of angiotensin converting enzyme.
The table includes only those consumed by ≥ 2 patients.
No significant between group differences were found (Student test, χ2 test).
The adherence to treatment was satisfactory and comparable between groups, since the patients consumed > 85% of the treatment that corresponded to them. Adherence to the treatment was confirmed by counting the remaining tablets, checking the bottles with suspension and interviewing the patients in the consultations.
Primary efficacy endpoints
Table 2 summarizes the effects of treatment on the evaluated GSRS survey score. At the beginning of the study, the groups presented similar scores in all domains of the scale. In both groups, a significantly decrease in the global score of the survey was observed with respect to the baseline level (p < 0.001) of 81.4% in the Abexol suspension group and 77.9% in the Abexol tablets group, showing no significantly differences in the comparisons between groups at different treatment times.
Table 2.
Effects on the scores of Gastrointestinal Symptom Rating Scale (GSRS)
| Abexol-treatment | Baseline | Week 2 | Week 4 | Week 6 | Week 8 | Changes (%) |
|---|---|---|---|---|---|---|
| Abdominal pain | ||||||
| Tablets | 0.5 ± 0.5 | 0.5 ± 0.5 | 0.1 ± 0.3** | 0.1 ± 0.4** | 0.1 ± 0.4** | −80.0 |
| Suspension | 0.8 ± 0.7 | 0.8 ± 0.7 | 0.1 ± 0.4** | 0.1 ± 0.3** | 0.1 ± 0.2** | −87.5 |
| Acidity/hearthburn | ||||||
| Tablets | 0.8 ± 0.7 | 0.8 ± 0.7 | 0.5 ± 0.6* | 0.3 ± 0.5** | 0.2 ± 0.5** | −75.0 |
| Suspension | 0.8 ± 0.8 | 0.8 ± 0.8 | 0.3 ± 0.5* | 0.2 ± 0.4** | 0.2 ± 0.4** | −75.0 |
| Acid regurgitation | ||||||
| Tablets | 0.9 ± 0.7 | 0.9 ± 0.8 | 0.3 ± 0.5* | 0.1 ± 0.4** | 0.0 ± 0.2** | −100.0 |
| Suspension | 0.6 ± 0.8 | 0.6 ± 0.8 | 0.1 ± 0.3* | 0.1 ± 0.3** | 0.1 ± 0.2** | −83.3 |
| Sensation of stomach emptiness | ||||||
| Tablets | 0.8 ± 0.7 | 0.8 ± 0.7 | 0.4 ± 0.5* | 0.2 ± 0.4** | 0.1 ± 0.3** | −87.5 |
| Suspension | 0.7 ± 0.7 | 0.7 ± 0.7 | 0.3 ± 0.5* | 0.2 ± 0.4** | 0.1 ± 0.3** | −85.7 |
| Nauseas & vomits | ||||||
| Tablets | 0.3 ± 0.5 | 0.4 ± 0.5 | 0.0 ± 0.2** | 0.0 ± 0.2** | 0.0 ± 0.2** | −100.0 |
| Suspension | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.1 ± 0.2** | 0.1 ± 0.2** | 0.1 ± 0.2* | −66.7 |
| Abdominal noises | ||||||
| Tablets | 0.8 ± 0.6 | 0.8 ± 0.6 | 0.3 ± 0.5* | 0.1 ± 0.4** | 0.1 ± 0.4** | −87.5 |
| Suspension | 0.6 ± 0.6 | 0.6 ± 0.6 | 0.2 ± 0.4* | 0.1 ± 0.4** | 0.1 ± 0.2** | −83.3 |
| Abdominal bloating | ||||||
| Tablets | 0.8 ± 0.7 | 0.8 ± 0.7 | 0.2 ± 0.4* | 0.1 ± 0.3** | 0.1 ± 0.3** | −87.5 |
| Suspension | 0.8 ± 0.8 | 0.8 ± 0.8 | 0.2 ± 0.4* | 0.1 ± 0.3** | 0.1 ± 0.3** | −87.5 |
| Eructation | ||||||
| Tablets | 0.9 ± 0.7 | 0.9 ± 0.7 | 0.6 ± 0.5* | 0.4 ± 0.5** | 0.3 ± 0.5** | −66.7 |
| Suspension | 0.6 ± 0.6 | 0.6 ± 0.6 | 0.4 ± 0.5* | 0.2 ± 0.4** | 0.2 ± 0.4** | −66.7 |
| Flatulence | ||||||
| Tablets | 1.0 ± 0.7 | 1.0 ± 0.7 | 0.5 ± 0.5* | 0.4 ± 0.5** | 0.4 ± 0.5** | −60.0 |
| Suspension | 0.8 ± 0.7 | 0.8 ± 0.7 | 0.4 ± 0.5* | 0.3 ± 0.5** | 0.3 ± 0.5** | −62.5 |
| Slow intestinal transit | ||||||
| Tablets | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.2 ± 0.5 | 0.2 ± 0.4 | 0.1 ± 0.4** | −66.7 |
| Suspension | 0.2 ± 0.5 | 0.2 ± 0.5 | 0.1 ± 0.2 | 0.1 ± 0.3 | 0.1 ± 0.2 | −50.0 |
| Accelerated intestinal transit | ||||||
| Tablets | 0.2± 0.6 | 0.2 ± 0.6 | 0.1 ± 0.4 | 0.1 ± 0.3* | 0.1 ± 0.3* | −50.0 |
| Suspension | 0.1 ± 0.4 | 0.1 ± 0.4 | 0.1 ± 0.3 | 0.1 ± 0.2 | 0.0 ± 0.2* | −100.0 |
| Soft feces | ||||||
| Tablets | 0.4 ± 0.7 | 0.4 ± 0.7 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.1 ± 0.4* | −75.0 |
| Suspension | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.2 ± 0.4 | 0.1 ± 0.2 | 0.0 ± 0.1* | −100.0 |
| Hard feces | ||||||
| Tablets | 0.3 ± 0.6 | 0.3 ± 0.6 | 0.1 ± 0.3 | 0.1 ± 0.4 | 0.0 ± 0.2* | −100.0 |
| Suspension | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.0 ± 0.3 | 0.1 ± 0.3 | 0.0 |
| Urgency for defecation | ||||||
| Tablets | 0.1 ± 0.4 | 0.2 ± 0.4 | 0.1 ± 0.4 | 0.1 ± 0.3 | 0.1 ± 0.2 | 0.0 |
| Suspension | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.0 ± 0.2 | 0.0 ± 0.1 | 0.0 ± 0.1 | −100.0 |
| Sensation of incomplete emptiness | ||||||
| Tablets | 0.4 ± 0.5 | 0.3 ± 0.5 | 0.1 ± 0.2** | 0.0 ± 0.2** | 0.1 ± 0.2** | −75.0 |
| Suspension | 0.8 ± 0.8 | 0.8 ± 0.8 | 0.2 ± 0.4** | 0.1 ± 0.3** | 0.1 ± 0.3** | −87.5 |
| Whole score | ||||||
| Tablets | 8.6 ± 3.3 | 8.6 ± 3.3 | 3.6 ± 2.5** | 2.5 ± 1.8** | 1.9 ± 2.7** | −77.9 |
| Suspension | 7.0 ± 3.6 | 6.9 ± 3.6 | 2.8 ± 1.9** | 1.7 ± 1.4** | 1.3 ± 1.2** | −81.4 |
Values are presented as mean ± standard deviation.
p < 0.0125,
p < 0.001 comparison with baseline (Student test, Bonferroni adjustment).
Secondary efficacy endpoints
Regarding the analysis of each of the items that are part of the GSRS scale, a significantly improvement was observe in most of the gastrointestinal-symptoms in relation to the baseline level in both treatment groups (Table 2).
The frequency of consumption of neutralizing antacids at least once during the study in the Abexol suspension 6/50 group (12.0%) was low and similar to that in the Abexol tablets 7/50 group (14.0%).
On the other hand, an analysis carried out at the end of the study on the effects of treatment in the perception of the state of health showed that the frequency of patients treated with Abexol suspension who report feeling better (46/50, 92.0%) was similar to that in the Abexol tablets group (48/50, 96.0%), while only 4 patients (8.0%) in the Abexol suspension group and 2 patients (4.0%) in the Abexol tablets group report feeling the same or no significantly improvement in gastrointestinal-symptoms at the end of the study.
Collateral variable
Treatment with Abexol suspension significantly improvement the score of some of the components of the SF-36 questionnaire related to physical health (physical role, body pain, and general health), as well as the score of some of the components related to mental health (vitality and social function), while in the Abexol tablets group this significant improvement was only observed in the score of some items corresponding to physical health (physical role and general health). However, the comparisons made between groups did not show significant differences (Table 3).
Table 3.
Effects on the scores of questionnaire of quality of life SF-36
| Abexol-treatment | Baseline | Week 8 |
|---|---|---|
| Physical function | ||
| Tablets | 88.8 ± 17.4 | 88.3 ± 18.0 |
| Suspension | 82.5 ± 22.4 | 85.3 ± 21.0 |
| Physical role | ||
| Tablets | 64.0 ± 20.3 | 82.0 ± 34.6** |
| Suspension | 60.5 ± 23.2 | 78.5 ± 37.8** |
| Corporal pain | ||
| Tablets | 75.6 ± 20.9 | 78.0 ± 21.0 |
| Suspension | 68.2 ± 28.3 | 75.3 ± 27.1** |
| General health | ||
| Tablets | 59.6 ± 9.6 | 81.3 ± 11.4** |
| Suspension | 60.5 ± 9.3 | 80.6 ± 10.6** |
| Vitality | ||
| Tablets | 62.1 ± 14.4 | 65.1 ± 16.8 |
| Suspension | 58.7 ± 17.7 | 62.5 ± 18.0* |
| Social function | ||
| Tablets | 48.0 ± 10.8 | 46.5 ± 13.1 |
| Suspension | 45.0 ± 11.6 | 48.0 ± 11.7* |
| Emotional role | ||
| Tablets | 88.7 ± 26.6 | 67.2 ± 11.3 |
| Suspension | 87.3 ± 30.8 | 65.4 ± 12.4 |
| Mental health | ||
| Tablets | 66.7 ± 11.3 | 66.7 ± 11.3 |
| Suspension | 67.4 ± 13.2 | 67.4 ± 13.2 |
Values are presented as mean ± standard deviation.
p < 0.05,
p < 0.01 comparison with baseline (Student test for paired samples).
Safety and tolerability
Both treatments were safe and well tolerated. In basal conditions, both groups were statistically similar. There were no significant changes in the physical and laboratory indicators investigated during the trial, with the exception of a small reduction in the values of hemoglobin, hematocrit, glucose and creatinine in the group treated with Abexol tablets compared to the baseline level. However, the comparisons made between groups did not show significant differences and all the individual values of the variables evaluated remained within the normal range (data not shown for simplicity).
One patient in the Abexol tablets group reported an adverse experience (low back pain), which was classified as moderate, as it required treatment with ibuprofen and not related to the investigational product.
DISCUSSION
This study shows that the effects induced by Abexol suspension on the primary and secondary response variables are significant with respect to baseline levels and similar to the effects induced by Abexol tablets, using half the dose.
The administration of Abexol suspension and Abexol tablets for eight weeks improved gastric symptoms assessed through the GSRS questionnaire, improved the general state of health and quality of life assessed through the SF-36 questionnaire in patients with gastrointestinal symptoms.
The study population was homogeneous, as evidenced by the similarity of their baseline characteristics, which indicates that the randomization process was satisfactory and that the effects on the efficacy variables demonstrated here are relate to the treatments evaluated.
Although all the patients presented gastric symptoms from the moment of their recruitment, the consumption of other gastroprotective drugs different from the study medications was not allow, with the exception of the use of antacids with neutralizing action, depending on the symptoms they presented. In this sense, the frequency of consumption of neutralizing antacids at least once during the study in the Abexol suspension group was low, only 6 patients/50 (12.0%) and similar to that of the Abexol tablets group where 7 patients/50 (14.0%) required their consumption.
The results of the present study show that Abexol suspension administered for eight weeks to patients with gastrointestinal symptoms significantly reduced the total score of the GSRS scale, as well as, certain symptoms evaluated as independent items in the survey, which is consistent with the effects obtained with Abexol tablets and those found in other short and medium-term studies where Abexol tablets were administered [26–29]. However, patients treated with the suspension received a dose of 75 mg/d, half the dose used with the tablets.
According to the above, it’s corroborated that the final form used is decisive for the effect and influences the potency of the substance [38]. In this case, the active ingredient in suspension form has greater bioavailability than in tablet form, therefore that the same effect obtained using less substance.
At the beginning, both groups presented similar scores in all domains of the scale and in the general score. In both groups treated with Abexol (suspension or tablets) after 4 weeks of treatment, a significant decrease in the overall score of the survey was observe with respect to the baseline level, a difference that became more marked at the end of treatment. At eight weeks, there is a change of 81.4% in the overall score in the group treated with Abexol suspension versus 77.9% in the Abexol tablets group, which coincides with what was report in previous studies with Abexol tablets, while the difference between groups was not statistically significant.
In the analysis by domains, in both groups, at the end of the trial, most of the symptoms disappeared and others were significantly reduced.
The individual variables most affected at the beginning of the study and that responded best to the treatments were abdominal pain, acidity/heartburn, acid regurgitation, sensation of stomach emptiness, abdominal noise, abdominal bloating, eructation and flatulence.
These results are in correspondence with those reported in short- and medium-term studies [26,27] in which the significant reduction in the score of various items of the GSRS scale in relation to the beginning corresponds mostly to the most frequent symptoms, as well as coincides with those of an open-label follow-up study in which a significant improvement of different gastrointestinal symptoms was observed in subjects taking Abexol tablets.
The significant improvement in the perception of the state of health obtained both in the Abexol suspension group and in the Abexol tablets group at the end of the study, is in correspondence with the improvement in the symptoms that these patients presented during the trial, as well as with the improvement achieved in some of the components evaluated in the SF-36 questionnaire and related to physical and mental health, which contributes to an improvement in the quality of life of these patients.
Gastrointestinal symptoms can affect the quality of life of patients, becoming a social problem, since they constitute a cause of decreased work capacity and sick leave. The effects on the quality of life depend on the intensity of the symptoms rather than their origin, so it is important to have tools for the evaluation of the symptoms and the follow-up of their repercussion on the perception of the quality of life of the patients.
The quality of life questionnaires in organic digestive diseases are a complement that allow an adequate clinical assessment and, above all, the quantification of the changes after a certain therapeutic intervention, within these, the GSRS is a scoring scale, validated internationally for the evaluation of gastrointestinal symptoms [39,40].
These results are in line with what was expect, since in previous studies Abexol (tablets) after eight weeks produced significant improvements in the GSRS questionnaire score and the general perception of health, as well as after six months of treatment the efficacy of the treatment persists [26,27].
Abexol has shown gastroprotective effects, preventing the formation of ulcers induced by indomethacin and ischemia/reperfusion while sequestering free radicals and increasing the activity of endogenous antioxidant enzymes, preventing protein and lipid oxidation [10,11]. It also prevents esophageal lesions in models of damage induced by gastroesophageal reflux and duodenum with concomitant antioxidant effects. Also, Abexol improves the symptoms of gastrointestinal discomfort in middle-aged and elderly subjects with an associated antioxidant effect [23].
Taking into account the above, the effects found here are consistent with previous studies, so it is logical to assume that the improvement in gastrointestinal symptoms evaluated through the GSRS scale in the study patients is due to its gastroprotective effect. However, it is plausible to think that the antioxidant effects may also contribute to its gastroprotective effects.
The efficacy of the suspension was similar to that of the tablets; however, the effect of the suspension was better than that of the tablet, considering that the dose in the suspension was half that of the tablet. This is logical since, in the case of the forms finished in solid form, they must disintegrate and dissolve, so the dissolution range can be limiting in absorption [38], especially in substances with low solubility in water, such as Abexol case. In the suspension, not only is prior disintegration not necessary, but the particle size of the active ingredient is smaller, so its absorption is favored. The fact that the suspension has a greater effect than the tablet suggests better absorption and therefore greater bioavailability of the substance when administered in this pharmaceutical form, although further studies should confirm this hypothesis.
The treatments were safe and well tolerated, as they did not affect the safety indicators investigated and no adverse experiences associated with their use were report.
A limitation of this study was the doses used are not same, the daily dose in the tablet treatment group was 150 mg/d while the patients treated with the suspension received 75 mg/d, without there being a group with the same dose. This makes comparison between groups difficult and prevents establishing the superiority of the suspension, with the same tablet dose; however, it is worth comparing the dosage unit indicated in each case, one tablet versus one teaspoon, and the effects in each case. However, subsequent studies should be design to establish the dose-effect relationship of the suspension and to establish the comparison between equal doses of both finished forms.
In conclusion, Abexol suspension showed similar efficacy, safety and tolerability to Abexol tablets in patients with gastrointestinal symptoms, using half the dose.
KEY MESSAGE
1. Abexol suspension showed efficacy, safety and tolerability similar to Abexol tablets in patients with gastrointestinal symptoms, but using half the dose.
Footnotes
CRedit authorship contributions
Alfredo Hierro González: data curation, writing - review & editing; Julio César Fernández Travieso: conceptualization, project administration, writing - original draft; Yoandy Hernández Casas: data curation, methodology; Susana Borges González: data curation, methodology; Maria de los Angeles Camacho Morales: data curation, methodology; Elena Ferrer Batallie: methodology; Anaisa Roja Carralera: methodology; Yenney Reyes Núñez: methodology, visualization; Sarahi Mendoza Castaño: writing - review & editing; Maytee Robaina García: formal analysis; Diana Margarita Rey Kaba: formal analysis
Conflicts of interest
The authors disclose no conflicts.
Funding
This study was support by the National Centre for Scientific Research, as part of its research-development projects.
REFERENCES
- 1.Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156:254–272e11. doi: 10.1053/j.gastro.2018.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik TF, Gnanapandithan K, Singh K. StatPearls [Internet] Traeasure Island, FL: StatPearls Publishing; 2022. Peptic Ulcer Disease. [PubMed] [Google Scholar]
- 4.Azer SA, Akhondi H. StatPearls [Internet] Traeasure Island, FL: StatPearls Publishing; 2022. Gastritis. [Google Scholar]
- 5.Fass R, Boeckxstaens GE, El-Serag H, Rosen R, Sifrim D, Vaezi MF. Gastro-oesophageal reflux disease. Nat Rev Dis Primers. 2021;7:55. doi: 10.1038/s41572-021-00287-w. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM. Diagnosis and treatment of peptic ulcer disease. Am J Med. 2019;132:447–456. doi: 10.1016/j.amjmed.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Más R. D-002: Beeswax alcohols. Drugs of the Future. 2001;26:731–744. [Google Scholar]
- 9.Menéndez R, Amor AM, González RM, Jiménez S, Más R. Inhibition of rat microsomal lipid peroxidation by the oral administration of D002. Braz J Med Biol Res. 2000;33:85–90. doi: 10.1590/s0100-879x2000000100012. [DOI] [PubMed] [Google Scholar]
- 10.Molina V, Valdés S, Carbajal D, Arruzazabala L, Menéndez R, Más R. Antioxidant effect of D-002 on gastric mucosa of rats with experimentally induced injury. J Med Food. 2001;4:79–83. doi: 10.1089/109662001300341734. [DOI] [PubMed] [Google Scholar]
- 11.Pérez Y, Oyárzabal A, Mas R, Molina V, Jiménez S. Protective effect of D-002, a mixture of beeswax alcohols, against indomethacin-induced gastric ulcers and mechanism of action. J Nat Med. 2013;67:182–189. doi: 10.1007/s11418-012-0670-y. [DOI] [PubMed] [Google Scholar]
- 12.Carbajal D, Molina V, Valdés S, Arruzazabala L, Más R. Anti-ulcer activity of higher primary alcohols of beeswax. J Pharm Pharmacol. 1995;47:731–73s3. doi: 10.1111/j.2042-7158.1995.tb06732.x. [DOI] [PubMed] [Google Scholar]
- 13.Molina V, Ravelo Y, Zamora Z, Mas R. Effects of D-002 on non-steroidal anti-inflammatory drugs-induced gastric ulcer in rats. Int J Pharm Sci Rev Res. 2015;30:253–257. [Google Scholar]
- 14.Carbajal D, Molina V, Valdés S, et al. Possible cytoprotective mechanism in rats of D-002, an anti-ulcerogenic product isolated from beeswax. J Pharm Pharmacol. 1996;48:858–860. doi: 10.1111/j.2042-7158.1996.tb03987.x. [DOI] [PubMed] [Google Scholar]
- 15.Carbajal D, Molina V, Noa M, et al. Effect of D-002 on gastric mucus composition in ethanol-induced ulcer. Pharmacol Res. 2000;42:329–332. doi: 10.1006/phrs.2000.0693. [DOI] [PubMed] [Google Scholar]
- 16.Zamora Z, Molina V, Más R, et al. D-002 treatment attenuates esophagitis in a model of chronic gastro-esophageal reflux in rats. IOSRPHR. 2015;5:36–40. [Google Scholar]
- 17.Noa M, Más R, Carbajal D, Valdés S. Effect of D-002 on acetic acid-induced colitis in rats at single and repeated doses. Pharmacol Res. 2000;41:391–395. doi: 10.1006/phrs.1999.0596. [DOI] [PubMed] [Google Scholar]
- 18.Pérez Y, Oyarzábal A, Ravelo Y, Mas R, Jiménez S, Molina V. Inhibition of cyclooxygenase and 5-lipooxygenase enzymes by D-002 (beeswax alcohols) Curr Top Nutraceutical Res. 2014;12:13–18. [Google Scholar]
- 19.Molina CV, Valle CM, Ravelo CY, Carbajal QD, Mas FR. Efectos del D-002 en la úlcera gástrica inducida por aspirina. Rev Cub Tox. 2012;1 [Google Scholar]
- 20.Menéndez R, Más R, Illnait J, et al. Effects of D-002 on lipid peroxidation in older subjects. J Med Food. 2001;4:71–77. doi: 10.1089/109662001300341725. [DOI] [PubMed] [Google Scholar]
- 21.López E, Illnait J, Molina V, et al. Effects of D-002 (Beeswax alcohols) on lipid peroxidation in middle-aged and older subjects. Lat Am J Pharm. 2008;27:695–703. [Google Scholar]
- 22.Menéndez R, Más R, Amor AM, et al. Antioxidant effects of D002 on the in vitro susceptibility of whole plasma in healthy volunteers. Arch Med Res. 2001;32:436–441. doi: 10.1016/s0188-4409(01)00315-0. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez I, Illnait J, Molina V, et al. Comparison of the antioxidant effects of D-002 (Beeswax alcohols) and grape seed extract (GSE) on plasma oxidative variables in healthy subjects. Lat Am J Pharm. 2010;29:255–262. [Google Scholar]
- 24.Hano O, Illnait J, Más R, Fernández L, Piñol F, Fernández JC. Effects of D-002, a product isolated from beeswax, on duodenal ulcer: a double-blind, placebo-controlled study. Curr Ther Res. 2001;62:394–407. [Google Scholar]
- 25.Illnait J, Terry H, Más R, Fernández L, Carbajal D. Effects of D-002, a product isolated from beeswax, on gastric symptoms of patients with osteoarthritis treated with piroxicam: a pilot study. J Med Food. 2005;8:63–68. doi: 10.1089/jmf.2005.8.63. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez I, Illnait J, Terry H, et al. Effects of Abexol® (beeswax alcohols) on gastrointestinal symptoms in middle-aged and older subjects. Rev CENIC Cien Biol. 2009;40:147–154. [Google Scholar]
- 27.Illnait J, Rodríguez I, Molina V, et al. Effects of D-002 (Beeswax alcohols) on gastrointestinal symptoms and oxidative markers in middle-aged and older subjects. Lat Am J Pharm. 2013;32:166–174. [Google Scholar]
- 28.Fernández JC, Rodríguez I, Illnait J, et al. Effects of Abexol (beeswax alcohols) in patients with gastric symptoms. Rev CENIC Cien Biol. 2012;43:9–16. [Google Scholar]
- 29.Fernández L, Terry H, Quiñones AM, et al. Effects of Abexol in middle-aged and older subjects: an open follow-up. Rev CENIC Ciencias Biológicas. 2008;39:3–8. [Google Scholar]
- 30.Rodeiro I, Alemán C, Noa M, et al. Preclinical oral toxicology in rats of D-002, a natural drug with antiulcer effects. Drug Chem Toxicol. 1998;21:151–162. doi: 10.3109/01480549809011644. [DOI] [PubMed] [Google Scholar]
- 31.Alemán C, Rodeiro I, Noa M, et al. One-year dog toxicity study of D-002, a mixture of aliphatic alcohols. J Appl Toxicol. 2001;21:179–184. doi: 10.1002/jat.705. [DOI] [PubMed] [Google Scholar]
- 32.Rodeiro I, Gámez R, Acosta P, Fernandez S, Mas R, Aleman C. Estudio genotóxico del D-002, un producto con actividad antiulcerosa. Rev Tox. 1998;15:117–121. [Google Scholar]
- 33.Gámez R, Fernández I, Acosta PC, et al. Evaluacion mutagénica del D-002 en el ensayo de micronúcleos en médula ósea en ratas Sprague Dawley de ambos sexos. Rev. CNIC Cienc Biol. 1999;30:70–72. [Google Scholar]
- 34.Asociación Médica Mundial . Principios éticos para las investigaciones con seres humanas. Fortaleza: Asociación Médica Mundial; 2013. Declaración de Helsinki. [Google Scholar]
- 35.Svedlund J, Sjödin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–134. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 36.Alonso J, Prieto L, Antó JM. [The Spanish version of the SF-36 Health Survey (the SF-36 health questionnaire): an instrument for measuring clinical results]. Med Clin (Barc) 1995;104:771–776. Spanish. [PubMed] [Google Scholar]
- 37.Centro para el Control Estatal de los Medicamentos, Equipos y Dispositivos Médicos (CECMED) Requerimientos para la notificación y el reporte de eventos adversos graves e inesperados en los ensayos clínicos. Habana La: Ministerio de Salud Publica; 2007. Regulación No. 45-2007. [Google Scholar]
- 38.Burton LL, Hilal-Dandan R, Knollman BC. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill Education; 2018. [Google Scholar]
- 39.Castellas Jorda F, López Vivancos J. Evaluación de la calidad de vida en las enfermedades digestivas. Gastroenterol Hepatol. 2004;27:58–68. doi: 10.1016/s0210-5705(03)79088-8. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs KH, Musial F, Eypasch E, Meining A. Gastrointestinal quality of life in gastroesophageal reflux disease: a systematic review. Digestion. 2022;103:253–260. doi: 10.1159/000524766. [DOI] [PubMed] [Google Scholar]