Abstract
Background/Aims
There may be many predictors of anticoagulation-related gastrointestinal bleeding (GIB), but until now, systematic reviews and assessments of the certainty of the evidence have not been published. We conducted a systematic review to identify all risk factors for anticoagulant-associated GIB to inform risk prediction in the management of anticoagulation-related GIB.
Methods
A systematic review and meta-analysis were conducted to search PubMed, EMBASE, Web of Science, and Cochrane Library databases (from inception through January 21, 2022) using the following search terms: anticoagulants, heparin, warfarin, dabigatran, rivaroxaban, apixaban, DOACs, gastrointestinal hemorrhage, risk factors. According to inclusion and exclusion criteria, studies of risk factors for anticoagulation-related GIB were identified. Risk factors for anticoagulant-associated GIB were used as the outcome index of this review.
Results
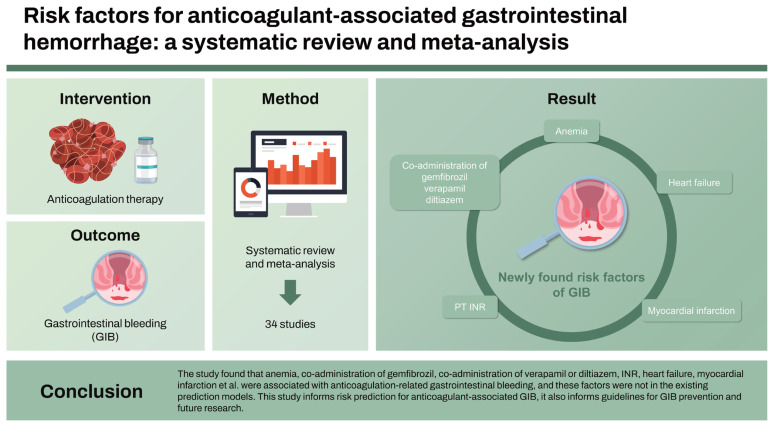
We included 34 studies in our analysis. For anticoagulant-associated GIB, moderate-certainty evidence showed a probable association with older age, kidney disease, concomitant use of aspirin, concomitant use of the antiplatelet agent, heart failure, myocardial infarction, hematochezia, renal failure, coronary artery disease, helicobacter pylori infection, social risk factors, alcohol use, smoking, anemia, history of sleep apnea, chronic obstructive pulmonary disease, international normalized ratio (INR), obesity et al. Some of these factors are not included in current GIB risk prediction models. such as anemia, co-administration of gemfibrozil, co-administration of verapamil or diltiazem, INR, heart failure, myocardial infarction, etc.
Conclusions
The study found that anemia, co-administration of gemfibrozil, co-administration of verapamil or diltiazem, INR, heart failure, myocardial infarction et al. were associated with anticoagulation-related GIB, and these factors were not in the existing prediction models. This study informs risk prediction for anticoagulant-associated GIB, it also informs guidelines for GIB prevention and future research.
Keywords: Gastrointestinal hemorrhage, Risk factor, Predict, Meta-analysis
Graphical abstract
INTRODUCTION
Anticoagulants including heparin, low molecular heparin, fondaparinux, warfarin and novel oral anticoagulants (NOACs) are effective against acute or chronic thromboembolic complications [1–3]. Anticoagulants increase the risk of bleeding while exerting their antithrombotic effect. The annual rate of major bleeding in patients taking warfarin is reported to be as high as 8%, with gastrointestinal bleeding (GIB) being the most common [4]. The incidence of GIB during antithrombotic therapy with vitamin K antagonists (VKAs) ranges from 1.5% to 4.5% [5,6] and may result in a 10–15% short-term mortality rate [7–9]. And with millions of patients currently receiving anticoagulation therapy worldwide, it is necessary to accurately predict the risk of GIB associated with anticoagulants.
The risk assessment models (RAMs) for anticoagulation-related GIB consists of a combination of multiple predictors. Risk for specific endpoints can be obtained based on relevant predictors, thus providing recommendations for patient stratification [10].
Although these models can prevent GIB to some extent, most were developed using existing data rather than based on a systematic review of all potential risk factors [11]. The risk factors included in existing models are not comprehensive and may reduce the predictive power of the model. Therefore, this review conducts a systematic review and meta-analysis of risk factors for GIB that may inform anticoagulation therapy, future guideline recommendations, and the development of RAMs.
METHODS
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [12]. The protocol for this systematic review was prospectively registered with PROSPERO (CRD 42022340867).
Patient and public involvement
No patient involved.
Search strategy
Data were reviewed from four databases: PubMed, EMBASE, Web of Science, and the Cochrane Library. Studies in English published before January 21, 2022 were included. To ensure a comprehensive literature search, we also identified additional studies by searching the reference list of the literature.
Supplementary Material 1 provides detailed descriptions of the search strategy.
Study selection
Studies that met the following criteria were included: Use of anticoagulants (e.g., heparin, VKAs, NOACs); Comparison between the GIB group and the non-GIB group; The outcome index was risk factors or predictors.
Studies that met the following criteria were excluded: Patients with GIB treated with non-anticoagulant medications; Incomplete data (including data related to risk factors not obtained, a study in design or recruitment phase, permission to use data not obtained, the corresponding author contacted but not responded to).
Data extraction
For all identified studies, RAMs, and prognostic factor studies, the data extracted included the name of the first author, year of publication, time frame, population, and their demographics (e.g., sample size, number of centers, age, and sex), study design (e.g., cohort or case-control), outcomes and measures of association (e.g., odds ratio [OR] or risk ratio [RR] or hazard ratio [HR], 95% confidence interval [CI] and p value). GIB was defined as a reduction in the Hb level ≥ 2 g/dL, or transfusion of at least 2 units of blood.
Quality assessment
Risk of bias assessment
We assessed the risk of bias in the included studies by using the Prediction model Risk Of Bias Assessment Tool (PROBAST) for RAM studies and the Quality in Prognosis Studies (QUIPS) tool for prognostic factor studies [13–15].
Certainty of evidence assessment
We performed an assessment of the certainty of the evidence for each of the prognostic factors per outcome based on the GRADE approach. The approach considers the following domains: risk of bias, indirectness, inconsistency, imprecision, and publication bias. We developed evidence profiles and rated the overall certainty of evidence as high, moderate, and low or very low, depending on the grading of the individual domains [16].
Statistical analysis
We standardized each risk factor by log transformation and unifying the direction of the predictors. In studies that reported the measure of association as a HR or RR, we converted them to OR using the baseline risk reported in the studies [17,18]. We used the Review Manager 5.3 software for meta-analysis. The statistical indicators were OR and 95% CI. The chi-square test (χ2) was used to test the heterogeneity of results. If p ≥ 0.1 and I2 ≤ 50%, the fixed-effect model was used for meta-analysis. The random-effect model was used when p < 0.1 and I2 > 50%.
RESULTS
The characteristics of included studies
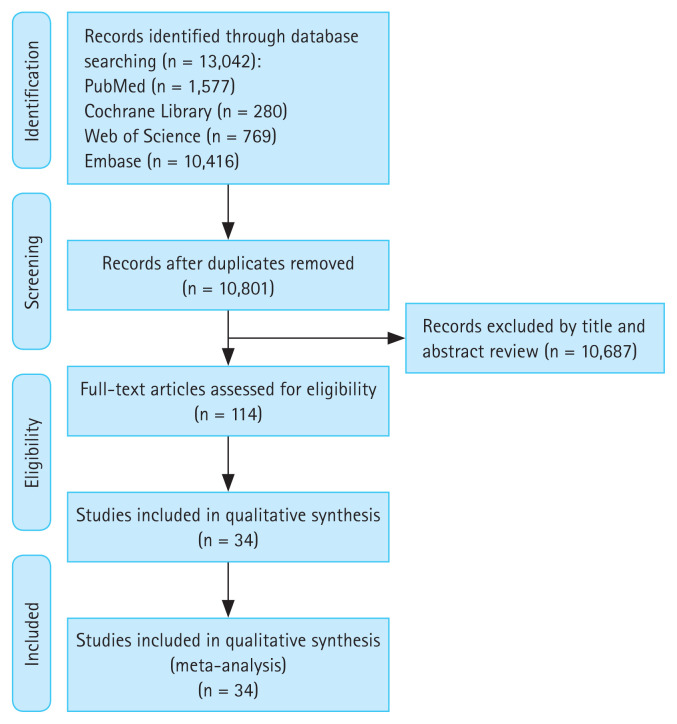
Our search identified 13,042 citations, of which we included 114 studies for full-text assessment. Finally, 34 articles fulfilled the inclusion criteria and were included in this study. Figure 1 is a PRISMA flowchart. Supplementary Table 1 describes the characteristics of the included studies reporting on the outcomes of GIB. Thirty-three studies were risk factor studies [19–50,51]. One study was a prediction model development study [52]. Twnety-seven studies were cohorts [19–21,23–25,27–29,31,34,36–49,51,52]: 1 of which was prospective cohort [40], 26 of which were retrospective cohorts [19–21,23–25,27–29,31,34,36–39,41–49,51,52]. Two studies were case-control studies [26,32], 5 were randomized controlled trials (RCTs) [22,30,33,35,50]. Among the 34 studies, the populations of 23 studies were only stroke patients [19–22,25,27–37,39–41,43,44,47,50], the composition of the population indications in the remaining 11 studies included atrial fibrillation, venous thromboembolism, pulmonary embolism, deep vein embolism, and stroke [23,24,26,38,42,45,46,48,49]. Most patients were between 50 and 80 years old, and most were male.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis flowchart.
Risk of bias assessment
The risk of bias was serious across all identified studies, each presenting risk of bias in at least 1 domain or item (Supplementary Table 2). Among the 34 included studies, 26 were retrospective, which may have introduced classification bias [19–21,23–25,27–29,31,34,36–49,51,52]. We detected evidence of publication bias through visual assessment of asymmetry of the funnel plot for each pooled predictor in those that included at least 10 studies (Supplementary Table 2). Certainty in evidence was downgraded for imprecision, given that the CI suggests that there may be no association. 2 of the 33 risk factors studies did not clearly describe appropriate outcome measurement [37,38]. Supplementary Table 3 and 4 provide detailed judgments for each risk of bias domain criteria.
Analysis of risk factors of anticoagulant-associated gastrointestinal bleeding
Investigated were 48 candidate risk factors for GIB from 34 studies. Supplementary Table 2 provides the evidence profile for anticoagulant-associated GIB risk factors. Supplementary Figure (S1–S48) provides the forest plots of the meta-analysis of each of the risk factors.
Demographic factors
We found moderate-certainty evidence that there is probably an association between risk of GIB and older age (OR, 1.95; 95% CI, 1.36–2.79) [19,23,24,28,33,36,45,47], age growth (age per year increase; OR, 1.03; 95% CI, 1.01–1.06; age increase per five years; OR, 1.11; 95% CI, 1.06–1.17) [40,50,51], and obesity (weight > 120 kg; OR, 1.44; 95% CI, 1.01–2.05) [25]. We found low-certainty evidence that there may be little to no association between risk of GIB and sex (male vs. female; OR, 0.95; 95% CI, 0.72–1.26) [20,26,28,36,39,40,47].
Functional factors
There was moderate-certainty evidence for a probable association between the risk of GIB and any international normalized ratio (INR) (OR, 2.43; 95% CI, 1.30–4.53) [24,41]. We found very-low-certainty evidence that there may be little to no association between the risk of GIB and Has-Bled-Score (≥ 3; OR, 1.20; 95% CI, 0.06–22.63) [19,41].
Medical illness and patient history factors
We identified moderate-certainty evidence for an association between the risk of GIB and kidney disease (OR, 1.69; 95% CI, 1.24–2.31) [19,36,45,46,52], cirrhosis (OR, 6.24; 95% CI, 2.63–14.83) [24,52], liver failure (OR, 7.01; 95% CI, 4.78–10.27) [26], and heart failure (HF) (OR, 1.30; 95% CI, 1.14–1.49) [28,36,46]. Subgroup analysis showed that congestive HF (OR, 1.29; 95% CI, 1.06–1.57) [28,36] and chronic HF (OR, 1.31; 95% CI, 1.09–1.58) [46] were statistically significant. We found moderate-certainty evidence that there is probably an association between the risk of GIB and history of bleeding (OR, 3.26; 95% CI, 1.86–5.73) [28], myocardial infarction (OR, 2.23; 95% CI, 1.12–4.43) [28], renal failure (OR, 3.18; 95% CI, 1.44–6.99) [34,47], coronary artery disease (OR, 1.36; 95% CI, 1.10–1.69) [36], Helicobacter pylori infection (OR, 4.75; 95% CI, 1.93–11.68) [36], anemia (OR, 1.48; 95% CI, 1.10–1.98) [36,50], history of sleep apnea (OR, 1.60; 95% CI, 1.22–2.10) [50], psychiatric illness, defined as schizophrenia, affective psychosis, paranoia, or other nonorganic psychosis (OR, 1.20; 95% CI, 1.03–1.39) [46], venous thromboembolism including deep vein thrombosis (OR, 1.21; 95% CI, 1.02–1.44) [36,46].
Furthermore, we identified low-certainty evidence that there may be little to no association between the risk of GIB and peripheral vascular disease including peripheral artery disease (OR, 2.33; 95% CI, 0.66–8.20) [28,36], mechanical valve implantation (OR, 1.97; 95% CI, 0.43–9.07) [45], liver disease (OR, 1.31; 95% CI, 0.99–1.74) [46], diabetes (OR, 1.08; 95% CI, 0.96–1.21) [36,46], and chronic obstructive pulmonary disease (OR, 2.01; 95% CI, 0.69–5.83) [50,52].
We found very-low-certainty evidence that there may be an association between the risk of GIB and history of peptic ulcer/GIB (OR, 5.26; 95% CI, 2.76–10.05) [19,23,24,28,30, 39,41,45,47,48,50–52].
Laboratory and physical examination factors
There was moderate certainty evidence of a probable association between the risk of GIB and creatinine level (per 1 mg/dL increase; OR, 1.38; 95% CI, 1.09–1.74) [40] and diastolic BP (decrease to < 80 mmHg; OR, 1.10; 95% CI, 1.05–1.16) [50]. We identified low-certainty evidence that there may be an association between the risk of GIB and creatinine clearance (< 60 mL/min; OR, 1.06; 95% CI, 1.01–1.12) [50].
Medication factors
We found moderate-certainty evidence that there is probably an association between the risk of GIB and concomitant use of aspirin (OR, 2.07; 95% CI, 1.17–3.66) [22,23,26,27,47] and concomitant with non-steroidal anti-inflammatory drugs (NSAIDs) (OR, 2.37; 95% CI, 1.61–3.50) [26,39,43,47]. Subgroup analysis showed that combination of paracetamol (OR, 1.47; 95% CI, 1.35–1.60) [26] and combination of COX-2 inhibitor (OR, 1.97; 95% CI, 1.59–2.40) [26] were statistically significant. We found moderate-certainty evidence that there is probably an association between the risk of GIB and antiplatelet therapy (OR, 1.45; 95% CI, 1.11–1.90) [19,27,36,39,42,47,48,50,51], concomitant use of dronedarone (OR, 1.29; 95% CI, 1.04–1.62) [29], concomitant use of CYP3A4 or P-glycoprotein inhibitors (OR, 1.47; 95% CI, 1.15–1.88) [31], combination of digoxin (OR, 1.50; 95% CI, 1.19–1.88) [36], combination of gemfibrozil (OR, 2.29; 95% CI, 1.61–3.25) [38], combination of verapamil or diltiazem (OR, 2.33; 95% CI, 1.82–2.98) [44], and long-term acetylsalicylic acid (ASA) use at screening (OR, 1.47; 95% CI, 1.26–1.72) [50].
However, low-quality evidence showed that there may be little to no association between the risk of GIB and combination of clopidogrel (OR, 2.37; 95% CI, 1–5.65) [19], combination of corticosteroid (OR, 2.14; 95% CI, 0.98–4.72) [19,41], combination of thienopyridines (OR, 2.37; 95% CI, 0.75–7.44) [47].
We identified low-certainty evidence that there may be an association between the risk of GIB and concomitant use of oral glucocorticoid (OR, 1.83; 95% CI, 1.30–2.59) [32].
Other factors
There was moderate-certainty evidence of a probable association between risk of GIB and social risk factors, defined as lack of housing, inadequate housing, inadequate material resources, persons living alone, no other household member able to render care, or non-compliance with medical treatment (OR, 1.29; 95% CI, 1.12–1.48) [46]. We also identified moderate-certainty evidence that there is probably an association between risk of GIB and alcohol use (OR, 3.46; 95% CI, 2.30–5.19) [26,36] and smoking (OR, 1.26; 95% CI, 1.18–1.35) [26,50], anticoagulant treatment time (≤ 100 d; OR, 4.94; 95% CI, 2.66–9.17) [47–49], and substance abuse, defined as alcohol dependence, drug dependence, or non-dependent abuse, excluding tobacco use disorder (OR, 1.41; 95% CI, 1.07–1.87) [46].
We identified low-certainty evidence that there may be an association between the risk of GIB and dabigatran 150 mg twice daily (OR, 1.53; 95% CI, 1.39–1.69) [21,35].
DISCUSSION
We evaluated 48 risk factors for anticoagulant-associated GIB. We also identified several statistically significant predictors, such as social risk factors, alcohol consumption, smoking, co-administration of aspirin, co-administration of NSAIDs, renal disease, cirrhosis, liver failure, INR, older age, age growth, obesity (weight > 120 kg) et al., which supported by moderate certainty of the evidence.
Therefore, in addition to anticoagulation therapy, which can affect GIB, other risk factors should also be noted. We can intervene in undesirable behaviors such as drinking and smoking through behavior-based education, minimize the combination of drugs such as aspirin, NSAIDs, antiplatelet drugs, verapamil or diltiazem, and anticoagulants, and actively treat kidney disease, cirrhosis, liver failure, and HF to reduce the occurrence of GIB.
Our study identified candidate risk factors for GIB, such as age, smoking, alcohol consumption, the combination of aspirin, the combination of NSAID, antiplatelet therapy, diabetes, cirrhosis, peripheral vascular disease, renal disease, etc. These risk factors have been considered in the analysis of some developed and widely used RAMs in daily practice, such as New Score, RIETE Score, Cuschieriet al. Score, and de Groot et al. Score [52–55]. However, some factors that we identified as having a probable association with GIB, based on our meta-analysis results, were not included or considered in the development of most of the RAMs, such as history of sleep apnea, co-administration of CYP3A4 or P-glycoprotein antagonists, co-administration of digoxin, co-administration of gemfibrozil, co-administration of verapamil or diltiazem, INR, HF, myocardial infarction, long-term ASA use at screening. This deserves our special concern. In addition, we found that antiplatelet theapy was associated with GIB risk. This observation was opposite to Nawarawong et al.’s study [42]. Antiplatelet theapy showed no association with GIB risk in their study. We believe that such reverse causation, given the study design, may be plausible. However, given the small sample size, the finding warrants further investigations in primary studies.
We found that dabigatran dose 150 mg is associated with GIB, which is an interesting point. Of note, in a previous meta-analysis by our research team, a higher risk of GIB with dabigatran than with warfarin had been demonstrated [56]. This result should draw clinicians’ attention to the possible benefit of monitoring patients’ risk of GIB after administration of dabigatran 150 mg.
In our meta-analyses, proton pump inhibitor (PPI) use decreased the risk of GIB by half (HR, 0.5), which closely reflects the findings of Ray et al. [57], who reported that PPI use was associated with a substantial reduction in the risk of warfarin-related upper GIB (HR, 0.76). Although PPI therapy was not included in either the HAS-BLED score, ATRIA score or ORBIT score, PPI use is an important means of preventing GIB in the long term.
We also did subgroup analyses to explore the sources of heterogeneity in history of peptic ulcer/GIB (I2 = 97%). Subgroup analysis by population, design type, sample size, and study quality showed that retrospective cohort studies were the main cause of heterogeneity, with little heterogeneity in the RCT group.
The greatest advantage of our study is the comprehensiveness of the study results, which may have some clinical significance in preventing the occurrence of anticoagulant-associated GIB.
The study also has some limitations. Since most of the studies included in this review were retrospective, classification and recall bias may lead to potential limitations. In addition, potential limitations of the included studies related to the inconsistency and variability across eligibility criteria in the original studies and variability in study design, study type, sample size, and definitions of the risk factors. For example, in our study, 22 studies included only atrial fibrillation indications, while others 11 studies included venous thromboembolism, pulmonary embolism, deep vein embolism, and stroke in addition to atrial fibrillation. In anticoagulation, different populations will influence the choice of drug as well as the dose and duration of drug therapy and significantly affect the outcome of GIB in each study. Study effect OR value is closely related to outcomes, and we found significant differences in OR value for the same variables across studies. Of note, the process of meta-analysis may cause variables that were originally risk factors to become nonsignificant, or even to become protective factors.
Research may be needed to reevaluate existing RAMs, as the developers of the models may not have been able to use the variables we identified, given the limitations in the existing databases. However, full development or improvement of a RAM that supports clinical practice requires further investigation of all the prognostic factors we identified in our study. Therefore, more rigorous and large-scale studies are needed to confirm our findings, and further analysis is necessary to provide a more reliable basis for clinical work.
KEY MESSAGE
1. In this systematic review, we identified all reported risk factors for anticoagulation-associated GIB (e.g., alcohol consumption, smoking, co-administration of aspirin).
2. Some risk factors not included in current GIB risk prediction models (e.g., anemia, history of sleep apnea, co-administration of digoxin).
3. Our findings will help inform experts in developing population-based guidelines and accurate, user-friendly RAMs to guide individual patient management better.
Footnotes
CRedit authorship contributions
Fuxin Ma: data curation, writing - original draft, writing - review & editing), Shuyi Wu: formal analysis, project administration; Shiqi Li: data curation, project administration; Zhiwei Zeng: conceptualization, project administration; Jinhua Zhang: methodology, writing - review & editing
Conflicts of interest
The authors disclose no conflicts.
Funding
None
Supplementary Information
REFERENCES
- 1.Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ. Antithrombotic and thrombolytic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):110S–112S. doi: 10.1378/chest.08-0652. [DOI] [PubMed] [Google Scholar]
- 2.Abraham NS, Noseworthy PA, Yao X, Sangaralingham LR, Shah ND. Gastrointestinal safety of direct oral anticoagulants: a large population-based study. Gastroenterology. 2017;152:1014–1022e1. doi: 10.1053/j.gastro.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Jingke W, Zhiyi L, Guiqing L, et al. [Current status and progress of oral combination antithrombotic drug therapy] Int J Cardiovasc Dis. 2017;44:132–135. Chinese. [Google Scholar]
- 4.Choudari CP, Palmer KR. Acute gastrointestinal haemorrhage in patients treated with anticoagulant drugs. Gut. 1995;36:483–484. doi: 10.1136/gut.36.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothberg MB, Celestin C, Fiore LD, Lawler E, Cook JR. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med. 2005;143:241–250. doi: 10.7326/0003-4819-143-4-200508160-00005. [DOI] [PubMed] [Google Scholar]
- 6.Sørensen R, Hansen ML, Abildstrom SZ, et al. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet. 2009;374:1967–1974. doi: 10.1016/S0140-6736(09)61751-7. [DOI] [PubMed] [Google Scholar]
- 7.van Leerdam ME, Vreeburg EM, Rauws EA, et al. Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol. 2003;98:1494–1499. doi: 10.1111/j.1572-0241.2003.07517.x. [DOI] [PubMed] [Google Scholar]
- 8.Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327–1335. doi: 10.1136/gut.2010.228437. [DOI] [PubMed] [Google Scholar]
- 9.Rosenstock SJ, Møller MH, Larsson H, et al. Improving quality of care in peptic ulcer bleeding: nationwide cohort study of 13,498 consecutive patients in the Danish Clinical Register of Emergency Surgery. Am J Gastroenterol. 2013;108:1449–1457. doi: 10.1038/ajg.2013.162. [DOI] [PubMed] [Google Scholar]
- 10.Darzi AJ, Karam SG, Charide R, et al. Prognostic factors for VTE and bleeding in hospitalized medical patients: a systematic review and meta-analysis. Blood. 2020;135:1788–1810. doi: 10.1182/blood.2019003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley RD, Moons KGM, Snell KIE, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364:k4597. doi: 10.1136/bmj.k4597. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang SW, Zhang Y, Tao BL, Yang ZR, Sun F, Zhan SY. [Risk of bias assessment: (7) assessing bias in studies of prognostic factors] Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39:1003–1008. doi: 10.3760/cma.j.issn.0254-6450.2018.07.026. Chinese. [DOI] [PubMed] [Google Scholar]
- 14.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 15.Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Including non-randomized studies. Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions Cochrane. 2008:391. [Google Scholar]
- 16.Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 17.Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. 2014;348:f7450. doi: 10.1136/bmj.f7450. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z. Converting odds ratio to relative risk in cohort studies with partial data information. J Stat Softw. 2013;55:1–11. [Google Scholar]
- 19.Agudo-Fernández S, Castaño Milla C, González Blanco A, Olmos Jerez JA, Calvo Morillas I, Sancho Del Val L. RHEDAR study: determination of the risk of gastrointestinal hemorrhage in treatment with dabigatran, acenocoumarol and rivaroxaban. J Gastroenterol Hepatol. 2021;36:2794–2802. doi: 10.1111/jgh.15547. [DOI] [PubMed] [Google Scholar]
- 20.Fanning L, Wong ICK, Li X, et al. Gastrointestinal bleeding risk with rivaroxaban vs aspirin in atrial fibrillation: a multinational study. Pharmacoepidemiol Drug Saf. 2020;29:1550–1561. doi: 10.1002/pds.5130. [DOI] [PubMed] [Google Scholar]
- 21.Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 22.Aisenberg J, Chatterjee-Murphy P, Friedman Flack K, et al. Gastrointestinal bleeding with edoxaban versus warfarin: results from the ENGAGE AF-TIMI 48 trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis In Myocardial Infarction) Circ Cardiovasc Qual Outcomes. 2018;11:e003998. doi: 10.1161/CIRCOUTCOMES.117.003998. [DOI] [PubMed] [Google Scholar]
- 23.Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology. 2015;149:586–595e3. doi: 10.1053/j.gastro.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Chen WC, Chen YH, Hsu PI, et al. Gastrointestinal hemorrhage in warfarin anticoagulated patients: incidence, risk factor, management, and outcome. Biomed Res Int. 2014;2014:463767. doi: 10.1155/2014/463767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coates J, Bitton E, Hendje A, et al. Clinical outcomes of dabigatran use in patients with non-valvular atrial fibrillation and weight >120 kg. Thromb Res. 2021;208:176–180. doi: 10.1016/j.thromres.2021.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Delaney JA, Opatrny L, Brophy JM, Suissa S. Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007;177:347–351. doi: 10.1503/cmaj.070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douros A, Renoux C, Yin H, Filion KB, Suissa S, Azoulay L. Concomitant use of direct oral anticoagulants with antiplatelet agents and the risk of major bleeding in patients with nonvalvular atrial fibrillation. Am J Med. 2019;132:191–199e12. doi: 10.1016/j.amjmed.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Ferroni E, Denas G, Gennaro N, Fedeli U, Pengo V. Gender related differences in gastrointestinal bleeding with oral anticoagulation in atrial fibrillation. J Cardiovasc Pharmacol Ther. 2022;27:10742484211054609. doi: 10.1177/10742484211054609. [DOI] [PubMed] [Google Scholar]
- 29.Gandhi SK, Reiffel JA, Boiron R, Wieloch M. Risk of major bleeding in patients with atrial fibrillation taking dronedarone in combination with a direct acting oral anticoagulant (from a U.S. Claims Database) Am J Cardiol. 2021;159:79–86. doi: 10.1016/j.amjcard.2021.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Garcia DA, Fisher DA, Mulder H, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with Apixaban or warfarin: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Am Heart J. 2020;221:1–8. doi: 10.1016/j.ahj.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Holm J, Mannheimer B, Malmström RE, Eliasson E, Lindh JD. Bleeding and thromboembolism due to drug-drug interactions with non-vitamin K antagonist oral anticoagulants-a Swedish, register-based cohort study in atrial fibrillation outpatients. Eur J Clin Pharmacol. 2021;77:409–419. doi: 10.1007/s00228-020-03015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holt A, Blanche P, Zareini B, et al. Gastrointestinal bleeding risk following concomitant treatment with oral glucocorticoids in patients on non-vitamin K oral anticoagulants. Heart. 2022;108:626–632. doi: 10.1136/heartjnl-2021-319503. [DOI] [PubMed] [Google Scholar]
- 33.Kato ET, Giugliano RP, Ruff CT, et al. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF-TIMI 48 trial. J Am Heart Assoc. 2016;5:e003432. doi: 10.1161/JAHA.116.003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalil RS, Kaboli PJ, Liu X, Vaughan-Sarrazin M. Association between renal function and bleeding risk for dabigatran after switching from warfarin. Am J Nephrol. 2016;44:11–18. doi: 10.1159/000446848. [DOI] [PubMed] [Google Scholar]
- 35.Kolb JM, Flack KF, Chatterjee-Murphy P, et al. Locations and mucosal lesions responsible for major gastrointestinal bleeding in patients on warfarin or dabigatran. Dig Dis Sci. 2018;63:1878–1889. doi: 10.1007/s10620-018-5007-6. [DOI] [PubMed] [Google Scholar]
- 36.Lauffenburger JC, Rhoney DH, Farley JF, Gehi AK, Fang G. Predictors of gastrointestinal bleeding among patients with atrial fibrillation after initiating dabigatran therapy. Pharmacotherapy. 2015;35:560–568. doi: 10.1002/phar.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SR, Choi EK, Jung JH, et al. Body mass index and clinical outcomes in asian patients with atrial fibrillation receiving oral anticoagulation. Stroke. 2021;52:521–530. doi: 10.1161/STROKEAHA.120.030356. [DOI] [PubMed] [Google Scholar]
- 38.Leonard CE, Brensinger CM, Bilker WB, et al. Gastrointestinal bleeding and intracranial hemorrhage in concomitant users of warfarin and antihyperlipidemics. Int J Cardiol. 2017;228:761–770. doi: 10.1016/j.ijcard.2016.11.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maruyama K, Yamamoto T, Aoyagi H, et al. Difference between the upper and the lower gastrointestinal bleeding in patients taking nonvitamin K oral anticoagulants. Biomed Res Int. 2018;2018:7123607. doi: 10.1155/2018/7123607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murata N, Okumura Y, Nagashima K, et al. SAKURA AF Registry Investigators Gastrointestinal bleeding from oral anticoagulant therapy among Japanese patients with atrial fibrillation identified from the SAKURA atrial fibrillation registry. Circ J. 2020;84:1475–1482. doi: 10.1253/circj.CJ-20-0090. [DOI] [PubMed] [Google Scholar]
- 41.Nantsupawat T, Soontrapa S, Nantsupawat N, et al. Risk factors and prevention of dabigatran-related gastrointestinal bleeding in patients with atrial fibrillation. J Arrhythm. 2017;34:30–35. doi: 10.1002/joa3.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nawarawong N, Pongprasobchai S, Tanwandee T. Acute gastrointestinal bleeding in anticoagulated patients: prevalence and predictors of significant endoscopic lesions and change of the management. J Med Assoc Thai. 2018;101:135. [Google Scholar]
- 43.Olsen AS, McGettigan P, Gerds TA, et al. Risk of gastrointestinal bleeding associated with oral anticoagulation and non-steroidal anti-inflammatory drugs in patients with atrial fibrillation: a nationwide study. Eur Heart J Cardiovasc Pharmacother. 2020;6:292–300. doi: 10.1093/ehjcvp/pvz069. [DOI] [PubMed] [Google Scholar]
- 44.Pham P, Schmidt S, Lesko L, Lip GYH, Brown JD. Association of oral anticoagulants and verapamil or diltiazem with adverse bleeding events in patients with nonvalvular atrial fibrillation and normal kidney function. JAMA Netw Open. 2020;3:e203593. doi: 10.1001/jamanetworkopen.2020.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pourafkari L, Ghaffari S, Zamani N, et al. Upper gastrointestinal bleeding in the setting of excessive warfarin anticoagulation: risk factors, and clinical outcome. Cor et Vasa. 2017;59:e128–e133. [Google Scholar]
- 46.Schauer DP, Moomaw CJ, Wess M, Webb T, Eckman MH. Psychosocial risk factors for adverse outcomes in patients with nonvalvular atrial fibrillation receiving warfarin. J Gen Intern Med. 2005;20:1114–1119. doi: 10.1111/j.1525-1497.2005.0242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherid M, Sifuentes H, Sulaiman S, et al. Gastrointestinal bleeding with dabigatran, a comparative study with warfarin: a multicenter experience. Korean J Gastroenterol. 2015;65:205–214. doi: 10.4166/kjg.2015.65.4.205. [DOI] [PubMed] [Google Scholar]
- 48.Sherid M, Sifuentes H, Sulaiman S, et al. Risk of gastrointestinal bleeding with dabigatran: a head-to-head comparative study with rivaroxaban. Digestion. 2014;90:137–146. doi: 10.1159/000365967. [DOI] [PubMed] [Google Scholar]
- 49.Sherid M, Sulaiman S, Samo S, et al. Risk of gastrointestinal bleeding with rivaroxaban: a comparative study with warfarin. Gastroenterol Res Pract. 2016;2016:9589036. doi: 10.1155/2016/9589036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J Am Coll Cardiol. 2015;66:2271–2281. doi: 10.1016/j.jacc.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 51.Youn SH, Lim H, Ju Y, et al. Effect of gastroprotective agents on upper gastrointestinal bleeding in patients receiving direct oral anticoagulants. Scand J Gastroenterol. 2018;53:1490–1495. doi: 10.1080/00365521.2018.1541478. [DOI] [PubMed] [Google Scholar]
- 52.Shimomura A, Nagata N, Shimbo T, et al. New predictive model for acute gastrointestinal bleeding in patients taking oral anticoagulants: a cohort study. J Gastroenterol Hepatol. 2018;33:164–171. doi: 10.1111/jgh.13830. [DOI] [PubMed] [Google Scholar]
- 53.López-Jiménez L, Montero M, González-Fajardo JA, et al. RIETE Investigators Venous thromboembolism in very elderly patients: findings from a prospective registry (RIETE) Haematologica. 2006;91:1046–1051. [PubMed] [Google Scholar]
- 54.Cuschieri JR, Drawz P, Falck-Ytter Y, Wong RC. Risk factors for acute gastrointestinal bleeding following myocardial infarction in veteran patients who are prescribed clopidogrel. J Dig Dis. 2014;15:195–201. doi: 10.1111/1751-2980.12123. [DOI] [PubMed] [Google Scholar]
- 55.de Groot NL, Hagenaars MP, Smeets HM, Steyerberg EW, Siersema PD, van Oijen MG. Primary non-variceal upper gastrointestinal bleeding in NSAID and low-dose aspirin users: development and validation of risk scores for either medication in two large Dutch cohorts. J Gastroenterol. 2014;49:245–253. doi: 10.1007/s00535-013-0817-y. [DOI] [PubMed] [Google Scholar]
- 56.Xu W, Lv M, Wu S, et al. Severe bleeding risk of direct oral anticoagulants versus vitamin K antagonists for stroke prevention and treatment in patients with atrial fibrillation: a systematic review and network meta-analysis. Cardiovasc Drugs Ther. 2023;37:363–377. doi: 10.1007/s10557-021-07232-9. [DOI] [PubMed] [Google Scholar]
- 57.Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105–1112e10. doi: 10.1053/j.gastro.2016.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.