Abstract
Background:
Current guidelines recommend evaluation for underlying heart disease and reversible conditions for patients with new-onset heart failure (HF). There are limited data on contemporary testing for coronary artery disease (CAD) in patients with new-onset HF.
Methods:
We performed an observational cohort study using the GWTG-HF registry linked to Medicare claims. All patients were aged ≥65 and hospitalized for new-onset HF from 2009-2015. We collected left ventricular ejection fraction (LVEF), prior HF history, and in-hospital CAD testing from the registry, as well as testing for CAD using claims from 90 days before to 90 days after index HF hospitalization.
Results:
Among 17,185 patients with new-onset HF, 6672 (39%) received testing for CAD, including 3997 (23%) during the index hospitalization. Testing for CAD differed by LVEF: 53% in HF with reduced EF (LVEF ≤40%), 42% in HF with borderline EF (LVEF 41-49%), and 31% in HF with preserved EF (LVEF ≥50%). After multivariable adjustment, patients who received testing for CAD, compared with those who did not, were younger and more likely to be male, have a smoking history, have hyperlipidemia, and have HFrEF or HFbEF (all P <0.05).
Conclusions:
The majority of patients hospitalized for new-onset HF did not receive testing for CAD either during the hospitalization or in the 90 days before and after. The rates of testing for CAD were higher in patients with LVEF ≤40% though remained low. These data highlight an opportunity to improve care by identifying appropriate candidates for optimal CAD medical therapy and revascularization.
Keywords: heart failure, left ventricular dysfunction, coronary artery disease, registries, diagnostic testing
Introduction
Heart failure (HF) is a common condition, affecting over 6 million adults in the United States alone.1 Despite advancements in medical therapy for cardiovascular disease, new-onset HF remains common.2 In patients with new-onset HF, patients are recommended to receive a thorough evaluation for underlying heart disease and for reversible predisposing conditions. Given that the prevalence of concomitant significant coronary artery disease (CAD) among HF patients is approximately 60%,3–6 current guidelines state that for patients presenting with new-onset HF it is reasonable to undergo noninvasive imaging to detect myocardial ischemia.7
Prior data suggest an underutilization of testing for CAD in patients with new-onset HF.8,9 For example, in the Truven Health MarketScan Commercial and Medicare Supplemental databases, the use of testing for CAD among patients hospitalized for new-onset HF was 18% during the index hospitalization, and increased to only 27% at 90 days after admission.9 This study relied on claims data alone and there were limited data available on left ventricular ejection fraction (LVEF) and other relevant information such as renal function. The current analysis was designed to address this gap in knowledge with a contemporary analysis and by determining how clinical variables, including LVEF, influence testing for CAD among patients with new-onset HF.
Methods
Data Sources
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. This observational cohort study utilized data obtained from the American Heart Association’s Get With The Guidelines - Heart Failure (GWTG-HF) registry, which was linked to Medicare claims. The GWTG-HF program is a voluntary, hospital-based quality improvement initiative initiated in 2005 to improve the care of patients with HF by translating guidelines into clinical practice.10 The program collects >188 variables including medical history, in-hospital laboratory studies and tests (e.g., LVEF assessments and stress testing), in-hospital procedures (e.g., coronary angiography), and discharge disposition. Quality measures are collected by and provided to institutions from IQVIA (Cambridge, MA) via the Patient Management Tool™.
Programs must comply with local government regulations and seek guidance from their respective institutional review boards. Since data provided at the local site are used for quality improvement, informed consent waivers are implemented per the Common Rule. IQVIA (Cambridge, MA) serves as the registry coordinating center and the Duke Clinical Research Institute (Durham, NC) acts as the data analysis center. The Institutional Review Board of Duke University Health System approved the current analysis.
Study Populations and Study Definitions
Primary data were obtained from 2009 to 2015 in the GWTG-HF registry for hospitalizations for a new diagnosis of HF. Additionally, patients linked with Medicare claims were evaluated 90 days before and after index hospitalization in order to assess for testing for CAD. Patients were excluded from the analysis if they had any of the following: a diagnosis of HF prior to admission, a transfer during the hospitalization to another medical facility, a referral to hospice upon discharge, or death within 90 days of the index hospitalization. HF with reduced ejection fraction (HFrEF) was defined as LVEF ≤40%, HF with borderline EF (HFbEF) as LVEF 41% to 49%), and HF with preserved EF (HFpEF) as LVEF ≥50%. Non-invasive testing for CAD constituted an assessment (or combination) of the following: exercise test without imaging, nuclear stress imaging, stress echocardiogram, cardiac magnetic resonance imaging (with or without stress), coronary artery calcium scoring, and coronary computed tomography angiography. Coronary angiography with or without percutaneous coronary intervention (PCI) was considered invasive testing for CAD. Testing for CAD was identified in the registry or through linked Medicare claims using billing codes as outlined in Supplemental Table 1.
Statistical Methods
Baseline patient and hospital characteristics were described by the presence of testing for CAD using proportions and medians with interquartile ranges (IQRs) for categorical and continuous variables, respectively. Standardized differences in proportions, means or ranked means were calculated for categorical, continuous, or ordinal categorical characteristics respectively, between patients with and without testing for CAD. A standardized difference of ≥10% was considered as a reference for meaningful difference between groups.
A multivariable logistic regression was used to identify factors associated with testing for CAD. Candidate variables were selected based on prior established models in GWTG-HF.11 Generalized estimating equations were used to account for the within-hospital clustering of patients. The model predicted a binary outcome for the instance of testing for CAD and included the following factors: age, sex, race, medical history (atrial fibrillation/flutter, chronic obstructive pulmonary disease, diabetes mellitus, dyslipidemia, hypertension, peripheral arterial disease, stroke, anemia, pacemaker or implantable cardioverter defibrillator, chronic kidney disease or chronic dialysis, depression, valvular heart disease, and smoking), LVEF category, vital signs at admission (heart rate, systolic blood pressure), laboratory studies at admission (serum creatinine), year of hospitalization and quarter, and hospital variables (number of hospital beds, geographic region, rural location, and teaching status). We also evaluated the interaction effects of LVEF category by age, LVEF category by history of chronic kidney disease, and LVEF category by presence of diabetes mellitus. Significant interactions were reported. Patient-level model covariates with >50% missing were not considered for the model. Covariates with <50% missing were imputed before entering into models. Hospital-level characteristics were not imputed. Patient continuous variables were imputed using multiple imputation methods with 10 datasets. For modeling purposes, P values of <0.05 were considered significant.
We then described the modality utilized to test for CAD, including if noninvasive or invasive strategies were utilized before, during, or after the hospitalization. We also reported the differences in modality utilized to test for CAD by LVEF categories, comparing HFrEF and HFbEF with HFpEF as the reference group. Cochran-Armitage tests were performed to assess temporal changes in ischemia evaluation within each LVEF category.
We also described in-hospital and discharge quality of care by testing for CAD using selected GWTG-HF achievement measures (e.g., angiotensin-converting enzyme inhibitor/angiotensin receptor blocker prescription at discharge in patients with HFrEF) and GWTG-HF quality metrics (e.g., cardiac resynchronization therapy placed or prescribed at discharge in patients with an LVEF ≤35% and a QRS ≥120ms without reason for exclusion). Standardized differences for care received by patients with and without testing for CAD were reported. As a sensitivity analysis, we repeated the analyses in patients with a prior history of CAD. Analyses were performed in SAS software version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Between 2009 and 2015 we identified 22,747 patients in the GWTG-HF registry with new-onset HF who were able to be linked to Medicare claims. Of these, we excluded patients for incomplete insurance coverage in the 90 days before or after hospitalization (599, 2.6%), discharge disposition such as in-hospital death or referral to hospice upon discharge (2535, 11%), death within 90 days of discharge (1978, 8.7%), and missing LVEF (450, 2.0%). The final study population was 17,185 patients from 352 hospitals across the United States.
Of these, 6672 (39%) received testing for CAD (Table 1). In univariate analyses, patients who received testing for CAD, compared with those who did not, were younger, more likely to be male, more likely to have HFrEF, and more likely to have traditional risk factors for CAD (i.e., diabetes and smoking in the last 12 months) (all standardized differences ≥10%). Patients who received testing for CAD were also more likely to receive care in a hospital that was larger as measured by number of beds and have access to PCI and adult cardiac surgery (all standardized differences ≥10%). Serum creatinine and treatment with aspirin and statins were similar in both groups (all standardized differences <10%). In the adjusted model including all clinical and hospital factors of interest (Table 2), the following factors were associated with testing for CAD: younger age, male sex, smoking in the last 12 months, hyperlipidemia, and LVEF category (all P values <0.05). Patients with HFrEF, compared with HFpEF, were more likely to receive testing for CAD, adjusted odds ratio 2.42 (95% confidence interval 2.21 to 2.65) (Supplemental Table 2). However, there was a signification interaction with LVEF category and age (P value <0.001). Patients with HFrEF were still more likely to receive testing compared with HFpEF though this attenuated with age ≥80. There no significant interaction effect with LVEF category and chronic kidney disease or history of diabetes (P values=0.18 and 0.65, respectively). Testing for CAD also increased over time in patients with HFrEF (from 50% in 2009 to 61% in 2015, P value <0.001) and HFbEF (from 38% in 2009 to 46% in 2015, P value=0.01). For HFpEF, testing for CAD slightly decreased over time (from 31% in 2009 to 30% in 2015, P value=0.03).
Table 1.
Patient Characteristics Stratified by Testing for Coronary Artery Disease
| Overall N = 17,185 |
CAD Testing N = 6736 |
No CAD Testing N = 10,449 |
Standardized Difference | |
|---|---|---|---|---|
| Age, years | 81 (73 - 87) | 77 (71 - 83) | 83 (76 - 89) | 65.5 |
| Female sex (%) | 57.0 | 50.8 | 60.9 | 20.4 |
| Race and ethnicity (%) | 8.7 | |||
| White | 83.5 | 81.7 | 84.6 | |
| Black | 8.2 | 9.3 | 7.5 | |
| Asian | 1. | 1.8 | 1.6 | |
| Other | 2.6 | 2.5 | 2.6 | |
| Hispanic (any race) | 4.1 | 4.7 | 3.8 | |
| Medical history (%) | ||||
| Atrial fib/flutter | 29.1 | 24.2 | 32.4 | 18.3 |
| Diabetes | 33.0 | 36.0 | 31.0 | 10.4 |
| Hypertension | 81.2 | 80.0 | 82.0 | 5.1 |
| Hyperlipidemia | 51.1 | 53.5 | 49.5 | 8.2 |
| History of CAD | 33.4 | 36.4 | 31.5 | 10.4 |
| Prior CVA or TIA | 13.8 | 11.8 | 15.1 | 9.7 |
| PAD | 9.7 | 9.9 | 9.5 | 1.4 |
| Smoking in last 12 mo | 9.7 | 12.2 | 8.1 | 13.7 |
| CKD (Cr >2 mg/dl) | 13.1 | 11.7 | 14.1 | 7.2 |
| Chronic dialysis | 1.5 | 1.7 | 1.4 | 2.0 |
| Asthma or COPD | 21.1 | 20.6 | 21.4 | 2.2 |
| Admission medications | ||||
| ACE inhibitor | 28.0 | 29.0 | 27.2 | 4.0 |
| ARB | 15.3 | 15.6 | 15.2 | 1.1 |
| β-blockers | 50.6 | 48.6 | 52.0 | 6.9 |
| Aldosterone antag | 2.8 | 2.9 | 2.7 | 1.6 |
| Aspirin | 41.8 | 43.5 | 40.7 | 5.6 |
| Statin | 42.8 | 45.2 | 41.2 | 8.2 |
| Laboratory data and imaging | ||||
| Serum Cr mg/dl | 1.1 (0.9 – 1.5) | 1.1 (0.9 – 1.4) | 1.1 (0.9 – 1.5) | 3.4 |
| LVEF (%) | 53 (35 – 60) | 45 (30 – 58) | 55 (41 – 60) | 44.6 |
| LVEF <30% | 15.6 | 23.0 | 10.7 | 33.2 |
| Hospital Characteristics | ||||
| Number of beds | 350 (221 – 483) | 369 (230 – 530) | 329 (217 – 483) | 13.0 |
| Geographic region | 11.8 | |||
| Western US | 12.0 | 12.0 | 12.0 | |
| Southern US | 29.5 | 29.3 | 29.7 | |
| Midwestern US | 25.7 | 28.6 | 23.9 | |
| Northeastern US | 32.8 | 30.2 | 34.4 | |
| Rural location | 4.1 | 3.5 | 4.6 | 5.6 |
| Teaching status | 71.2 | 72.8 | 70.2 | 5.9 |
| PCI-capable | 87.3 | 91.3 | 84.7 | 20.2 |
| Adult cardiac surgery | 78.6 | 83.6 | 75.3 | 20.5 |
Values shown are medians with interquartile ranges and percentages
We considered standardized differences ≥10% as statistically significant
ACE, angiotensin-converting enzyme; Aldosterone Antag, aldosterone antagonist; ARB, angiotensin-receptor blockers; Atrial fib, fibrillation; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; Cr, creatinine; CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction; mo, months; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; TIA, transient ischemic attack; US, United States
Table 2.
Patient and Hospital Characteristics Associated with Testing for Coronary Artery Disease
| Adjusted OR (95% CI) | P value | |
|---|---|---|
| Age, per year incr | 0.92 (0.91, 0.93) | <0.001 |
| Age ≥80 years | 1.18 (1.02, 1.35) | 0.022 |
| Female sex | 0.91 (0.84, 0.97) | 0.008 |
| Race and ethnicity (vs. white) | ||
| Black | 0.92 (0.78, 1.07) | 0.27 |
| Hispanic | 1.13 (0.93, 1.38) | 0.21 |
| Asian | 1.46 (1.02, 2.09) | 0.037 |
| Other | 0.97 (0.76, 1.24) | 0.79 |
| Medical History | ||
| Atrial fib/flutter | 0.80 (0.74, 0.86) | <0.001 |
| Diabetes | 0.96 (0.89, 1.05) | 0.39 |
| Hypertension | 1.10 (0.97, 1.26) | 0.14 |
| Hyperlipidemia | 1.18 (1.09, 1.28) | <0.001 |
| Ischemic etiology | 1.12 (1.02, 1.24) | 0.017 |
| Prior CVA or TIA | 0.87 (0.78, 0.97) | 0.016 |
| PAD | 1.06 (0.94, 1.19) | 0.34 |
| Smoking in last 12 months | 0.86 (0.76, 0.97) | 0.014 |
| CKD (Cr >2 mg/dl) | 0.84 (0.75, 0.95) | 0.004 |
| Chronic dialysis | 0.91 (0.68, 1.22) | 0.53 |
| Asthma or COPD | 0.85 (0.77, 0.93) | <0.001 |
| Anemia | 0.80 (0.72, 0.89) | <0.001 |
| Depression | 0.91 (0.80, 1.03) | 0.13 |
| Prior PCI | 1.25 (1.10, 1.43) | <0.001 |
| Valvular heart disease | 1.21 (1.07, 1.38) | 0.003 |
| Pacemaker | 1.00 (0.88, 1.14) | 0.97 |
| CRT-P | 1.09 (0.64, 1.84) | 0.76 |
| CRT-D | 0.57 (0.32, 1.01) | 0.05 |
| Laboratory data and imaging | ||
| Creatinine, per 10 unit incr | 1.00 (0.99, 1.01) | 0.49 |
| Left ventricular ejection fraction | ||
| HFrEF vs HFpEF | 2.42 (2.21, 2.65) | <0.001 |
| HFbEF vs HFpEF | 1.65 (1.44, 1.90) | <0.001 |
| Admission SBP, per 10 unit incr | 1.00 (0.99, 1.02) | 0.52 |
| Admission heart rate, per 10 unit incr | 0.99 (0.97, 1.01) | 0.53 |
| Hospitalization year, per quarter incr | 1.01 (1.00, 1.01) | 0.013 |
| Hospital characteristics | ||
| Bed size, per 100 unit incr | 1.02 (0.97, 1.07) | 0.48 |
| Geographic region (vs Northeast) | ||
| Midwestern | 1.20 (0.99, 1.47) | 0.07 |
| South | 0.96 (0.82, 1.12) | 0.62 |
| West | 0.92 (0.75, 1.14) | 0.47 |
| Rural location | 0.81 (0.56, 1.19) | 0.29 |
| Teaching status | 0.98 (0.86, 1.11) | 0.73 |
Atrial fib, fibrillation; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; Cr, creatinine; CRT, cardiac resynchronization therapy (P, pacemaker, D, defibrillator); CVA, cerebrovascular accident; incr, increase; HFbEF, heart failure with borderline ejection fraction; HFpEF heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; TIA, transient ischemic attack
Non-invasive modalities (24%) were as common as invasive (23%) modalities for testing for CAD (Table 3). The rates of PCI were relatively low during the time period (4.7%), as were rates of coronary artery bypass surgery (2.9%). More patients received testing in the hospital (24%) compared with before (8.3%) or after (11.5%) (Figure). Patients who received testing for CAD, compared with those who did not, were more likely to receive other recommended care (Table 4). For example, patients who received testing for CAD were more likely to receive aldosterone antagonists at discharge and anticoagulation for atrial fibrillation when indicated (standardized differences ≥10%).
Table 3:
Testing Modality for Coronary Artery Disease Stratified by Left Ventricular Ejection Fraction
| Standardized Differences | ||||||
|---|---|---|---|---|---|---|
| Overall N = 17,185 |
HFrEF N=5,475 |
HFbEF N=1,487 |
HFpEF N=10,223 |
HFrEF vs HFpEF |
HFbEF vs HFpEF |
|
| Any Testing for CAD | 39.2 | 54.1 | 42.5 | 30.8 | 48.5 | 24.6 |
| Noninvasive | 23.9 | 27.4 | 27.4 | 21.6 | 13.7 | 13.5 |
| ECG stress | 20.9 | 23.3 | 23.8 | 19.3 | 9.8 | 11.1 |
| Nuclear | 20.4 | 22.8 | 22.6 | 18.8 | 9.9 | 9.4 |
| Stress echo | 1.5 | 1.6 | 2.2 | 1.2 | 3.4 | 7.5 |
| Cardiac MRI | 0.7 | 1.4 | 0.5 | 0.4 | 10.9 | 2.0 |
| Coronary CTA | 0.4 | 0.4 | 0.5 | 0.4 | 0.6 | 0.5 |
| Invasive | 22.6 | 38.9 | 23.9 | 13.6 | 60.1 | 26.7 |
| Angiography | 22.5 | 38.8 | 23.7 | 13.6 | 59.9 | 26.3 |
| Angiography & PCI | 4.7 | 7.8 | 6.3 | 2.8 | 22.4 | 16.8 |
| Both noninvasive & invasive | 7.3 | 12.3 | 8.8 | 4.4 | 28.8 | 17.8 |
In this table, patients can be counted more than once if they received >1 test for CAD
We considered standardized differences ≥10% as statistically significant
CAD, coronary artery disease; CTA, computed tomography angiography; ECG, electrocardiogram; echo, echocardiogram; MRI, magnetic resonance imaging, PCI, percutaneous coronary intervention
Figure.
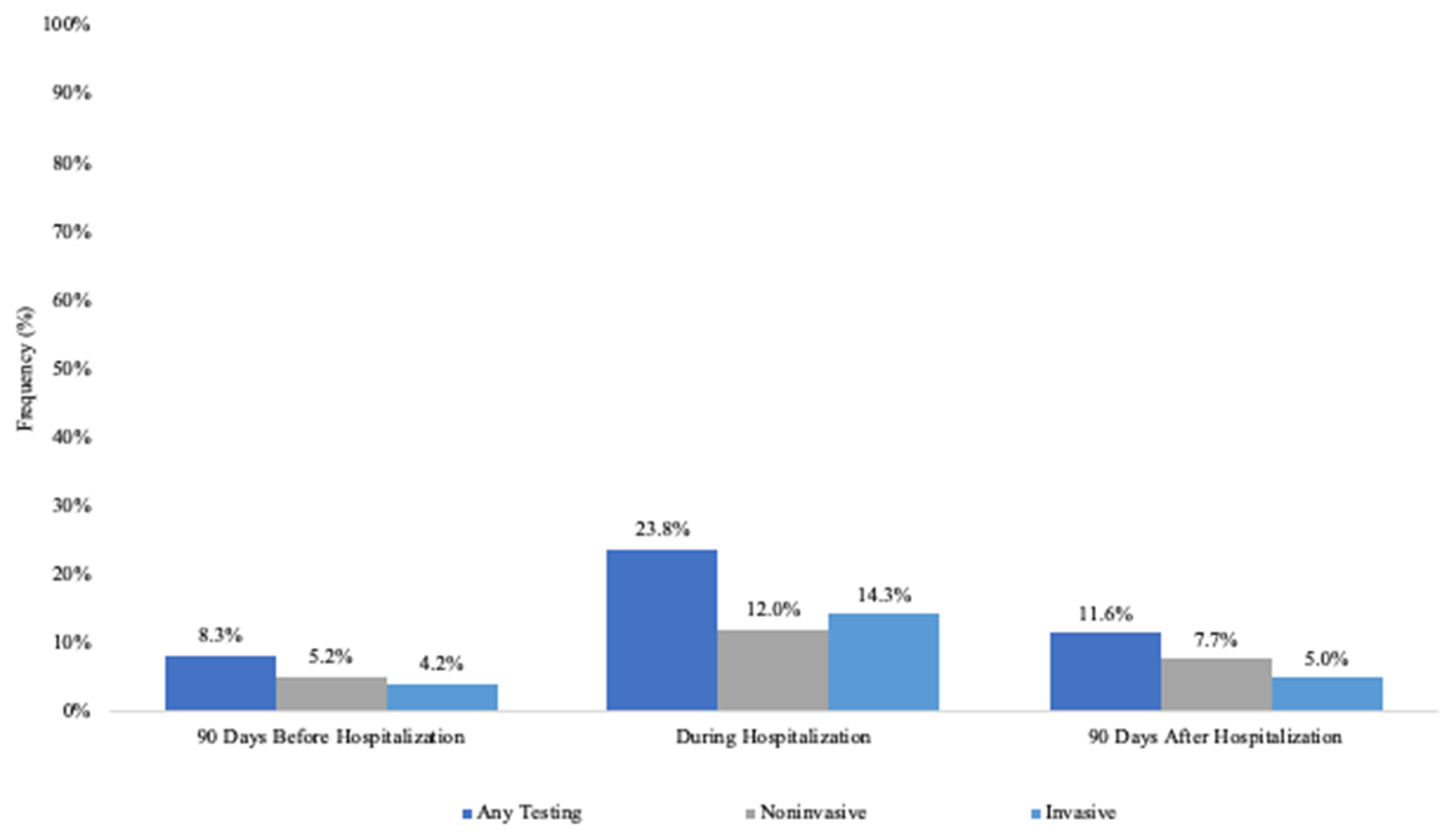
Timing of Testing for Coronary Artery Disease
Table 4.
Adherence to Get With The Guidelines-Heart Failure Quality Metrics Stratified by Testing for Coronary Artery Disease
| CAD Testing N = 6736 |
No CAD Testing N = 10,449 |
Standardized Differences | |
|---|---|---|---|
| In-hospital care | |||
| DVT prophylaxis | 71.7 | 73.7 | 3.2 |
| Influenza vaccination | 75.5 | 74.1 | 3.4 |
| Pneumococcal vaccination | 73.2 | 72.8 | 0.8 |
| Discharge care | |||
| ACEI/ARB | 97.1 | 95.3 | 9.4 |
| Evidence-based β-blockers | 85.4 | 79.6 | 7.0 |
| Aldosterone antag | 31.5 | 20.8 | 24.6 |
| Hydralazine & nitrate | 16.4 | 13.1 | 9.3 |
| Hypertension control | 75.1 | 72.2 | 6.5 |
| Lipid lowering medications | 51.3 | 44.0 | 14.6 |
| Diabetes treatment | 53.2 | 50.1 | 6.3 |
| Anticoagulation for atrial fib/flutter | 82.4 | 77.8 | 11.7 |
| CRT placed or prescribed | 30.4 | 22.2 | 18.6 |
| ICD counseling, placed, or prescribed | 24.4 | 18.6 | 14.3 |
| Follow-up visit ≤7 days | 73.5 | 76.2 | 6.2 |
We considered standardized differences ≥10% as statistically significant
Atrial fib, atrial fibrillation, ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; Aldosterone Antag, aldosterone antagonist; Atrial fib, fibrillation; CAD, coronary artery disease; DVT, deep vein thrombosis; CRT, cardiac resynchronization therapy, ICD, implantable cardioverter-defibrillator
We repeated the analysis excluding the 5124 patients with a prior history of CAD and found similar rates of testing for CAD. Among 12,061 patients with new-onset HF and no prior history of CAD, 4520 (37%) received testing for CAD and testing again differed by LVEF category: 54% in HFrEF, 42% in HFbEF, and 28% in HFpEF (standardized difference 51.5%).
Discussion
In this study, we assessed testing for CAD among older patients hospitalized for new-onset HF. The key findings can be summarized as follows 1) testing for CAD occurs in <50% of patients with new-onset HF, 2) testing for CAD is more common in patients with greater severity of LV dysfunction, 3) testing for CAD is more common in patients with traditional clinical risk factors for CAD (e.g., hyperlipidemia) but was also more common in patients receiving other recommended care (e.g., anticoagulation for atrial fibrillation). The observed low rates of testing for CAD are surprising given that many hospitals in the US have the ability to evaluate for CAD through multiple non-invasive as well as invasive diagnostic modalities. The reasons for low rates of testing for CAD in patients hospitalized with new-onset HF, despite guideline recommendations, are unknown and not apparent from our data.
Our study builds upon two prior observational analyses. First, among 5878 patients with new-onset HF participating in the Cardiovascular Research Network HF study, only 37% had testing for CAD from 14 days prior to hospitalization to 6 months after hospital discharge.8 This study primarily utilized claims data and included patients hospitalized from 2005 to 2008. Second, among patients with new-onset HF in the Truven Health MarketScan Commercial and Medicare Supplemental databases, only 27% had testing for CAD within 90 days after admission to the hospital. This study also utilized claims data and included patients hospitalized from 2011 to 2013.9 Our study was able to include data from the GWTG-HF registry, including LVEF and laboratory studies, and found notable variation in testing by LVEF. We also observed that elevated creatine during the hospitalization did not explain low rates of testing for CAD. Notably, patients in all 3 of these studies, including ours, had medical insurance and the rates of testing for CAD for patients without insurance are unknown. The proportion of patients presenting with new-onset HF that would benefit from testing for CAD is also unknown. However, the current observed rates of testing for CAD in our study are less than the expected prevalence, approximately 60%, of CAD in this population.3–6
Outcomes for patients hospitalized for HF remain suboptimal.12 Treatment of comorbid conditions, including CAD, is recommended in the current HF guidelines for patients, regardless of LVEF.7,13 Treatment of CAD is also a unique therapeutic pathway for patients with HF as medical therapy for CAD may improve overall outcomes. Treatment of CAD may also address an underlying contributing factor of the cardiomyopathy and/or exacerbating factor for acute HF. A prior analysis of OPTIMIZE-HF showed that patients hospitalized with HF who underwent angiography were more likely to be treated with medical therapies for CAD and undergo revascularization.14 These patients also had significantly lower rates of death and rehospitalization during the first 60-90 days follow-up post discharge. In our study, patients that underwent testing for CAD were significantly more likely to be discharged on lipid lowering medications. Identifying patients with CAD also affords the opportunity to identify appropriate candidates for revascularization. This is currently a minority of patients with CAD and HF, such as those with an LVEF of ≤35% and coronary anatomy amenable to bypass surgery.15,16 The role of revascularization in other patients with HF is less clear.13, 17–19
In our study, patients receiving testing for CAD also received other aspects of HF guideline-recommended care unrelated to CAD (Table 4). These data suggest this gap in care may be amenable to quality improvement campaigns, both at a practice/hospital level and national level. Similar to tools (e.g., HF checklist) designed to ensure patients with HFrEF are evaluated for appropriate medical therapy prior to discharge, similar efforts could be applied to encourage routine evaluations for CAD.
Our study has limitations. First, our study was limited to Medicare beneficiaries with a median age of 81 years old admitted at hospitals participating in GWTG-HF age 65 years and older in order to have access to pre- and post-discharge claims data. While this cohort is a subset of those with HF, it does represent an insured population with a high prevalence of both HF and CAD. Second, evaluations for other causes of HF and/or etiology of cardiomyopathy that may explain new-onset HF are unknown (e.g., evaluation for a familial cardiomyopathy). Third, we did not analyze CAD diagnostic testing that may have taken place more than 90 days prior or 90 days after the index hospitalization. Fourth, many different factors affect the reasons for clinicians to evaluate for CAD. We adjusted for many of these factors, but residual measured and unmeasured confounding may have persisted. Finally, the impact of additional testing for CAD in terms of costs and potentially unnecessary downstream testing is unknown.
Conclusions
Among older patients with new-onset HF, the rates of testing for CAD are below expected threshold recommended by current HF guidelines. The rates of testing for CAD were higher in patients with HFrEF though remained low. The current study highlights an opportunity to test the impact of prospective interventions designed to improve the rates of testing for CAD in patients with HF and assess the potential impact on outcomes.
Supplementary Material
What is new
In this observational study from the GWTG-HF registry linked to Medicare claims, we assessed testing for coronary artery disease among older patients hospitalized for new-onset heart failure.
Testing occurs in <50% of patients with new-onset heart failure
Testing is more common in patients with traditional clinical risk factors for coronary artery disease and greater left ventricular systolic dysfunction but was also more common in patients receiving other recommended care (e.g., anticoagulation for atrial fibrillation).
What are the clinical implications?
This study identifies an opportunity to improve heart failure care by identifying appropriate candidates for treatment of coronary artery disease with optimal medical therapy and revascularization
Sources of Funding
This work was supported in part by an American Heart Association grant award #16SFRN30180010. The GWTG-HF program is provided by the American Heart Association. Quintiles is the data collection coordination center for the American Heart Association/American Stroke Association GWTG programs. The GWTG-HF program is provided by the American Heart Association. GWTG-HF is sponsored, in part, by Amgen and has been funded in the past through support from Medtronic, GlaxoSmithKline, Ortho-McNeil, and the American Heart Association Pharmaceutical Roundtable.
Conflict of Interest Disclosures
The following relationships exist related to this presentation:
Gregg Fonarow reports research funding from the National Institutes of Health and consulting for Abbott, Amgen, AstraZeneca, CHF Solutions, Janssen, Medtronic, Merck, and Novartis. Ron Blankstein reports research support from Amgen Inc, and Astellas Inc. Nancy Albert reports consulting for Amgen, AstraZeneca and Novartis. Adrian Hernandez reports research funding from American Regent, AstraZeneca, Bristol Myers Squibb, GlaxoSmithKline, Merck, Novartis, Verily, and consulting with AstraZeneca, Bayer, Boehringer-Ingelheim, Boston Scientific, Merck and Novartis. Adam DeVore reports research funding from the American Heart Association, Amgen, AstraZeneca, Bayer, Intra-Cellular Therapies, Luitpold Pharmaceuticals, Merck, the NHLBI, Novartis and PCORI. He also provides consulting services for Amgen, AstraZeneca, Bayer, InnaMed, LivaNova, Mardil Medical, Novartis, Procyrion, scPharmaceuticals, and Zoll.
Abbreviations:
- GWTG-HF
Get With the Guidelines–Heart Failure registry
- HFrEF
Heart failure with reduced ejection fraction
- HFbEF
Heart failure with borderline ejection fraction
- HFpEF
Heart failure with preserved ejection fraction
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2019 Update: A Report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM, Vasan RS. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail. 2018;6:678–685. doi: 10.1016/j.jchf.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Sopko G, De Luca L, Velazquez EJ, Parker JD, Binkley PF, Sadowski Z, Golba KS, Prior DL, Rouleau JL, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006;114:1202–1213 [DOI] [PubMed] [Google Scholar]
- 4.Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, Cheng ML, Wynne J; ADHERE Scientific Advisory Committee and Investigators; ADHERE Study Group. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57–64. [DOI] [PubMed] [Google Scholar]
- 5.Mentz RJ, Allen BD, Kwasny MJ, Konstam MA, Udelson JE, Ambrosy AP, Fought AJ, Vaduganathan M, O’Connor CM, Zannad F, et al. Influence of documented history of coronary artery disease on outcomes in patients admitted for worsening heart failure with reduced ejection fraction in the EVEREST trial. Eur J Heart Fail. 2013;15:61–8. doi: 10.1093/eurjhf/hfs139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shore S, Grau-Sepulveda MV, Bhatt DL, Heidenreich PA, Eapen ZJ, Hernandez AF, Yancy CW, Fonarow GC. Characteristics, Treatments, and Outcomes of Hospitalized Heart Failure Patients Stratified by Etiologies of Cardiomyopathy. JACC Heart Fail. 2015;3:906–916. [DOI] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–52. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 8.Farmer SA, Lenzo J, Magid DJ, Gurwitz JH, Smith DH, Hsu G, Sung SH, Go AS. Hospital-level variation in use of cardiovascular testing for adults with incident heart failure: findings from the cardiovascular research network heart failure study. JACC Cardiovasc Imaging. 2014;7:690–700. doi: 10.1016/j.jcmg.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doshi D, Ben-Yehuda O, Bonafede M, Josephy N, Karmpalitotis D, Parikh M, Moses JW, Stone GW, Leon MB, Schwartz A. Underutilization of Coronary Artery Disease Testing Among Patients Hospitalized With New-Onset Heart Failure. J Am Coll Cardiol. 2016;68:450–458. doi: 10.1016/j.jacc.2016.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellrodt AG, Fonarow GC, Schwamm LH, Albert N, Bhatt DL, Cannon CP, Hernandez AF, Hlatky MA, Luepker RV, Peterson PN, et al. Synthesizing lessons learned from get with the guidelines: the value of disease-based registries in improving quality and outcomes. Circulation. 2013;128:2447–60. doi: 10.1161/01.cir.0000435779.48007.5c. [DOI] [PubMed] [Google Scholar]
- 11.Ziaeian B, Hernandez AF, DeVore AD, Wu J, Xu H, Heidenreich PA, Matsouaka RA, Bhatt DL, Yancy CW, Fonarow GC. Long-term outcomes for heart failure patients with and without diabetes: From the Get With The Guidelines-Heart Failure Registry. Am Heart J. 2019;211:1–10. doi: 10.1016/j.ahj.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol. 2017;70:2476–2486. doi: 10.1016/j.jacc.2017.08.074. [DOI] [PubMed] [Google Scholar]
- 13.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 14.Flaherty JD, Rossi JS, Fonarow GC, Nunez E, Stough WG, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, Yancy CW, et al. Influence of coronary angiography on the utilization of therapies in patients with acute heart failure syndromes: Findings from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J. 2009;157:1018–25. doi: 10.1016/j.ahj.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, et al. ; STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–16. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, et al. ; STICHES Investigators. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. doi: 10.1056/NEJMoa1602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(25 Pt A):2817–27. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 18.Wolff G, Dimitroulis D, Andreotti F, Kołodziejczak M, Jung C, Scicchitano P, Devito F, Zito A, Occhipinti M, Castiglioni B, et al. Survival benefits of invasive versus conservative strategies in heart failure in patients with reduced ejection fraction and coronary artery disease: a meta-analysis. Circ Heart Fail. 2017;10:e003255. doi: 10.1161/CIRCHEARTFAILURE.116.003255. [DOI] [PubMed] [Google Scholar]
- 19.DeVore AD, Yow E, Krucoff MW, Sherwood MW, Shaw LK, Chiswell K, O’Connor CM, Ohman EM, Velazquez EJ. Percutaneous coronary intervention outcomes in patients with stable coronary disease and left ventricular systolic dysfunction. ESC Heart Fail. 2019;6:1233–1242. doi: 10.1002/ehf2.12510 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


