Abstract
Immunoglobulins (Ig) from healthy, nonimmune individuals bind to the surfaces of Plasmodium falciparum-infected erythrocytes (RBC). In order to investigate the presence of this parasite phenotype in wild isolates and its potential association with malarial anemia, we conducted a study of 207 anemic or nonanemic children with malaria in Gabon. Surface Ig binding to infected RBC was detected for 83% of the isolates. No difference in Ig binding between the groups was observed, but all isolates which exhibited extensive Ig binding were found in a group of moderately anemic children.
Severe and life-threatening Plasmodium falciparum malaria, primarily characterized by severe anemia, cerebral involvement, and respiratory distress, causes two million deaths annually (7). Hemolysis of the infected erythrocyte by the parasite is the most likely cause of anemia, but it alone cannot explain the dramatic drop in hemoglobin (Hb) level frequently apparent in malarious children. Dyserythropoiesis, autoimmune mechanisms, and excessive phagocytosis may also be involved in the multifactorial pathogenesis (1, 4, 13).
P. falciparum-infected erythrocytes with a capacity to bind other erythrocytes (RBC), to form rosettes, are more often encountered in patients with severe malaria than in patients with mild disease (2, 8). RBC infected by such rosetting strains carry fibrillar structures, containing nonimmune immunoglobulin (Ig), on their surfaces, which are partly responsible for RBC binding in rosettes (9, 12). Phagocytic cells of malaria patients engulf more uninfected RBC than infected RBC (6). These findings led us to hypothesize that infected RBC in a rosette sensitize bound RBC with Ig remaining on the membrane attached to the RBC after shizogony, making them recognizable targets for excessive clearance from the circulation by phagocytic cells. Therefore, Ig-binding parasites would be more likely to cause anemia than nonbinding cells. To determine whether this is the case, we studied the frequency of Ig binding and rosetting of P. falciparum isolates from children with various degrees of malarial anemia at the Albert Schweitzer Hospital in Lambaréné, Gabon.
Peripheral blood samples from 294 randomly selected children between 5 months and 15 years of age with P. falciparum infection were collected at the Albert Schweitzer Hospital during February and March 1995 and from November 1995 to February 1996 after informed parental consent was obtained. Patients homozygous for the sickle cell gene were excluded by microscopic examination of the samples. On the basis of a small number of samples collected during a pilot study period the patients were divided into two groups, anemic (Hb, ≤90 g/liter) (A) or nonanemic (Hb, >90 g/liter) (NA). Hb was determined by the absorbance of visible light or UV light in a spectrophotometer after centrifugation of blood in an acridine orange-coated capillary tube (QBC system; Becton Dickinson).
Samples were washed three times in RPMI 1640 and propagated according to standard procedures (10) at 5% hematocrit with 10% heat-inactivated AB+ Rh+ nonimmune serum from Swedish blood donors added to the buffered medium (pH 7.2). Isolates from 92% (270 of 294) of the children grew satisfactorily. The level of parasitemia was calculated from the number of infected RBC relative to the number of uninfected RBC from an average of 200 cells. An isolate was included in the study only if more than 50% of the parasites had developed to late stages (trophozoites and early schizonts) and if the parasitemia level was 0.5% or more. Isolates with mostly schizonts were excluded since they rupture during the assays. A total of 207 isolates met these criteria and were subsequently studied for their ability to form rosettes and to bind Ig.
Unfixed RBC infected with asexual late-stage parasites were surface labelled with fluorescein isothiocyanate-labelled anti-Ig reagents as previously described (9). The fluorescence rate was expressed as the number of fluorescent late-stage-infected RBC relative to the total number of late-stage-infected RBC.
Assessment of rosetting was made as previously described (2) at 12 to 18, 24 to 30, and 42 to 46 h. The rosetting rate was expressed as the number of infected RBC in rosettes relative to the total number of late-stage-infected RBC.
Spearman’s rank correlation test, with appropriate corrections for ties, was used for the analysis of an association between the rosetting rate and the Ig binding rate of the individual isolates. The Mann-Whitney U test was used for comparison of the Ig binding rates or the rosetting rates and the Hb values of the two groups of patients.
The mean age of the 207 patients was 5.5 years, that of the 73 A patients was 3.4 years (4 months to 11 years), and that of the 135 NA patients was 6.6 years (7 months to 15 years). The mean Hb values were 99 g/liter for the whole group, 71 g/liter for the A children, and 115 g/liter for the NA children. The parasite densities were the same in the two groups.
Surface Ig binding to infected RBC was detected for 83% (173 of 207) of the isolates, with a mean fluorescence rate of 13% (range, 0.6 to 87%) when antibodies to total Ig were used, while detection of IgM and IgG binding was less frequent, 73% (143 of 196 isolates available) and 61% (116 of 189 available), respectively. Most isolates bound both Igs, but 38 isolates bound only IgM and 14 bound only IgG.
One hundred thirty-four (65%) of the 207 isolates formed rosettes, with a mean rosetting rate of 13% (range, 0.5 to 88%). Although highly statistically significant, the correlations between rosetting and Ig binding (rho 0.41, P < 0.0001), rosetting and IgM binding (rho 0.41, P < 0.0001), and rosetting and IgG binding (rho 0.20, P < 0.01) were very weak.
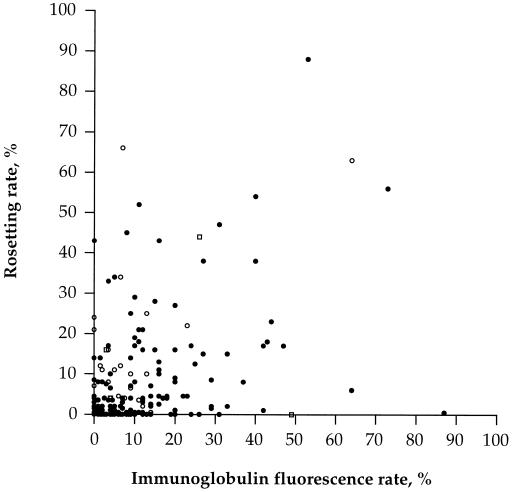
The isolates could be grouped on the basis of the Ig binding and the rosetting capacity of the individual isolates (Fig. 1). Most of the isolates bound Ig and formed rosettes, while some lacked both phenotypes. Many isolates bound Ig without forming rosettes, and a few isolates formed rosettes without binding Ig.
FIG. 1.
Ig binding and rosetting of P. falciparum-infected erythrocytes from 207 fresh parasite isolates. Each symbol represents one isolate. ○, isolates binding IgM alone; □, isolates binding IgG alone; •, isolates binding both Igs.
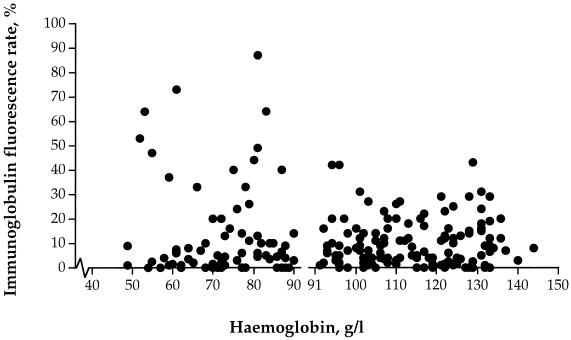
No difference was observed in a comparison of all isolates from the A (Hb, ≤90 g/liter) and the NA (Hb, >90 g/liter) patients for Ig binding (Fig. 2) (Z = 0.098, P = 0.92), but isolates with extensive Ig binding were detected in children with a moderately low Hb. No difference in the overall rosetting rate was apparent for the A and the NA patients.
FIG. 2.
Ig binding of P. falciparum-infected erythrocytes in 207 fresh parasite isolates from a group of children with moderate anemia (Hb, >50 to ≤90 g/liter; n = 73) and a group of children without anemia (Hb, >90 g/liter; n = 134). Each symbol represents one isolate.
We have here examined the capacity of P. falciparum-infected RBC to bind nonimmune Ig in a group of Gabonese children with malaria and demonstrate that high-level Ig binding occurs in some isolates from moderately anemic children. Rosetting rates did not differ for the A and NA groups, but isolates with high rosetting rates (≥15%) were obtained from children with anemia (not shown). It remains unclear whether extensive Ig binding is important in malarial anemia. Recent findings suggest, however, that a high rosetting capacity in at least some isolates is linked to a panadhesive phenotype including Ig binding, autoagglutination, endothelial binding to CD36 (5) and CD31 (11), and binding to the ABO blood group antigens (3, 5). This may also involve augmented recognition by multiple receptors on phagocytic cells, enhanced by Ig sensitization, making both infected and uninfected RBC recognizable targets for excessive clearance from the circulation.
Our finding of Ig-binding infected RBC in children with moderate anemia is potentially interesting and has to be further explored in the context of the complex interaction between the parasite and the host.
Acknowledgments
We thank Marcel Nkeyi and Anselme Ndzenguet for excellent technical assistance; Leo Lehman, Bertrand Lell, and Ruprecht Schmidt-Ott for their help during the study period at the Albert Schweitzer Hospital; Robert Harris for editorial comments; and Staffan Ekblom of the Statistical Research Group, Stockholm University, for statistical advice.
This study was supported by grants from the Swedish Medical Research Council, the Swedish International Development Authority (SIDA), Stiftelsen Sigurd och Elsa Goljes Minne, Stiftelsen Clas Groschinskys Minnesfond, Martin Rinds Stiftelse, and Fortüne grant 227 (Medical Faculty of the University of Tübingen).
REFERENCES
- 1.Abdalla S, Weatherall D J, Wickramasinghe S N, Hughes M. The anaemia of P. falciparum malaria. Br J Haematol. 1980;46:171–183. doi: 10.1111/j.1365-2141.1980.tb05956.x. [DOI] [PubMed] [Google Scholar]
- 2.Carlson J, Helmby H, Hill A V S, Brewster D, Greenwood B M, Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 3.Carlson J, Wahlgren M. Plasmodium falciparum erythrocyte rosetting is mediated by promiscuous lectin-like interactions. J Exp Med. 1992;176:1311–1317. doi: 10.1084/jem.176.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Facer C A. Direct antiglobulin reactions in Gambian children with P. falciparum malaria. III. Expression of IgG subclass determinants and genetic markers and association with anaemia. Clin Exp Immunol. 1980;41:81–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez, V., C. J. Treutiger, G. B. Nash, and M. Wahlgren. Multiple adhesive phenotypes linked to rosetting-binding of erythrocytes in Plasmodium falciparum malaria. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 6.Knüttgen H J. Das Verhalten der Makrophagen und die Phagozytose von Parasiten im Knochenmark bei Malaria tropica. Z Tropenmed Parasitol. 1961;12:161–171. [PubMed] [Google Scholar]
- 7.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, Pasvol G, Snow R. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 8.Rowe A, Obeiro J, Newbold C I, Marsh K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholander C, Treutiger C J, Hultenby K, Wahlgren M. Novel fibrillar structure confers adhesive property to malaria-infected erythrocytes. Nat Med. 1996;2:204–208. doi: 10.1038/nm0296-204. [DOI] [PubMed] [Google Scholar]
- 10.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 11.Treutiger, C. J., A. Heddini, V. Fernandez, W. A. Muller, and M. Wahlgren. PECAM-1/CD31, an endothelial receptor for rosetting Plasmodium falciparum-infected erythrocytes. Nat. Med., in press. [DOI] [PubMed]
- 12.Treutiger, C. J., C. Scholander, J. Carlson, K. P. McAdam, J. G. Raynes, L. Falksveden, and M. Wahlgren. Adhesive serum proteins cause stable rosetting-binding of Plasmodium falciparum-infected erythrocytes. Submitted for publication. [DOI] [PubMed]
- 13.Turrini F, Mannu F, Cappadoro M, Ulliers D, Giribaldi G, Arese P. Binding of naturally occurring antibodies to oxidatively and nonoxidatively modified band 3. Biochim Biophys Acta. 1994;1190:297–303. doi: 10.1016/0005-2736(94)90087-6. [DOI] [PubMed] [Google Scholar]