Abstract
Migraine is commonly reported in patients with temporomandibular disorders (TMDs), but little is known about the mechanisms underlying the comorbid condition. Here we prepared a mouse model to investigate this comorbidity, in which masseter muscle tendon ligation (MMTL) was performed to induce a myogenic TMD and the pre-existing TMD enabled a subthreshold dose of nitroglycerin (NTG) to produce migraine-like pain in mice. RNA sequencing followed by real-time quantitative PCR confirmation showed that MMTL plus NTG treatment increased prodynorphin (Pdyn) mRNA expression in the spinal trigeminal nucleus caudalis (Sp5C) of female mice, but not in male mice. Chemogenetic inhibition of Pdyn-expressing neurons or microinjection of anti-dynorphin antiserum in the Sp5C alleviated MMTL-induced masseter hypersensitivity and diminished the MMTL-enabled migraine-like pain in female mice, but not in male mice. Moreover, chemogenetic activation of Pdyn-expressing neurons or microinjection of dynA(1–17) peptide in the Sp5C enabled a subthreshold dose of NTG to induce migraine-like pain in female mice, but not in male mice. Taken together, our results suggest that trigeminal dynorphin has a female-specific role in the modulation of comorbid TMDs and migraine.
Keywords: Orofacial pain, Comorbidity, Dynorphin, Sp5C, Sex difference
1. Introduction
Temporomandibular disorders (TMDs) and migraine are highly debilitating orofacial pain conditions that are often comorbid and occur more in women than men. Patients with TMDs frequently experience headaches in addition to the pain in the temporomandibular joint (TMJ) [5,6,15]. Migraine is prevalent among TMD patients with a prevalence of up to 58%, especially in women with myogenic TMD [14,20–23,32]. However, the mechanisms underlying such comorbidity as well as its sex dimorphism remain poorly understood. In our previous study [51], we combine masseter muscle tendon ligation (MMTL) with systemic injection of nitroglycerin (NTG) to develop a comorbid TMDs and migraine-like pain mouse model. We use this model to further investigate the underlying mechanism for this comorbidity in the present study.
The prodynorphin (Pdyn) gene encodes a precursor protein that is biologically inactive and can be post-translationally cleaved to form following opioid neuropeptides: dynorphin A (dynA), dynorphin B (dynB), neoendorphins, and bio-active/inactive fragments [17,25]. Although dynB and neoendorphin have been shown to involve in antinociception after sensory stimulation [49], dynA plays an important role in the development and maintenance of chronic pain. For instance, a single intrathecal administration of dynA induces chronic allodynia [38,57] and intrathecal injection of anti-dynA antiserum inhibits chronic pain [26]. Previous studies have shown that the dynorphin level in the spinal cord is increased in both chronic neuropathic and inflammatory pain models [27,61,62], which indicates the involvement of spinal dynorphin in chronic pain. Interestingly, knockout of Pdyn gene blocks chronic pain without effect on acute nociception [61], and spinal dynorphin level can fluctuate during the menstrual cycle of females [7]. However, it is unknown whether Pdyn gene and trigeminal dynorphin contribute to the comorbid TMDs and migraine-like pain. Here we use multidisciplinary approaches to address this knowledge gap and identify therapeutic targets for such comorbidity condition.
In the present study, we conducted RNA sequencing (RNA-Seq) followed by real-time quantitative PCR (RT-qPCR) confirmation to reveal that dynorphin in the spinal trigeminal nucleus caudalis (Sp5C) could be a potential therapeutic target for the comorbid TMDs and migraine-like pain. Using Pdyn-Cre mice and chemogenetic manipulation approach, we further investigated the sex-selective role of trigeminal dynorphin in the pathogenesis of this comorbidity. Intra-Sp5C injection of anti-dynorphin antiserum and dynA peptide were also used to verify our findings in this study.
2. Materials and Methods
2.1. Animals
Male and female adult (8–10 weeks old) C57BL/6J mice (Jackson Lab, Strain #000664), Pdyn-Cre mice (Jackson Lab, Strain #027958), and Ai14 mice (Jackson Lab, Strain #007914) were used in this study. Homozygous Pdyn-Cre mice were crossed with homozygous Ai14 mice to produce Pdyn-Cre;Ai14 mice. Animals were housed under standard conditions on a 12-hour light/dark cycle with ad libitum access to food and water. All behavioral tests were performed during the light phase and carried out by an investigator who was blinded to the treatment groups. The estrous cycle of female mice were determined by cytological evaluation of vaginal smears [8,63] when needed. All experimental procedures were carried out in accordance with the ethical guidelines of the National Institutes of Health and the International Association for the Study of Pain. All experimental protocols were approved by Texas A&M University School of Dentistry Institutional Animal Care and Use Committee.
2.2. Comorbid TMDs and migraine-like pain mouse model
Mice were randomly assigned to the unilateral MMTL or sham surgery. The MMTL was performed as described in our previous study [51]. In brief, mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p., Cat. #P3761, Sigma-Aldrich). The left anterior superficial part of masseter muscle tendon was exposed through an intraoral incision, gently freed from the surrounding connective tissue, then tied with two 6.0 chromic gut ligatures. Tissue adhesive (Cat. #1469SB, 3M) was used to close the incision. The mice with sham surgery received only tendon exposure without ligation. Our previous study [51] has shown that MMTL produces long-lasting mechanical allodynia in trigeminal nerve V3 branch-innervated masseter region and causes oral dysfunction, which indicates that it can be used to study myogenic TMD and TMJ pain in such TMD patients. On day 8 post-MMTL, the mice received a single subthreshold dose of NTG (1 mg/kg, i.p.) or vehicle at a volume of 1 ml/kg. NTG solution was freshly prepared by diluting a stock solution (5 mg/ml, NDC #0517-4810-25, American Regent) with saline to 1 mg/ml. Because the stock solution contains 30% alcohol and 30% propylene glycol as dissolvent, the vehicle control was prepared by diluting the dissolvent with saline. A systemic injection of NTG has been used extensively to induce migraine pain, since NTG causes immediate headache in both healthy people and patients with migraine [1,12,45].
2.3. RNA sequencing (RNA-Seq)
On day 8 post-MMTL/sham, female mice at the diestrus phase were selected to receive NTG (1 mg/kg, i.p.) or vehicle. One day after NTG/vehicle administration, mice were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and Sp5C tissues were rapidly removed and stabilized in RNAlater tissue storage reagent (Cat. #R0901, Sigma-Aldrich). Total RNA was extracted from the Sp5Cs (ipsilateral to surgery side) using RNeasy Micro Kit (Cat. #74004, Qiagen). Sequencing libraries were generated from 1 μg of RNA per sample using NEBNext UltraTM RNA Library Prep Kit for Illumina (Cat. #E7530L, NEB) following manufacturer’s protocol and index codes were added to attribute sequences to each sample. Paired-end reads (150 bp, 30 million reads) per sample were generated on an Illumina Hiseq platform. Index of the reference genome was built using Bowtie v2.2.3 [36,37] and paired-end clean reads were aligned to the reference genome using TopHat v2.0.12 [54]. HTSeq v0.6.1 [2] was used to count the reads numbers mapped to each gene. Fragments per kilobase of transcript per million fragments mapped (FPKM) was calculated for each transcript [55].
2.4. Real-time quantitative PCR (RT-qPCR)
On day 8 post-MMTL/sham, mice received NTG or vehicle. Sp5C samples were obtained on day one after NTG/vehicle administration. Next, cDNA was prepared by using the QuantiTect Reverse Transcription Kit (Cat. #205311, Qiagen) and RT-qPCR was performed using the CFX96 real-time PCR detection system (Bio-Rad) with the dye-based qPCR mix (Cat. #A6001, Promega). All samples were analyzed in triplicate. The primer pair for Pdyn was designed using Primer3 [56]: forward, 5’-ATGATGAGACGCCATCCTTC-3’; reverse, 5’-TTAATGAGGGCTGTGGGAAC-3’. Gapdh was used as an internal control [3]. The primer pair for Gapdh is: forward, 5’-TGAAGGTCGGTGTGAACGGA-3’; reverse, 5’-ACAAGCTTCCCATTCTCGGC-3’ [39]. Relative expression of Pdyn was calculated using the ΔΔCt method (2−ΔΔCt) [43]. After the PCR run, we performed melting-curve analysis and agarose gel electrophoresis to verify the PCR product specificity.
2.5. Immunofluorescence staining
Deeply anesthetized Pdyn-Cre;Ai14 mice were perfused with 4% paraformaldehyde. The brainstem tissues (containing Sp5C) were harvested, post-fixed at 4°C overnight, and then cryoprotected in 30% sucrose for two days. The tissues were sectioned at a thickness of 20 μm. After being blocked with PBS containing 5% normal goat serum (Cat. #5425, Cell Signaling Technology) and 0.3% Triton X-100 (Cat. #1610407, Bio-Rad) at room temperature for 1 h, the free-floating sections were incubated with each primary antibody at 4°C overnight, and then washed and incubated with respective secondary antibody for 1 h at room temperature. Primary antibodies we used: mouse anti-NeuN (1:500, Cat. #ab104224, Abcam), rabbit anti-ionized calcium binding adapter molecule 1 (anti-Iba1, 1:400, Cat. #019-19741, FUJIFILM Wako Chemicals), chicken anti-glial fibrillary acidic protein (anti-GFAP, 1:800, Cat. #AB5541, EMD Millipore), rabbit anti-glutaminase (1:500, Cat. #Ab131554, Abcam), rabbit anti-paired box 2 (anti-PAX2, 1:500, Cat. #71-6000, Invitrogen). Secondary antibodies we used: donkey anti-mouse 488 (1:500, Cat. #715-545-150, Jackson ImmunoResearch), donkey anti-rabbit 488 (1:500, Cat. #715-545-152, Jackson ImmunoResearch), donkey anti-chicken 488 (1:500, Cat. #715-545-155, Jackson ImmunoResearch). All images were acquired using a Leica DMi8 fluorescence microscope.
2.6. Orofacial mechanical hypersensitivity test
Orofacial mechanical hypersensitivity was measured with von Frey filaments as described in our previous study [51]. Each mouse was allocated in a plexiglass cylinder (10 cm long, 3 cm internal diameter) with the head and forepaws leaving outside and allowed to move freely. Mice were habituated to the apparatus for 3 days (30 min per day) before the first test and 10 min before each test. A series of calibrated von Frey filaments (0.07, 0.16, 0.4, 0.6, 1.0, 1.4, and 2.0 g) were applied to the trigeminal nerve V3-innervated masseter region for measuring MMTL-produced TMJ pain and applied to the trigeminal nerve V1-innervated periorbital region (skin over the rostral portion of the eye) for measuring NTG-induced migraine pain [16,19,48]. A positive response was defined as a sharp head withdrawal. The head withdrawal threshold was recorded as the force at which the positive response occurred three times out of five stimuli.
2.7. Chemogenetic manipulation of Pdyn-expressing neurons in the Sp5C
DREADDs-based chemogenetic activation or inhibition of Pdyn-expressing neurons was conducted to reveal the role of trigeminal dynorphin in the comorbid TMDs and migraine-like pain condition. To infuse the DREADD or control virus, Pdyn-Cre mice were anesthetized with 2–3% inhaled isoflurane, and then 0.5 μl of the virus was injected into unilateral Sp5C (ipsilateral to MMTL) (−8.0 mm AP; 1.5 mm ML; 4.5 mm DV) [41,42] using a 33-gauge needle at a rate of 0.1 μl/min, after which the injection needle was left in place for 10 min prior to being withdrawn. Viruses we used: (1) Cre-inducible DREADD virus expressing hM3D for neuronal activation (AAV5-hSyn-DIO-hM3D (Gq)-mCherry, Cat. #44361, Addgene); (2) Cre-inducible DREADD virus expressing hM4D for neuronal inhibition (AAV5-hSyn-DIO-hM4D (Gi)-mCherry, Cat. #44362, Addgene); (3) control virus (AAV5-hSyn-DIO-mCherry, Cat. #50459, Addgene). Compound 21 (C21, Cat. #HB4888, Hello Bio) dissolved in saline was given at 1 mg/kg (i.p.) to activate DREADDs [31,53].The virus infusion site was confirmed histologically after experiments.
2.8. Microinjection of drugs into the Sp5C
Under anesthesia with 2–3% inhaled isoflurane, mice received a single injection of drugs into Sp5C. The microinjection method is the same as the virus infusion method described above. For anti-dynorphin antiserum injection, rabbit anti-dynA antiserum (Cat. #T-4279.0400, Peninsula Laboratories) dissolved in saline was given at 0.25 μg or 0.5 μg at a volume of 0.5 μl [24]. The control group of mice received 0.5 μg of rabbit IgG (Cat. #BE0095, Bio X Cell) dissoved in saline at a volume of 0.5 μl. For dynA(1–17) peptide injection, 1 nmol of dynA(1–17) peptide (Cat. #AS-24297, AnaSpec Inc.) [38] dissolved in saline was given at a volume of 0.5 μl. The control group of mice received saline.
2.9. Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). All statistical analyses were performed using GraphPad PRISM 8 software. The results from RNA-Seq and RT-qPCR were analyzed with one-way ANOVA, and the results from behavioral tests were analyzed with two-way repeated-measures ANOVA. The Sidak or Tukey’s post hoc test was conducted following ANOVA. Statistical significance was accepted for a P value less than 0.05.
3. Results
3.1. Comorbid TMDs and migraine-like pain increases Pdyn expression in the Sp5C of female but not male mice
We have previously developed a comorbid TMDs and migraine-like pain mouse model and demonstrated that in the comorbid model, MMTL enhances NTG-induced migraine-like hypersensitivity [51].To investigate the underlying mechanism of this comorbidity, we conducted RNA-Seq to analyze the mRNA profile in the Sp5C following MMTL and/or NTG treatment, and we found that “MMTL+NTG” treatment caused significant upregulation of Pdyn expression compared with the control groups in the Sp5C (ipsilateral to MMTL side) of female mice at the diestrus phase (Fig. 1A). We confirmed the upregulation of ipsilateral Pdyn expression using RT-qPCR (Fig. 1B) and further found that the expression of Pdyn in the contralateral Sp5C had no significant difference among all the treatment groups (Fig. 1C).
Figure 1. Expression of Pdyn increases in the Sp5C of female but not male mice in “MMTL+NTG”-induced comorbid TMDs and migraine-like pain.
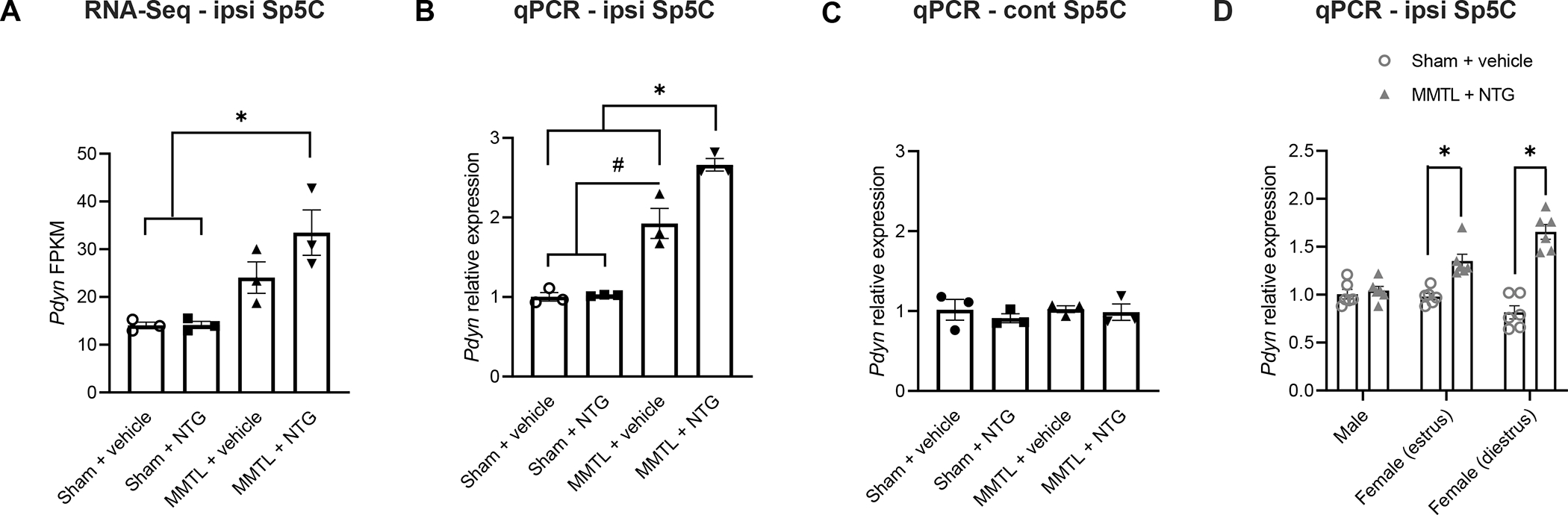
(A) RNA-Seq analysis showed that FPKM of Pdyn in the ipsilateral (ipsi) Sp5C (ipsilateral to MMTL) was significantly upregulated in the “MMTL+NTG” group of female mice at the diestrus phase (n = 3 for each group). (B and C) RT-qPCR confirmed the increase of Pdyn expression in the ipsilateral (ipsi) Sp5C in the “MMTL+NTG” group of female mice (B) and showed that the increase of Pdyn expression also occurred in the “MMTL+vehicle” group (B); however, the expression of Pdyn in the contralateral (cont) Sp5C had no significant difference among all the treatment groups (C) (n = 3 for each group). (D) RT-qPCR further showed that “MMTL+NTG” treatment had no effect on the expression of Pdyn in the ipsilateral (ipsi) Sp5C of male mice compared with the “Sham+vehicle” control group and that the increase of Pdyn expression following “MMTL+NTG” treatment occurred in both estrus and diestrus phases of the estrous cycle in female mice (n = 5−6 for each group). All data are expressed as mean ± SEM. *P < 0.05, #P < 0.05 as indicated in the figure. FPKM: fragments per kilobase of transcript per million fragments mapped.
To find out whether there is sex difference in the upregulation of Pdyn expression after the “MMTL+NTG” treatment, we performed the RT-qPCR with Sp5C tissues from male mice and found that the “MMTL+NTG” treatment had no effect on Pdyn expression in the ipsilateral Sp5C of male mice compared with the “Sham+vehicle” control group (Fig. 1D). We further revealed that the upregulation of Pdyn expression following “MMTL+NTG” treatment occurred in both estrus and diestrus phases of the estrous cycle in female mice (Fig. 1D).
3.2. Pdyn is primarily expressed in excitatory glutamatergic neurons in the Sp5C
To determine the cellular distribution of Pdyn in the Sp5C, we crossed Pdyn-Cre mice with Ai14 mice to generate Pdyn-Cre;Ai14 mice, in which Pdyn-expressing cells show tdTomato-positive red color. We cut brainstem sections containing Sp5C to perform immunofluorescence staining. We observed that Pdyn-expressing cells in the Sp5C were co-labeled with NeuN (a specific neuronal marker), but not co-labeled with Iba1 (a specific microglial marker) and GFAP (a specific astrocytic marker) in both control and “MMTL+NTG” treatment groups (Fig. 2). Our results indicate that Pdyn is predominantly expressed in Sp5C neurons and that “MMTL+NTG” treatment did not alter the cellular distribution of Pdyn in the Sp5C. We further performed immunofluorescence staining with antibodies against glutaminase (a marker for excitatory neurons) and PAX2 (a marker for inhibitory neurons) and we observed that Pdyn-expressing neurons in the Sp5C are primarily positive with glutaminase and only a few of them were positive with PAX2 (Fig. 3). Thus, the majority of Pdyn-expressing neurons in the Sp5C are excitatory glutamatergic neurons.
Figure 2. Pdyn is predominantly expressed in the Sp5C neurons but not glia and its cellular distribution is not altered in comorbid TMDs and migraine-like pain condition.
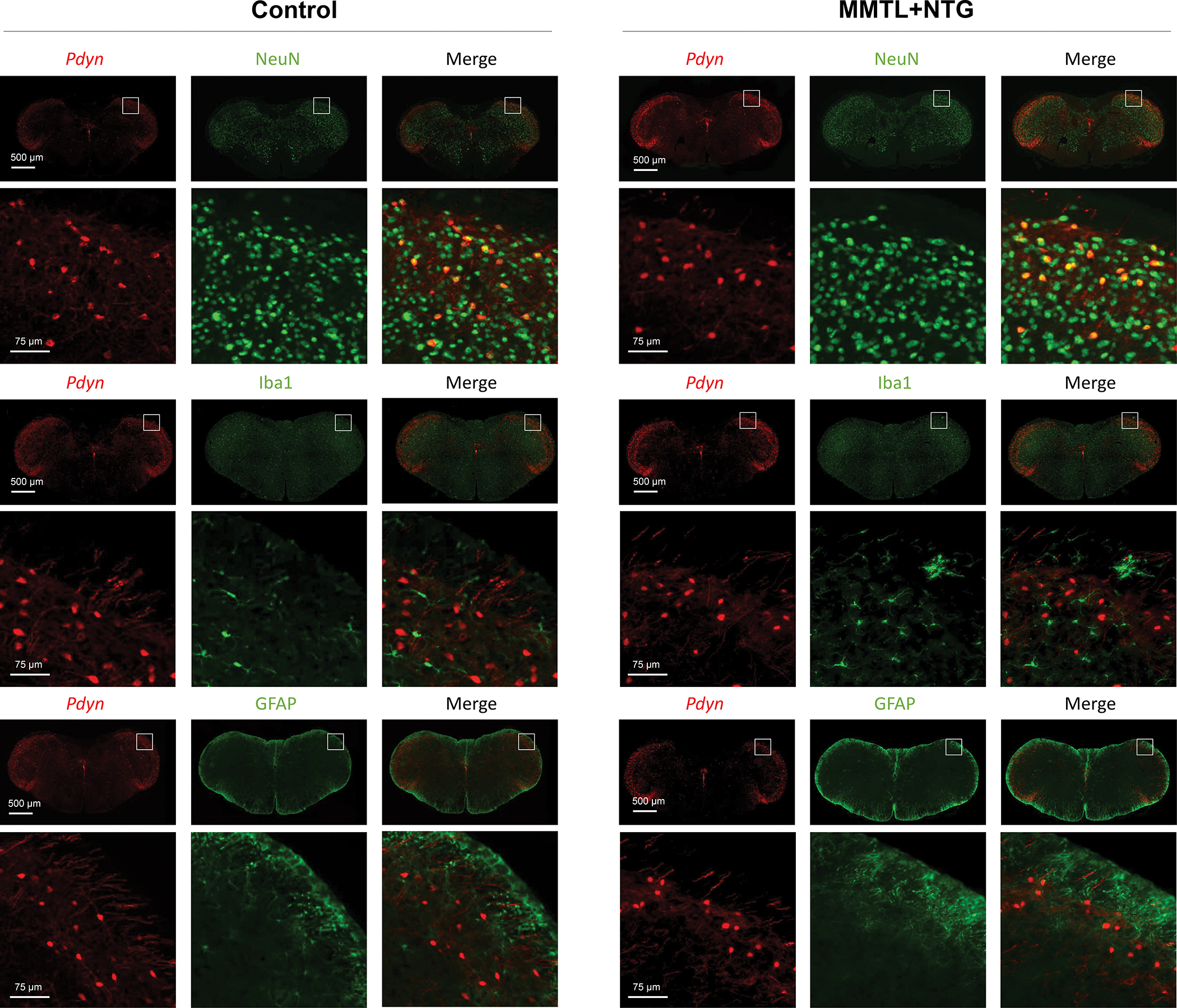
The brainstem sections containing Sp5C from Pdyn-Cre;Ai14 mice were used for immunofluorescence staining. In the transgenic mice, Pdyn-expressing cells in the Sp5C are labeled with tdTomato (red fluorescence). The staining data showed that Sp5C Pdyn-expressing cells were co-labeled with NeuN (a specific neuronal marker) but not with Iba1 (a specific microglia marker) or GFAP (a specific astrocytic marker) in both control and “MMTL+NTG” groups. The immunofluorescence staining experiments were repeated three times.
Figure 3. Pdyn is primarily expressed in Sp5C excitatory glutamatergic neurons.
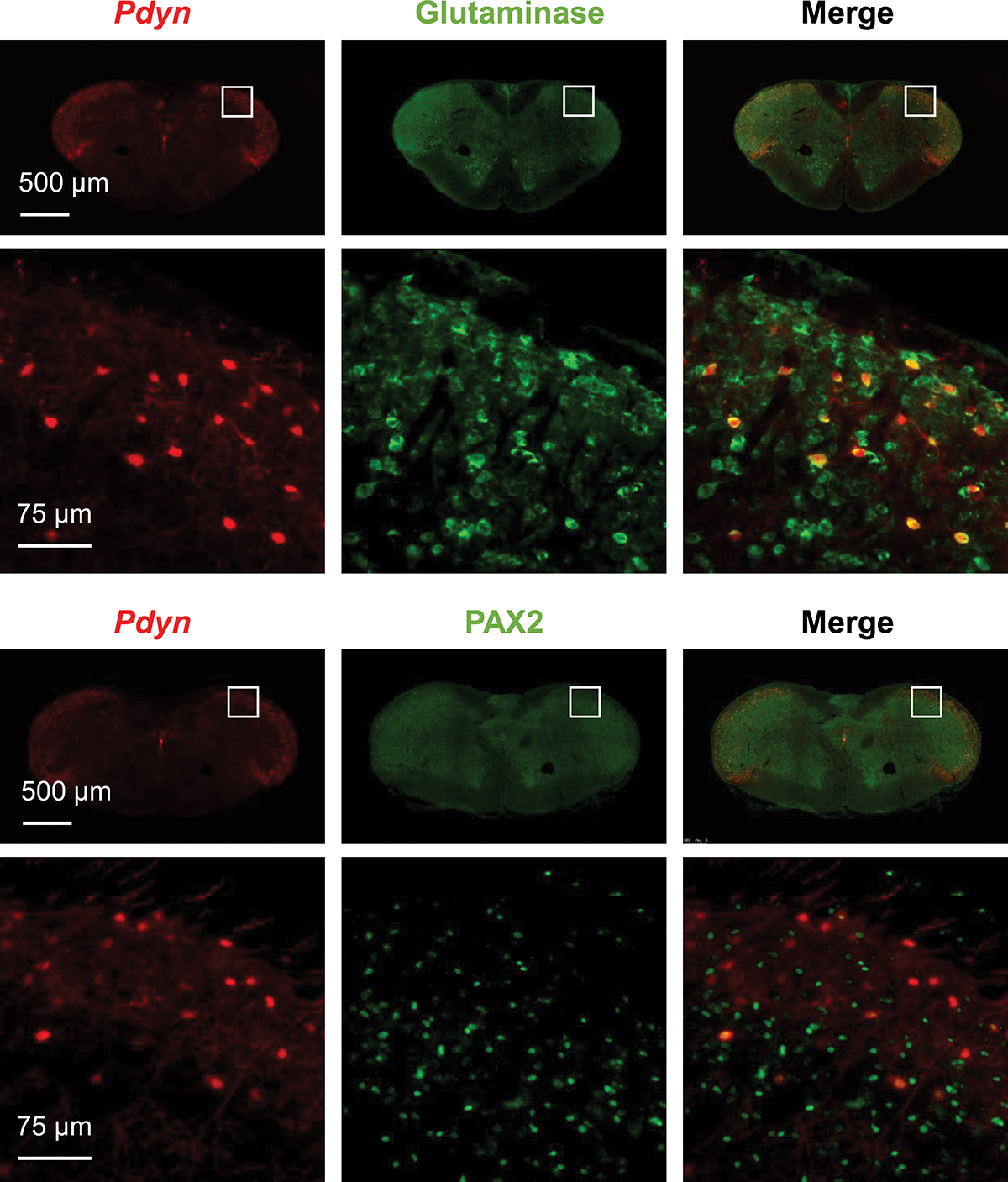
The brainstem sections containing Sp5C from Pdyn-Cre;Ai14 mice were used for immunofluorescence staining. The staining data showed that Pdyn-expressing cells in the Sp5C were co-labeled with glutaminase (a marker for excitatory neurons) and only a few co-labeled with PAX2 (a marker for inhibitory neurons). The immunofluorescence staining experiments were repeated three times.
3.3. Specific inhibition of Pdyn-expressing neurons in the Sp5C attenuates MMTL-induced masseter hypersensitivity and diminishes MMTL-enabled migraine-like pain in female but not male mice
To reveal the role of trigeminal dynorphin in the comorbid TMDs and migraine-like pain, we performed chemogenetic inhibition of Pdyn-expressing neurons in the Sp5C of Pdyn-Cre mice and carried out orofacial pain testing (see timeline in Fig. 4A). We observed that in female mice, specific inhibition of Pdyn-expressing neurons in the Sp5C not only markedly attenuated the MMTL-induced masseter hypersensitivity (Fig. 4B), but also significantly diminished the MMTL-enabled migraine-like pain in the periorbital area (Fig. 4C) compared with the mCherry control group. However, the specific inhibition of Pdyn-expressing neurons had no effects in male mice (Fig. 4D and E).
Figure 4. Specific inhibition of Pdyn-expressing neurons in the Sp5C attenuates MMTL-induced masseter hypersensitivity and diminishes MMTL-enabled migraine-like pain in female but not male mice.
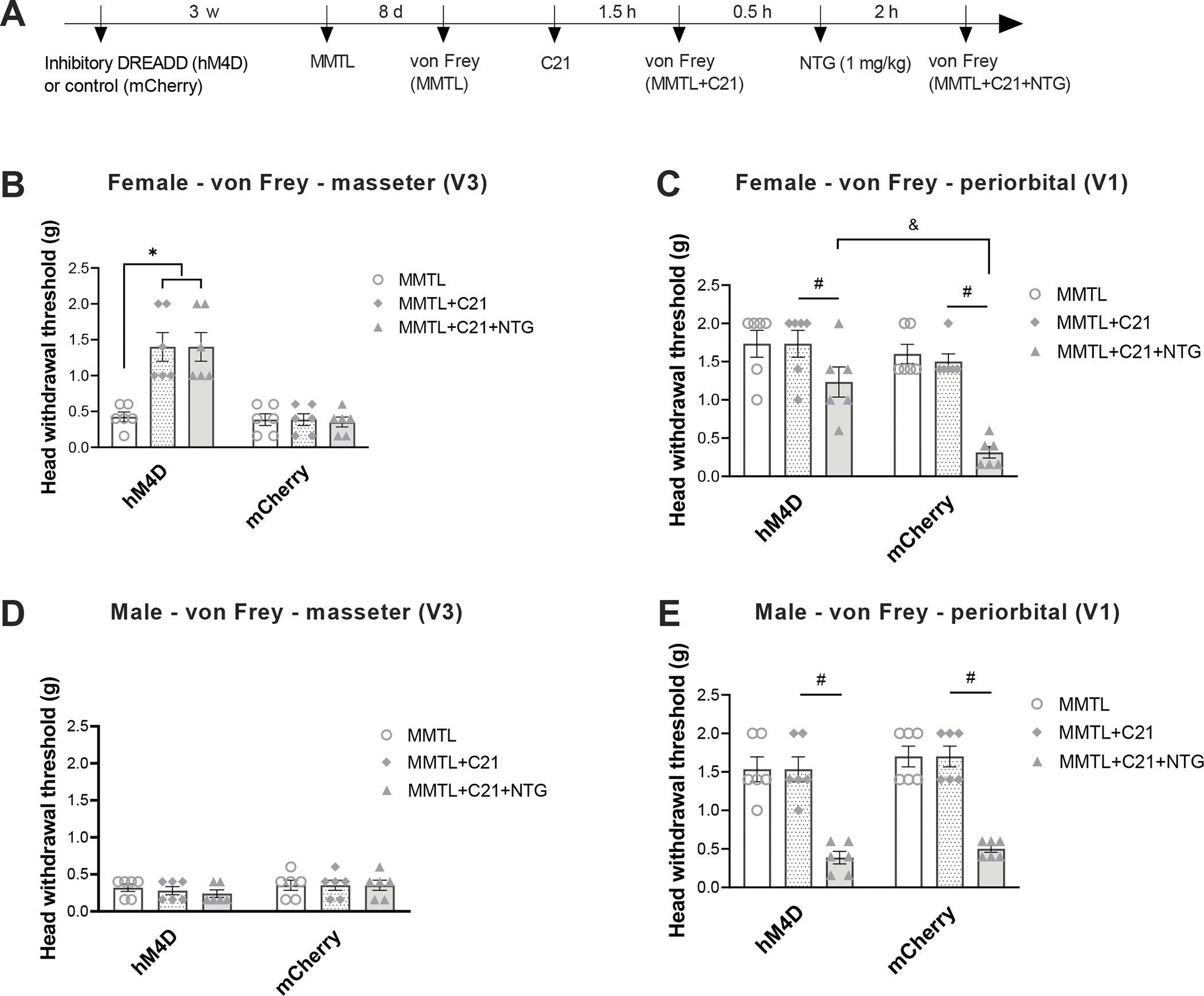
(A) Timeline of the experiment. Cre-inducible inhibitory DREADD (AAV5-hSyn-DIO-hM4D (Gi)-mCherry) or its control (AAV5-hSyn-DIO-mCherry) was injected into Sp5C (ipsilateral to MMTL) three weeks before MMTL, and C21 was administered (1 mg/kg, i.p.) to activate the DREADD. (B and C) In Pdyn-Cre female mice, inhibitory DREADD (hM4D)-produced chemogenetic inhibition of Pdyn-expressing neurons in the Sp5C significantly increased head withdrawal thresholds in trigeminal nerve V3 branch-innervated masseter area (B) and in trigeminal nerve V1 branch-innervated periorbital area (C), but the control (mCherry) treatment had no effects on MMTL-induced masseter hypersensitivity (B) and MMTL-enabled migraine-like pain (C) (n = 6 mice for each group). (D and E) In Pdyn-Cre male mice, the chemogenetic inhibition of Pdyn-expressing neurons in the Sp5C had no effects on MMTL-induced masseter hypersensitivity (D) and MMTL-enabled migraine-like pain (E) (n = 6 mice for each group). All data are expressed as mean ± SEM. *P < 0.05, #P < 0.05, &P < 0.05 as indicated in the figure.
To verify whether the chemogenetic inhibition-produced effect is directly related to trigeminal dynorphin, we performed intra-Sp5C injection of anti-dynorphin antiserum on day one post-NTG in the comorbid TMDs and migraine-like pain mouse model and carried out orofacial pain testing (see timeline in Fig. 5A). We observed that a single injection of 0.25 μg of anti-dynA antiserum significantly attenuated the MMTL-induced masseter hypersensitivity and diminished the MMTL-enabled migraine-like pain in the periorbital area at 45 min post-injection compared with the IgG control group (Fig. 5B and C), whereas treatment with 0.5 μg of the antiserum produced its effect at 30 min post-injection and the inhibitory effect lasted for at least 30 min (Fig. 5B and C). However, the injection of anti-dynorphin antiserum had no effects in male mice (Fig. 5D and E).
Figure 5. Intra-Sp5C injection of anti-dynorphin antiserum alleviates MMTL-induced masseter hypersensitivity and diminishes MMTL-enabled migraine-like pain in female but not male mice.
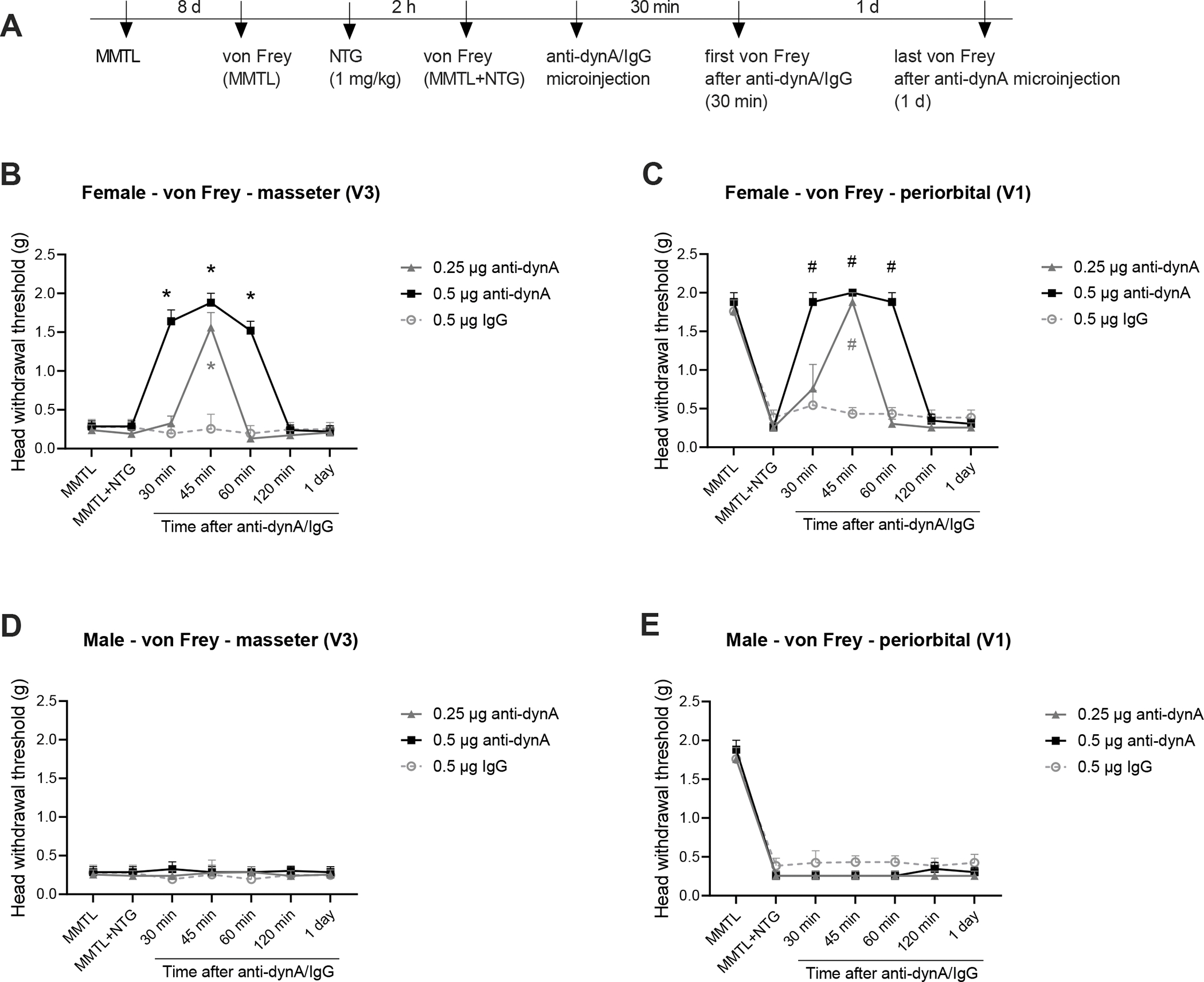
(A) Timeline of the experiment. (B and C) In wild-type female mice, intra-Sp5C injection of anti-dynA antiserum (0.25 μg) significantly increased head withdrawal thresholds in trigeminal nerve V3 branch-innervated masseter area (B) and in trigeminal nerve V1 branch-innervated periorbital area (C) at 45 min post-injection compared with the IgG control group, whereas treatment with 0.5 μg of the antiserum produced its effect at 30 min post-injection and the inhibitory effect lasted for at least 30 min (B and C) (n = 6 mice for each group). (D and E) In wild-type male mice, the intra-Sp5C injection of anti-dynA antiserum had no effects on head withdrawal thresholds in the masseter (D) and periorbital (E) areas (n = 6 mice for each group). All data are expressed as mean ± SEM. *P < 0.05; #P < 0.05 vs the respective IgG control group.
3.4. Specific activation of Pdyn-expressing neurons in the Sp5C without MMTL enables a subthreshold dose of NTG to induce migraine-like pain in female but not male mice
To further determine the role of trigeminal dynorphin in the comorbid TMDs and migraine-like pain, we performed chemogenetic activation of Pdyn-expressing neurons in the Sp5C of Pdyn-Cre mice and carried out orofacial pain testing (see timeline in Fig. 6A). We observed that in female mice, specifical activation of Pdyn-expressing neurons in the Sp5C alone did not affect mechanical hypersensitivity in the masseter and periorbital areas (Fig. 6B and C), but combining the chemogenetic activation of Pdyn-expressing neurons in the Sp5C with single injection of NTG (1 mg/kg, i.p.) not only produced TMD-like masseter hypersensitivity (Fig. 6B), but also enabled a subthreshold dose of NTG to induce migraine-like pain in the periorbital area (Fig. 6C) compared with the mCherry control group. However, the specific activation of Pdyn-expressing neurons had no effects in male mice (Fig. 6D and E).
Figure 6. Specific activation of Pdyn-expressing neurons in the Sp5C without MMTL enables a subthreshold dose of NTG to induce migraine-like pain in female but not male mice.
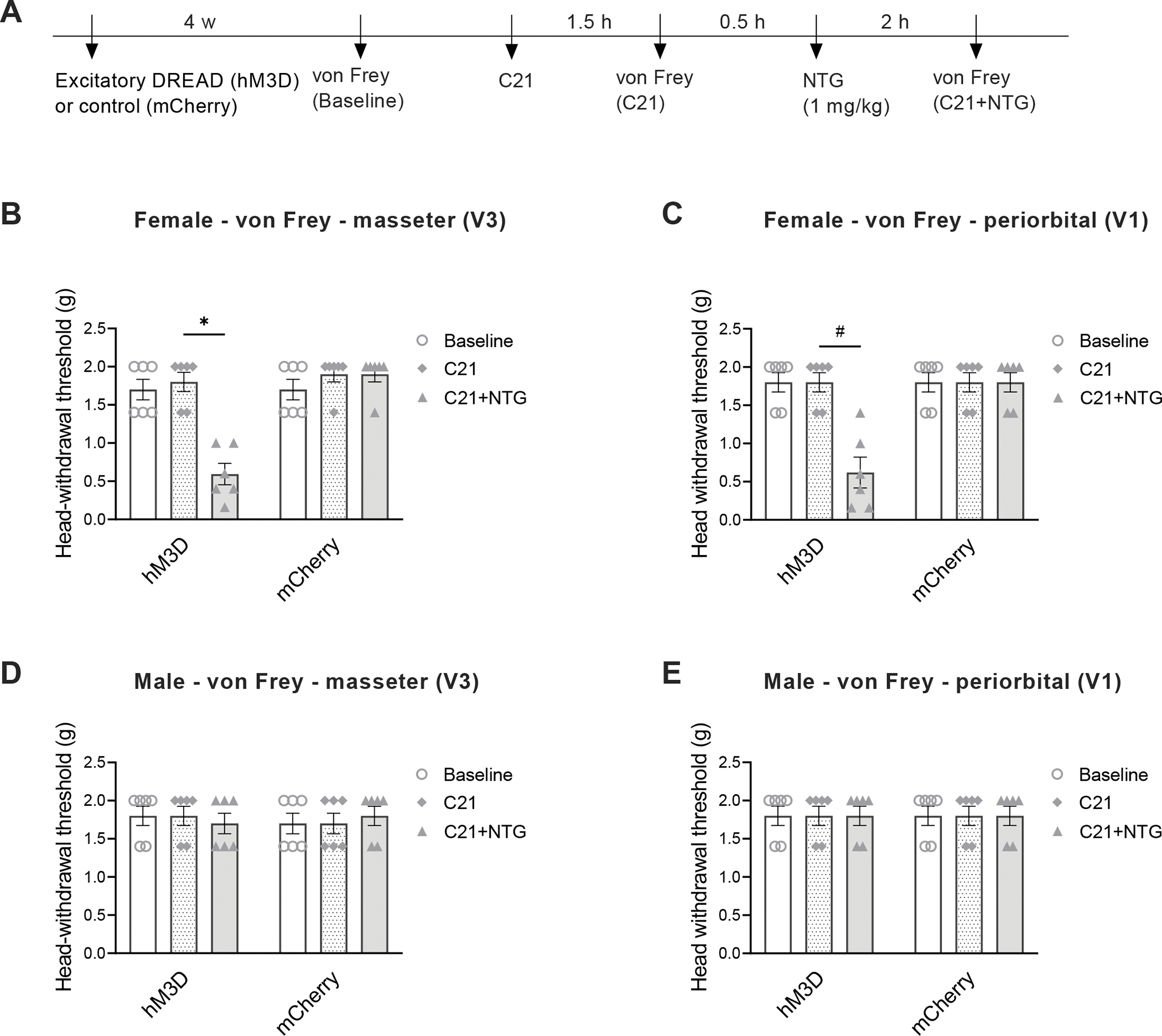
(A) Timeline of the experiment. Cre-inducible excitatory DREADD (AAV5-hSyn-DIO-hM3D (Gq)-mCherry) or its control (AAV5-hSyn-DIO-mCherry) was injected into unilateral Sp5C four weeks before behavioral testing, and C21 was administered (1 mg/kg, i.p.) to activate the DREADD. (B and C) In Pdyn-Cre female mice, excitatory DREADD (hM3D)-produced chemogenetic activation of Pdyn-expressing neurons in the Sp5C alone did not affect head mechanical thresholds in trigeminal nerve V3 branch-innervated masseter area (B) and in trigeminal nerve V1 branch-innervated periorbital area (C), but combining the chemogenetic activation of Pdyn-expressing neurons in the Sp5C with a single injection of NTG (1 mg/kg, i.p.) significantly decreased head withdrawal thresholds in both masseter (B) and periorbital (C) areas (n = 6 mice for each group). (D and E) In Pdyn-Cre male mice, the chemogenetic activation of Pdyn-expressing neurons in the Sp5C had no effects on head withdrawal thresholds in the masseter (D) and periorbital (E) areas (n = 6 mice for each group). All data are expressed as mean ± SEM. *P < 0.05, #P < 0.05 as indicated in the figure.
To verify whether the chemogenetic activation-produced effect is directly related to dynorphin in the Sp5C, we performed intra-Sp5C injection of dynA(1–17) peptide in wild-type mice and carried out orofacial pain testing (see timeline in Fig. 7A). We observed that in female mice, a single injection of dynA(1–17) peptide (1 nmol) alone did not affect mechanical hypersensitivity in the masseter and periorbital areas (Fig. 7B and C), but combining the peptide treatment with a single injection of NTG (1 mg/kg, i.p.) at 45 min post-peptide not only produced TMD-like masseter hypersensitivity (Fig. 7B), but also enabled a subthreshold dose of NTG to induce migraine-like pain in the periorbital area (Fig. 7C) compared with the control group. The TMD-like masseter hypersensitivity and migraine-like pain lasted for at least 10 days. However, the peptide treatment had no effects in male mice (Fig. 7D and E).
Figure 7. Intra-Sp5C injection of dynA(1–17) peptide without MMTL enables a subthreshold dose of NTG to induce migraine-like pain in female but not male mice.
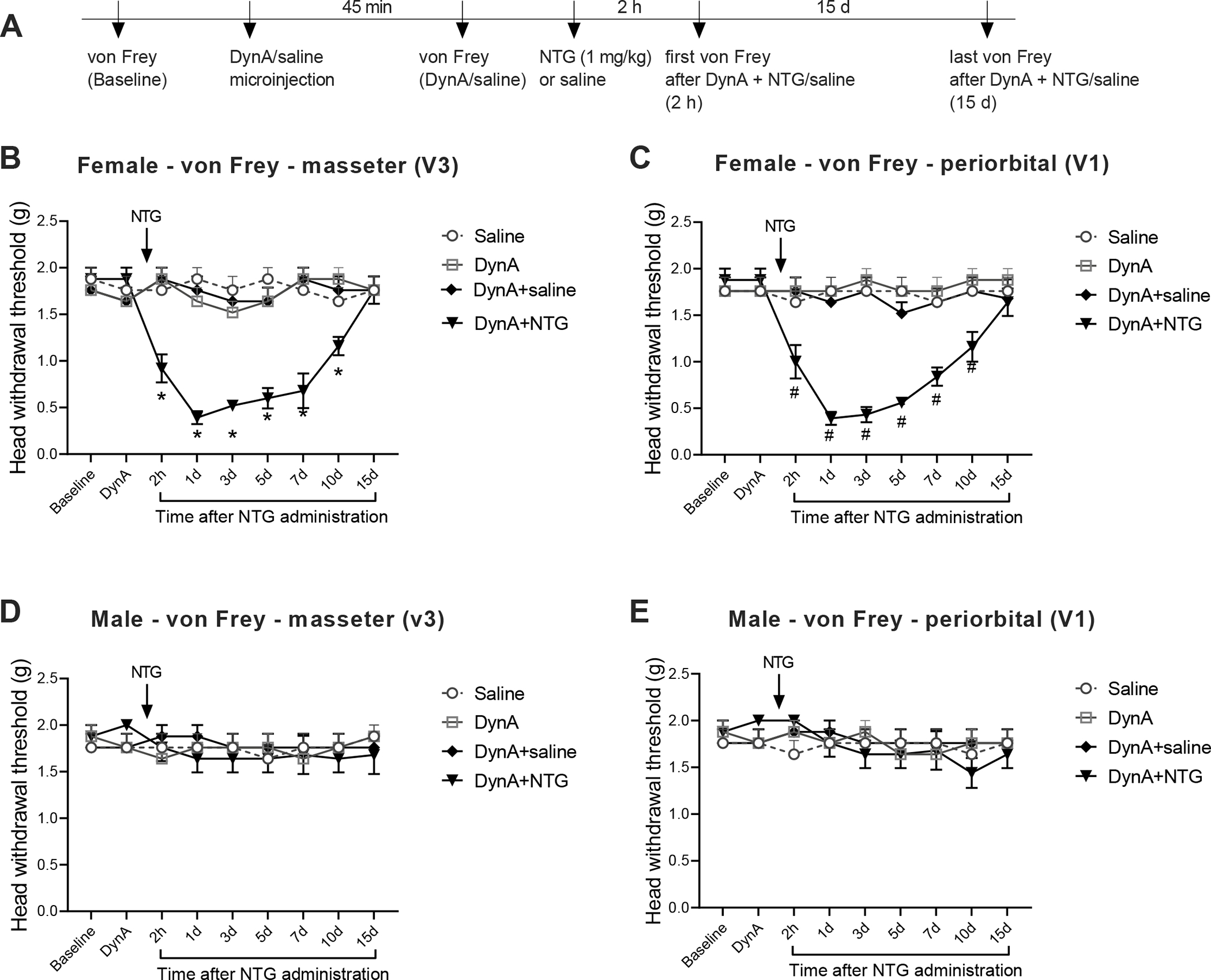
(A) Timeline of the experiment. (B and C) In wild-type female mice, intra-Sp5C injection of dynA(1–17) peptide (1 nmol) alone did not affect head withdrawal thresholds in trigeminal nerve V3 branch-innervated masseter area (B) and in trigeminal nerve V1 branch-innervated periorbital area (C), but combining the intra-Sp5C injection of dynA(1–17) peptide with a single injection of NTG (1 mg/kg, i.p.) significantly decreased head withdrawal thresholds in both masseter (B) and periorbital (C) areas (n = 6 mice for each group). The TMD-like mechanical hypersensitivity in the masseter area and migraine-like pain in the periorbital area lasted for at least 10 days. (D and E) In wild-type male mice, the intra-Sp5C injection of dynA(1–17) peptide had no effects on head withdrawal thresholds in the masseter (D) and periorbital (E) areas (n = 5 mice for each group). All data are expressed as mean ± SEM. *P < 0.05; #P < 0.05 vs the respective control group.
4. Discussion
In the present study, our RNA-Seq followed by RT-qPCR confirmation shows that in the comorbid TMDs and migraine-like pain mouse model, MMTL plus NTG treatment increases Pdyn expression in the Sp5C of female mice, but not in male mice. Chemogenetic inhibition of Pdyn-expressing neurons or microinjection of anti-dynorphin antiserum in the Sp5C alleviates MMTL-induced masseter hypersensitivity and diminishes the MMTL-enabled migraine-like pain in female but not male mice. Moreover, chemogenetic activation of Pdyn-expressing neurons or microinjection of dynA(1–17) peptide in the Sp5C can enable a subthreshold dose of NTG to induce migraine-like pain in female but not male mice.
In the RNA-Seq experiment, we selected female mice at diestrus phase to harvest Sp5C tissues, so that all the mice have similar hormone levels. The estrous cycle in rodents, as an analogous to the menstrual cycle in humans, is regulated by hormones including estrogens. Estrogen levels in high-receptive phases (estrus and proestrus) are typically higher than those in low-receptive phases (diestrus and metestrus) [44,63,64]. A rapid decline of serum estrogen level during the diestrus phase has been linked to triggering migraine [10]. A previous study reported that female mice in the diestrus phase are more prone to cortical spreading depression [18], which is the underlying pathophysiology of migraine aura [11]. Our RNA-Seq data show that Pdyn expression increases significantly in the “MMTL+NTG” group compared with other groups, which has been confirmed by the results from RT-qPCR. We further investigate whether the increase of Pdyn expression also occurs in male mice and whether Pdyn expression is regulated differentially at different estrous phases of female mice in the comorbid TMDs and migraine-like pain mouse model, and our results demonstrate that Pdyn upregulation is specifically produced in female mice (both estrus and diestrus phases) but not in male mice.
Previous studies have reported that preprodynorphin gene transcription and dynorphin expression significantly increase in the spinal trigeminal nucleus of male rats after inflammation or injury [28,29,34]. In our comorbid orofacial pain model, the increased expression of trigeminal dynorphin is female-specific. This discrepancy may be due to the following factors: 1) We used mice in this study and those previous studies used rats; 2) The effect produced by the combination of MMTL with NTG injection in our comorbidity model could be different compared with those caused by inflammation or injury.
Dynorphin has been suggested as a mediator of central sensitization [57]. Whole-cell patch clamp recordings have shown that dynorphin has contrasting influences on γ-aminobutyric acid (GABA) input to excitatory and inhibitory neurons in the ventral pallidum by binding to κ-opioid receptors [30]. Another study using ex vivo electrophysiological recording shows that bath application of dynorphin decreases the excitability of GABAergic neurons of insular cortex in both male and female mice, but dynorphin increases the Excitation/Inhibition ratio only in male mice [46]. In addition, positron emission tomography scanning in a human study has revealed that males has a significantly higher availability of κ-opioid receptors than women in many brain regions including pain-related anterior cingulate cortex and insular cortex [58]. In an animal study, sex differences in the expression of κ-opioid receptors and the receptor-mediated G protein activation in brain regions are observed [60].
Pdyn can give rise to several different endogenous opioid peptides, including dynA, dynB, α- and β-neoendorphin, and bio-active/inactive fragments [17,25]. These peptides have been found to be present in neurons, microglia, and astrocytes in the spinal cord [26,33,59], but their expression in the Sp5C remains unknown. In the present study, we crossed homozygous Pdyn-Cre mice with homozygous Ai14 reporter mice to generate Pdyn-Cre;Ai14 mice. Because Ai14 reporter mice express robust tdTomato fluorescence signals when Cre recombinase exists, Pdyn-Cre;Ai14 mice express tdTomato in Pdyn-expressing cells. By staining with markers for different cell types, we reveal that Pdyn is primarily expressed in excitatory glutamatergic neurons of Sp5C and the cellular distribution has no change after “MMTL+NTG” treatment.
The endogenous opioid peptides derived from Pdyn have been demonstrated to show analgesic effects through activating inhibitory opioid receptors [47]. Previous studies have shown that there are sex differences in opioid-produced analgesia [4,9,13,35,40,52]. For instance, it is observed that the μ opioid-mediated analgesic effect is stronger in male rats than female rats [13], while in another study, morphine exhibits greater analgesic potency in female mice than male mice [35]. However, dynA has also been reported to facilitate chronic pain development in pathological conditions [50]. In the present study, we provide more evidence to demonstrate the role of trigeminal dynorphin (especially dynA) in the comorbid TMDs and migraine-like pain. Using Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)-based chemogenetic manipulation, we reveal that specific inhibition of Pdyn-expressing neurons in the Sp5C can attenuate the MMTL-induced masseter hypersensitivity and diminish the MMTL-enabled migraine-like pain in our comorbid mouse model. Importantly, the chemogenetic inhibition-produced effects are only observed in female mice, but not in male mice. To confirm the effects are directly related to trigeminal dynorphin, we performed intra-Sp5C injection of anti-dynorphin antiserum and we observed a similar effect on the comorbidity in female but not male mice. These results suggest that trigeminal dynorphin could be targeted to treat such comorbid pain condition and the therapeutic target is female-specific.
To further determine the role of trigeminal dynorphin in the comorbid TMDs and migraine-like pain, we performed DREADDs-based chemogenetic activation of Pdyn-expressing neurons in the Sp5C. Although the specific activation of Pdyn-expressing neurons alone does not alter mechanical hypersensitivity in masseter and periorbital areas, combining it with single injection of a subthreshold dose of NTG (1 mg/kg, i.p.) not only produces TMD-like masseter hypersensitivity, but also enables a subthreshold dose of NTG to induce migraine-like pain in the periorbital area. All these effects are only observed in female but not male mice. To confirm the effects are directly related to trigeminal dynorphin, we performed intra-Sp5C injection of dynA(1–17) peptide, and our results demonstrate that the dynA treatment produced a similar effect on the comorbidity in female but not male mice, which suggests that the effects of chemogenetic activation of Sp5C Pdyn-expressing neurons in female mice could be mediated by increasing dynorphin release in the Sp5C. These results support our hypothesis that trigeminal dynorphin is critical for the comorbid TMDs and migraine-like pain in females and blocking trigeminal dynorphin signaling can be developed into a novel female-specific therapy for this comorbid pain condition.
In summary, our work reveals a female-specific role for trigeminal dynorphin in the comorbidity of TMDs and migraine-like pain. We provide evidence to show that trigeminal dynorphin can modulate TMD-like masseter hypersensitivity and enable the development of migraine-like pain in a sex-dependent manner. Therefore, trigeminal dynorphin may represent a therapeutic target for the comorbid TMDs and migraine-like pain condition.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Grants K02 DE023551, R01 DE031255, and R01 DE032061. The authors thank Drs. Larry Bellinger, Phillip Kramer and Steven Bender for stimulating discussions and their comments.
Footnotes
Conflict of interest statement: The authors have no conflict of interest to declare.
Data availability statement:
The data generated in this study are available from the corresponding author on reasonable request.
References
- [1].Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, Goadsby PJ. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 2005;128(Pt 4):932–939. [DOI] [PubMed] [Google Scholar]
- [2].Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015;31(2):166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bangaru ML, Park F, Hudmon A, McCallum JB, Hogan QH. Quantification of gene expression after painful nerve injury: validation of optimal reference genes. J Mol Neurosci 2012;46(3):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bartok RE, Craft RM. Sex differences in opioid antinociception. J Pharmacol Exp Ther 1997;282(2):769–778. [PubMed] [Google Scholar]
- [5].Bender SD. Orofacial Pain and Headache: A Review and Look at the Commonalities. Current Pain and Headache Reports 2014;18. [DOI] [PubMed] [Google Scholar]
- [6].Bevilaqua Grossi D, Lipton RB, Bigal ME. Temporomandibular disorders and migraine chronification. Curr Pain Headache Rep 2009;13(4):314–318. [DOI] [PubMed] [Google Scholar]
- [7].Bradshaw H, Miller J, Ling Q, Malsnee K, Ruda MA. Sex differences and phases of the estrous cycle alter the response of spinal cord dynorphin neurons to peripheral inflammation and hyperalgesia. Pain 2000;85(1–2):93–99. [DOI] [PubMed] [Google Scholar]
- [8].Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci 2009;Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Candido J, Lutfy K, Billings B, Sierra V, Duttaroy A, Inturrisi CE, Yoburn BC. Effect of adrenal and sex hormones on opioid analgesia and opioid receptor regulation. Pharmacol Biochem Behav 1992;42(4):685–692. [DOI] [PubMed] [Google Scholar]
- [10].Chai NC, Peterlin BL, Calhoun AH. Migraine and estrogen. Current opinion in neurology 2014;27(3):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol 2013;9(11):637–644. [DOI] [PubMed] [Google Scholar]
- [12].Christiansen I, Thomsen LL, Daugaard D, Ulrich V, Olesen J. Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia 1999;19(7):660–667. [DOI] [PubMed] [Google Scholar]
- [13].Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology (Berl) 2000;150(4):430–442. [DOI] [PubMed] [Google Scholar]
- [14].Dahan H, Shir Y, Velly A, Allison P. Specific and number of comorbidities are associated with increased levels of temporomandibular pain intensity and duration. J Headache Pain 2015;16:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dando WE, Branch MA, Maye JP. Headache disability in orofacial pain patients. Headache 2006;46(2):322–326. [DOI] [PubMed] [Google Scholar]
- [16].De Logu F, Landini L, Janal MN, Li Puma S, De Cesaris F, Geppetti P, Nassini R. Migraine-provoking substances evoke periorbital allodynia in mice. J Headache Pain 2019;20(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Douglass J, McMurray CT, Garrett JE, Adelman JP, Calavetta L. Characterization of the rat prodynorphin gene. Molecular endocrinology (Baltimore, Md) 1989;3(12):2070–2078. [DOI] [PubMed] [Google Scholar]
- [18].Ebine T, Toriumi H, Shimizu T, Unekawa M, Takizawa T, Kayama Y, Shibata M, Suzuki N. Alterations in the threshold of the potassium concentration to evoke cortical spreading depression during the natural estrous cycle in mice. Neurosci Res 2016;112:57–62. [DOI] [PubMed] [Google Scholar]
- [19].Elliott MB, Oshinsky ML, Amenta PS, Awe OO, Jallo JI. Nociceptive neuropeptide increases and periorbital allodynia in a model of traumatic brain injury. Headache 2012;52(6):966–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Florencio LL, de Oliveira AS, Carvalho GF, Dach F, Bigal ME, Fernandez-de-Las-Penas C, Bevilaqua-Grossi D. Association Between Severity of Temporomandibular Disorders and the Frequency of Headache Attacks in Women With Migraine: A Cross-Sectional Study. J Manipulative Physiol Ther 2017;40(4):250–254. [DOI] [PubMed] [Google Scholar]
- [21].Franco AL, Goncalves DA, Castanharo SM, Speciali JG, Bigal ME, Camparis CM. Migraine is the most prevalent primary headache in individuals with temporomandibular disorders. J Orofac Pain 2010;24(3):287–292. [PubMed] [Google Scholar]
- [22].Goncalves DA, Bigal ME, Jales LC, Camparis CM, Speciali JG. Headache and symptoms of temporomandibular disorder: an epidemiological study. Headache 2010;50(2):231–241. [DOI] [PubMed] [Google Scholar]
- [23].Goncalves DA, Camparis CM, Speciali JG, Franco AL, Castanharo SM, Bigal ME. Temporomandibular disorders are differentially associated with headache diagnoses: a controlled study. Clin J Pain 2011;27(7):611–615. [DOI] [PubMed] [Google Scholar]
- [24].Hamann SR, Martin WR. Analgesic actions of dynorphin A(1–13) antiserum in the rat brain stem. Brain Res Bull 1992;29(5):605–607. [DOI] [PubMed] [Google Scholar]
- [25].Horikawa S, Takai T, Toyosato M, Takahashi H, Noda M, Kakidani H, Kubo T, Hirose T, Inayama S, Hayashida H, et al. Isolation and structural organization of the human preproenkephalin B gene. Nature 1983;306(5943):611–614. [DOI] [PubMed] [Google Scholar]
- [26].Huang Q, Mao XF, Wu HY, Li TF, Sun ML, Liu H, Wang YX. Bullatine A stimulates spinal microglial dynorphin A expression to produce anti-hypersensitivity in a variety of rat pain models. J Neuroinflammation 2016;13(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Iadarola MJ, Douglass J, Civelli O, Naranjo JR. Differential activation of spinal cord dynorphin and enkephalin neurons during hyperalgesia: evidence using cDNA hybridization. Brain research 1988;455(2):205–212. [DOI] [PubMed] [Google Scholar]
- [28].Imbe H, Ren K. Orofacial deep and cutaneous tissue inflammation differentially upregulates preprodynorphin mRNA in the trigeminal and paratrigeminal nuclei of the rat. Brain Res Mol Brain Res 1999;67(1):87–97. [DOI] [PubMed] [Google Scholar]
- [29].Imbe H, Ren K. The up-regulation of preprodynorphin mRNA in trigeminoparabrachial neurons after inflammation. Neuroreport 2000;11(4):845–847. [DOI] [PubMed] [Google Scholar]
- [30].Inbar K, Levi LA, Bernat N, Odesser T, Inbar D, Kupchik YM. Cocaine Dysregulates Dynorphin Modulation of Inhibitory Neurotransmission in the Ventral Pallidum in a Cell-Type-Specific Manner. J Neurosci 2020;40(6):1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jendryka M, Palchaudhuri M, Ursu D, van der Veen B, Liss B, Katzel D, Nissen W, Pekcec A. Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci Rep 2019;9(1):4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kang JK, Ryu JW, Choi JH, Merrill RL, Kim ST. Application of ICHD-II criteria for headaches in a TMJ and orofacial pain clinic. Cephalalgia 2010;30(1):37–41. [DOI] [PubMed] [Google Scholar]
- [33].Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber HR, Todd AJ, Ross SE. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 2014;82(3):573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kato J, Wakisaka S, Tabata MJ, Itotagawa T, Kurisu K. Appearance of dynorphin in the spinal trigeminal nucleus complex following experimental tooth movement in the rat. Arch Oral Biol 1995;40(1):79–81. [DOI] [PubMed] [Google Scholar]
- [35].Kest B, Wilson SG, Mogil JS. Sex differences in supraspinal morphine analgesia are dependent on genotype. J Pharmacol Exp Ther 1999;289(3):1370–1375. [PubMed] [Google Scholar]
- [36].Langmead B. Aligning short sequencing reads with Bowtie. Curr Protoc Bioinformatics 2010;Chapter 11:Unit 11 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 2012;9(4):357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Laughlin TM, Vanderah TW, Lashbrook J, Nichols ML, Ossipov M, Porreca F, Wilcox GL. Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain 1997;72(1–2):253–260. [DOI] [PubMed] [Google Scholar]
- [39].Laumet G, Garriga J, Chen SR, Zhang Y, Li DP, Smith TM, Dong Y, Jelinek J, Cesaroni M, Issa JP, Pan HL. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci 2015;18(12):1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lee CW, Ho IK. Sex differences in opioid analgesia and addiction: interactions among opioid receptors and estrogen receptors. Mol Pain 2013;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu S, Shu H, Crawford J, Ma Y, Li C, Tao F. Optogenetic Activation of Dopamine Receptor D1 and D2 Neurons in Anterior Cingulate Cortex Differentially Modulates Trigeminal Neuropathic Pain. Mol Neurobiol 2020;57(10):4060–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu S, Tang Y, Shu H, Tatum D, Bai Q, Crawford J, Xing Y, Lobo MK, Bellinger L, Kramer P, Tao F. Dopamine receptor D2, but not D1, mediates descending dopaminergic pathway-produced analgesic effect in a trigeminal neuropathic pain mouse model. Pain 2019;160(2):334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- [44].McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp 2012(67):e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacology & Therapeutics 2008;120(2):157–171. [DOI] [PubMed] [Google Scholar]
- [46].Pina MM, Pati D, Hwa LS, Wu SY, Mahoney AA, Omenyi CG, Navarro M, Kash TL. The kappa opioid receptor modulates GABA neuron excitability and synaptic transmission in midbrainprojections from the insular cortex. Neuropharmacology 2020;165:107831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Podvin S, Yaksh T, Hook V. The Emerging Role of Spinal Dynorphin in Chronic Pain: A Therapeutic Perspective. Annu Rev Pharmacol Toxicol 2016;56:511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Romero-Reyes M, Ye Y. Pearls and pitfalls in experimental in vivo models of headache: conscious behavioral research. Cephalalgia 2013;33(8):566–576. [DOI] [PubMed] [Google Scholar]
- [49].Rosén A, Lund I, Lundeberg T, Nylander I. Antinociceptive effects of sensory stimulation involve dynorphin B supraspinally in rats. Acupuncture and Related Therapies 2013;1(4):35–41. [Google Scholar]
- [50].Schwarzer C 30 years of dynorphins--new insights on their functions in neuropsychiatric diseases. Pharmacol Ther 2009;123(3):353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Shu H, Liu S, Tang Y, Schmidt BL, Dolan JC, Bellinger LL, Kramer PR, Bender SD, Tao F. A Pre-Existing Myogenic Temporomandibular Disorder Increases Trigeminal Calcitonin Gene-Related Peptide and Enhances Nitroglycerin-Induced Hypersensitivity in Mice. Int J Mol Sci 2020;21(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].South SM, Wright AW, Lau M, Mather LE, Smith MT. Sex-related differences in antinociception and tolerance development following chronic intravenous infusion of morphine in the rat: modulatory role of testosterone via morphine clearance. J Pharmacol Exp Ther 2001;297(1):446–457. [PubMed] [Google Scholar]
- [53].Thompson KJ, Khajehali E, Bradley SJ, Navarrete JS, Huang XP, Slocum S, Jin J, Liu J, Xiong Y, Olsen RHJ, Diberto JF, Boyt KM, Pina MM, Pati D, Molloy C, Bundgaard C, Sexton PM, Kash TL, Krashes MJ, Christopoulos A, Roth BL, Tobin AB. DREADD Agonist 21 Is an Effective Agonist for Muscarinic-Based DREADDs in Vitro and in Vivo. ACS Pharmacol Transl Sci 2018;1(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009;25(9):1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 2010;28(5):511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3--new capabilities and interfaces. Nucleic Acids Res 2012;40(15):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, Ossipov MH, Malan TP Jr., Porreca F. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain 1996;68(2–3):275–281. [DOI] [PubMed] [Google Scholar]
- [58].Vijay A, Wang S, Worhunsky P, Zheng MQ, Nabulsi N, Ropchan J, Krishnan-Sarin S, Huang Y, Morris ED. PET imaging reveals sex differences in kappa opioid receptor availability in humans, in vivo. Am J Nucl Med Mol Imaging 2016;6(4):205–214. [PMC free article] [PubMed] [Google Scholar]
- [59].Wahlert A, Funkelstein L, Fitzsimmons B, Yaksh T, Hook V. Spinal astrocytes produce and secrete dynorphin neuropeptides. Neuropeptides 2013;47(2):109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang YJ, Rasakham K, Huang P, Chudnovskaya D, Cowan A, Liu-Chen LY. Sex difference in kappa-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5’-O-(3-[35S]thiotriphosphate) binding in the guinea pig. J Pharmacol Exp Ther 2011;339(2):438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP Jr., Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci 2001;21(5):1779–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Weihe E, Millan MJ, Hollt V, Nohr D, Herz A. Induction of the gene encoding pro-dynorphin by experimentally induced arthritis enhances staining for dynorphin in the spinal cord of rats. Neuroscience 1989;31(1):77–95. [DOI] [PubMed] [Google Scholar]
- [63].Wood GA, Fata JE, Watson KL, Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction 2007;133(5):1035–1044. [DOI] [PubMed] [Google Scholar]
- [64].Zysow BR, Kauser K, Lawn RM, Rubanyi GM. Effects of estrus cycle, ovariectomy, and treatment with estrogen, tamoxifen, and progesterone on apolipoprotein(a) gene expression in transgenic mice. Arterioscler Thromb Vasc Biol 1997;17(9):1741–1745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available from the corresponding author on reasonable request.


