Abstract
Essential hypertension is a notable threat for the older (age, ≥65 years) population. However, to the best of our knowledge, a real-world study assessing olmesartan medoxomil-amlodipine besylate (OM-AML) tablets in older Chinese patients with essential hypertension has not been performed. Therefore, the present study aimed to evaluate the efficacy and safety of OM-AML tablets in these patients. A total of 463 older Chinese patients with essential hypertension treated with OM-AML (20/5 mg) tablets (Sevikar®) were analyzed in a prospective, single-arm, multi-center, real-world study. Seated systolic blood pressure (SeSBP) and seated diastolic blood pressure (SeDBP) at baseline, and at week (W)4 and W8 after OM-AML tablet administration were measured. The mean ± standard error change of SeSBP/SeDBP was -10.3±0.8/-4.6±0.5 and -12.5±0.8/-5.6±0.5 mmHg at W4 and W8, respectively. At W4, 74.1 and 26.8% of patients achieved BP target according to the China and American Heart Association (AHA) criteria, while at W8, 78.0 and 38.7% of patients reached these BP targets accordingly. Finally, 76.5 and 80.5% of patients achieved BP response at W4 and W8, respectively. Furthermore, home-measured SeSBP and SeDBP were significantly decreased from W1 to W8 (both P<0.001). Additionally, the satisfaction of both patients and physicians was elevated at W8 compared with at W0 (both P<0.001). The medication possession rate from baseline to W4 and W8 was 95.5 and 92.5%. The most common drug-associated adverse events by system organ classes were nervous system disorder (4.5%), vascular disorder (2.8%), and general disorder and administration site conditions (2.6%), which were generally mild. In conclusion, OM-AML tablets may be considered effective and safe in lowering BP, enabling the achievement of guideline-recommended BP targets in older Chinese patients with essential hypertension.
Keywords: olmesartan medoxomil-amlodipine besylate tablet, essential hypertension, blood pressure target, adverse event
Introduction
Essential hypertension is a highly prevalent chronic disease with >30% of adults having hypertension in 2010 globally; the disease is associated with cardio- and cerebrovascular diseases, such as stroke, myocardial infarction and heart failure (1,2). It has been reported that essential hypertension is more prevalent in the older (age ≥65 years) population compared with young adults or middle-aged subjects, partially due to arterial stiffness, worse renal function and comorbidities observed in older individuals (3,4). Considering the aging population and the increase in life expectancy, essential hypertension in older adults may pose a critical burden on the public health system in the future (5-7). Regarding the pharmacological management of essential hypertension in older patients, numerous factors should be taken into consideration, including contraindications due to comorbidity, frailty and ability to follow medical instructions. Therefore, more alternative routes of pharmacological management are needed for these patients (3,8,9).
Olmesartan medoxomil-amlodipine besylate (OM-AML) tablets are a dose-fixed antihypertensive drug, containing an angiotensin receptor blocker (OM) and a calcium channel blocker (AML) (10,11). Compared with combined administration of OM and AML tablets, dose-fixed OM-AML tablets are more convenient and can promote drug adherence (12). Currently, dose-fixed antihypertensive drugs are recommended by several guidelines, including guidelines from the World Health Organization, American College of Cardiology and American Heart Association, and European Society Of Hypertension-European Society Of Cardiology (13,14). According to previous studies, OM-AML tablets exhibit better efficacy in controlling blood pressure (BP) compared with OM or AML monotherapy (15-17). This could be due to the fact that OM-AML tablets not only combine two effective antihypertensive drugs, but also improve patient compliance due to convenience (18). However, the majority of studies evaluating the efficacy and safety of OM-AML tablets have been performed in Western countries, with Caucasian, Hispanic and Black individuals being the primary study subjects (15-17). Since China accounts for a large proportion of hypertensive individuals globally (7), it is necessary to evaluate OM-AML tablets in Chinese patients with essential hypertension.
Therefore, the current prospective, multicenter, real-world study aimed to evaluate the efficacy and safety of OM-AML tablets in older (age, ≥65 years) Chinese patients with essential hypertension.
Materials and methods
Study population
A subgroup analysis of 463 older patients with essential hypertension from the Sevikar® (SVK) study was performed. The SVK study was a prospective, single-arm, multicenter, real-world study aiming to investigate the efficacy and safety of SVK in patients with essential hypertension in China. A detailed description of the SVK study design is available in the Chinese Clinical Trial Registry (chictr.org.cn/; registration no., ChiCTR1900026574). A total of 463 older patients were screened from the SVK study based on the following criteria: i) Patients diagnosed with essential hypertension; ii) aged ≥65 years; iii) treated with SVK as antihypertensive therapy; iv) with at least one follow-up BP measurement in addition to baseline measurement and v) signed informed consent. The present study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University (approval no. B2019-174R2; Shanghai, China).
Administration of medication
SVK [Daiichi Sankyo (Shanghai) Holdings Co., Ltd.] was a compound preparation; each SVK tablet contained 20 mg OM and 5 mg AML. The dose of SVK recommended by the physicians was one oral tablet once a day.
Measurement
The seated diastolic BP (SeDBP) and seated systolic BP (SeSBP) of patients were measured at baseline (week 0, W0) and then at W4±7 days (W4) and W8±7 days (W8) in outpatient clinics. From the first day of medication, the patients measured their BP every day (home-measured BP). Furthermore, the daily medication-taking of patients and adverse events (AEs) were recorded to determine the medication possession rate (MPR) and safety profiles. Additionally, both attending physicians and patients scored satisfaction with the current hypertension treatment at W0 and W8 using a 10-cm visual analogue scale (VAS) (19); a higher score indicated higher satisfaction.
Outcomes and definitions
The outcomes included mean change in SeDBP and SeSBP from W0 to W8, proportion of patients achieving American Heart Association (AHA) and China BP targets (20,21), proportion of patients achieving BP response, changes in home-measured BP from W0 to W8, change in physician and patient satisfaction with hypertension treatment (VAS) from W0 to W8, MPR and onset of AEs. The AHA BP target was defined as SeSBP <130 mmHg and SeDBP <80 mmHg (20). The China BP target was defined as SeSBP and SeDBP <140 and <90 mmHg, respectively (21). The BP response rate was defined as proportion of patients who achieved SeSBP <140 mmHg (or a decrease of ≥20 mmHg) and SeDBP of <90 mmHg (or a decrease of ≥10 mmHg). MPR was calculated as follows: MPR=actual days of medication use/total number of days.
Statistical analysis
Statistical analysis was performed using R version 4.0.5 (r-project.org) and SPSS version 26.0 (IBM Corp.). Categorical data are expressed as number and percentage, and were analyzed using χ2 or Fisher's exact test. Measured data are expressed as the mean ± SD or SEM, or median and interquartile range. Comparisons of the measured data were carried out by Mann Whitney U test or Kruskal-Wallis test. Data on blood pressure are usually presented as the mean ± SD in the field of hypertension, so this convention has been followed. Post hoc comparison for multiple groups was conducted by Bonferroni test. Related factors were screened using a logistic regression model. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
The mean ± SD age of patients was 70.4±4.1 years. In addition, a total of 238 (51.4%) female patients (mean age, 70.5±4.2 years) and 225 (48.6%) male patients (mean age, 70.4±4.0 years) were included. The median (IQR) time since hypertension diagnosis was 13.1 (6.1-21.2) years, while 259 (55.9%) patients had a family history of hypertension. At baseline, mean ± SD SeSBP and SeDBP were 142.8±16.7 and 82.1±10.2 mmHg, respectively. A total of 264 (57.0%) and 108 (23.3%) patients had abnormal SeSBP and SeDBP, respectively (defined as SeSBP ≥140 mmHg and SeDBP ≥90 mmHg, accordingly). Furthermore, 349 (75.4%) patients received OM-AML tablets without lipid-modifying agents or other medication (any medication apart from antihypertensive agents and lipid-modifying agents), 33 (7.1%) patients were co-treated with OM-AML tablets and lipid-modifying agents, while 81 (17.5%) patients received OM-AML tablets and lipid-modifying agents and other drugs. The main characteristics of patients are listed in Table I.
Table I.
Baseline characteristics (n=463).
| Characteristic | Value |
|---|---|
| Mean age, years | 70.4±4.1 |
| Sex, n (%) | |
| Female | 238 (51.4) |
| Male | 225 (48.6) |
| Mean BMI, kg/m2 | 25.2±3.1 |
| Highest completed education level, n (%) | |
| Primary school or less | 83 (17.9) |
| High school | 269 (58.1) |
| Undergraduate or above | 111 (24.0) |
| Smoker, n (%) | |
| No | 339 (73.2) |
| Yes | 124 (26.8) |
| Alcohol intake, n (%) | |
| No | 408 (88.1) |
| Yes | 55 (11.9) |
| Median (IQR) time since hypertension diagnosis, years | 13.1 (6.1-21.2) |
| Family history of hypertension, n (%) | |
| No | 185 (40.0) |
| Yes | 259 (55.9) |
| Unknown | 19 (4.1) |
| History of allergy, n (%) | |
| No | 410 (88.6) |
| Yes | 47 (10.2) |
| Unknown | 6 (1.3) |
| History of respiratory disease, n (%) | |
| No | 416 (89.8) |
| Yes | 45 (9.7) |
| Unknown | 2 (0.4) |
| History of kidney disease, n (%) | |
| No | 432 (93.3) |
| Yes | 30 (6.5) |
| Unknown | 1 (0.2) |
| History of diabetes, n (%) | |
| No | 358 (77.3) |
| Yes | 102 (22.0) |
| Unknown | 3 (0.6) |
| History of CCVD, n (%) | |
| No | 256 (55.3) |
| Yes | 207 (44.7) |
| History of dyslipidemia, n (%) | |
| No | 266 (57.5) |
| Yes | 188 (40.6) |
| Unknown | 9 (1.9) |
| Mean baseline respiratory rate, breaths/min | 17.7±1.9 |
| Mean heart rate, beats/min | 73.6±9.6 |
| Mean SeSBP, mmHg | 142.8±16.7 |
| Abnormal SeSBP, n (%) | 264 (57.0) |
| Mean SeDBP, mmHg | 82.1±10.2 |
| Abnormal SeDBP, n (%) | 108 (23.3) |
| Hypertension severity, n (%) | |
| No | 188 (40.6) |
| Mild | 197 (42.5) |
| Moderate | 67 (14.5) |
| Severe | 11 (2.4) |
| History of hypertension treatment, n (%) | |
| Yes | 446 (96.3) |
| No | 17 (3.7) |
| History of antihypertensive drugs, n (%) | |
| Monotherapy | 237 (51.2) |
| Double combination | 164 (35.4) |
| Triple combination | 37 (8.0) |
| Unknown | 25 (5.4) |
| History of antihypertensive medication, n (%) | |
| Calcium channel blocker | 281 (60.7) |
| Angiotensin II antagonist | 338 (73.0) |
| Angiotensin-converting enzyme inhibitor | 36 (7.8) |
| Combination, n (%) | |
| No combination | 349 (75.4) |
| Lipid-modifying agent | 33 (7.1) |
| Lipid-modifying agent and othersa | 81 (17.5) |
aAny medications apart from antihypertensive agents and lipid-modifying agent. Data are presented as n (%), mean ± SD or median ± IQR. BMI, body mass index; CCVD, cardiovascular and cerebrovascular disease; SeSBP, seated systolic blood pressure; SeDBP, seated diastolic blood pressure.
SeSBP and SeDBP are reduced after OM-AML treatment
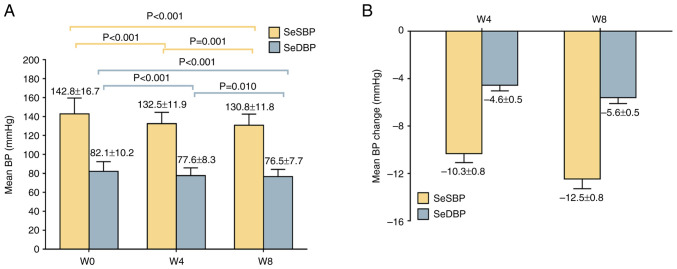
The mean ± SD SeSBP and SeDBP values at W4 were 132.5±11.9 and 77.6±8.3 mmHg, respectively, which were decreased compared with at W0. At W8, mean ± SD SeSBP and SeDBP values were 130.8±11.8 and 76.5±7.7 mmHg, respectively, which were decreased compared with those recorded at W4 and W0 (Fig. 1A). In addition, the mean ± SEM change of SeSBP and SeDBP was -10.3±0.8 and -4.6±0.5 mmHg at W4 and -12.5±0.8 and -5.6±0.5 mmHg at W8 (Fig. 1B).
Figure 1.
SeSBP and SeDBP following treatment. (A) SeSBP and SeDBP at W0, W4 and W8. (B) Changes in SeSBP and SeDBP at W4 and W8. Data are presented as mean ± SD. SeSBP, seated systolic blood pressure; SeDBP, seated diastolic blood pressure; W, week.
Comparison revealed greater changes in SeSBP or SeDBP in patients with shorter time since diagnosis of hypertension, patients with a history of allergy and kidney disease, patients without history of cardiovascular disease or dyslipidemia, patients with abnormal SeSBP and SeDBP at baseline, patients with moderate or severe hypertension, or patients without history of hypertension treatment and patients treated with OM-AML tablets and lipid-modifying agents (Table SI). Patients who continued their existing antihypertensive therapy (n=118) showed the most significant changes in SeSBP and SeDBP compared with patients without history of antihypertensive drugs (n=17) and those who discontinued existing antihypertensive therapy (n=328; Table SII).
BP target achievement was satisfactory after OM-AML treatment
At W4, 343 (74.1%) and 124 (26.8%) patients achieved BP targets according to the China or AHA criteria, respectively. Additionally, at W8 (n=431 due to lack of assessment data at W8 for some patients), 336 (78.0%) and 167 (38.7%) patients achieved the China and AHA criteria of BP target, respectively (Fig. 2A). A total of 355 (76.7%) and 434 (93.7%) patients at W4, and 347 (80.5%) and 410 (95.1%) patients at W8, met the China criteria of SeSBP and SeDBP target, respectively (Fig. 2B). A total of 183 (39.5%) and 255 (55.1%) patients at W4, as well as 204 (47.3%) and 274 (63.6%) patients at W8, achieved SeSBP and SeDBP targets according to AHA criteria, respectively (Fig. 2C). BP response rates of 76.5 and 80.5% were recorded at W4 and W8, respectively (Fig. 2D).
Figure 2.
Achievement of BP target. (A) Proportion of patients who achieved BP target according to Chinese or AHA criteria at W4 and W8. Proportion of patients who achieved SeSBP or SeDBP targets according to (B) Chinese and (C) AHA criteria at W4 and W8. (D) BP response rate at W4 and W8. AHA, American Heart Association; W, week; SeSBP, seated systolic blood pressure; SeDBP, seated diastolic blood pressure.
Subgroup analysis showed that female patients or patients with shorter time since hypertension diagnosis, normal SeSBP or SeDBP at baseline, a history of monotherapy of antihypertensive drugs or those treated with OM-AML tablets alone more significantly achieved China or AHA BP targets or BP response rate at W8 (Table II). Furthermore, male patients (vs. females), time since hypertension diagnosis of ≥10 were associated with a lower probability of achieving AHA BP target at W8. Abnormal SeSBP at baseline (vs. normal) and treatment with OM-AML tablets and lipid-modifying agents and other drugs (vs. OM-AML tablets without lipid-modifying agent or other drugs) were also independently associated with lower probability of achieving AHA BP target at W8 (Table SIII). In addition, abnormal SeSBP at baseline (vs. normal) and patient treatment with OM-AML tablets and lipid-modifying agents and other drugs (vs. OM-AML tablets without lipid-modifying agent or other drugs) were independently associated with lower probability of achieving China BP target at W8 (Table SIV). At W8 after the initiation of OM-AML tablet administration, the history of double combination of antihypertensive drugs (vs. monotherapy) and treatment with OM-AML tablets and lipid-modifying agents and other drugs (vs. OM-AML tablets without lipid-modifying agent or other drugs) were independently associated with lower BP response rate (Table SV).
Table II.
Achievement of BP target rate and BP response rate at week 8 (n=431).
| Characteristic | N | AHA BP target, n (%) | P-value | China BP target, n (%) | P-value | BP response, n (%) | P-value |
|---|---|---|---|---|---|---|---|
| Sex | 0.002 | 0.096 | 0.069 | ||||
| Female | 223 | 102 (45.7) | 181 (81.2) | 187 (83.9) | |||
| Male | 208 | 65 (31.3) | 155 (74.5) | 160 (76.9) | |||
| BMIa, kg/m2 | 0.878 | 0.767 | 0.371 | ||||
| <30 | 394 | 152 (38.6) | 306 (77.7) | 315 (79.9) | |||
| ≥30 | 30 | 12 (40.0) | 24 (80.0) | 26 (86.7) | |||
| Highest completed education level | 0.306 | 0.515 | 0.530 | ||||
| Primary school or less | 80 | 37 (46.3) | 60 (75.0) | 62 (77.5) | |||
| High school | 249 | 93 (37.3) | 199 (79.9) | 205 (82.3) | |||
| Undergraduate or above | 102 | 37 (36.3) | 77 (75.5) | 80 (78.4) | |||
| Smoker | 0.067 | 0.199 | 0.297 | ||||
| No | 317 | 131 (41.3) | 252 (79.5) | 259 (81.7) | |||
| Yes | 114 | 36 (31.6) | 84 (73.7) | 88 (77.2) | |||
| Alcohol intake | 0.078 | 0.176 | 0.126 | ||||
| No | 380 | 153 (40.3) | 300 (78.9) | 310 (81.6) | |||
| Yes | 51 | 14 (27.5) | 36 (70.6) | 37 (72.5) | |||
| Time since hypertension diagnosis, years | 0.016 | 0.307 | 0.668 | ||||
| <5 | 87 | 45 (51.7) | 73 (83.9) | 73 (83.9) | |||
| 5-9 | 55 | 22 (40.0) | 43 (78.2) | 44 (80.0) | |||
| ≥10 | 289 | 100 (34.6) | 220 (76.1) | 230 (79.6) | |||
| Family history of hypertension | 0.657 | 0.676 | 0.803 | ||||
| No | 178 | 70 (39.3) | 140 (78.7) | 144 (80.9) | |||
| Yes | 234 | 87 (37.2) | 180 (76.9) | 187 (79.9) | |||
| History of allergy | 0.845 | 0.813 | 0.840 | ||||
| No | 382 | 148 (38.7) | 296 (77.5) | 306 (80.1) | |||
| Yes | 43 | 16 (37.2) | 34 (79.1) | 35 (81.4) | |||
| History of respiratory disease | 0.436 | 0.556 | 0.565 | ||||
| No | 386 | 147 (38.1) | 299 (77.5) | 309 (80.1) | |||
| Yes | 43 | 19 (44.2) | 35 (81.4) | 36 (83.7) | |||
| History of kidney disease | 0.210 | 0.701 | 0.451 | ||||
| No | 402 | 153 (38.1) | 314 (78.1) | 325 (80.8) | |||
| Yes | 28 | 14 (50.0) | 21 (75.0) | 21 (75.0) | |||
| History of diabetes | 0.814 | 0.637 | 0.735 | ||||
| No | 332 | 127 (38.3) | 260 (78.3) | 268 (80.7) | |||
| Yes | 96 | 38 (39.6) | 73 (76.0) | 76 (79.2) | |||
| History of CCVD | 0.596 | 0.713 | 0.923 | ||||
| No | 234 | 88 (37.6) | 184 (78.6) | 188 (80.3) | |||
| Yes | 197 | 79 (40.1) | 152 (77.2) | 159 (80.7) | |||
| History of dyslipidemia | 0.743 | 0.060 | 0.105 | ||||
| No | 245 | 98 (40.0) | 200 (81.6) | 205 (83.7) | |||
| Yes | 177 | 68 (38.4) | 131 (74.0) | 137 (77.4) | |||
| Respiratory rate | 1.000 | 1.000 | 1.000 | ||||
| Normal | 356 | 146 (41.0) | 283 (79.5) | 294 (82.6) | |||
| Abnormal | 5 | 2 (40.0) | 4 (80.0) | 4 (80.0) | |||
| Heart rate (%) | 0.246 | 0.193 | 0.441 | ||||
| Normal | 397 | 153 (38.5) | 308 (77.6) | 319 (80.4) | |||
| Abnormal | 26 | 13 (50.0) | 23 (88.5) | 23 (88.5) | |||
| SeSBP | <0.001 | <0.001 | <0.001 | ||||
| Normal | 180 | 90 (50.0) | 162 (90.0) | 168 (93.3) | |||
| Abnormal | 251 | 77 (30.7) | 174 (69.3) | 179 (71.3) | |||
| SeDBP | 0.001 | 0.034 | 0.017 | ||||
| Normal | 330 | 142 (43.0) | 265 (80.3) | 274 (83.0) | |||
| Abnormal | 101 | 25 (24.8) | 71 (70.3) | 73 (72.3) | |||
| Hypertension severity | 0.950 | 0.097 | 0.034 | ||||
| Mild | 189 | 57 (30.2) | 138 (73.0) | 143 (75.7) | |||
| Moderate or severe | 72 | 22 (30.6) | 45 (62.5) | 45 (62.5) | |||
| History of hypertension treatment | 0.420 | 0.774 | 0.754 | ||||
| Yes | 414 | 162 (39.1) | 323 (78.0) | 334 (80.7) | |||
| No | 17 | 5 (29.4) | 13 (76.5) | 13 (76.5) | |||
| History of antihypertensive drugs | 0.566 | 0.005 | 0.070 | ||||
| Monotherapy | 224 | 92 (41.1) | 187 (83.5) | 189 (84.4) | |||
| Double combination | 146 | 54 (37.0) | 106 (72.6) | 109 (74.7) | |||
| Triple combination | 36 | 12 (33.3) | 23 (63.9) | 29 (80.6) | |||
| History of calcium channel blockers | 0.192 | 0.814 | 0.209 | ||||
| No | 64 | 30 (46.9) | 53 (82.8) | 56 (87.5) | |||
| Yes | 271 | 103 (38.0) | 221 (81.5) | 219 (80.8) | |||
| History of angiotensin II antagonists | 0.119 | 1.000 | 1.000 | ||||
| No | 7 | 5 (71.4) | 6 (85.7) | 6 (85.7) | |||
| Yes | 328 | 128 (39.0) | 268 (81.7) | 269 (82.0) | |||
| History of angiotensin-converting enzyme inhibitors | 0.736 | 0.058 | 0.140 | ||||
| No | 302 | 119 (39.4) | 251 (83.1) | 251 (83.1) | |||
| Yes | 33 | 14 (42.4) | 23 (69.7) | 24 (72.7) | |||
| Combination | 0.009 | 0.001 | 0.002 | ||||
| No combination | 325 | 139 (42.8) | 266 (81.8) | 273 (84.0) | |||
| Lipid-modifying agent | 32 | 10 (31.3) | 24 (75.0) | 25 (78.1) | |||
| Lipid-modifying agent and other | 74 | 18 (24.3) | 46 (62.2) | 49 (66.2) |
aSeven patients had no BMI data, thus the comparison was made in 424 patients. AHA, American Heart Association; BMI, body mass index; CCVD, cardiovascular and cerebrovascular disease; SeSBP, seated systolic blood pressure; SeDBP, seated diastolic blood pressure.
Home-measured BP is reduced after OM-AML treatment
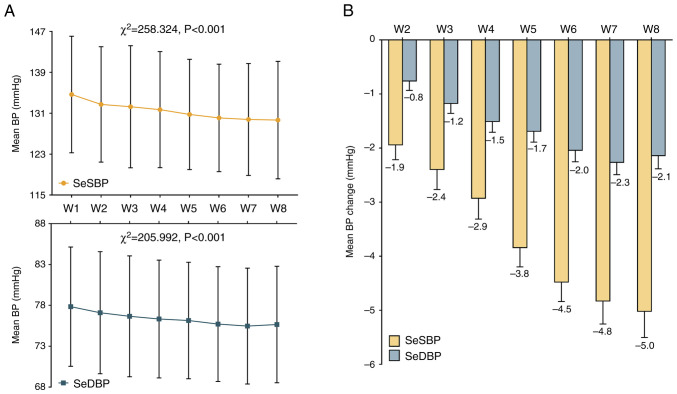
Home-measured SeSBP and SeDBP were significantly decreased from W1 to W8 (Fig. 3A). The mean changes of weekly home-measured SeSBP from W2 to W8 were -1.9, -2.4, -2.9, -3.8, -4.5, -4.8 and -5.0, respectively. Additionally, the mean changes of weekly home-measured SeDBP from W2 to W8 were -0.8, -1.2, -1.5, -1.7, -2.0, -2.3 and -2.1, respectively (Fig. 3B). The post hoc comparisons of home-measured BP are shown in Table SVI.
Figure 3.
Home-measured BP following treatment. (A) Home-measured SeSBP and SeDBP from W1 to W8. Data are presented as mean ± SD. (B) Changes in home-measured SeSBP and SeDBP from W2 to W8. Data are presented as mean ± SEM. W, week; SeSBP, seated systolic blood pressure; SeDBP, seated diastolic blood pressure.
Satisfaction is improved and medication possession is high after OM-AML treatment
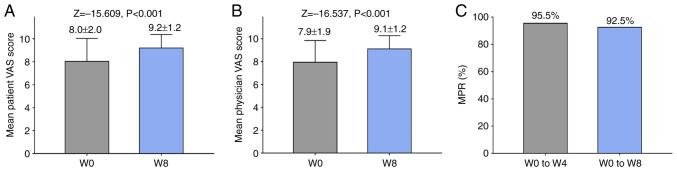
The satisfaction of both patients and physicians was significantly increased at W8 compared with W0 (Fig. 4A and B). MPR for W0-W4 and W0-W8 was 95.5 and 92.5%, respectively (Fig. 4C).
Figure 4.
Satisfaction and MPR following treatment. The satisfaction of (A) patients and (B) physicians at W0 and W8 was evaluated. (C) MPR at W4 and W8 was determined. W, week; MPR, medication possession rate; VAS, visual analogue scale.
OM-AML treatment is generally tolerable
The most common AEs were nervous system disorder (13.4%), vascular disorder (9.7%), general disorder and administration site conditions (6.5%) and cardiac disorder (4.5%). Additionally, severe AEs (grade 3-4 AEs) included vascular disorder (0.6%), cardiac disorder (0.4%), respiratory, thoracic and mediastinal disorder (0.2%), general disorders and administration site conditions (0.2%), and reproductive system and breast disorders (0.2%). Furthermore, the most common drug-associated AEs (AEs that were associated with the drug use, as evaluated by the investigators) were nervous system disorder (4.5%), vascular disorder (2.8%), and general disorder and administration site conditions (2.6%; Table III).
Table III.
AEs by system organ class.
| System organ class | Any AE, n (%) | Severe AE, n (%) | Drug-associated AE, n (%) |
|---|---|---|---|
| Nervous system disorder | 62 (13.4) | 0 (0.0) | 21 (4.5) |
| Vascular disorder | 45 (9.7) | 3 (0.6) | 13 (2.8) |
| General disorder and administration site conditions | 30 (6.5) | 1 (0.2) | 12 (2.6) |
| Cardiac disorder | 21 (4.5) | 2 (0.4) | 9 (1.9) |
| Gastrointestinal disorder | 18 (3.9) | 0 (0.0) | 6 (1.3) |
| Respiratory, thoracic and mediastinal disorder | 15 (3.2) | 2 (0.4) | 1 (0.2) |
| Metabolism and nutrition disorder | 12 (2.6) | 0 (0.0) | 0 (0.0) |
| Psychiatric disorder | 8 (1.7) | 0 (0.0) | 6 (1.3) |
| Skin and subcutaneous tissue disorder | 7 (1.5) | 0 (0.0) | 2 (0.4) |
| Musculoskeletal and connective tissue disorder | 7 (1.5) | 0 (0.0) | 0 (0.0) |
| Investigations | 4 (0.9) | 0 (0.0) | 0 (0.0) |
| Eye disorder | 3 (0.6) | 0 (0.0) | 1 (0.2) |
| Reproductive system and breast disorder | 3 (0.6) | 1 (0.2) | 0 (0.0) |
| Renal and urinary disorder | 3 (0.6) | 0 (0.0) | 0 (0.0) |
| Endocrine disorder | 2 (0.4) | 0 (0.0) | 0 (0.0) |
| Immune system disorder | 1 (0.2) | 0 (0.0) | 1 (0.2) |
| Hepatobiliary disorder | 1 (0.2) | 0 (0.0) | 0 (0.0) |
AE, adverse event.
Discussion
OM-AML tablets are an effective antihypertensive agent not only for the general population, but also for older patients, and patients with diabetes mellitus or obesity (22,23). Regarding the effect of OM-AML tablets on older patients with essential hypertension, a previous study demonstrated that the mean change of SeSBP/SeDBP was -14.5/-7.8 mmHg in older patients with uncontrolled hypertension who had previously received monotherapy followed by administration of OM-AML tablets for 20 weeks (24). Another study showed that after treatment with OM-AML tablets for 36 months, SeSBP/SeDBP decreased from 157.2/84.6 to 132.6/72.6 mmHg in older patients with hypertension (25), resulting in a mean change of -24.6/-12.0 mmHg for SeSBP/SeDBP. To the best of our knowledge, however, no similar studies have been performed in China. Due to differences in ethnicity, as well as lifestyle factors of Chinese patients, including high sodium and low potassium intake, low levels of physical exercise and high levels of alcohol abuse, evaluating the efficacy of OM-AML tablets in older patients with essential hypertension in China is of marked importance. The present study revealed that the mean change of SeSBP/SeDBP in older patients with essential hypertension was -12.5/-5.6 mmHg. The change of SeSBP/SeDBP was lower compared with that reported in previous studies (20 weeks and 36 months, respectively) (24,25). This may be due to the different duration of treatment, which was 8 weeks in the present study. However, OM-AML tablets could effectively lower BP in older patients with essential hypertension.
Decreasing BP to a particular threshold is the main objective of antihypertensive treatment. A previous study showed that 62.5% of older patients with resistant hypertension achieved the goal of SeSBP/SeDBP <140/90 mmHg following treatment with OM-AML tablets for 8 weeks (26) Additionally, a BP threshold of <140/90 mmHg was achieved by 86.8% of older patients with essential hypertension receiving OM-AML tablets for 20 weeks (24). Furthermore, another study reported that 51.4% of older patients with essential hypertension achieved a BP goal of <140/90 mmHg after treatment with OM-AML tablets for 10 weeks (27). In the present study, 78.0 and 38.7% of older patients achieved a BP target of <140/90 and <130/80 mmHg, based on the China and AHA criteria, respectively. The aforementioned results were consistent with those reported in previous studies, which used a BP goal of <140/90 mmHg (24,26,27). Additionally, compared with previous studies on older patients with hypertension treated with OM or AML monotherapy (22,28), the present study revealed that a higher proportion of patients achieved a BP target of <140/90 mmHg. This could be due to the fact that OM-AML tablets combine two antihypertensive drugs with high efficacy, thus displaying superior treatment efficacy.
During the treatment of essential hypertension, both patient and physician satisfaction should be considered. Satisfaction is commonly associated with treatment efficacy, convenience of treatment and cost (8,9,18). Consistent with a previous study (29), satisfaction of both patients and physicians in the present study was increased at W8 compared with at W0. This may be because OM-AML tablets were effective in controlling BP, thus enhancing both patient and physician satisfaction and OM-AML tablets were convenient to take due to their single-pill, dose-fixed design, reducing the probability of missing doses, thus also increasing the satisfaction of both patients and physicians. Additionally, the present study reported a MPR of 92.5% at W8, which was similar to that reported in Korean patients with essential hypertension treated with a dose-fixed OM/AML/hydrochlorothiazide regimen (29).
Due to comorbidities and frailty, the safety of antihypertensive drugs is a key issue during the treatment of older patients with essential hypertension (3,5). The present study revealed that the incidence of OM-AML-associated AEs in older patients with essential hypertension was similar to that reported in previous studies (24-27). In addition, the incidence of severe AEs was relatively low, indicating that OM-AML tablets could be considered a safe antihypertensive drug.
The present study had some limitations. Firstly, the present study was a prospective, observational, single-cohort study that evaluated the efficacy and safety of OM-AML tablets in older patients with essential hypertension. However, further randomized, controlled trials should be performed to provide more evidence for the administration of OM-AML tablets in these patients. Secondly, a 10-cm VAS scale was used to assess the satisfaction of both patients and physicians. This scale is characterized by ease of assessment; however, this leads to an increased risk of bias. Thirdly, the long-term efficacy and safety of OM-AML tablets in older patients with essential hypertension should be further explored in the future.
In conclusion, the present study indicated that OM-AML tablets were an effective and safe antihypertensive drug, facilitating the achievement of BP targets in older patients with essential hypertension.
Supplementary Material
Acknowledgements
The authors thank all the investigating groups of the SVK study, as listed in Table SVII, for their contributions.
Funding Statement
Funding: The present study was supported by Daiichi Sankyo (China) Holdings Co., Ltd, Shanghai, China.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JG, ZC and JD contributed to conception and design of study. ZC, ZQ, WH, GM, YL, RW, LC, LF, MH, YH, MW, JX, YX, ZW, XJ, JH, DF, LT, WC, XCa, YJ, YZ, LH, PB, XCh, PD, XH, MLL, XW, KH, YLL, YWL, DL, JuW, JiW, GF, LW and MQL were responsible for acquisition of data. JG, ZC, ZQ, WC, WH, GM, JD, LW and ML performed data analysis and interpreted data. JG and ZC confirm the authenticity of all the raw data. JG, ZC, JD, LW and ML drafted the manuscript and all other authors provided critical revision. All authors read and approved the final version of manuscript.
Ethics approval and consent to participate
A detailed description of the SVK study design is available in Chinese Clinical Trial Registry (chictr.org.cn/; registration no. ChiCTR1900026574). The present study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University (approval no. B2019-174R2; Shanghai, China). All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
JD, LW and MLi are employees of Daiichi Sankyo (China) Holdings Co., Ltd., the company that makes SVK. The other authors declare that they have no competing interests.
References
- 1.Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18:785–802. doi: 10.1038/s41569-021-00559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128:864–886. doi: 10.1161/CIRCRESAHA.121.318061. [DOI] [PubMed] [Google Scholar]
- 3.Oliveros E, Patel H, Kyung S, Fugar S, Goldberg A, Madan N, Williams KA. Hypertension in older adults: Assessment, management, and challenges. Clin Cardiol. 2020;43:99–107. doi: 10.1002/clc.23303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124:1045–1060. doi: 10.1161/CIRCRESAHA.118.313236. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda S, Miyamoto Y, Ogawa H. Current status of cardiovascular medicine in the aging society of Japan. Circulation. 2018;138:965–967. doi: 10.1161/CIRCULATIONAHA.118.035858. [DOI] [PubMed] [Google Scholar]
- 6.Mohsen Ibrahim M. Hypertension in developing countries: A major challenge for the future. Curr Hypertens Rep. 2018;20(38) doi: 10.1007/s11906-018-0839-1. [DOI] [PubMed] [Google Scholar]
- 7.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas NF, Dunn KS. Self-transcendence and medication adherence in older adults with hypertension. J Holist Nurs. 2014;32:316–326. doi: 10.1177/0898010114528379. [DOI] [PubMed] [Google Scholar]
- 9.Lo SH, Chau JP, Woo J, Thompson DR, Choi KC. Adherence to antihypertensive medication in older adults with hypertension. J Cardiovasc Nurs. 2016;31:296–303. doi: 10.1097/JCN.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdine S. Olmesartan/amlodipine: Blood pressure lowering and beyond in special populations. Ther Adv Cardiovasc Dis. 2012;6:31–44. doi: 10.1177/1753944711432902. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Zhang H, Ma Y, Che W, Hamblin MR. Management of hypertension using olmesartan alone or in combination. Cardiol Ther. 2017;6:13–32. doi: 10.1007/s40119-017-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parati G, Kjeldsen S, Coca A, Cushman WC, Wang J. Adherence to Single-Pill versus free-equivalent combination therapy in hypertension: A systematic review and meta-analysis. Hypertension. 2021;77:692–705. doi: 10.1161/HYPERTENSIONAHA.120.15781. [DOI] [PubMed] [Google Scholar]
- 13.Al-Makki A, DiPette D, Whelton PK, Murad MH, Mustafa RA, Acharya S, Beheiry HM, Champagne B, Connell K, Cooney MT, et al. Hypertension pharmacological treatment in adults: A World Health organization guideline executive summary. Hypertension. 2022;79:293–301. doi: 10.1161/HYPERTENSIONAHA.121.18192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakris G, Ali W, Parati G. ACC/AHA Versus ESC/ESH on hypertension guidelines: JACC guideline comparison. J Am Coll Cardiol. 2019;73:3018–3026. doi: 10.1016/j.jacc.2019.03.507. [DOI] [PubMed] [Google Scholar]
- 15.Derosa G, Mugellini A, Pesce RM, D'Angelo A, Maffioli P. Olmesartan combined with amlodipine on oxidative stress parameters in type 2 diabetics, compared with single therapies: A randomized, controlled, clinical trial. Medicine (Baltimore) 2016;95(e3084) doi: 10.1097/MD.0000000000003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chrysant SG, Melino M, Karki S, Lee J, Heyrman R. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety study. Clin Ther. 2008;30:587–604. doi: 10.1016/j.clinthera.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Volpe M, Brommer P, Haag U, Miele C. Efficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: A randomized, double-blind, parallel-group, multicentre study. Clin Drug Investig. 2009;29:11–25. doi: 10.2165/0044011-200929010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Levi M, Pasqua A, Cricelli I, Cricelli C, Piccinni C, Parretti D, Lapi F. Patient adherence to olmesartan/amlodipine combinations: Fixed versus extemporaneous combinations. J Manag Care Spec Pharm. 2016;22:255–262. doi: 10.18553/jmcp.2016.22.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas C, Romatet S, Mekiès C, Allaf B, Lantéri-Minet M. Stability, responsiveness, and reproducibility of a visual analog scale for treatment satisfaction in migraine. Headache. 2012;52:1005–1018. doi: 10.1111/j.1526-4610.2012.02157.x. [DOI] [PubMed] [Google Scholar]
- 20.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AA PA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of Cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/CIR.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 21.Wu S, Xu Y, Zheng R, Lu J, Li M, Chen L, Huo Y, Xu M, Wang T, Zhao Z, et al. Hypertension Defined by 2017 ACC/AHA guideline, ideal cardiovascular health metrics, and risk of cardiovascular disease: A nationwide prospective cohort study. Lancet Reg Health West Pac. 2022;20(100350) doi: 10.1016/j.lanwpc.2021.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chrysant SG, Lee J, Melino M, Karki S, Heyrman R. Efficacy and tolerability of amlodipine plus olmesartan medoxomil in patients with difficult-to-treat hypertension. J Hum Hypertens. 2010;24:730–738. doi: 10.1038/jhh.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oparil S, Lee J, Karki S, Melino M. Subgroup analyses of an efficacy and safety study of concomitant administration of amlodipine besylate and olmesartan medoxomil: Evaluation by baseline hypertension stage and prior antihypertensive medication use. J Cardiovasc Pharmacol. 2009;54:427–436. doi: 10.1097/FJC.0b013e3181bad74e. [DOI] [PubMed] [Google Scholar]
- 24.Weir MR, Shojaee A, Maa JF. Efficacy of amlodipine/olmesartan medoxomil +/- hydrochlorothiazide in patients aged >/=65 or <65 years with uncontrolled hypertension on prior monotherapy. Postgrad Med. 2013;125:124–134. doi: 10.3810/pgm.2013.03.2646. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa H, Kim-Mitsuyama S, Matsui K, Jinnouchi T, Jinnouchi H, Arakawa K. Angiotensin II receptor blocker-based therapy in Japanese elderly, high-risk, hypertensive patients. Am J Med. 2012;125:981–990. doi: 10.1016/j.amjmed.2011.12.010. OlmeSartan and Calcium Antagonists Randomized (OSCAR) Study Group. [DOI] [PubMed] [Google Scholar]
- 26.Ding S, Liu J, Fu Q, Zheng Y. Clinical effects of combined olmesartan medoxomil and amlodipine on clinic and ambulatory blood pressure in elderly patients with resistant hypertension. Arch Gerontol Geriatr. 2013;57:423–427. doi: 10.1016/j.archger.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Kreutz R, Ammentorp B, Laeis P, de la Sierra A. Efficacy and tolerability of triple-combination therapy with olmesartan, amlodipine, and hydrochlorothiazide: A subgroup analysis of patients stratified by hypertension severity, age, sex, and obesity. J Clin Hypertens (Greenwich) 2014;16:729–740. doi: 10.1111/jch.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu JR, Zhang SY, Gao PJ. Efficacy and safety of olmesartan medoxomil/amlodipine fixed-dose combination for hypertensive patients uncontrolled with monotherapy. Arch Pharm Res. 2014;37:1588–1598. doi: 10.1007/s12272-014-0446-x. [DOI] [PubMed] [Google Scholar]
- 29.Sohn IS, Ihm SH, Kim GH, Park SM, Hong BK, Lee CH, Lee SH, Chang DI, Joo SP, Lee SC, et al. Real-world evidence on the strategy of olmesartan-based triple single-pill combination in Korean hypertensive patients: A prospective, multicenter, observational study (RESOLVE-PRO) Clin Hypertens. 2021;27(21) doi: 10.1186/s40885-021-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.