Abstract
Following the EFSA commodity risk assessment of Malus domestica plants imported from Türkiye into the EU, the EFSA Panel on Plant Health performed a pest categorisation of Pratylenchus loosi (Nematoda: Pratylenchidae) for the EU. Pratylenchus loosi belongs to the order Rhabditida, subfamily Pratylenchidae. This nematode is not known to be present in the EU. The species is not included in the EU Commission Implementing Regulation 2019/2072. The pest occurs primarily in tropical, subtropical and warm temperate areas. It is widely distributed in Asian countries, with tea plants (Camellia sinensis) as the main host. The pest was reported from more than 60 plant species, but reports from hosts other than C. sinensis, e.g. citrus (Citrus spp.) and banana (Musa spp.), are associated with high uncertainty due to doubtful pest identification. Morphological and molecular methods are available for the identification of the pest. Pathways of entry are host plants for planting except seeds, as well as soil attached to plants for planting, machinery or footwear. Soil import to the EU is prohibited from third countries. The climatic preferences of P. loosi are compatible with the microclimatic conditions occurring in the areas of the EU where tea is grown outside. The impact of the nematode is primarily known for Asian countries, where it is a devastating pathogen on tea plants, but there is a key uncertainty on impacts on hosts other than tea. Considering the strong pathogenicity of the pest, its establishment in tea producing areas would have negative consequences for tea producers. Therefore, the Panel concludes that P. loosi satisfies all the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest.
Keywords: geographic distribution, host range, intraspecific diversity, pest risk, plant pest, polyphagous nematodes, tea cultivation
1. INTRODUCTION
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The new Plant Health Regulation (EU) 2016/2031, on the protective measures against pests of plants, is applying from 14 December 2019. Conditions are laid down in this legislation in order for pests to qualify for listing as Union quarantine pests, protected zone quarantine pests or Union regulated non‐quarantine pests. The lists of the EU regulated pests together with the associated import or internal movement requirements of commodities are included in Commission Implementing Regulation (EU) 2019/2072. Additionally, as stipulated in the Commission Implementing Regulation 2018/2019, certain commodities are provisionally prohibited to enter in the EU (high risk plants, HRP). EFSA is performing the risk assessment of the dossiers submitted by exporting to the EU countries of the HRP commodities, as stipulated in Commission Implementing Regulation 2018/2018. Furthermore, EFSA has evaluated a number of requests from exporting to the EU countries for derogations from specific EU import requirements.
In line with the principles of the new plant health law, the European Commission with the Member States are discussing monthly the reports of the interceptions and the outbreaks of pests notified by the Member States. Notifications of an imminent danger from pests that may fulfil the conditions for inclusion in the list of the Union quarantine pest are included. Furthermore, EFSA has been performing horizon scanning of media and literature.
As a follow‐up of the above‐mentioned activities (reporting of interceptions and outbreaks, HRP, derogation requests and horizon scanning), a number of pests of concern have been identified. EFSA is requested to provide scientific opinions for these pests, in view of their potential inclusion by the risk manager in the lists of Commission Implementing Regulation (EU) 2019/2072 and the inclusion of specific import requirements for relevant host commodities, when deemed necessary by the risk manager.
1.1.2. Terms of reference
EFSA is requested, pursuant to Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinions in the field of plant health.
EFSA is requested to deliver 53 pest categorisations for the pests listed in Annex 1A, 1B, 1D and 1E (for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Additionally, EFSA is requested to perform pest categorisations for the pests so far not regulated in the EU, identified as pests potentially associated with a commodity in the commodity risk assessments of the HRP dossiers (Annex 1C; for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Such pest categorisations are needed in the case where there are not available risk assessments for the EU.
When the pests of Annex 1A are qualifying as potential Union quarantine pests, EFSA should proceed to phase 2 risk assessment. The opinions should address entry pathways, spread, establishment, impact and include a risk reduction options analysis.
Additionally, EFSA is requested to develop further the quantitative methodology currently followed for risk assessment, in order to have the possibility to deliver an express risk assessment methodology. Such methodological development should take into account the EFSA Plant Health Panel Guidance on quantitative pest risk assessment and the experience obtained during its implementation for the Union candidate priority pests and for the likelihood of pest freedom at entry for the commodity risk assessment of High Risk Plants.
1.2. Interpretation of the Terms of Reference
Pratylenchus loosi is one of a number of pests listed in Annex 1C to the Terms of Reference (ToRs) to be subject to pest categorisation to determine whether it fulfils the criteria of a potential Union quarantine pest (QP) for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores, and so inform EU decision making as to its appropriateness for potential inclusion in the lists of pests of Commission Implementing Regulation (EU) 2019/2072. If a pest fulfils the criteria to be potentially listed as a Union QP, risk reduction options will be identified.
1.3. Additional information
This pest categorisation was carried out following the commodity risk assessment of Malus domestica plants from Türkiye performed by EFSA (EFSA PLH Panel, 2022), in which P. loosi was identified as a relevant non‐regulated EU pest which could potentially enter the EU on M. domestica.
2. DATA AND METHODOLOGIES
2.1. Data
2.1.1. Information on pest status from NPPOs
In the context of the current mandate, EFSA is preparing pest categorisations for new/emerging pests that are not yet regulated in the EU. When official pest status is not available in the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, online), EFSA consults the NPPOs of the relevant MSs. To obtain information on the official pest status for P. loosi, EFSA has consulted the NPPO of Bulgaria. The results of this consultation are presented in Section 3.2.2.
2.1.2. Literature search
A literature search on P. loosi was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as search term. Papers relevant for the pest categorisation were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.3. Database search
Pest information, on host(s) and distribution, was retrieved from the EPPO Global Database (EPPO, online), the CABI databases and scientific literature databases as referred above in Section 2.1.1.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt and TRACES databases were consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission as a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. TRACES is the European Commission's multilingual online platform for sanitary and phytosanitary certification required for the importation of animals, animal products, food and feed of non‐animal origin and plants into the European Union, and the intra‐EU trade and EU exports of animals and certain animal products. Up until May 2020, the Europhyt database managed notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States and the phytosanitary measures taken to eradicate or avoid their spread. The recording of interceptions switched from Europhyt to TRACES in May 2020.
GenBank was searched to determine whether it contained any nucleotide sequences for P. loosi which could be used as reference material for molecular diagnosis. GenBank® (www.ncbi.nlm.nih.gov/genbank/) is a comprehensive publicly available database that as of August 2019 (release version 227) contained over 6.25 trillion base pairs from over 1.6 billion nucleotide sequences for 450,000 formally described species (Sayers et al., 2020).
2.2. Methodologies
The Panel performed the pest categorisation for P. loosi, following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018), the EFSA guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee et al., 2017) and the International Standards for Phytosanitary Measures No. 11 (FAO, 2013).
The criteria to be considered when categorising a pest as a potential Union QP is given in Regulation (EU) 2016/2031 Article 3 and Annex I, Section 1 of the Regulation. Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. In judging whether a criterion is met the Panel uses its best professional judgement (EFSA Scientific Committee et al., 2017) by integrating a range of evidence from a variety of sources (as presented above in Section 2.1) to reach an informed conclusion as to whether or not a criterion is satisfied.
TABLE 1.
Pest categorisation criteria under evaluation, as derived from Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column).
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest (Article 3) |
|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed. |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways for entry and spread. |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests' introduction have an economic or environmental impact on the EU territory? |
| Available measures (Section 3.6 ) | Are there measures available to prevent pest entry, establishment, spread or impacts? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. |
The Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, deemed to be a risk management decision, the Panel will present a summary of the observed impacts in the areas where the pest occurs, and make a judgement about potential likely impacts in the EU. Whilst the Panel may quote impacts reported from areas where the pest occurs in monetary terms, the Panel will seek to express potential EU impacts in terms of yield and quality losses and not in monetary terms, in agreement with the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Article 3 (d) of Regulation (EU) 2016/2031 refers to unacceptable social impact as a criterion for QP status. Assessing social impact is outside the remit of the Panel.
3. PEST CATEGORISATION
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
P. loosi Loof, 1960 belongs to the order Rhabditida, family Pratylenchidae, subfamily Pratylenchinae. The genus Pratylenchus contains 68 species (Castillo & Vovlas, 2007). Molecular sequences are available for the identification of this species. The Gene Bank lists 128 accessions for this pest (https://www.ncbi.nlm.nih.gov/nuccore).
Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and/or to be transmissible?
Yes, the identity of the pest is clearly defined based on both morphology and molecular sequences.
The EPPO code 1 (EPPO, 2019; Griessinger & Roy, 2015) for this species is: PRATLO (EPPO, online).
3.1.2. Biology of the pest
P. loosi is a migratory (i.e. it is able to move inside the roots) endoparasitic root nematode with sexual reproduction (Table 2). The life cycle consists of eggs, four juvenile and one adult stages, either female or male. The pest occurs in tropical, subtropical, and warm temperate areas. It is primarily reported from tea plants (Camellia sinensis) and some other hosts such as citrus (Citrus spp.) and banana (Musa spp.) (Brooks, 2004; Goodey et al., 1965; Gnanapragasham & Mohotti, 2018) (Appendix A). The nematode is widespread and a devastating parasite of tea in Asian countries (Amarasena et al., 2016, 2020; CABI, 2021; Mohotti et al., 2023).
TABLE 2.
Important features of the life history strategy of Pratylenchus loosi.
| Life stage | Phenology and relation to host | Other relevant information |
|---|---|---|
| Egg | Eggs are laid in soil and/or in the root tissue (mainly cortex). | – |
| Juvenile | There are four juvenile stages (J). The first stage J1 moults in the egg. The J2 stage hatches from the egg. The stages J2‐J4 can attack the root from the outside or infect the root tissue (mainly cortex). | The juveniles move freely in the soil water films and in the root tissues. |
| Adult | Adults feed in the root cortex causing root lesions and cavities. |
The pest reproduces sexually and has several generations per year. For all stages, the optimum temperatures are 18–24°C (Gnanapragasham & Mohotti, 2018). Highest population densities may occur in spring and autumn (Seraji et al., 2006). Nematodes move only short distances over a year. Longer dispersal is possible only by movement of soil, water and plants. P. loosi tolerates dry soil conditions. |
3.1.3. Host range/species affected
Camellia sinensis is the main host of P. loosi, as much of the literature on this nematode is related to tea. The nematode has also been reported on Citrus spp. (citrus) and Musa spp. (banana), but there are only a few reports on these hosts. The pest was reported from more than 60 plant species (Appendix A) but studies on nematode populations reported from hosts other than tea, e.g. citrus and banana, have revealed much variation in morphology raising doubts on the correct nematode identification (Gnanapragasham & Mohotti, 2018) and, thus, the host range.
This pest categorisation will focus on the documented hosts of P. loosi that are relevant for the EU (tea, citrus and banana).
3.1.4. Intraspecific diversity
In Sri Lanka, a considerable intraspecific diversity in P. loosi was reported (Amarasena et al., 2020).
In addition, the ability of the pest to reproduce sexually could potentially enhance its genomic plasticity and adaptation to various adverse environmental conditions.
3.1.5. Detection and identification of the pest
P. loosi can be identified based on morphological characters, i.e. head with two head annules, oval/rectangular spermatheca, female tail with a conical tail with a smooth tip. However, molecular tools seem important because of morphological similarities with closely related species.
Are detection and identification methods available for the pest?
Yes, both morphological and molecular methods are available for its identification.
Molecular techniques (18S and 28S rRNA gene, ITS region, mitochondrial COI and partial nuclear hsp90 gene) are available for the identification of the pest (Haji et al., 2007; Mirghasemi et al., 2019; Uehara et al., 1998). Laboratory testing without root incubation may fail in detecting early infections.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
The pest was reported from North (Florida, Kansas, Louisiana), Central (Guadeloupe) and South (Brazil, Chile) America, Africa (Kenya, Senegal), Asia (Bangladesh, China, India, Iran, Japan, South Korea, Sri Lanka, Taiwan, Türkiye) and Oceania (American Samoa, Australia, Cook Islands) (Figure 1, Appendix B).
FIGURE 1.
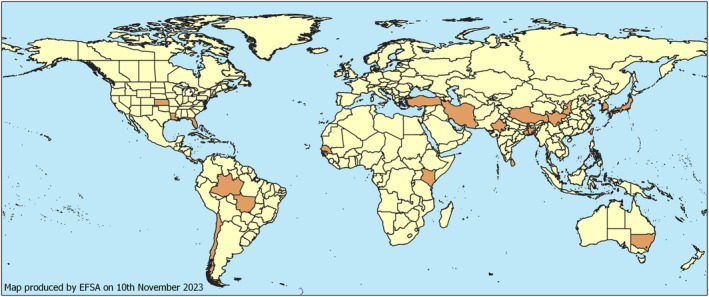
Global distribution of Pratylenchus loosi (Data source: CABI (2021) and literature).
As many reports rely on morphological identification of the pest only, there is uncertainty about the worldwide distribution and native range of P. loosi.
3.2.2. Pest distribution in the EU
The pest has been reported in Bulgaria (Katalan‐Gateva & Nedelchev, 1983), but there is uncertainty about this report because the report is only based on morphological identification. Indeed, the Bulgarian NPPO considers the status of the pest in Bulgaria as absent, unreliable record.
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed.
No, the pest is not known to be present in the EU.
3.3. Regulatory status
3.3.1. Commission Implementing Regulation 2019/2072
Pratylenchus loosi is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072, an implementing act of Regulation (EU) 2016/2031, or in any emergency plant health legislation.
3.3.2. Hosts or species affected that are prohibited from entering the union from third countries
None of the main hosts identified in Section 3.1.3 are included in Commission Implementing Regulation 2019/2072. A list of commodities included in Annex VI of Commission Implementing Regulation (EU) 2019/2072 is provided in Table 3. High‐risk plants which are reported as hosts of P. loosi are: Acacia Mill., Cassia L., Diospyros L., Malus Mill. and Prunus L., but there is uncertainty about the host status of these species (see Section 3.1.3).
TABLE 3.
List of plants, plant products and other objects that are Pratylenchus loosi hosts whose introduction into the Union from certain third countries is prohibited (Source: Commission Implementing Regulation (EU) 2019/2072, Annex VI).
| List of plants, plant products and other objects whose introduction into the union from certain third countries is prohibited | |||
|---|---|---|---|
| Description | CN code | Third country, group of third countries or specific area of third country | |
| 11. | Plants of Citrus L., […] Poncirus Raf., and their hybrids, other than fruits and seeds |
ex 0602 10 90 ex 0602 20 200,602 20 30 ex 0602 20 80 ex 0602 90 45 ex 0602 90 46 ex 0602 90 47 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 ex 0604 20 90 ex 1404 90 00 |
All third countries |
| 19. | Soil as such consisting in part of solid organic substances | ex 2530 90 00 ex 3824 99 93 | Third countries other than Switzerland |
| 20. | Growing medium as such, other than soil, consisting in whole or in part of solid organic substances, other than that composed entirely of peat or fibre of Cocos nucifera L., previously not used for growing of plants or for any agricultural purposes | ex 2530 10 00 ex 2530 90 00 ex 2703 00 00 ex 3101 00 00 ex 3824 99 93 | Third countries other than Switzerland |
3.4. Entry, establishment and spread in the EU
3.4.1. Entry
The entry pathways (Table 4) are:
host plants for planting, except seeds
soil and growing media, as well as
soil attached to plants for planting, machinery, or footwear.
Is the pest able to enter into the EU territory? If yes, identify and list the pathways.
Yes, P. loosi could enter the EU on host plants for planting, except seeds, soil and growing media, and soil as a contaminant.
Comment on plants for planting as a pathway.
Plants for planting are a main pathway of entry of the pest into the EU.
TABLE 4.
Potential pathways for Pratylenchus loosi into the EU.
| Pathways (e.g. host/intended use/source) | Life stage | Relevant mitigations [e.g. prohibitions (Annex VI), special requirements (Annex VII) or phytosanitary certificates (Annex XI) within implementing regulation 2019/2072] |
|---|---|---|
| Hosts plants for planting, other than seeds | All | Plants for planting, other than seeds, that are hosts of P. loosi and are prohibited from being imported from third countries [Annex VI of Commission Implementing Regulation (EU) 2019/2072] are listed in Table 3. There is a temporary prohibition for high‐risk plants (Regulation 2018/2019). High‐risk plants which are reported as hosts of P. loosi are: Acacia Mill., Cassia L., Diospyros L., Malus Mill. and Prunus L. |
| Soil/growing medium as such | All | The introduction into the Union from third countries, other than Switzerland, of soil/growing medium is banned [Annex VI (19) of Commission Implementing Regulation (EU) 2019/2072]. |
| Growing media carrying infected plant debris | All | A phytosanitary certificate is required for the introduction into the Union from third countries, other than Switzerland, of growing medium attached to or associated with plants, intended to sustain the vitality of the plants [Annex XI, Part A (1) of Commission Implementing Regulation (EU) 2019/2072]. Special requirements also exist for this commodity [Annex VII (1) of Commission Implementing Regulation (EU) 2019/2072]. |
| Machinery and vehicles which have been operated for agricultural purposes in infested areas | All | Official statement that machinery and vehicles are cleaned and free from soil and plant debris. (Annex VII 2) |
Notifications of interceptions of harmful organisms began to be compiled in Europhyt in May 1994 and in TRACES in May 2020. As of Sep 2023, there were no records of interception of P. loosi in the Europhyt and TRACES databases.
3.4.2. Establishment
P. loosi occurs in tropical, subtropical and warm temperate areas of the world (Figure 1). In the EU, suitable areas for establishment for the pest occur in southern Spain, southern Portugal, southern France, Italy and Greece.
Is the pest able to become established in the EU territory?
Yes, both the biotic (host availability) and abiotic (climate suitability) conditions in the EU suggest that P. loosi could establish in parts of the EU territory where hosts are grown.
In the EU, suitable areas for establishment of the pest coincide with the presence of hosts (Table 5).
TABLE 5.
Production data of two of the Pratylenchus loosi main hosts in the EU, 2017–2021 (1000 ha/tonnes).
| Crop | Unit | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| Citrus | 1000 ha | 502,9 | 509,0 | 512,8 | 520,0 | 515,7 |
| Banana | 1000 tonnes | 613,7 | 586,8 | 624,4 | 634,5 | 638,4 |
Source: EUROSTAT (accessed on 25/07/2023; for individual Member States see Appendix C).
In the EU, tea (Camellia sinensis) is cultivated in farms in Belgium, France, Germany, Italy, the Netherlands, Portugal, Spain and Sweden (Hardin, 2020). The Tea Grown in Europe Association currently has tea‐growing members in the Azores, Belgium, France, Georgia, Germany, Ireland, Italy, Jersey, Portugal, Scotland, Switzerland, the Netherlands, Ukraine and Wales, and there is increasing interest in growing tea in these countries (Anonymous, 2023). Moreover, Camellia sinensis is commonly grown as ornamental in private and public gardens.
3.4.2.1. EU distribution of main host plants
3.4.2.2. Climatic conditions affecting establishment
Based on the data available in the literature on the geographical coordinates of the locations from where P. loosi has been reported, the pathogen is present in non‐EU areas with BSh, BSk, Cfa, Cfb, Cfc, Csa, Csb, Csc, Dfb and Dfc Köppen–Geiger climate zones. These climate zones also occur in the EU territory, where susceptible hosts of P. loosi are grown (Figure 2).
FIGURE 2.
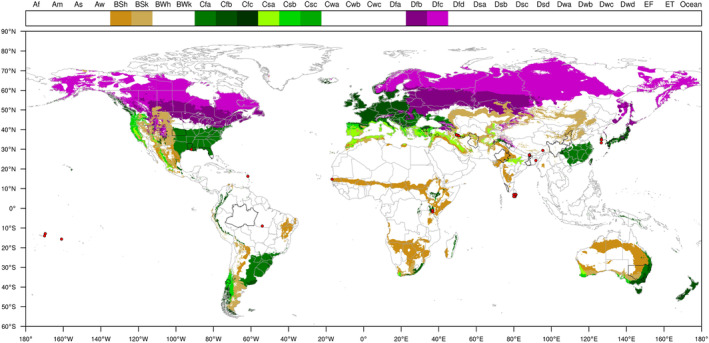
Distribution of 10 Köppen–Geiger climate types, i.e. BSh, BSk, Cfa, Cfb, Cfc, Csa, Csb, Csc, Dfb and Dfc that occur in the EU and in third countries where Pratylenchus loosi has been reported. The legend shows the list of Köppen–Geiger climates. Red dots indicate point locations where P. loosi was reported.
3.4.3. Spread
The pest would spread in the EU with host plants for planting, but also with movement of infested soil, flooding, run‐off water and soil from infested fields associated with machinery and footwear.
Describe how the pest would be able to spread within the EU territory following establishment?
The pest would spread in the EU by natural and human‐assisted means.
Plants for planting are one of the main means of spread.
3.5. Impacts
P. loosi is well recognised as a devastating pest of tea plants. Typical field symptoms of damage caused by P. loosi, in both young and mature tea plants, include patches of plants showing stunted growth with sparse and yellowish foliage, because of the reduced nutrient uptake by the damaged root system (Sivapalan, 1971).
Would the pests' introduction have an economic or environmental impact on the EU territory?
Yes, the pest introduction would have an economic impact on tea in the EU. There is a lack of information on the impact on citrus and banana.
P. loosi is the most serious pest of tea in Sri Lanka, mainly at altitudes between 900 and 1800 m. The pest affects tea in other countries such as Japan, Iran, Bangladesh, China and Korea (Amarasena et al., 2016).
P. loosi is a persistent soil pest attacking roots of tea plants of all ages and is thus problematic in tea nurseries, new plantations and mature tea fields. The damage of P. loosi to tea crops was estimated to be between 4% and 40% (Gnanapragasham & Mohotti, 2018).
There is increasing interest in growing tea in the EU and Europe, with production reported from Belgium, France, Germany, Italy, the Netherlands, Portugal, Spain, Sweden, Switzerland and the UK (Anonymous, 2023; Hardin, 2020).
The pest has been associated with growth reduction of Unshiu oranges on trifoliate stocks (Ushiyama & Ogaki, 1970). There is a key uncertainty on the impact overall, given the lack of impact reports in hosts other than tea. Given the generally polyphagous nature of the genus Pratylenchus, impacts can be expected on various hosts, e.g. citrus and banana.
3.6. Available measures and their limitations
3.6.1. Identification of potential additional measures
Phytosanitary measures (prohibitions) are currently applied to some host plants for planting (see Section 3.3.2).
Are there measures available to prevent pest entry, establishment, spread or impacts such that the risk becomes mitigated?
Yes. Although not specifically targeted against P. loosi, existing phytosanitary measures (see Sections 3.3.2 and 3.4.1) help in mitigating the likelihood of the pest to enter and spread into the EU territory on plants for planting. Potential additional measures are also available to further mitigate the risk of entry, establishment, spread and impacts of the pest in the EU (see Section 3.6.1).
Additional potential risk reduction options and supporting measures are shown in Sections 3.6.1.1 and 3.6.1.2.
3.6.1.1. Additional potential risk reduction options
Potential additional control measures are listed in Table 6.
TABLE 6.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways. Control measures are measures that have a direct effect on pest abundance.
| Control measure/Risk reduction option (Blue underline = Zenodo doc, Blue = WIP) | RRO summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Require pest freedom | Plants from pest free areas and pest free production sites would be unlikely to disseminate the pest. | Entry/Spread |
| Growing plants in isolation | Growing plants in physical isolation would help in reducing infection by the pest. | Entry (reduce contamination/infestation)/Spread |
| Managed growing conditions | Plants grown without contact with soil would reduce infections by the pest, and this also relates to plants grown in nurseries under official control. | Entry (reduce contamination/infestation)/Spread |
| Crop rotation, associations and density, weed/volunteer control |
Crop rotation, associations and density, weed/volunteer control are used to prevent problems related to pests and are usually applied in various combinations to make the habitat less favourable for pests. The measures deal with (1) allocation of crops to field (over time and space) (multi‐crop, diversity cropping) and (2) to control weeds and volunteers as hosts of pests/vectors. Weed control of basket grass Oplismenus compositus would reduce field infestation of the pest (Gnanapragasham & Mohotti, 2018) |
Entry/Establishment/Impact |
| Use of resistant and tolerant plant species/varieties | Resistant plants are used to restrict the growth and development of a specified pest and/or the damage they cause when compared to susceptible plant varieties under similar environmental conditions and pest pressure.
|
Entry/Establishment/Impact |
| Chemical treatments on crops including reproductive material |
Treatment with neem Treatment with Furadan (carbofuran) was reported from Iran (Sivapalan et al., 1980). This pesticide was banned in the EU in 2008 (Kitowski et al., 2020). |
Entry/Establishment/Impact |
| Chemical treatments on consignments or during processing |
Use of chemical compounds that may be applied to plants or to plant products after harvest, during process or packaging operations and storage. The treatments addressed in this information sheet are:
|
Entry/Spread |
| This information sheet deals with the following categories of physical treatments: irradiation; ionisation; mechanical cleaning (brushing, washing); sorting and grading, and; removal of plant parts (e.g. debarking wood). This information sheet does not address: heat and cold treatment (information sheet 1.14); roguing and pruning (information sheet 1.12). | Entry/Spread | |
|
Cleaning and disinfection of facilities, tools and machinery |
The physical and chemical cleaning and disinfection of facilities, tools, machinery, transport means, facilities and other accessories (e.g. boxes, pots, pallets, palox, supports, hand tools). The measures addressed in this information sheet are: washing, sweeping and fumigation. This would be helpful in reducing nematode infestation. |
Entry/Spread |
| Limits on soil | This has only a limited effect on endoparasitic nematodes like P. loosi. | Entry/Spread |
| Soil treatment |
Pre‐planting steaming of soil would reduce infestation of plants. Although there is no specific information on P. loosi, soil solarization and fumigation are used to control other Pratylenchus species (Castillo & Vovlas, 2007). |
Entry/Establishment/Impact |
| Use of non‐contaminated water |
Chemical and physical treatment of water to eliminate waterborne microorganisms. The measures addressed in this information sheet are: chemical treatments (e.g. chlorine, chlorine dioxide, ozone); physical treatments (e.g. membrane filters, ultraviolet radiation, heat); ecological treatments (e.g. slow sand filtration). Using clean water would be helpful to avoid the introduction and spread of the pest. |
Entry/Spread |
| Waste management | Although there is no specific information for P. loosi, waste management can generally be helpful to reduce infection of Pratylenchus species. | Establishment/Spread |
| Heat and cold treatments | No information was found on the effect of hot water treatment against P. loosi, but this would reduce infestation. | Entry/Spread |
| Controlled atmosphere |
Treatment of plants by storage in a modified atmosphere (including modified humidity, O2, CO2, temperature, pressure). No known effect on P. loosi. |
Entry/Spread (via commodity) |
| Post‐entry quarantine and other restrictions of movement in the importing country |
This information sheet covers post‐entry quarantine (PEQ) of relevant commodities; temporal, spatial and end‐use restrictions in the importing country for import of relevant commodities; Prohibition of import of relevant commodities into the domestic country. ‘Relevant commodities’ are plants, plant parts and other materials that may carry pests, either as infection, infestation, or contamination. Symptoms develop gradually, so post‐entry quarantine would be helpful in detecting infected plants. |
Establishment/Spread |
3.6.1.2. Additional supporting measures
Potential additional supporting measures are listed in Table 7.
TABLE 7.
Selected supporting measures (a full list is available in EFSA PLH Panel, 2018) in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance.
| Supporting measure (Blue underline = Zenodo doc, Blue = WIP) | Summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Inspection and trapping |
Inspection is defined as the official visual examination of plants, plant products or other regulated articles to determine if pests are present or to determine compliance with phytosanitary regulations (ISPM 5). The effectiveness of sampling and subsequent inspection to detect pests may be enhanced by including trapping and luring techniques. This would only be effective on developed infections. Early infections would not show symptoms. |
Establishment/Spread |
| Laboratory testing |
Examination, other than visual, to determine if pests are present using official diagnostic protocols. Diagnostic protocols describe the minimum requirements for reliable diagnosis of regulated pests. Laboratory tests would detect asymptomatic infections. |
Entry/Establishment/Spread |
| Sampling |
According to ISPM 31, it is usually not feasible to inspect entire consignments, so phytosanitary inspection is performed mainly on samples obtained from a consignment. It is noted that the sampling concepts presented in this standard may also apply to other phytosanitary procedures, notably selection of units for testing. For inspection, testing and/or surveillance purposes the sample may be taken according to a statistically based or a non‐statistical sampling methodology. Important to sample symptomatic plants if detected. |
Entry/Establishment/Spread |
| Phytosanitary certificate and plant passport | An official paper document or its official electronic equivalent, consistent with the model certificates of the IPPC, attesting that a consignment meets phytosanitary import requirements (ISPM 5)
|
Entry/Spread |
| Certified and approved premises |
Mandatory/voluntary certification/approval of premises is a process including a set of procedures and of actions implemented by producers, conditioners and traders contributing to ensure the phytosanitary compliance of consignments. It can be a part of a larger system maintained by the NPPO in order to guarantee the fulfilment of plant health requirements of plants and plant products intended for trade. Key property of certified or approved premises is the traceability of activities and tasks (and their components) inherent the pursued phytosanitary objective. Traceability aims to provide access to all trustful pieces of information that may help to prove the compliance of consignments with phytosanitary requirements of importing countries. This would be helpful in reducing infection by the pest. |
Entry/Spread |
| Certification of reproductive material (voluntary/official) |
Plants come from within an approved propagation scheme and are certified pest free (level of infestation) following testing; Used to mitigate against pests that are included in a certification scheme. This would be helpful in reducing infection by the pest. |
Entry/Spread |
| Delimitation of Buffer zones |
ISPM 5 defines a buffer zone as “an area surrounding or adjacent to an area officially delimited for phytosanitary purposes in order to minimise the probability of spread of the target pest into or out of the delimited area, and subject to phytosanitary or other control measures, if appropriate” (ISPM 5). The objectives for delimiting a buffer zone can be to prevent spread from the outbreak area and to maintain a pest free production place (PFPP), site (PFPS) or area (PFA). This would be helpful in reducing infection by the pest, especially if buffer zones are kept free of host plants such as Tephrosia vogelii, Sesbania cinerascens, Cassia elata and Acacia spp., which support population build‐up of the pest (Gnanapragasham & Mohotti, 2018). |
Spread |
| Surveillance |
Surveillance to guarantee that plants and produce originate from a Pest Free Area could be an option. This would be helpful in reducing infection by the pest. |
Spread |
3.6.1.3. Biological or technical factors limiting the effectiveness of measures
Plants may be asymptomatic, and symptoms may develop gradually so frequent inspections are needed to detect infested plants.
Laboratory testing without root incubation may fail in detecting early infections.
The pest may be overlooked by morphological identification only.
The polyphagous nature of the nematode limits the effectiveness of surveillance.
Root washings that are frequently used against nematodes are ineffective against P. loosi because it is an endoparasite.
3.7. Uncertainty
There is a key uncertainty about impact on hosts other than tea.
4. CONCLUSIONS
Pratylenchus loosi is well known as a key pest of tea. Its climatic preferences are compatible with the microclimatic conditions occurring in areas of the EU where tea is produced outside (see section 3.4.2). Considering the strong pathogenicity of the pest, its establishment in these areas would have negative consequences for tea producers.
Pratylenchus loosi therefore meets all the criteria that are within the remit of EFSA to assess this species to be regarded as a potential Union quarantine pest (Table 8).
TABLE 8.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column).
| Criterion of pest categorisation | Panel's conclusions against criterion in regulation (EU) 2016/2031 regarding Union quarantine pest | Key uncertainties |
|---|---|---|
| Identity of the pest (Section 3.1 ) |
The identity of Pratylenchus loosi is clearly defined. The pathogen has been shown to produce consistent symptoms and to be transmissible. |
None |
| Absence/presence of the pest in the EU (Section 3.2 ) | The pest is not known to be present in the EU. | None |
| Pest potential for entry, establishment and spread in the EU (Section 3.4 ) | The pest could enter, establish and spread in the EU. Host plants for planting, soil adhering to plants, machinery and footwear are the main pathways. | None |
| Potential for consequences in the EU (Section 3.5 ) | Negative effects are expected on tea production in the EU. | There is uncertainty about potential impacts on hosts other than tea. |
| Available measures (Section 3.6 ) | Measures are available to reduce the risk of entry, establishment, spread and impacts of the pest in the EU. | None |
| Conclusion (Section 4 ) |
Pratylenchus loosi meets all the criteria that are within the remit of EFSA to assess this pest to be regarded as a potential Union quarantine pest. |
|
| Aspects of assessment to focus on/scenarios to address in future if appropriate | The potential host status of non‐tea crops grown in the EU could be further investigated. | |
ABBREVIATIONS
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
GLOSSARY
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 2022)
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 2022)
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2022)
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2022)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2022)
- Greenhouse
A walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment
- Hitchhiker
An organism sheltering or transported accidentally via inanimate pathways including with machinery, shipping containers and vehicles; such organisms are also known as contaminating pests or stowaways (Toy & Newfield, 2010)
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2022)
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2022)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2022)
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2022)
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2022)
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2023‐00347
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A. Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L. Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà
NOTE
The designations employed and the presentation of material on any maps included in this scientific output do not imply the expression of any opinion whatsoever on the part of the European Food Safety Authority concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
ACKNOWLEDGEMENTS
EFSA wishes to acknowledge the contribution to the literature review and climate suitability analysis of Giovanni Del Frari and Oresteia Sfyra (ISA experts).
APPENDIX A. Pratylenchus loosi host plants reported/species affected
A.1.
Source: CABI (2021) and literature.
| Host status | Host name | Plant family | Common name | ReferenceA |
|---|---|---|---|---|
| Cultivated hosts | Abelmoschus esculentus | Malvaceae | Okra | CABI (2021) |
| Acacia decurrens | Fabaceae | Green wattle | CABI (2021) | |
| Acacia pruinosa | Fabaceae | Frost wattle | Goodey et al. (1965) | |
| Albizia summatrana | Mimosaceae | Albizia | Goodey et al. (1965) | |
| Artemisia vulgaris | Asteraceae | Mugwort | CABI (2021) | |
| Brassica oleracea var. capitata | Brassicaceae | Cabbage | Waceke (2007); CABI (2021) | |
| Camellia sinensis | Theaceae | Tea | Mirghasemi et al. (2014) | |
| Cassia alata | Fabaceae | Ringworm senna | CABI (2021) | |
| Cassia didymobotrya | Fabaceae | Cassia | Goodey et al. (1965) | |
| Catharanthus roseus | Apocynaceae | Madagascar periwinkle | CABI (2021) | |
| Cestrum | Solanaceae | Jessamine | CABI (2021) | |
| Cinnamomum camphora | Lauraceae | Camphor laurel | CABI (2021) | |
| Citrus | Rutaceae | Divsalar et al. (2012); CABI (2021) | ||
| Convallaria majalis | Liliaceae | Lily of the valley | Goodey et al. (1965); CABI (2021) | |
| Crocus sativus | Iridaceae | Saffron | Mahdikhani and Alvani (2013), cited by CABI (2021) | |
| Cyperus | Cyperaceae | Flatsedge | CABI (2021) | |
| Cyperus rotundus | Cyperaceae | Purple nutsedge | CABI (2021) | |
| Desmodium motorium | Fabaceae | Telegraph plant | Goodey et al. (1965) | |
| Dioscorea | Dioscoreaceae | Yam | CABI (2021) | |
| Dioscorea rotundata | Dioscoreaceae | CABI (2021) | ||
| Diospyros kaki | Ebenaceae | Persimmon | CABI (2021) | |
| Dipteryx odorata | Fabaceae | Tonka bean | Azad and Seraji (2016) | |
| Fragaria ananassa | Rosaceae | Strawberry | Goodey et al. (1965); CABI (2021) | |
| Gossypium hirsutum | Malvaceae | Cotton | Gnanapragasham and Mohotti (2018) | |
| Grevillea robusta | Proteaceae | Silky oak | CABI (2021) | |
| Hibiscus rosa‐sinensis | Malvaceae | Chinese rose | CABI (2021); Gnanapragasham and Mohotti (2018) | |
| Malus domestica | Rosaceae | Apple | Gnanapragasham and Mohotti (2018); CABI (2021) | |
| Mangifera indica | Anacardiaceae | Mango | Gnanapragasham and Mohotti (2018); CABI (2021) | |
| Musa x paradisiaca | Musaceae | Plantain | CABI (2021) | |
| Musa sp. | Musaceae | Banana | Goodey et al. (1965) | |
| Pisum sativum | Fabaceae | Pea | CABI (2021) | |
| Poncirus trifoliata | Rutaceae | Trifoliate orange | Gotoh and Ohshima (1963); CABI (2021) | |
| Prunus avium | Rosaceae | Sweet cherry | CABI (2021) | |
| Pyrus communis | Rosaceae | European pear | Azad and Seraji (2016) | |
| Saccharum officinarum | Poaceae | Sugarcane | CABI (2021) | |
| Sesbania cannabina | Fabaceae | Corkwood tree | CABI (2021) | |
| Sesbania cinerascens | Fabaceae | Sesbania | Goodey et al. (1965); Gnanapragasham and Mohotti (2018) | |
| Solanum nigrum | Solanaceae | Black nightshade | CABI (2021) | |
| Solanum tuberosum | Solanaceae | Potato | Akgul et al. (2010), cited by CABI (2021) | |
| Sorghum bicolor | Poaceae | Sorghum | Gnanapragasham and Mohotti (2018); CABI (2021) | |
| Tagetes spp. | Asteraceae | Marigold | CABI (2021) | |
| Tecoma stans | Bignoniaceae | Yellow bells | CABI (2021) | |
| Tephrosia vogelii | Fabaceae | Hoary‐pea | Goodey et al. (1965); Gnanapragasham and Mohotti (2018); CABI (2021) | |
| Tithonia diversifolia | Asteraceae | Mexican sunflower | CABI (2021) | |
| Triticum aestivum | Poaceae | Wheat | Șahİn et al. (2009); CABI (2021) | |
| Vigna unguiculata | Fabaceae | Cowpea | Gnanapragasham and Mohotti (2018); CABI (2021) | |
| Vitis vinifera | Vitaceae | Grapevine | Gnanapragasham and Mohotti (2018) | |
| Zea mays | Poaceae | Maize | Goodey et al. (1965); CABI (2021) | |
| Wild weed hosts | Alternanthera sessilis | Amaranthaceae | Sessile joyweed | CABI (2021) |
| Cymbopogon citratus | Poaceae | Lemongrass | CABI (2021) | |
| Imperata cylindrica | Poaceae | Cogon grass | CABI (2021) | |
| Oplismenus compositus | Poaceae | Mirghasemi and Seraji (2010); CABI (2021) | ||
| Panicum repens | Poaceae | Torpedo grass | Gnanapragasham and Mohotti (2018); CABI (2021) | |
| Paspalum notatum | Poaceae | Bahia grass | Gnanapragasham and Mohotti (2018); CABI (2021) | |
| Artificial/experimental host |
APPENDIX B. Distribution of Pratylenchus loosi
B.1.
Distribution records based on CABI CPC (CABI, 2021) and literature.
| Region | Country | Sub‐national (e.g. state) | Status | References |
|---|---|---|---|---|
| North America | Guadeloupe | Present, no details | Van den Berg and Quénéhervé (2000); CABI (2021) | |
| United States | Present, no details | CABI (2021) | ||
| Florida | Present, no details | Inserra (1996), cited by CABI (2021) | ||
| Kansas | Present, no details | Handoo et al. (2008) | ||
| Louisiana | Present, no details | Waeyenberge et al. (2000) | ||
| South America | Brazil | Present, no details | Silva et al. (2008); CABI (2021) | |
| Amazonas | Present, no details | Silva et al. (2008) | ||
| Mato Grosso | Present, no details | CABI (2021) | ||
| Chile | Present, no details | Rubilar and Aballay (2006), cited by CABI (2021) | ||
| Africa | Kenya | Present, no details | Waceke (2007); CABI (2021) | |
| Senegal | Present, no details | Baujard et al. (1990); CABI (2021) | ||
| Asia | Bangladesh | Present, no details | CABI (2021) | |
| China | Present, no details | CABI (2021) | ||
| Shaanxi | Present, no details | Wang (1993), cited by CABI (2021) | ||
| Sichuan | Present, no details | Li (1985), cited by CABI (2021) | ||
| Tibet | Present, no details | Yu et al. (2017) | ||
| India | Present, no details | CABI (2021) | ||
| Delhi | Present, no details | Sethi and Swarup (1971), cited by CABI (2021) | ||
| Himachal Pradesh | Present, no details | Sethi and Swarup (1971), cited by CABI (2021) | ||
| Kerala | Present, no details | Reni Varghese and Mohandas (2004) | ||
| Rajasthan | Present, no details | Sethi and Swarup (1971), cited by CABI (2021) | ||
| Sikkim | Present, no details | Baqri (1991), cited by CABI (2021) | ||
| West Bengal | Present, no details | Mukherjee and Dasgupta (1982); CABI (2021) | ||
| Iran | Present, no details | Azad and Seraji (2016) | ||
| Japan | Present, no details | CABI (2021) | ||
| Honshu | Present, no details | Gotoh (1976), CABI (2021) | ||
| Kyushu | Present, no details | Gotoh (1976); CABI (2021) | ||
| Ryukyu Islands | Present, no details | Gotoh (1976); CABI (2021) | ||
| Shikoku | Present, no details | Gotoh (1976); CABI (2021) | ||
| South Korea | Present, no details | Park et al. (2002) | ||
| Sri Lanka | Present, no details | Hutchinson and Vythilingam (1963); CABI (2021) | ||
| Taiwan | Present, no details | CABI (2021) | ||
| Turkey | Present, no details | Kasapoğlu et al. (2014); CABI (2021) | ||
| Oceania | American Samoa | Present, no details | CABI (2021) | |
| Australia | Present, no details | CABI (2021) | ||
| New South Wales | Present, no details | CABI (2021) | ||
| Cook Islands | Present, no details | CABI (2021) |
APPENDIX C. EU and member state cultivation/harvested/production area of Pratylenchus loosi hosts (in 1000 ha/tonnes)
C.1.
Source: Eurostat accessed on 25/7/2023.
Citrus (1000 ha).
| 2017 | 2018 | 2019 | 2020 | 2021 | |
|---|---|---|---|---|---|
| Croatia | 2,1 | 2,0 | 2,2 | 2,1 | 2,1 |
| Cyprus | 2,9 | 3,1 | 3,2 | 3,0 | 3,0 |
| France | 4,3 | 4,4 | 4,6 | 4,7 | 3,2 |
| Greece | 43,5 | 46,3 | 44,2 | 45,6 | 40,5 |
| Italy | 135,4 | 134,6 | 140,7 | 145,1 | 144,7 |
| Malta | 0,0 | 0,0 | 0,0 | 0,0 | 0,1 |
| Portugal | 20,5 | 21,1 | 21,4 | 21,5 | 21,7 |
| Spain | 294,3 | 297,6 | 296,5 | 298,0 | 300,5 |
Banana (1000 tonnes).
| 2017 | 2018 | 2019 | 2020 | 2021 | |
|---|---|---|---|---|---|
| Greece | 1,6 | 2,3 | 1,5 | 1,4 | 1,4 |
| Spain | 423,1 | 387,9 | 399,7 | 422,7 | 409,1 |
| France | 162,1 | 173,6 | 197,6 | 184,4 | 203,0 |
| Cyprus | 3,1 | 3,8 | 2,3 | 2,6 | 2,6 |
| Portugal | 23,7 | 19,1 | 23,2 | 23,4 | 22,2 |
EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Baptista, P. , Chatzivassiliou, E. , Di Serio, F. , Gonthier, P. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Stefani, E. , Thulke, H.‐H. , Van der Werf, W. , Vicent Civera, A. , Yuen, J. , … Reignault, P. L. (2024). Pest categorisation of Pratylenchus loosi . EFSA Journal, 22(1), e8548. 10.2903/j.efsa.2024.8548
Adopted: 15 December 2023
Notes
An EPPO code, formerly known as a Bayer code, is a unique identifier linked to the name of a plant or plant pest important in agriculture and plant protection. Codes are based on genus and species names. However, if a scientific name is changed the EPPO code remains the same. This provides a harmonised system to facilitate the management of plant and pest names in computerised databases, as well as data exchange between IT systems (EPPO, 2019; Griessinger & Roy, 2015).
REFERENCES
- Akgul, H. C. , Bayram, S. , & Erdogus, F. D. (2010). Two new records of Turkish nematode fauna: Ditylenchus equalis and Pratylenchus pseudopratensis . Pakistan Journal of Nematology, 28(2), 285–293. [Google Scholar]
- Amarasena, P. G. , Mohotti, K. M. , & De Costa, D. M. (2016). Effects of changing rainfall and soil temperature on population density of Pratylenchus loosi in tea lands at different elevations. Tropical Agricultural Research, 27(3), 265–276. [Google Scholar]
- Amarasena, P. G. , Mohotti, K. M. , De Costa, D. M. , & Fosu‐Nyarko, J. (2020). Morphometric and molecular characterization of isolates of the root lesion nematode, Pratylenchus loosi infecting tea in Sri Lanka. Tropical Agricultural Research, 31(1), 57–71. [Google Scholar]
- Anonymous. (2023). Tea Grown in Europe Association ‐ 2023 focus. Tea Grown in Europe Association, 28 pp. https://tea‐grown‐in‐europe.eu/wp‐content/uploads/2023/06/EuT‐2023‐Leaflet‐digital‐version.pdf
- Azad, Y. , & Seraji, A. (2016). Shade tree Tonka (Dipteryx odorata) new host for Pratylenchus loosi in tea plantation. Iranian. Journal of Plant Pathology, 52(2), 295–296. (abstract). [Google Scholar]
- Baqri, Q. H. (1991). Contribution to the fauna of Sikkim. Nematodes associated with citrus from Sikkim, India. Records of the Zoological Survey of India, Nr 128, 103 pp.
- Baujard, P. , Mounport, D. , & Martiny, B. (1990). Scanning electron microscopy study of four species of the genus Pratylenchus Filip'ev 1936 (Nemata: Pratylenchidae). Revue de Nématologie, 13(2), 203–210. [Google Scholar]
- Brooks, F. E. (2004). Plant‐parasitic nematodes of banana in American Samoa. Nematropica, 34, 65–72. [Google Scholar]
- CABI . (2021). Pratylenchus loosi (root lesion nematode). CABI Crop Protection Compendium. 10.1079/cabicompendium.43898 [DOI]
- Castillo, P. , & Vovlas, N. (2007). Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, biology, pathogenicity and management. Nematology Monographs and Perspectives, 6, 529. [Google Scholar]
- Divsalar, N. , Jamali, S. , Pedramfar, H. , & Taheri, H. (2012). Root lesion nematodes (Pratylenchus spp.) on citrus in south‐west of Caspian Sea. Journal of Agricultural Technologies, 8(7), 2227–2238. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Baptista, P. , Chatzivassiliou, E. , Gonthier, P. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Stefani, E. , Thulke, H.‐H. , Van der Werf, W. , Civera, A. V. , Zappala, L. , … Yuen, J. (2022). Scientific Opinion on the commodity risk assessment of Malus domestica plants from Turkey. EFSA Journal, 20(5), 7301. 10.2903/j.efsa.2022.7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger, M. , Bragard, C. , Caffier, D. , Candresse, T. , Chatzivassiliou, E. , Dehnen‐Schmutz, K. , Gregoire, J.‐C. , Jaques Miret, J. A. , Mac Leod, A. , Navajas Navarro, M. , Niere, B. , Parnell, S. , Potting, R. , Rafoss, T. , Rossi, V. , Urek, G. , Van Bruggen, A. , Van Der Werf, W. , … Gilioli, G. (2018). Guidance on quantitative pest risk assessment. EFSA Journal, 16(8), 5350. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy, A. , Benford, D. , Halldorsson, T. , Jeger, M. J. , Knutsen, H. K. , More, S. , Naegeli, H. , Noteborn, H. , Ockleford, C. , Ricci, A. , Rychen, G. , Schlatter, J. R. , Silano, V. , Solecki, R. , Turck, D. , Benfenati, E. , Chaudhry, Q. M. , Craig, P. , … Younes, M. (2017). Scientific opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal, 15(8), 4971. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO . (2019). EPPO codes. https://www.eppo.int/RESOURCES/eppo_databases/eppo_codes
- EPPO (European and Mediterranean Plant Protection Organization) . (online). EPPO Global Database. https://gd.eppo.int
- FAO (Food and Agriculture Organization of the United Nations) . (2013). ISPM (international Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014‐04‐30_201405121523‐494.65%20KB.pdf
- FAO (Food and Agriculture Organization of the United Nations) . (2022). International Standards for Phytosanitary Measures. ISPM 5 Glossary of phytosanitary terms. FAO, Rome. https://www.fao.org/3/mc891e/mc891e.pdf
- Gnanapragasham, N. C. , & Mohotti, K. M. (2018). Nematode parasites of tea. In Sikora R. A., Coyne D., Hallmann J., & Timper P. (Eds.), Plant parasitic nematodes in subtropical and tropical agriculture (pp. 584–616). CAB International. [Google Scholar]
- Goodey, J. B. , Franklin, M. T. , & Hooper, D. J. (1965). The nematode parasites of plants catalogued under their hosts (Third ed.). Commonwealth Agricultural Bureaux. [Google Scholar]
- Gotoh, A. (1976). A review of the plant‐parasitic nematodes in warm and subtropical regions of Japan. In: Miscellaneous Bulletin, Kyushu Agricultural Experiment Station, 61 pp.
- Gotoh, A. , & Oshima, Y. (1963). Pratylenchus species and their geographical distribution in Japan (Nematoda: Tylenchida). Japanese Journal of Applied Entomology and Zoology, 7, 187–199. [Google Scholar]
- Griessinger, D. , & Roy, A.‐S. (2015). EPPO codes: a brief description. https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Haji, E. B. , Mohammadi, M. , Kheyri, A. , & Tanha, M. Z. (2007). A comparison of different populations of tea root lesion nematode, Pratylenchus loosi Loof, 1960, from Guilan province, Iran's tea gardens based on ITS rDNA and D2/D3 LSU‐rDNA genomic regions PCR‐RFLPs. Iranian Journal of Agricultural Sciences, 37(6), Pe965‐en962. [Google Scholar]
- Handoo, Z. A. , Carta, L. K. , & Skantar, A. M. (2008). Taxonomy, morphology and phylogenetics of coffee‐associated root‐lesion nematodes, Pratylenchus spp. In Souza R. M. (Ed.), Plant‐parasitic nematodes of coffee (pp. 29–50). Springer. [Google Scholar]
- Hardin, J. G. (2020). Where tea grows in Europe. https://www.killgreen.io/main/where‐tea‐grows‐europe
- Hutchinson, M .T. , & Vythilingam, M. K. (1963). The distribution of Pratylenchus loosi Loof among tea estates in Ceylon, with particular reference to altitude. Tea Quarterly, 34, 68–84. [Google Scholar]
- Inserra, R. (1996). Nematology Section. Triology Technical Report, 35(3), 10–11. [Google Scholar]
- Kasapoğlu, E. B. , Imren, M. , & Elekcioglu, İ. H. (2014). Plant parasitic nematode species found on important cultivated plants in Adana. Turkish Journal of Entomology, 38(3), 333–350. [Google Scholar]
- Katalan‐Gateva, S. D. , & Nedelchev, S. L. (1983). New species for the phytonematode fauna of Bulgaria. Acta Zoologica Bulgarica, 22, 76–81. [Google Scholar]
- Kitowski, I. , Łopucki, R. , Stachniuk, A. , & Fornal, E. (2020). A pesticide banned in the European Union over a decade ago is still present in raptors in Poland. Environmental Conservation, 47(4), 310–314. [Google Scholar]
- Li, D. Z. (1985). Description of some species of parasitic nematodes of the genus Pratylenchus on plant roots in Sichuan Province. Journal of Southwest Agricultural College, 2, 49–51. [Google Scholar]
- Mahdikhani, E. , & Alvani, S. (2013). Plant parasitic nematodes from rhizosphere of saffron (Crocus sativus L.) with two new records of Geocenamus squamatus and Filenchus pratensis from Iran. Pakistan Journal of Nematology, 31(2), 99–103. [Google Scholar]
- Mirghasemi, S. N. , Fanelli, E. , Troccoli, A. , Jamali, S. , Sohani, M. M. , & De Luca, F. (2019). Molecular variability of the root‐lesion nematode, Pratylenchus loosi (Nematoda: Pratylenchidae), from tea in Iran. European Journal of Plant Pathology, 155, 557–569. [Google Scholar]
- Mirghasemi, S. N. , Seraji, A. , Jamali, S. , & Choshali, A. H. (2014). Reported some new species of plant parasitic nematodes from rhizosphere of tea plantation in Iran. International Journal of Biosciences, 5(9), 37–46. [Google Scholar]
- Mirghasemi, T. , & Seraji, A. (2010). Basket grass (Oplismenus compositus) as a new host for tea root lesion nematode, Pratylenchus loosi . Iranian Journal of Plant Pathology, 46(1), 91–92. [Google Scholar]
- Mohotti, K. , Amarasena, D. , & Al Mamun, M. S. (2023). Nematode problems in tea and their sustainable management. In Rahman Khan M. & Quintanilla M. (Eds.), Nematode diseases of crops and their sustainable management (pp. 597–621). Academic Press. [Google Scholar]
- Mukherjee, B. , & Dasgupta, M. K. (1982). Community analyses of plant‐parasitic nematodes in tea plantations of West Bengal, India. Nematologia Mediterranea, 10, 1–7. [Google Scholar]
- Park, B. , Choi, D. , Lee, J. , Choi, Y. , & Shin, G. (2002). An unrecorded species of Pratylenchus loosi Loof (Tylenchida: Pratylenchidae) from tea in Korea. Korean Journal of Applied Entomology, 41(4), 299–303. [Google Scholar]
- Rubilar, P. , & Aballay, E. (2006). Morphometrics of two Pratylenchus species associated with sugar beet in the Eighth Region (Bio‐Bio), Chile. Fitopatología, 41(2), 81–85. [Google Scholar]
- Șahİn, E. , Nıcol, J. M. , Elekçioğlu, İ. H. , Yorgancılar, O. , Yıldırım, A. F. , Tülek, A. , Hekimhan, H. , et al. (2009). Frequency and diversity of cereal nematodes on the Central Anatolian Plateau of Turkey. In Cereal cyst nematodes: Status, research and outlook. Proceedings of the First Workshop of the International Cereal Cyst Nematode Initiative (pp. 100–105). International Maize and Wheat Improvement Centre (CIMMYT). [Google Scholar]
- Sayers, E. W. , Cavanaugh, M. , Clark, K. , Ostell, J. , Pruitt, K. D. , & Karsch‐Mizrachi, I. (2020). Genbank. Nucleic Acids Research, 48(Database issue), D84–D86. 10.1093/nar/gkz956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seraji, A. , Porjam, E. , Tanha Maafi, Z. , & Safaie, N. (2006). Biology and population dynamics of tea root lesion nematode (Pratylenchus loosi) in Iran. Iranian Journal of Plant Pathology, 43, 42–43. [Google Scholar]
- Sethi, C. L. , & Swarup, G. (1971). Plant parasitic nematodes of North‐Western India. III. The Genus Pratylenchus. Indian Phytopathology, 24(2), 410–412. [Google Scholar]
- Silva, R. A. , Oliveira, C. M. , & Inomoto, M. M. (2008). Fauna de fitonematóides em áreas preservadas e cultivadas da floresta amazônica no Estado de Mato Grosso. Tropical Plant Pathology, 33, 204–211. [Google Scholar]
- Sivapalan, P. (1971). The effects of infection by Pratylenchus loosi, and of fumigation, on the growth of young tea plants in different soil types. Tea Quarterly, 42(3), 131–137. [Google Scholar]
- Sivapalan, P. , Gnanapragasam, N. C. , & Jebamalai, A. S. (1980). Chemical control of the root‐lesion nematode, Pratylenchus loosi, in new tea plantings. Tea Quarterly, 49(2), 30–36. [Google Scholar]
- Toy, S. J. , & Newfield, M. J. (2010). The accidental introduction of invasive animals as hitchhikers through inanimate pathways: A New Zealand perspective. Revue Scientifique et Technique (International Office of Epizootics), 29(1), 123–133. [DOI] [PubMed] [Google Scholar]
- Uehara, T. , Mizukubo, T. , Kushida, A. , & Momota, Y. (1998). Identification of Pratylenchus coffeae and P. loosi using specific primers for PCR amplification of ribosomal DNA. Nematologica, 44(4), 357–368. [Google Scholar]
- Ushiyama, K. , & Ogaki, C. (1970). Studies on the replant problem in Unshiu orange orchards. II.(I) The effects of citrus root nematodes, Tylenchulus semi‐pénétrons, and root lesion nematodes, Pratylenchus loosi, on citrus trees and fruits.(II) Effects of grass fallow and fumigation with nematicides on the growth of trees replanted in old citrus soils. Bulletin of the Horticultural Branch, Kanagawa Agricultural Experiment Station, 18, 46–56. [Google Scholar]
- Van Den Berg, E. , & Quénéhervé, P. (2000). Hirschmanniella caribbeana sp. n. and new records of Pratylenchus spp. (Pratylenchidae: Nematoda) from Guadeloupe, French West Indies. Nematology, 2(2), 179–190. [Google Scholar]
- Varghese, R. , & Mohandas, C. (2004). Pratylenchus loosi, a new record on Dioscorea rotundata . Indian Journal of Nematology, 34(2), 221–222. [Google Scholar]
- Waceke, J. W. (2007). Plant parasitic nematodes associated with cabbages in Kenya. African Crop Science Conference Proceedings, 8, 1071–1074. [Google Scholar]
- Waeyenberge, L. , Ryss, A. , Moens, M. , Pinochet, J. , & Vrain, T. C. (2000). Molecular characterisation of 18 Pratylenchus species using rDNA restriction fragment length polymorphism. Nematology, 2, 135–142. [Google Scholar]
- Wang, R. X. (1993). The identification of nematodes on fruit trees in Shaanxi Province. Acta Agriculturae Boreali‐Occidentalia Sinica, 2, 81–86. [Google Scholar]
- Yu, J. , Jin, X. , Qin, M. , Wu, W. , Xu, C. , & Xie, H. (2017). Identification and description of three species of genus Pratylenchus from crops in Tibet. Journal of Huazhong Agricultural University, 36, 20–24. [Google Scholar]


