Abstract
Background and Objectives
Distal myopathies are a heterogeneous group of primary muscle disorders with recessive or dominant inheritance. ADSSL1 is a muscle-specific adenylosuccinate synthase isoform involved in adenine nucleotide synthesis. Recessive pathogenic variants in the ADSSL1 gene located in chromosome 14q32.33 cause a distal myopathy phenotype. In this study, we present the clinical and genetic attributes of 6 Indian patients with this myopathy.
Methods
This was a retrospective study describing on Indian patients with genetically confirmed ADSSL1 myopathy. Details were obtained from the medical records.
Results
All patients presented in their first or early second decade. All had onset in the first decade with a mean age at presentation being 17.7 ± 8.4 years (range: 3–27 years) and M:F ratio being 1:2. The mean disease duration was 9.3 ± 5.2 years ranging from 2 to 15 years. All patients were ambulant with wheelchair bound state in 1 patient due to respiratory involvement. The median serum creatine kinase (CK) level was 185.5 IU/L (range: 123–1564 IU/L). In addition to salient features of ptosis, cardiac involvement, bulbar weakness, and proximo-distal limb weakness with fatigue, there were significant seasonal fluctuations and decremental response to repetitive nerve stimulation, which have not been previously reported. Muscle histopathology was heterogenous with the presence of rimmed vacuoles, nemaline rods, intracellular lipid droplets along with chronic myopathic changes. Subtle response to pyridostigmine treatment was reported. While 5 of 6 patients had homozygous c.781G>A (p.Asp261Asn) variation, 1 had homozygous c.794G>A (p.Gly265Glu) in ADSSL1 gene.
Discussion
This study expands the phenotypic spectrum and variability of ADSSL1 myopathy with unusual manifestations in this rare disorder. Because the variant c.781G>A (p.Asp261Asn) is the most common mutation among Indian patients similar to other Asian cohorts, this finding could be useful for genetic screening of suspected patients.
Introduction
Distal myopathies are a heterogenous group of inherited primary muscle diseases, which are variously classified based on the onset, clinical features, and muscle histopathology. With the advent of next-generation sequencing, there has been an expansion of data on causative/pathogenic variants causing distal myopathies. The common autosomal recessive distal myopathies include GNE, dysferlinopathies (DYSF), nebulin (NEB), anoctamin 5 (ANO5), and ADSSL1 myopathies. Pathogenic variants in ADSSL1 gene leading to deficiency of adenylosuccinate synthetase–like 1 muscle-specific isozyme causes a type of distal myopathy.1 It catalyzes the conversion of inosine monophosphate to adenylosuccinate, which is the initial step in adenine nucleotide synthesis.2 The functions of ADSSL1 are the processes involved in energy transfer, synthesis of nucleic acids, and regulation of metabolism. The previously reported clinical phenotype of ADSSL1 myopathy includes adolescent-onset distal leg weakness with mild facial weakness, mild creatine kinase (CK) elevation,3 and proximo-distal nemaline myopathy.4 This report describes Indian patients with ADSSL1 myopathy and their characteristic clinicopathologic features.
Methods
This retrospective descriptive study was conducted at a quaternary hospital for neurologic disorders in India on patients with genetically diagnosed ADSSL1 myopathy. The patients were evaluated between 2016 and 2022. The baseline demographic details, laboratory findings including muscle MRI, histopathology details were collected from the medical records and analyzed. Genetic testing was performed in patients and available family members as follows: PI-1, PI-2, patients and parents underwent clinical exome sequencing (CES) at Children's Hospital, Los Angeles; P-II and parents—whole-exome sequencing (WES) was performed by the genomics platform at the Broad Institute of MIT and Harvard, Cambridge. The exome data were then processed at the Centro Nacional de Análisis Genómico, Barcelona, Spain, and variant prioritization was conducted on the RD-Connect GPAP (platform.rd-connect.eu)5; P-III underwent WES at Stanford Health care, California; P-IV CES underwent WES at Sandor diagnostics, India; and P-V and parents underwent WES at Institute of Human genetics, Hamburg, Germany. ADSSL1 variants reported were classified as per ACMG guidelines and annotated based on muscle-specific Ref seq transcript NM_152328.4 Informed consent was obtained from all patients, and ethical approval was obtained from the Institution ethics committee. Permission was obtained for unmasking of faces in the photographs.
Data Availability
Anonymized data can be made available for qualified investigators on request to the corresponding author. Except for P-II, other patients came with genetic results obtained from other centers.
Results
Clinical Features
We included 6 patients from 5 families. All patients had onset of symptoms in the first decade with a mean age at presentation of 17.7 ± 8.4 years (range: 3–27 years). The M:F ratio was 1:2. All had onset of symptoms in early childhood with slow walking and difficulty in running. The major clinical features are given in eFigure 1 (links.lww.com/NXG/A669). Weakness of distal lower limbs (LL) with frequent tripping was noted in 3 patients, proximal upper limb (UL) weakness in 3, and significant limb fatigability in 4, among whom 1 had prominent seasonal fluctuations (worsening of limb weakness during summer) (P-II). Two patients had dysphagia with chewing difficulty. Exertion-induced palpitations were seen in 3 (P I-2, P-III, and P-V). Patient PV had significant dyspnea and orthopnea requiring nocturnal noninvasive ventilation from the age of 15 years. All patients were born of nonconsanguineous parentage except P-II. There was no significant family history among other patients. The mean disease duration was 9.3 ± 5.2 years ranging from 2 to 15 years. All patients were ambulant with wheelchair bound state in 1 patient (PV) due to respiratory involvement. Examination showed all with thin slender habitus and eyelid ptosis in 3 patients with divergent squint in 1. Prominent facial weakness was seen in 3. Other features such as distal joint laxity and atrophic scar were seen in 1 patient—PIII (Figure 1). The muscle power was scored as per modified Medical Research Council (MRC) grading: neck flexion was grade 3 (n = 3), proximal UL—3 to 4 (n = 3), and ankle dorsiflexion—3 to 4 (n = 4). One patient showed differential proximal LL weakness with predominant involvement of hip flexors and adductors (PII). All patients had hypoactive tendon reflexes with waddling gait. The major clinical possibilities considered in these patients were congenital myopathy, mitochondrial myopathy, or congenital myasthenic syndromes due to prominent limb fatigue with seasonal fluctuations and presence of ptosis (Table).
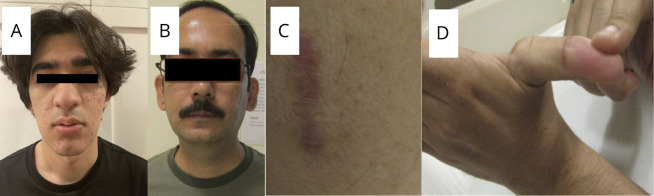
Figure 1. Clinical Images of Patients.
(A) Clinical image of patient I-2 showing elongated myopathic facies. (B) Clinical image of patient III showing absent nasolabial folds. (C) Atrophic scar of skin of patient III. (D) Distal laxity of joints of patient III.
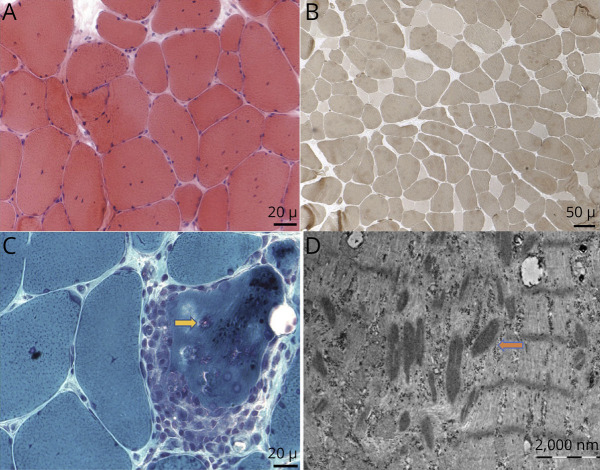
Table.
Clinical and Laboratory Features of Patients With ADSSL1 Myopathy
| Characteristics | Families/patients | |||||
| I-1 | I-2 | II | III | IV | V | |
| Sex | F | M | F | M | F | F |
| Age at onset (y) | 7 | 10 | 13 | 17 | 1 | 13 |
| Age at presentation (y) | 19 | 15 | 24 | 27 | 3 | 18 |
| Presenting symptoms | LL proximal weakness | LL proximal weakness | LL proximal weakness and tripping | LL proximal weakness and tripping | LL proximal weakness | LL proximal weakness and tripping |
| Limb fatigue | + | + | + | − | − | + |
| Ptosis | − | + | + (along with divergent squint) | + | − | − |
| Dysphagia/chewing difficulty | − | + | + | − | − | − |
| Facial weakness | − | − | + | + | − | + |
| Muscle power (MRC) | ||||||
| Proximal UL | 5 | 5 | 3 | 4 | 5 | 4 |
| Distal UL | 5 | 5 | 5 | 5 | 5 | 5 |
| Proximal LL | 4 | 4 | 1 | 4 | 4 | 2 |
| Distal LL | 4 | 4 | 3 | 4 | 5 | 4 |
| Tendon reflexes | Hypoactive | Hypoactive | Hypoactive | Hypoactive | Hypoactive | Hypoactive |
| Serum CK (U/L) (normal: 20–200) | 150 | 123 | 129 | 1,564 | 932 | 221 |
| RNS | Positive | Positive | Negative | Negative | Not done | Negative |
| MRI muscle | Not done | Fatty infiltration of gluteus maximus and gastrocsoleus | Not done | Fatty infiltration of gluteus maximus and semimembranosus | Normal | Not done |
| Muscle biopsy | Not done | Not done | Intracellular lipid vacuoles | Rimmed vacuoles with nemaline rods | Not done | CFTD, intracellular lipid vacuoles, ragged red fibres |
| Pathogenic variants ADSSL1 gene | c.781G>A (p.Asp261Asn) | c.781G>A (p.Asp261Asn) | c.794G>A (p.Gly265Glu) | c.781G>A (p.Asp261Asn) | c.781G>A (p.Asp261Asn) | c.781G>A (p.Asp261Asn) |
Abbreviations: CFTD = congenital fiber-type disproportion; CK = creatine kinase; F = female; LL = lower limb; M = male; MRC = Medical Research Council; RNS = repetitive nerve stimulation; UL = upper limb.
Laboratory Findings
The median serum CK level was 185.5 IU/L (range: 123–1,564 IU/L– normal to 7 times elevated). Nerve conduction studies and EMG revealed normal findings except PIII who showed myopathic pattern with fibrillations and positive sharp waves. Repetitive nerve stimulation was performed in 5 patients of whom 2 siblings in the first family showed significant decremental response of 15%–20% in trapezius and facial muscles. Electrocardiography and 2D echocardiography were normal in all except evidence of sinus tachycardia with mild QTc prolongation and biventricular dysfunctioning in P-III. MRI of muscles was performed in 3 patients (P I-2, P-III, and P-IV). Two patients showed fatty infiltration of gluteus maximus (PI-2, P-III), semimembranosus (P-III), and gastrocsoleus (PI-2), while 1 patient had normal findings (PIV) because of young age with minimal symptoms. Muscle biopsy was performed in 3 patients (P-II, P-III, and P-V). P-II showed prominent fiber size variation with positive Oil red-O stained intracellular lipid vacuoles. P-III displayed multiple internalized nuclei, splitting, focal inflammation, few fibers, rimmed vacuoles, and nemaline rods in electron microscopy along with fiber size variation and type 2 fiber predominance (Figure 2). The inflammation observed was focal, and the significance of this in the clinical and pathologic context is uncertain because further screening was not possible owing to inability in retrieving and loss of sample. P-V showed features of congenital fiber-type disproportion, intracellular lipid inclusions, ragged red fibers, and cytochrome c oxidase (COX)–deficient fibers. The ragged red fibers and cytochrome oxidase deficiency were seen in only a few scattered fibers (approximately 5%). P-V also had reduction in respiratory chain enzymes I-IV on mitochondrial assays.
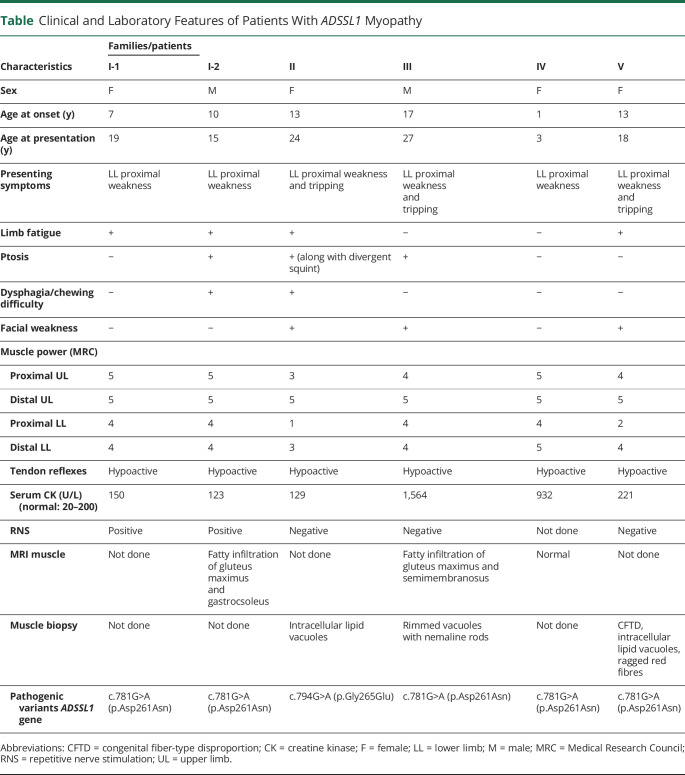
Figure 2. Muscle Histopathology of Patient-III.
(A) Hematoxylin and eosin—fiber size variability, internally placed nuclei, and fiber splitting (×400). (B) ATPase pH 9.4—type 2 predominance (×200). (C) Modified Gomori trichrome—inflammatory foci and rimmed vacuoles (yellow arrow) (×400). (D) Electron microscopy—varying degrees of disorganization of myofibrils with loss of their normal striation pattern and nemaline rods (X 37,500) (orange arrow).
Genetic analysis revealed that patients I-1, I-2, III, IV, and V had a homozygous missense ADSSL1 variant c.781G>A (p.Asp261Asn). While P-II had a homozygous missense variant c.794G>A (p.Gly265Glu). Both p.Asp261Asn ad p.Gly265Glu have been previously reported in patients with ADSSL1 myopathy in Korea and Japan3,4,6 and have been classified as pathogenic according to ACMG classification. Patients I-1 I-2, II, and V were given a trial of tablet pyridostigmine with the dose of 30 mg twice a day in patients I-1 and I-2 and 60 mg twice a day in P-II and P-V, and they showed subtle but definite response.
Discussion
ADSSL1 myopathy was first reported among Korean patients as a rare form of distal myopathy.6 ADSSL1 gene resides in the chromosome 14q32.33 and has a strong expression in skeletal muscles.2 Pathogenic variants in ADSSL1 results in disordered myocyte metabolism and apoptosis. All our patients had symptom onset in early childhood and were slower than their peers in motor abilities, which was followed by predominant proximal LL weakness in the first decade of life. This is in contrast to previous studies from Korean cohorts where the initial symptom was distal weakness progressing to proximal muscles.6 However, a subsequent report describing 2 patients, one each from Turkey and India, has expanded the phenotypic spectrum to include proximal myopathy with joint contractures.7 A study done in 2020, reported a cohort of 63 Japanese patients with recessive ADSSL1 pathogenic variants presenting with features of nemaline myopathy with proximodistal phenotype and concluded that ADSSL1 myopathy is the most frequent form of nemaline myopathy in Japan.4 Our cohort further reiterates that proximodistal pattern of weakness is most common in ADSSL1 myopathy. Both Korean and Japanese patients were also slow runners since early childhood similar to the present cohort. We also report a patient who presented at 3 years of age, which is the youngest patient reported till now. In vivo studies in zebra fish have shown that ADSSL1 pathogenic variants result in congenital myoseptal defects, which can explain very early disease onset in these patients.3 Another distinctive feature noted in our patients was prominent fatigability with seasonal fluctuations and ptosis with squint. This led to the initial consideration of congenital myasthenic syndrome. Moreover, the siblings from family-1 (patients I-1 and I-2) showed significant decremental response on repetitive nerve stimulation. Two of these patients (P I-1 and P-II) also showed partial but definite response to pyridostigmine, with improvement in fatigability of limbs. Though earlier reports from Japanese cohort4 have shown nonspecific fatigue, ocular involvement and electrophysiologic evidence of neuromuscular junction impairment have not been reported. While fatigability in ADSSL1 myopathy has been attributed to the defective purine synthesis and reduced ATP synthesis, the exact mechanism of NMJ dysfunction is unclear.4 This in turn results in secondary changes in myofibrils and mitochondria, which are seen as myofibrillar disarray and lipid droplets in histopathology.8 Thus, the partial response with oral pyridostigmine noted in 4 patients needs further dedicated trials. Dysphagia with chewing difficulty was noted only in 1 patient. However, 38% of Japanese cohort showed dysphagia with difficulty in mastication.4,6 Prominent cardiac symptoms with exertion-induced palpitations were seen in 3 patients with one of them showing ECG changes with QTc prolongation. Though the Japanese cohort4 have shown ventricular hypertrophy, rhythm disturbances have not been previously reported in ADSSL1 myopathy. Respiratory involvement in ADSSL1 myopathy is very rare. Most of the previously reported patients had subclinical restrictive pattern in pulmonary function tests.4,7 However, one of our patients has shown moderate restrictive pattern requiring nocturnal ventilatory support. CK was mildly elevated in 3 of our patients and ranged from 2 to 7 times above normal limit (range: 123–1,564 U/L). In the Japanese cohort, the serum CK level ranged 20–2006 U/L and showed inverse relation with age. However, this study did not show such relation between age and CK levels because congenital myopathies can have normal to mildly elevated CK values. Muscle MRI showed predominant involvement of semimembranosus and leg muscles. This is in contrast to the Korean and Japanese cohorts who showed predominant vastus lateralis, adductor group, and gastrocsoleus involvement.4,6 However, MRI muscle of an Indian patient reported by Mroczek et al., showed involvement of both anterior and posterior thigh muscles.7 Thus, involvement of posterior thigh muscles may be a unique finding in the Indian cohort. Muscle MRI in nemaline myopathy due to ACTA1 mutation shows predominant involvement of sartorius and adductor magnus in thigh and tibialis anterior/posterior and peronei in legs. Whereas in nemaline myopathy due to NEB mutation, vastus intermedius and adductor magnus in thigh and tibialis anterior and gastrocsoleus in legs are involved showing overall sparing of posterior thigh muscles.9
Muscle biopsy findings were heterogenous. Similar to previous reports in both Korean and Japanese cohorts, patients with muscle biopsy (PII, PIII, and PV) showed chronic myopathic changes with type 1 fiber predominance.3,4 The presence of focal inflammatory infiltrate in PIII were also reported in an Indian patient by Mroczek et al.7 Intracellular lipid inclusions noted in patients PII and PV were also reported in all patients of Japanese cohort.4 The proportion of fibers with nemaline rods and lipid inclusions noted in ADSSL1 myopathy is significantly less when compared with genetically confirmed nemaline myopathy and lipid storage myopathy with causative genes, respectively.4,10,11 In our cohort, 1 patient (PIII) was found to have few nemaline rods, while lipid inclusions were a more common finding in other 2 patients biopsied. Nemaline rod formation in ADSSL1 myopathy (defective adenine nucleotide biosynthesis) may be due to a different mechanism because defects in thin filament formation result in nemaline rods in other nemaline myopathies.12 In addition, presence of ragged red fibers with few COX-deficient fibers were not previously reported and may indicate secondary mitochondrial dysfunction.
The most common pathogenic variant c.781G>A (p.Asp261Asn) identified in our patients is also previously reported as the most frequent ADSSL1 myopathy–associated mutation from Korea and Japan.4,6 Only 1 patient (PII) had c.794G>A (p.Gly265Glu) pathogenic variant. It was also noted that all patients with recurrent p.Asp261Asn mutation were born of nonconsanguineous parentage. In contrast to homozygous mutations noted in all 4 patients, only 9.5% of Japanese cohort4 and none of Korean cohort5 had homozygous mutations. While a possibility of founder haplotype previously suggested due to shared ancestry among Asian populations cannot be ruled out, we hypothesize that it is likely a recurrent mutation due to the presence of CpG island (chr14:104741244-104741847and chr14:104741117-104741243) as per Weizmann Evolutionary CpG Island database (Evo CpG track, UCSC). This is further supported by the observation that p.Asp261Asn has been reported in gnomAD database among African, East Asian, and South Asian populations (8 heterozygotes and no homozygotes), suggesting a more wider geographical prevalence.4,6,13 However, a detailed haplotype analysis was not performed in the families, and we consider this as a limitation of the study. The c.794G>A (p.Gly265Glu) variant present as homozygous in our PII has also been previously reported in a Japanese patient as compound heterozygous with another frameshift pathogenic variant.4 Both p.Asp261Asn and p.Gly265Glu are located in highly conserved sites of adenylsuccinate synthetase domain structurally adjacent to critical IGF2 mRNA-binding proteins binding residues.4
This report describes the phenotypic presentations of Indian patients with ADSSL1 myopathy who manifested with ptosis, proximodistal weakness, prominent limb fatigability with seasonal fluctuations, and cardiac rhythm abnormalities. This further expands the phenotypic spectrum and geographical prevalence of ADSSL1 myopathy beyond East Asia. The high frequency of c.781G>A (p.Asp261Asn) in our Indian patients similar to Korean and Japanese patients suggests that p.Asp261Asn is probably the most common mutation associated with ADSSL1 myopathy and can be useful for genetic screening of suspected patients. While ADSSL1 myopathy currently appears to be limited to Asian populations, further studies might be necessary to determine the global prevalence.
Acknowledgment
The authors thank all the families for agreeing to be part of this article.
Glossary
- CES
clinical exome sequencing
- CK
creatine kinase
- LL
lower limb
- UL
upper limb
- WES
whole-exome sequencing
Appendix. Authors
| Name | Location | Contribution |
| Dipti Baskar, MD | Department of Neurology, National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Kiran Polavarapu, PhD | Children's Hospital of Eastern Ontario Research Institute, Ottawa, Canada | Analysis or interpretation of data |
| Veeramani Preethish-Kumar, PhD | Department of Neurology, Swansea University, Wales, United Kingdom | Analysis or interpretation of data |
| Seena Vengalil, MD | Department of Neurology, National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India | Major role in the acquisition of data; analysis or interpretation of data |
| Saraswati Nashi, MD | Department of Neurology, National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India | Analysis or interpretation of data |
| Ana Töpf, PhD | John Walton Muscular Dystrophy Research Centre, Translational and Clinical Research Institute, Newcastle University and Newcastle Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK | Analysis or interpretation of data |
| Aneesha Thomas, MD | Department of Neurology, National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India | Major role in the acquisition of data |
| Sai Bhargava Sanka, MBBS | Department of Neurology, National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India | Major role in the acquisition of data |
| Deepak Menon, MD | Department of Neurology, National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India | Analysis or interpretation of data |
| Kosha Srivastava, MBBS | Department of Neurology, National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India | Major role in the acquisition of data |
| Gautham Arunachal, MD | Department of Human Genetics, National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India | Analysis or interpretation of data |
| Bevinahalli N. Nandeesh, MD | Department of Neuropathology, National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India | Analysis or interpretation of data |
| Hanns Lochmüller, MD, FAAN | Children's Hospital of Eastern Ontario Research Institute; Brain and Mind Research Institute, University of Ottawa; Division of Neurology, Department of Medicine, The Ottawa Hospital, Canada; Centro Nacional de Análisis Genómico (CNAG-CRG), Center for Genomic Regulation, Barcelona Institute of Science and Technology (BIST), Catalonia, Spain; 7. Department of Neuropediatrics and Muscle Disorders, Medical Center–University of Freiburg, Faculty of Medicine, Germany | Study concept or design; analysis or interpretation of data |
| Atchayaram Nalini, MD, PhD | Department of Neurology, National Institute of Mental Health and Neuro Sciences (NIMHANS), Bengaluru, India | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
Footnotes
Editorial, page e200126
Study Funding
The authors report no targeted funding.
Disclosure
HL receives support from the Canadian Institutes of Health Research (Foundation Grant FDN-167281), the Canadian Institutes of Health Research and Muscular Dystrophy Canada (Network Catalyst Grant for NMD4C), the Canada Foundation for Innovation (CFI-JELF 38412), and the Canada Research Chairs program (Canada Research Chair in Neuromuscular Genomics and Health, 950-232279). AT has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No. 779257 (Solve-RD). All other authors report no disclosures relevant to the manuscript. Go to Neurology.org/NG for full disclosures.
References
- 1.Lipps G, Krauss G. Adenylosuccinate synthase from Saccharomyces cerevisiae: homologous overexpression, purification and characterization of the recombinant protein. Biochem J. 1999;341(Pt 3):537-543. [PMC free article] [PubMed] [Google Scholar]
- 2.Sun H, Li N, Wang X, et al. Molecular cloning and characterization of a novel muscle adenylosuccinate synthetase, ADSSL1, from human bone marrow stromal cells. Mol Cell Biochem. 2005;269(1-2):85-94. doi: 10.1007/s11010-005-2539-9 [DOI] [PubMed] [Google Scholar]
- 3.Park HJ, Hong YB, Choi YC, et al. ADSSL1 mutation relevant to autosomal recessive adolescent onset distal myopathy. Ann Neurol. 2016;79(2):231-243. doi: 10.1002/ana.24550 [DOI] [PubMed] [Google Scholar]
- 4.Saito Y, Nishikawa A, Iida A, et al. ADSSL1 myopathy is the most common nemaline myopathy in Japan with variable clinical features. Neurology. 2020;95(11):e1500-e1511. doi: 10.1212/WNL.0000000000010237 [DOI] [PubMed] [Google Scholar]
- 5.Laurie S, Piscia D, Matalonga L, et al. The RD-Connect Genome-Phenome Analysis Platform: accelerating diagnosis, research, and gene discovery for rare diseases. Hum Mutat. 2022;43(6):717-733. doi: 10.1002/humu.24353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HJ, Shin HY, Kim S, et al. Distal myopathy with ADSSL1 mutations in Korean patients. Neuromuscul Disord. 2017;27(5):465-472. doi: 10.1016/j.nmd.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 7.Mroczek M, Durmus H, Bijarnia-Mahay S, et al. Expanding the disease phenotype of ADSSL1-associated myopathy in non-Korean patients. Neuromuscul Disord. 2020;30(4):310-314. doi: 10.1016/j.nmd.2020.02.006 [DOI] [PubMed] [Google Scholar]
- 8.Operti MG, Vincent MF, Brucher JM, van den Berghe G. Enzymes of the purine nucleotide cycle in muscle of patients with exercise intolerance. Muscle Nerve. 1998;21(3):401-403. doi: [DOI] [PubMed] [Google Scholar]
- 9.Quijano-Roy S, Carlier RY, Fischer D. Muscle imaging in congenital myopathies. Semin Pediatr Neurol. 2011;18(4):221-229. doi: 10.1016/j.spen.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Sanoudou D, Beggs AH. Clinical and genetic heterogeneity in nemaline myopathy--a disease of skeletal muscle thin filaments. Trends Mol Med. 2001;7(8):362-368. doi: 10.1016/s1471-4914(01)02089-5 [DOI] [PubMed] [Google Scholar]
- 11.Liang WC, Nishino I. State of the art in muscle lipid diseases. Acta Myol. 2010;29(2):351-356. [PMC free article] [PubMed] [Google Scholar]
- 12.Clarkson E, Costa CF, Machesky LM. Congenital myopathies: diseases of the actin cytoskeleton. J Pathol. 2004;204(4):407-417. doi: 10.1002/path.1648 [DOI] [PubMed] [Google Scholar]
- 13.Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434-443. doi: 10.1038/s41586-020-2308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data can be made available for qualified investigators on request to the corresponding author. Except for P-II, other patients came with genetic results obtained from other centers.