One sentence summary:
Specialized epithelium restrains gut Candida albicans pathogenicity via an antimicrobial peptide.
Intestinal epithelial cells (IECs) line the inner surface of the intestinal tract and play crucial roles in digestion, absorption, and defense. By interacting with gut microbiota, IECs are essential for maintaining gut homeostasis and regulating immune responses. In this issue, Pierre et al. (1) report an antimicrobial function of the peptide YY (PYY1-36), which is secreted by Paneth cells that are embedded in the intestinal epithelium instead of the typical producer, enteroendocrine cells (EECs), which produce a smaller PYY peptide that functions as a hormone. PYY1-36 exhibits antifungal activity in the gastrointestinal (GI) tract of mice but does not affect other mucosal barriers. These findings demonstrate that intestinal peptides with hormonal function can adopt antimicrobial properties through spatial-specific secretion and diversified proteolytic cleavage.
Through functional specialization, IECs possess various regulatory functions to maintain intestinal homeostasis. Goblet cells produce the protective mucus layer and EECs secrete several hormones into the circulation in response to stimuli in the GI tract. Paneth cells sense microbes (2) and produce antimicrobial peptides (AMPs), such as defensins, cathelicidins, and lysozyme to directly kill or inhibit a wide range of intestinal microbes (3); intraepithelial lymphocytes and basolaterally positioned immune cells form a multi-layered defense system.
Dysfunctional autophagy-related 16-like 1 (ATG16L1), encoded by a risk allele of Crohn’s disease (a form of inflammatory bowel disease), led to impaired autophagy, altered granule morphology in exocytosis, and reduced production of AMPs in mice (4). Deletion of X-box binding protein-1 (XBP1), a transcription factor of the unfolded protein response, in Paneth cells is activated byled to endoplasmic reticulum (ER) stress and caused increased cell death in intestinal crypts of mice. ATG16L1 deficiency together with XBP1 impairment further result in severe spontaneous Crohn’s-disease-like ileitis in mice (5). Therefore, malfunctioning Paneth cells result in an increased susceptibility to intestinal pathologies, primarily due to the impairment of packaging and secretion of AMPs caused by ATG16L1 deficiency (4, 6).
It is increasingly being recognized that imbalance (dysbiosis) of fungal microbiota is a risk factor in the pathophysiology of mucosal surfaces and has been associated with inflammatory bowel disease. One prominent opportunistic fungus, Candida albicans, damages macrophages and intestinal epithelium (7, 8), which aggravates intestinal inflammation via a hyphae-produced toxin called candidalysin in a strain-dependent manner (7). Therefore, several mechanisms exist to limit C. albicans pathogenicity in the GI tract. Secretory immunoglobulin A (sIgA), which is secreted into the intestinal lumen, mediates regulation of C. albicans hyphal growth and shapes the gut mycobiota during homeostasis (9, 10), but this regulation is lost in Crohn’s disease (9). In addition, secreted mucin O-glycans (which form the mucus layer) suppress C. albicans hyphal morphogenesis by inducing the expression of NRG1 (11), a transcriptional repressor of filamentation. AMPs such as LL-37 and β-defensin 3 promote resistance to C. albicans colonization in the non-inflamed gut or the oral mucosa (12, 13).
Pierre et al. made the surprising observation that Paneth cells in both mouse and human small intestinal epithelium produce the peptide PYY1-36. The shorter version, PYY3-36, generated after N-terminal proteolysis of PYY1-36, is an appetite control hormone produced by EECs, so the presence of PYY1-36 in Paneth cells was unexpected. PYY1-36 exhibited broad antimicrobial activity against various Candida species and less pronounced activity against diverse bacterial species (see the figure). Consistent with previous findings that highlight the yeast-to-hyphae transition as a key virulence process in C. albicans (7–11), the authors found that PYY1-36 mitigates fungal pathogenesis by influencing hyphal formation. Further investigation revealed that PYY1-36 induces concentration-dependent membrane permeabilization and disruption of hyphal growth, with less impact on yeast morphotypes. Cationic PYY1-36 interacts with the anionic surface charge of fungal hyphae, further elucidating its mechanism of action.
Figure.
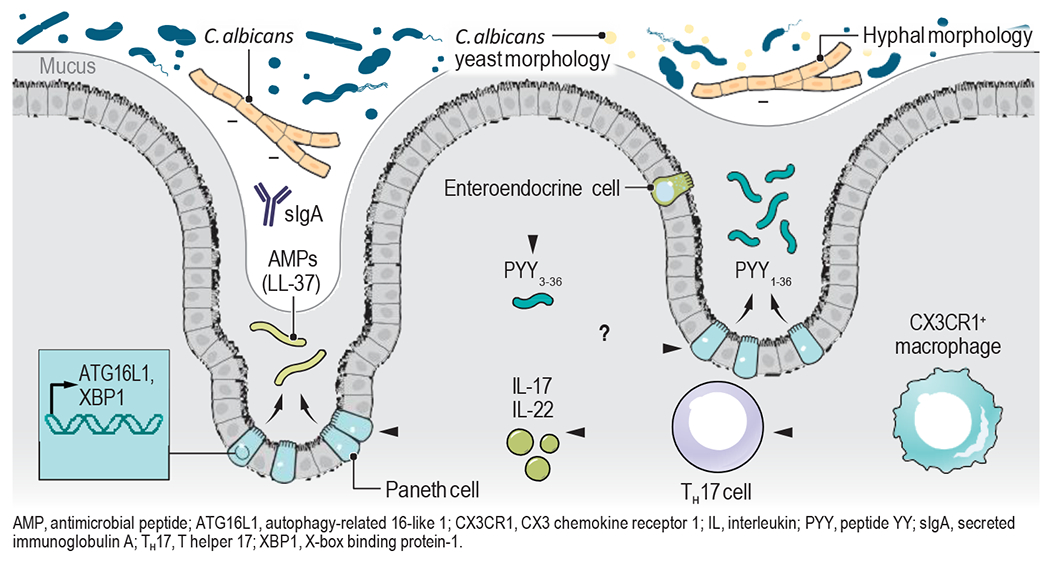
Mechanisms of gut mycobiota regulation. LL-37, an antimicrobial peptide (AMP), restrains Candida colonization in the presence of commensal Bacteroidetes. Mucin O-glycans secreted by goblet cells (GCs) suppress Candida hyphal morphogenesis by upregulating the expression of a major transcriptional repressor of filamentation. CX3CR1+CD64+ macrophages and Type 17 immunity play a role in host protection against intestinal fungi and their products. sIgA-mediated regulation of C. albicans hyphal growth shapes the gut mycobiota. Th17 cells promote AMP production at the mucosal barriers. PCs secretory function is dependent on ATG16L1 and XBP1-dpendent pathways. While peptide YY (PYY3-36) after proteolysis has been primarily characterized as a gut hormone produced by enteroendocrine cells (EECs) controlling appetite, Pierre et al. discovered a novel antimicrobial function of full-length PYY1-36, which is secreted by Paneth cells (PCs). This peptide exhibits selective antifungal activity against C. albicans hyphal morphotypes in the gut.
To investigate the release of luminal PYY1-36 in response to C. albicans hyphae, Pierre et al. conducted experiments using ex vivo mouse distal ileal loops exposed to C. albicans. They discovered that PYY1-36 is retained in the mucus layer owing to its charge affinity, protecting it from enzymatic cleavage into PYY3-36. In mice with prominent C. albicans gut colonization due to antibiotic treatment, administration of PYY1-36 reduced C. albicans burden. Analysis of the mycobiota in the mucus-associated layer of the gut revealed increased C. albicans abundance in PYY-deficient mice compared to wild-type mice, highlighting the role of PYY1-36 in modulating the gut fungal community. Overall, this study reveals the antimicrobial properties of PYY1-36 against C. albicans hyphae and provides valuable insights into how IECs regulate the gut mycobiota.
Several outstanding questions remain. IEC XBP1 and ER stress promote sIgA secretion that prevents pathogen access to the epithelial surface of the GI tract (14). How molecular mechanisms, such as ATG16L1-dependent autophagy and XBP1-regulated unfolded protein response, mediate Paneth cell secretory functions and might affect the secretion of PYY1-36 remains to be determined. Interleukin-17 (IL-17) is critical for antifungal mucosal immunity and induction of AMPs (13). In the gut, C. albicans colonization promotes T helper 17 (TH17) immune responses through the interaction with CX3C chemokine receptor 1 (CX3CR1)-expressing mononuclear phagocytes (MNPs), which express several antifungal receptors in a SYK-dependent manner (15). Whether PYY1-36 secretion is induced directly by C. albicans or indirectly via CX3CR1+ MNPs and IL-17 remains unknown.
Although Pierre et al. demonstrate the in vitro inhibition of C. albicans hyphal formation, it remains unclear whether PYY1-36 directly affects the yeast-to-hyphae program or whether this is a function of C. albicans impaired growth upon exposure to the peptide. Furthermore, because bacteria do not produce hyphae, it is unclear how PYY1-36 exerts antibacterial effects. Investigation into the cellular targets of PYY1-36 in fungi and bacteria could help elucidate differential efficacy. Given the observation by Pierre et al. of bacterial composition alterations in PYY-deficient mice, the indirect suppression of hyphal morphogenesis through inter-kingdom interactions could also contribute to the PYY1-36 mode of action. Nonetheless, understanding the evolution that led to the production of such antifungal molecules adds to our understanding of the intricate host-microbe interactions at the mucosal surfaces of the GI tract.
ACKNOWLEDGMENTS
The authors were supported by NIH grants (DK113136, DK121977, AI163007), BWF PATH and CIFAR awards.
REFERENCES AND NOTES
- 1.Pierre JF et al. , Science 381, 502 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaishnava S et al. , Proc. Natl. Acad. Sci 105, 20858 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salzman NH et al. , Nat. Immunol 11, 76 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadwell K et al. , Nature 456, 259 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adolph TE et al. , Nature 503, 272 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stappenbeck TS et al. , Gastroenterology 152, 322 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X et al. , Nature 603, 672 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasper L et al. , Nat. Commun 9, 4260 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doron I et al. , Nat. Microbiol 6, 1493 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ost KS et al. , Nature 596, 114 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takagi J et al. , Nat. Chem. Biol 18, 762 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan D et al. , Nat. Med 21, 808 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conti HR et al. , Cell Host Microbe 20, 606 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grootjans J et al. , Science 363, 993 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonardi I et al. , Science 10.1126/science.aao1503 (2018). [DOI] [Google Scholar]


