Introduction
The pursuit of optimal health and longevity is a long-standing human aspiration [1, 2]. Advances in public health policies and population medicine have significantly extended life expectancy, which has tripled from 20–30 years a century and a half ago. However, this has also led to an increase in age-related diseases. As we age, stem cells, the foundation of every organ and tissue in our body, lose their regenerative capacity, leading to tissue decline and age-related diseases. Environmental conditions and disease treatments can also negatively impact the quality of life and health span [3].
With an aging society (e.g., China’s population of people aged over 60 years is predicted to reach 28 % by 2040), there is a growing need for interventions to manage aging as a risk factor for chronic diseases. Recent developments in the biomarkers of aging can help identify and evaluate health span and longevity interventions [4], potentially reshaping the regulatory landscape for stem cell therapy, especially in China [2]. Stem cells offer the potential to regenerate or repair damaged tissues and organs, providing therapeutics for various degenerative [5], autoimmune, hematological [6], and neurodegenerative diseases, as well as organ failures and other chronic disorders. The US Food and Drug Administration (FDA) has recently approved a clinical trial to test patient-derived induced pluripotent stem cells (iPSCs) in patients with Parkinson’s disease.
A three-phase approach to stem cell research and therapy may involve first targeting the translational aspects (“research to target” or R2T), then moving to bedside translation to patients for clinical efficiency (“bench to bedside” or B2B; Phase 1–3 clinical trials for clinical science and knowledge), and finally clinical practice education and rational quantitative assessment of treatment for clinical effects (“treat to target” or T2T; Phase 3–4 clinical trials and beyond), This approach aims to promote the remission of disease and the ability to quantitatively increase health-/lifespan and/or longevity, measured with differentiation-insensitive but authentic aging-related epigenetic clocks, and to provide optional recommendations or potential prescriptions and formulations. Ultimately, we can move gerontology from the bench to clinical care and health policies [7].
Stem cell medicine: updated research highlights
We may honor and love all of our elderly and children with the same care that we give to our own parents and children (“老吾老以及人之老, 幼吾幼以及人之幼”) [2]. China’s population health strategy, central to the Healthy China 2030 agenda, includes improving the health of the elderly and children. Chinese scientists are investigating reproductive health, with this special issue highlighting human stem cell models of neuropsychiatric disorders, stem cell-derived embryo models, and stem cell models of non-human primates for the development of treatments for reproductive issues, neurodegenerative diseases, and diabetes. Continued advancements in stem cells and their derivatives, such as exosomes and organoids, will lead to a deeper understanding of the mechanisms of disease, the discovery of effective drugs, novel therapeutic strategies, and improved patient outcomes [8–10].
Chemical reprogramming offers a unique opportunity to control the fate of somatic cells and generate the desired cell types, including pluripotent stem cells for potential medical applications [11]. Human somatic cells chemically reprogrammed by activating a regeneration-like program may provide an alternative way of producing stem cells for clinical use. Understanding the epigenetic determinants of stemness is crucial for fully realizing the potential of cells reprogrammed in vitro. Landmark achievements include growing whole models of human embryos from stem cells without sperm or eggs [12], and hybrid embryos of humans and animals. China’s research force and its scale of funding are catching up with the United States, but China offers more significant resources of patient data for medical research and innovation [13].
However, a rush to the clinic is cautioned against, as ethical issues surrounding cancer immunotherapy and CRISPR/CAS9 genome editing have impacted cell therapy. Chinese State Food and Drug Administration (SFDA) may sometimes adapt policies from the FDA, focusing on data first and renovating their own policies to suit Chinese patients and medical research. Comparing the effects of existing treatments and no treatment is crucial, with digital toolkits and large databases of Chinese patient cohorts enabling better precision medicine. Combining Chinese resources, biolab-robots, and artificial intelligence (AI) offers advantages in this field, as mentioned by the CEO of In Silico Medicine.
Many diseases result from systematic failures [14, 15] and the aging of multiple organs rather than one [3]. The key switches and master regulators are hubs for stem cell medicine [16], with early warning signals of the diseases’ tipping points detecting imminent critical transitions [17].
The eight functional “R”s of “R2T” stem cell medicine
The eight functional “R”s of “R2T” stem cell medicine are self-Renewal, Reprogramming, Regeneration, Rejuvenation, Repair, Reversal, Rehabilitation, and Remodeling of animal use. To achieve the optimal human health span, thriving research programs on stem cell medicine targeting landmark clinical questions and longevity are essential. Some R2T examples are the following:
Ethical concerns surrounding embryonic stem cells led Professor Shinya Yamanaka, a Nobel Prize laureate, to focus on alternative sources of stem cells, such as iPSCs [18].
Organ-on-a-chip (organ chip) research targets the failure of animal models to predict therapeutic responses in humans. Organ chips are microfluidic devices lined with living cells cultured under a fluid flow that can recapitulate organ-level physiology and pathophysiology with high fidelity. Single and multiple human organ chip systems have been used to model complex diseases and inter-organ physiology, and to reproduce human clinical responses to drugs [9]. Like organ chips, organoid technology also aims to replace animal models [8].
Key issues, opportunities and advances
Biosafety concerns in stem cell therapy
Despite rapid progress, stem cell therapy is surrounded by ethical issues and risks of tumorigenesis [14–16, 18]. Stem-cell-derived exosomes may provide similar clinical benefits without the biosafety concerns associated with living cell transplants. However, challenges such as large-scale manufacturing, purification, batch-to-batch variation, and complex cargo analysis need to be addressed for clinical applications [10].
Large-scale manufacturing and purification of stem cells
Stem cells offer the potential and bring demands for reversing various diseases and conditions. Progress is being made towards the clinically meaningful expansion of human hematopoietic stem cells (HSCs) to supply the large demand for cells required by certain clinical applications such as gene therapy/editing and cord blood (CB) transplantation [19].
Organoids and organ chips
There is still a gap in the field of organoids and/or organ chips for their application in the quantitative study of aging and longevity [8, 9]. Hopefully soon, the holistic characteristics of humanoids may be successfully applied in patient settings and/or longevity settings to test Traditional Chinese Medicine, which claims to benefit many centennial villagers, (e.g., Yao medicine in Hezhou, known as the City of Longevity).
Alternative sources
Alternative sources such as iPSCs present challenges related to genetic and epigenetic stability. We also need to overcome the challenges of making mini-humanoids or “all-organs” chips so that the use of human all-organ chips for drug development, artificial organ-transplantation and personalized medicine will be thus closer to realization.
The epi/genetics, autophagy, and longevity
Research on epi/genetics, such as studies of Mi-2/NuRD [20], mTOR as the master regulator of autophagy(a mechanism for which Dr. Yoshinori Ohsumi was awarded 2016 Nobel Prize in Physiology or Medicine) [21], telomere [22] and beyond, aims to delay the aging process and prevent age-related diseases [1, 20, 23, 24]. Vitamin D receptor (Daf-12) as an evolutionarily conserved upstream master regulator for both Mi-2/LET-418 and mTOR/LET-363, acts as a “capacitor” like Hsp90 (chaperone-mediated autophagy) [25]. Detecting degeneration during the early phases of age-related diseases could potentially stop or delay them [17], including cancer. Potentially rejuvenating aged stem cells [26] through combining the use of chemical molecules, such as rapalogs, metformin, senolytics, nicotinamide riboside (NR), nicotinamide mononucleotide (NMN), histone deacetylase (HDAC) inhibitors and exogenous stem cells [10] or stem cell derivatives [8] and the like [9], could maximize the positive effects of each individual component while minimizing the side effects of drugs with the potential of “hit-and-run” style.
Stem cell, microbiota, and longevity
A distinct gut microbiome ecology is associated with longevity and has been implicated in the prevention of aging-related diseases [27]. Microbiota and their metabolites influence the fate and survival of intestinal stem cells. Fecal microbiota transplantation from young mice rejuvenates aged hematopoietic stem cells by suppressing inflammation [28]. Research on the gut microbiota in intestinal and hematopoietic stem cells aging may provide insights into therapeutic strategies for aging-related disorders [29]. Inflammaging diminishes adaptive immunity [26] and chronic inflammation is responsible for chronic diseases across the lifespan.
New regulatory horizons in stem cell medicine in China
China’s 2022 R&D budget was RMB 3000 billion. To promote translational and clinical research, cooperation by the industry, hospitals, and academia aims to improve stem cell medicine in basic, translational, and clinical practice. Embracing a suitable free market and innovation is essential in facing new challenges, creating new training programs, and fostering frontiers in medical research. The SFDA may creatively develop its own plans, directions, guidelines, standards, and policies. Moreover, there is probably extensive room for stem cell medicine and longevity research and policies to develop in China. For example, one step is that Hengrui Pharmaceutics HRS-1893 investigational new drug (IND) has been recently approved as the first IND using preclinical data based on Avatar organ-chips.
Finally, in addition to data-driven biomarkers [2], stem cell medicine and other methods could target the quantitative health-span and longevity after interventions, for example, based on the abovementioned approaches and epidemiologic surveys. A simplified formula for prolonging lifespan includes daily sun exposure for half an hour for Vitamin D (life span extension: 5–10 years), regular exercise for half an hour daily with a companion (lifespan extension: 5–10 years), restricting the diet to two-thirds with sufficient vegetables and fruits daily (life span extension: 5–10 years), hobby with sleeping for 7 hours while abstaining from liquor and tobacco (life span extension: 7–10 years), regular standard physical examination and stem cell therapy (still pending for years, urgent for its effects-quantification because of the distinct qualities and quantities), and attention to preventing injuries. Therefore, it should be feasible for most people to live another “extra” 10 years by 2040, which may be in China’s next new 5-year plan. Hypothetically, it should not be a challenge to reach 101.
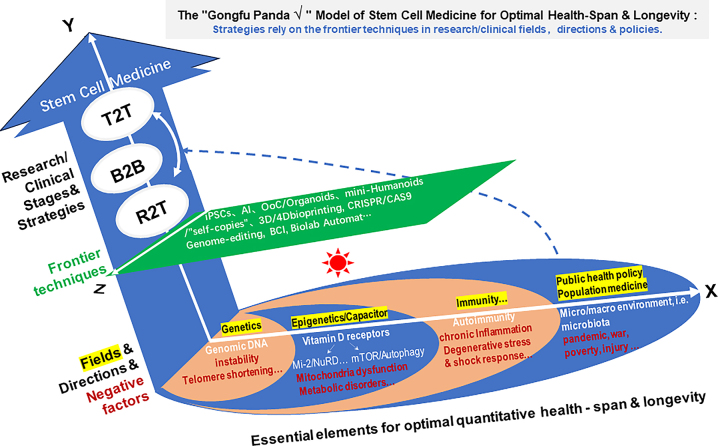
Indeed, stem cell medicine is a wonderful field for accelerating optimal health-span by pushing the boundaries of knowledge and collaborating across disciplines to fully realize the potential of stem cell-based therapies and beyond (Figure 1). However, like the Red Queen in Alice’s Looking-Glass Wonderland, we can say: “My dear, here we must run as fast as we can, just to stay in one place. And if you wish to go somewhere else, you must run twice as fast”.
Figure 1:
A simplified “√” model illustrating the role of 8R/T2T stem cell medicine and cutting-edge technology in promoting optimal health span and longevity. Tier 1: Genomic DNA (DNA repair): Key genetic elements [30] (e.g., instability, telomere shortening), highlighted in red as a key example of negative factors. Tier 2: Epigenetics [31] and capacitors: Examples include the involvement of Mi-2/NuRD in genetics and epigenetics; mTOR [32] as a master regulator of autophagy and metabolism (mitochondrial dysfunction, metabolic disorders, and other negative factors); and the Vitamin D receptor as an evolutionarily conserved upstream master regulator for both Mi-2 and mTOR, acting as a “capacitor” after sun exposure for Vitamin D. Tier 3: Immunity and autoimmunity: Chronic inflammation and degenerative responses to stress or shock are among the major contributors to age-related diseases and suboptimal health. Tier 4: Public health policy and population medicine [33]. The importance of a good regulatory system, natural micro-/macro-environments, and other elements (negative factors include injury, war, poverty, pandemics such as COVID-19). Y-axis: The interaction of R2T, B2B, and T2T strategies promotes the advancement of 8R stem cell medicine. The “treat to target” (T2T) approach has been used in various fields of medicine, including traditional Chinese medicine (TCM) for pre-disease stages, but relies heavily on expert experience and prescriptions. The “bench to bedside” (B2B) paradigm of translational medicine aims to transform clinical therapeutic models and advance clinical science and knowledge. In integrative medicine, the T2T strategy tailors treatments based on specific targets within diseased cells to improve the patients’ outcomes. More recently, the focus has shifted to exploring specific targets and pathways at a spatiotemporal multi-omics system level within diseased cells and pre-disease niches for addressing key clinical questions for “Reserach to target” (R2T). The current T2T approach includes Phase 3–4 clinical trials, medical education, and clinical practice to assess the clinical impacts and the healthcare-related decisions, with the goal of more effectively targeting disease, pre-disease, and suboptimal health. Z-axis: Powerful toolkits such as induced pluripotent stem cells (iPSCs), artificial intelligence (AI), CRISPR/CAS9 genome editing, organs-on-a-chip (OoC)/organoids, brain–computer interfacing (BCI), biolab automation and potential future derivatives such as mini-humanoids/“self-copies” accelerate optimal health span and longevity alongside 8R/T2T stem cell medicine [34, 35].
Acknowledgement
The authors are indebted to Dr. Tongju Li, Dr. Kunyan He, and Dr. Wuwei Wu for insightful discussions.
Footnotes
Research funding: The study was supported by grants from the National Natural Science Foundation of China (No. 81771748), the Shenzhen Science and Technology Project (No. JCYJ20180504170414637), Futian Healthcare Research Project (No. FTWS2021005) and the Sanming Project of Medicine in Shenzhen (No. SZSM201602087) to Yue Zhang.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Informed consent: Not applicable.
Ethical approval: The local Institutional Review Board deemed the study exempt from review.
Contributor Information
Yue Zhang, Email: humanoids101@163.com.
Cibo Huang, Email: huangcibo1208@139.com.
References
- 1.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 2.Ren J, Song M, Zhang W, Cai JP, Cao F, Cao Z, et al. The Aging Biomarker Consortium represents a new era for aging research in China. Nat Med. 2023;29:2162–5. doi: 10.1038/s41591-023-02444-y. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186:243–78. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Moqri M, Herzog C, Poganik JR, Justice J, Biomarkers of Aging Consortium, Belsky DW, et al. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell. 2023;186:3758–75. doi: 10.1016/j.cell.2023.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Z, Zhang Z, Li Z, Huang C, Zhang Y. Toward wiping out osteoarthritis in China: research highlights. Chin Med J. 2020;133:883–5. doi: 10.1097/cm9.0000000000000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Fang J, Liu B, Shao C, Shi Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell. 2022;29:1515–30. doi: 10.1016/j.stem.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Sierra F, Caspi A, Fortinsky RH, Haynes L, Lithgow GJ, Moffitt TE, et al. Moving geroscience from the bench to clinical care and health policy. J Am Geriatr Soc. 2021;69:2455–63. doi: 10.1111/jgs.17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendriks D, Brouwers JF, Hamer K, Geurts MH, Luciana L, Massalini S, et al. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis. Nat Biotechnol. 2023;41:1567–81. doi: 10.1038/s41587-023-01680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingber DE. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet. 2022;23:467–91. doi: 10.1038/s41576-022-00466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K, Cheng K. Stem cell-derived exosome versus stem cell therapy. Nat Rev Bioeng. 2023:1–2. doi: 10.1038/s44222-023-00064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Sun S, Deng H. Chemical reprogramming for cell fate manipulation: methods, applications, and perspectives. Cell Stem Cell. 2023;30:1130–47. doi: 10.1016/j.stem.2023.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Oldak B, Wildschutz E, Bondarenko V, Comar MY, Zhao C, Aguilera-Castrejon A, et al. Complete human day 14 post-implantation embryo models from naive ES cells. Nature. 2023 doi: 10.1038/s41586-023-06604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan W, Sipp D, Wang Z, Deng H, Pei D, Zhou Q, et al. Stem cell science on the rise in China. Cell Stem Cell. 2012;10:12–5. doi: 10.1016/j.stem.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Bar-Yam Y, Harmon D, de Bivort B. Systems biology. Attractors and democratic dynamics. Science. 2009;323:1016–7. doi: 10.1126/science.1163225. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Moriguchi H. Chromatin remodeling system, cancer stem-like attractors, and cellular reprogramming. Cell Mol Life Sci. 2011;68:3557–71. doi: 10.1007/s00018-011-0808-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y. Cancer embryonic stem cell-like attractors alongside deficiency of regulatory restraints of cell-division and cell-cycle. J Genet Syndr Gene Ther. 2013;4:1000130 [Google Scholar]
- 17.Li L, Xu Y, Yan L, Li X, Li F, Liu Z, et al. Dynamic network biomarker factors orchestrate cell-fate determination at tipping points during hESC differentiation. Innovation. 2023;4:100364. doi: 10.1016/j.xinn.2022.100364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamanaka S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 2020;27:523–31. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai M, Ishitsuka K, Ito R, Wilkinson AC, Kimura T, Mizutani E, et al. Chemically defined cytokine-free expansion of human haematopoietic stem cells. Nature. 2023;615:127–33. doi: 10.1038/s41586-023-05739-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y. Biology of the mi-2/NuRD complex in SLAC (stemness, longevity/ageing, and cancer) Gene Regul Syst Biol. 2011;5:1–26. doi: 10.4137/grsb.s6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amano H, Chaudhury A, Rodriguez-Aguayo C, Lu L, Akhanov V, Catic A, et al. Telomere dysfunction induces sirtuin repression that drives telomere-dependent disease. Cell Metabol. 2019;29:1274–90. doi: 10.1016/j.cmet.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Vasheghani F, Li YH, Blati M, Simeone K, Fahmi H, et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann Rheum Dis. 2015;74:1432–40. doi: 10.1136/annrheumdis-2013-204599. [DOI] [PubMed] [Google Scholar]
- 24.Sharp Z, Strong R. Rapamycin, the only drug that has been consistently demonstrated to increase mammalian longevity. An update. Exp Gerontol. 2023;176:112166. doi: 10.1016/j.exger.2023.112166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hochbaum D, Zhang Y, Stuckenholz C, Labhart P, Alexiadis V, Martin R, et al. DAF-12 regulates a connected network of genes to ensure robust developmental decisions. PLoS Genet. 2011;7:e1002179. doi: 10.1371/journal.pgen.1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girotra M, Chiang Y, Charmoy M, Ginefra P, Hope H, Bataclan C, et al. Induction of mitochondrial recycling reverts age-associated decline of the hematopoietic and immune systems. Nat Aging. 2023;3:1057–66. doi: 10.1038/s43587-023-00473-3. [DOI] [PubMed] [Google Scholar]
- 27.Johansen J, Atarashi K, Arai Y, Hirose N, Sorensen SJ, Vatanen T, et al. Centenarians have a diverse gut virome with the potential to modulate metabolism and promote healthy lifespan. Nat Microbiol. 2023;8:1064–78. doi: 10.1038/s41564-023-01370-6. [DOI] [PubMed] [Google Scholar]
- 28.Zeng X, Li X, Li X, Wei C, Shi C, Hu K, et al. Fecal microbiota transplantation from young mice rejuvenates aged hematopoietic stem cells by suppressing inflammation. Blood. 2023;141:1691–707. doi: 10.1182/blood.2022017514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad Sophien AN, Jusop AS, Tye GJ, Tan YF, Wan Kamarul Zaman WS, Nordin F. Intestinal stem cells and gut microbiota therapeutics: hype or hope? Front Med. 2023;10:1195374. doi: 10.3389/fmed.2023.1195374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Xie W, Li N, Li W, Zhang Z, Fan N, et al. Generation of a humanized mesonephros in pigs from induced pluripotent stem cells via embryo complementation. Cell Stem Cell. 2023;30:1235–45.e6. doi: 10.1016/j.stem.2023.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y. From “Old” Cloning to “Young” Cellular Reprogramming: Nobel Prize 2012 Spotlighted the Stem Cell Work. Clon Transgen. 2012;01:1–3. doi: 10.4172/2168-9849.1000e101. [DOI] [Google Scholar]
- 32.Zhulyn O, Rosenblatt H, Shokat L, Dai S, Kuzuoglu-Öztürk D, Zhang Z, et al. Evolutionarily divergent mTOR remodels translatome for tissue regeneration. Nature. 2023;620:163–71. doi: 10.1038/s41586-023-06365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, Chen S, Shan G, Leng Z, Bärnighausen T, Yang W. Strengthening population medicine to promote public health. Chin Med J (Engl) 2022;135:1135–37. doi: 10.1097/CM9.0000000000002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao Q, Chen W, Yu Y, Gao F, Zhou J, Wu J, et al. Human Placental Mesenchymal Stem Cells Relieve Primary Sclerosing Cholangitis via Upregulation of TGR5 in Mdr2(-/-) Mice and Human Intrahepatic Cholangiocyte Organoid Models. Research (Wash D C) . 2023;6:0207. doi: 10.34133/research.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma W, Zhang X, Liu Y, Fan L, Gan J, Liu W, et al. Polydopamine Decorated Microneedles with Fe-MSC-Derived Nanovesicles Encapsulation for Wound Healing. Advanced science (Weinheim, Baden-Wurttemberg, Germany) . 2022;9:e2103317. doi: 10.1002/advs.202103317. [DOI] [PMC free article] [PubMed] [Google Scholar]