Abstract
Nontypeable Haemophilus influenzae is a common cause of human disease and initiates infection by colonizing the upper respiratory tract. In previous work we identified high-molecular-weight adhesins referred to as HMW1 and HMW2, expressed by nontypeable strain 12, and determined that most strains of nontypeable H. influenzae express one or two antigenically related proteins. More recently, we determined that some strains lack HMW1- and HMW2-like proteins and instead express an adhesin called Hia. In the present study, we determined the prevalence and distribution of the hmw and hia genes in a collection of 59 nontypeable strains previously characterized in terms of genetic relatedness. Based on Southern analysis, 47 strains contained sequences homologous to the hmw1 and hmw2 genes and nine strains contained homologs to hia. No strain harbored both hmw and hia, and three strains harbored neither. Although the hmw and hia genes failed to define distinct genetic divisions, the hmw-deficient strains formed small clusters or lineages within the larger population structure. Additional analysis established that the IS1016 insertion element was uniformly absent from strains containing hmw sequences but was present in two-thirds of the hmw-deficient strains. As IS1016 is associated with the capsule locus (cap) in most encapsulated strains of H. influenzae, we speculate that hmw-deficient nontypeable strains evolved more recently from an encapsulated ancestor.
Haemophilus influenzae is a gram-negative bacterium that commonly inhabits the human upper respiratory tract. Isolates of H. influenzae are subdivided into encapsulated and nonencapsulated forms (11). Encapsulated strains express one of six structurally and antigenically distinct capsular polysaccharides, designated serotypes a to f (11). Nonencapsulated strains are defined on the basis of their failure to agglutinate with typing antisera against the known H. influenzae capsular structures and are referred to as nontypeable (11).
Analysis by multilocus enzyme electrophoresis indicates that encapsulated strains are clonal and can be segregated into genetically related clusters, which are grouped into two major phylogenetic divisions (9, 10). Division I includes clusters of serotypes a, b, c, d, and e strains, and division II includes clusters of serotypes a, b, and f strains. All encapsulated strains of H. influenzae have genes necessary for encapsulation (cap genes). These genes are organized into functionally distinct regions, with serotype-specific DNA flanked by DNA common to all serotypes (7). In division I strains, the cap gene cluster lies between direct repeats of an insertion element referred to as IS1016 (5). Division II strains also contain one or more copies of IS1016, but in these strains IS1016 is not associated with cap genes (5).
The population structure of nontypeable H. influenzae is less well defined. Musser et al. examined 65 epidemiologically distinct isolates by multilocus enzyme electrophoresis and found considerable heterogeneity, with each isolate corresponding to a unique electromorph type (8). Furthermore, comparison with 177 type b isolates revealed no overlap, suggesting that nontypeable strains are not simply phenotypic variants of common type b strains (8). More recently we studied a series of 123 pharyngeal isolates of nontypeable H. influenzae collected from healthy 3-year-old Finnish children (19). Among these isolates, one was a capsule-deficient type b strain. Interestingly, of the remaining 122 isolates, 31% hybridized with a probe containing the type b cap locus. These findings suggested that a subgroup of nontypeable strains might be more recent descendents of an encapsulated ancestor.
In previous work directed at understanding the mechanism by which strains of nontypeable H. influenzae colonize the respiratory tract, we identified two high-molecular-weight adhesins referred to as HMW1 and HMW2 (2, 18). Both of these proteins are expressed by nontypeable H. influenzae 12 and show significant amino acid sequence similarity to one another (2). Preliminary studies suggested that most nontypeable strains express one or two proteins that are antigenically related to HMW1 and HMW2. Further analysis revealed that strains lacking HMW1- and HMW2-like proteins remain capable of efficient attachment to cultured epithelial cells (3). Consistent with this observation, we recently identified another nontypeable H. influenzae high-molecular-weight adhesin referred to as Hia (3). Based on Southern hybridization studies, encapsulated strains of H. influenzae lack hmw1- and hmw2-like genes but uniformly possess an allelic variant of hia (16). In serotype b strains, the counterpart to hia is associated with expression of short, thin surface appendages referred to as fibrils and is called hsf (13, 16).
In the present study, we sought to determine the prevalence of the hmw and hia genes in a collection of nontypeable H. influenzae strains. In addition, we addressed the hypothesis that strains can be separated into genetically distinct divisions according to the adhesin they possess. Finally, we examined the possibility that strains lacking hmw genes are more likely to contain cap-specific sequences or IS1016 elements and might be more recent derivatives of an encapsulated line.
In performing this study, we exploited the collection of nontypeable H. influenzae strains previously characterized by Musser et al. and defined in terms of genetic relatedness (8). Four biotype 4 strains originally isolated from the blood of newborn infants or women with obstetrical infections were excluded because DNA hybridization studies indicate that they represent a separate Haemophilus species (12), a fifth strain was excluded because it is now known to express a serotype e capsule (14), and a sixth strain was missing from the collection. The remaining 59 strains included in our analysis were recovered between 1937 and 1985 during episodes of otitis media, bacteremia, or sinusitis in children throughout the United States and Canada (8). Control strains included nontypeable H. influenzae 12 and 11 and H. influenzae type b strain Eagan. Strain 12 is the strain from which the hmw1 and hmw2 genes were originally cloned (2), and strain 11 is the strain from which hia was originally cloned (3). Strain Eagan contains an allelic variant of hia (referred to as hsf) and an intact type b cap locus (13, 15).
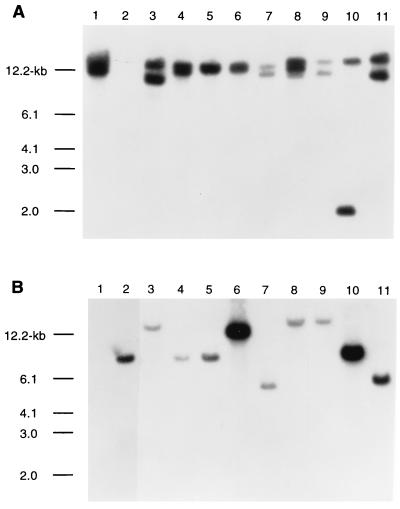
In initial experiments, we examined the 59 strains by Southern analysis, probing with a 3.2-kb SpeI-EcoRI fragment that corresponds to the promoter and 5′ coding sequence of the H. influenzae 12 HMW1 structural gene (hmw1A). Hybridization conditions were as previously described (17). As summarized in Fig. 1, 47 strains demonstrated specific high-molecular-weight bands of hybridization. In some cases, only a single band was discernible, while in others two bands were clearly present. Of note, in strain 12 the chromosomal fragments harboring hmw1A and hmw2A comigrated, giving the impression of a single band of hybridization. Figure 2A depicts a representative sampling of these strains. Nontypeable H. influenzae 11, known to lack hmw genes, served as a negative control.
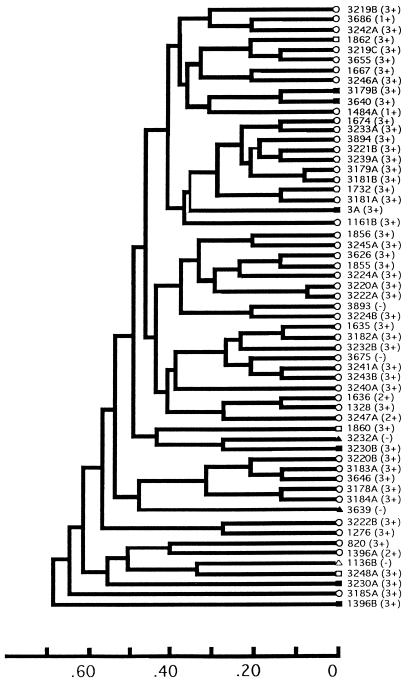
FIG. 1.
Genetic relationships between the 59 strains examined in this study. The dendrogram was generated previously by Musser et al. (8) by the average-linkage method of clustering from a matrix of coefficients of genetic distance, based on 15 metabolic enzymes. Circles indicate strains that hybridized with hmw but not hia, squares indicate strains that hybridized with hia but not hmw, and triangles indicate strains that failed to hybridize with either hia or hmw. Blackened symbols indicate strains that hybridized with pUO38 and IS1016. The numbers and letters to the right of the symbols are strain designations. Numbers in parentheses refer to level of adherence.
FIG. 2.
Southern blotting performed with probes for hmw sequences and hia. Chromosomal DNA was digested with BglII, separated by agarose electrophoresis, and subjected to Southern analysis. (A) Southern analysis with a probe corresponding to the promoter and 5′ coding sequence of hmw1A. Strains by lane: 1, 12; 2, 11; 3, 3219B; 4, 3686; 5, 3242A; 6, 3219C; 7, 3655; 8, 1667; 9, 3246A; 10, 1484A; 11, 1674. Less chromosomal DNA was inadvertently loaded in lanes 7 and 9. (B) Southern analysis with a probe that represents an intragenic fragment of hia. Strains by lane: 1, 12; 2, 11; 3, 1862; 4, 3179B; 5, 3640; 6, 3A; 7, 1860; 8, 3230B; 9, 3248A; 10, 3230A; 11, 1396B. Comparable quantities of DNA were loaded in each lane.
Next we probed chromosomal DNA from the 59 strains with a 1.6-kb SspI-StyI fragment that corresponds to an intragenic region of the H. influenzae 11 hia locus. Nontypeable H. influenzae 12, lacking an hia homolog, served as a negative control, while strain 11 was the positive control. As summarized in Fig. 1 and illustrated in Fig. 2B, nine strains demonstrated specific bands of hybridization. Interestingly, the 47 strains with hmw sequences and the nine strains with an hia homolog were mutually exclusive but failed to segregate into genetically distinct divisions (Fig. 1). Nevertheless, the strains containing an hia homolog formed small clusters within the larger population structure.
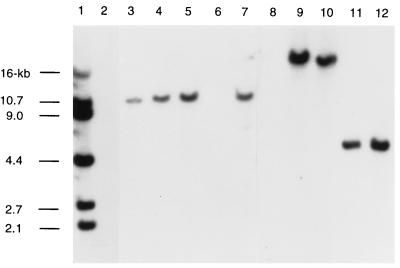
To gain additional insight into the evolutionary relationship between typeable and nontypeable strains of H. influenzae, we performed Southern analysis with pUO38, a pBR322 derivative that contains a complete set of the cap genes, including one copy of IS1016, from a phylogenetic division I H. influenzae type b strain (6). We refer to this set of genes, which includes sequences common to all encapsulated H. influenzae strains, as the cap locus. Of the 47 strains with hmw genes, none demonstrated hybridization with pUO38 (Fig. 1). In contrast, 8 of the remaining 12 strains, including 6 of the 9 strains with an hia gene, showed a single band of hybridization (Fig. 3). Further analysis revealed that in all strains, hybridization with IS1016 accounted entirely for the hybridization seen with pUO38 (not shown). H. influenzae type b strain Eagan served as a control for these blots and gave the predicted pattern of hybridizing bands, including bands of 2.1, 2.7, 4.4, 9.0, 10.7, and 16 kb with pUO38 and bands of 9.0, 10.7, and 16 kb with IS1016 (5, 19). Hybridization studies performed with radiolabeled pBR322 were negative for all strains (not shown).
FIG. 3.
Southern blotting performed with radiolabeled pUO38. Chromosomal DNA was digested with EcoRI, separated by agarose electrophoresis, and subjected to Southern analysis. Strains by lane: 1, Eagan; 2, 1862; 3, 3179B; 4, 3640; 5, 3A; 6, 1860; 7, 3230B; 8, 3248A; 9, 3230A; 10, 1396B; 11, 3232A; 12, 3639.
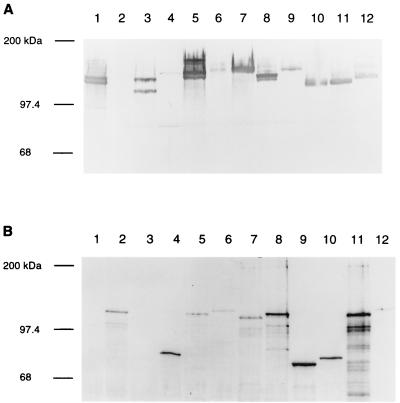
To determine whether the presence of hmw and hia sequences correlated with specific protein expression, whole-cell lysates of all 59 strains were examined by Western immunoblot analysis with a rabbit polyclonal antiserum reactive with HMW1 and HMW2 and a rabbit polyclonal antiserum raised against Hia. Forty-five of the 47 strains with hmw genes expressed one or two proteins that reacted with serum 25G (2), with the reactive proteins ranging in size between ∼105 and ∼160 kDa. Figure 4A shows a representative set of these strains. Among the 12 strains that failed to hybridize with hmw1A, none expressed proteins reactive with serum 25G. Control strains for these immunoblots included strain 12 (expressing HMW1 and HMW2) and strain 11 (lacking HMW proteins).
FIG. 4.
Western immunoblotting performed with serum 25G against HMW1 and HMW2 or serum 36B against Hia. Whole-cell lysates were prepared, and proteins were separated on a 7.5% polyacrylamide gel. (A) Western analysis with serum 25G. Strains by lane: 1, 12; 2, 11; 3, 3219B; 4, 3686; 5, 3242A; 6, 3219C; 7, 3655; 8, 1667; 9, 3246A; 10, 1484A; 11, 1674; 12, 3894. (B) Western analysis with serum 36B. Strains by lane: 1, 12; 2, 11; 3, 11hia::kan; 4, 1862; 5, 3179B; 6, 3640; 7, 3A; 8, 1860; 9, 3230B; 10, 3248A; 11, 3230A; 12, 1396B.
Analysis of the 59 strains with serum 36B (3), directed against Hia, revealed reactivity with all 9 strains containing an hia gene but with none of the remaining 50 isolates. Interestingly, in six of the nine reactive strains, multiple high-molecular-weight bands were detected, suggesting formation of multimers or aggregates (not shown). Formic acid treatment (4) resulted in elimination of the high-molecular-weight bands and generation of a predominant band ranging in size between ∼80 and ∼120 kDa (Fig. 4B). Control strains for these blots again included strain 12 and strain 11, along with a strain 11 mutant deficient in expression of Hia.
To examine whether expression of HMW or Hia proteins correlated with a capacity for adherence to human epithelial cells, we performed adherence assays with Chang epithelial cells. HMW1, HMW2, and Hia originally were identified as adhesins based on the ability to promote attachment to Chang cells. In the present study, adherence was measured by staining samples with Giemsa and then examining with light microscopy and counting the number of bacteria associated with 25 cells. Adherence was considered 3+ if the average number of bacteria per cell was greater than 25, 2+ if the average number was 10 to 25, and 1+ if the average number was 1 to 10. Strains were considered nonadherent if the average number of bacteria per cell was less than one. As summarized in Fig. 1, 45 of the 47 strains with hmw genes, including all 45 containing proteins reactive with serum 25G, demonstrated appreciable in vitro adherence. In 43 of these strains, the level of adherence was moderate to high (2+ to 3+). All nine of the strains with an hia homolog demonstrated high-level adherence (3+). Interestingly, the three strains that were negative by Southern blotting with both hmw1A and hia were nonadherent.
To summarize, Southern analysis of the 59 strains included in this study revealed three subsets, including 80% with an hmw1A or hmw2A homolog, 15% lacking hmw1A and hmw2A genes but harboring an hia homolog, and 5% lacking both hmw and hia sequences. Strains containing an hmw gene were uniformly devoid of IS1016, an insertion element associated with the cap locus in division I encapsulated H. influenzae. In contrast, nearly 70% of the hmw-deficient strains possessed one copy of this element.
Based on our earlier observations that a subset of nontypeable strains contain the hia gene, which is allelic to the hsf locus found in all encapsulated H. influenzae strains (3, 16), and that 31% of pharyngeal isolates of nontypeable H. influenzae from healthy 3-year-old Finnish children demonstrated hybridization with the cap locus (19), we hypothesized that strains possessing an hia homolog would contain cap sequences as well, reflecting more recent evolution from an encapsulated ancestor. Indeed, in the present study we found that six of nine strains that hybridized with hia also hybridized with pUO38. Further analysis revealed that in all six of these strains, the hybridization with pUO38 was due entirely to the presence of an IS1016 element. It is possible that these strains experienced a deletion of capsule genes due to recombination between duplicated copies of IS1016, leaving one intact copy of IS1016. Such precedent exists in the form of the longtime laboratory strain Rd, which originally contained capsule genes flanked by IS1016 elements and expressed a serotype d capsule but now contains a single copy of IS1016 with no capsule genes and is nonencapsulated (5).
Examination of the genetic relatedness between the 47 strains with hmw sequences and the 12 strains without such sequences revealed the absence of separable divisions. Nevertheless, the hmw-deficient strains were found to form clusters within the larger population structure. For example, strains 3179B and 3640, both of which hybridized with hia and IS1016, are more closely related to each other than they are to other strains in the collection. Similarly, strains 1860 (containing hia [hia+] but lacking IS1016 [IS1016−]), 3232A (hia−, IS1016+), and 3230B (hia+, IS1016+) form a second cluster of closely related strains, and strains 1136B (hia−, IS1016−), 3248A (hia+, IS1016−), and 3230A (hia+, IS1016+) form a third such cluster. Consistent with the evidence that strains 3179B and 3640 are closely related and the possibility that they have evolved from the same encapsulated ancestor, in both strains an ∼10-kb EcoRI fragment hybridizes with IS1016.
Division I and division II encapsulated H. influenzae strains are separated from one another by a genetic distance of 0.66 (9, 10). As shown in Fig. 1, the first 58 nontypeable strains in the collection we studied are separated by genetic distances that range between 0.08 and 0.64. Furthermore, the analysis by Musser et al. revealed that strains in positions 1 to 3 (strains 3219B, 3686, and 3242A [Fig. 1]) lie between type b strains belonging to clonal groups A1a and A2a, and strains in positions 4 to 22 (including strains 1862, 3179B, 3640, and 3A) lie between type b strains in clonal groups A2a and B1b (8, 9). Thus, 58 of the 59 nontypeable strains we examined, including 11 of the 12 hmw-deficient strains, are likely to be more closely related to division I encapsulated strains than they are to division II strains. One conclusion consistent with our observations and the dendrogram that exists for encapsulated strains is that strains 1862, 3179B, and 3640 all evolved from a type b ancestor. Similarly, superimposition of the dendrogram for encapsulated H. influenzae suggests that strain 3A might have evolved from a cluster B type b, type d, or type a strain; strains 1860, 3232A, and 3230B might have evolved from a cluster C type b or a cluster D type c strain; and strain 3639 might have evolved from a cluster E type c strain. The origins of strains 1136B, 3248A, 3230A, and 1396B are less clear, except that strain 1396B is more closely related to division II encapsulated strains, suggesting possible evolution from a type a, type b, or type f strain.
To examine the correlation between the presence of hmw sequences and expression of HMW protein, we performed Western immunoblot analysis with an antiserum raised against HMW1 and reactive with both HMW1 and HMW2. All but two of the 47 strains with an hmw1A or hmw2A homolog reacted with the antiserum. Of the two nonreactive strains, neither demonstrated appreciable adherence to Chang epithelial cells, thus confirming the absence of significant protein expression. One possibility suggested from a recent study by Barenkamp (1) is that protein expression in these strains was turned off during the course of infection, perhaps related to antibody directed against the HMW proteins, which are known to be immunogenic (2). Whether these strains have intact hmw loci is presently under investigation.
To conclude, our results indicate that 95% of nontypeable H. influenzae strains contain either hmw or hia sequences. Furthermore, it appears that hmw-deficient strains might have evolved more recently from an encapsulated progenitor. One possible model is that the primordial H. influenzae strain was nonencapsulated and spawned two separate but overlapping lineages. The first such lineage acquired hmw genes and remained nonencapsulated, while the second acquired hia and cap genes and became encapsulated. Subsequent mutations within the cap locus resulted in evolution of a relatively restricted set of nonencapsulated strains. Studies of additional strain collections may contribute to a better understanding of the prevalence of the hmw and hia genes and provide further insights into the evolutionary relationships between nontypeable and encapsulated forms.
Acknowledgments
We thank J. M. Musser for critical reading of the manuscript and for useful discussions.
This work was supported by Public Health Service grant 1RO1 DC-02873-01 from the National Institute on Deafness and Other Communication Disorders and by an American Heart Association grant-in-aid to J.W.S.
REFERENCES
- 1.Barenkamp S J. Immunization with high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae modifies experimental otitis media in chinchillas. Infect Immun. 1996;64:1246–1251. doi: 10.1128/iai.64.4.1246-1251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barenkamp S J, Leininger E. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992;60:1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barenkamp S J, St. Geme J W., III Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol Microbiol. 1996;19:1215–1223. doi: 10.1111/j.1365-2958.1996.tb02467.x. [DOI] [PubMed] [Google Scholar]
- 4.Klingman K L, Murphy T F. Purification and characterization of a high-molecular-weight outer membrane protein of Moraxella (Branhamella) catarrhalis. Infect Immun. 1994;62:1150–1155. doi: 10.1128/iai.62.4.1150-1155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroll J S, Loynds B M, Moxon E R. The Haemophilus influenzae capsulation gene cluster: a compound transposon. Mol Microbiol. 1991;5:1549–1560. doi: 10.1111/j.1365-2958.1991.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 6.Kroll J S, Moxon E R. Capsulation and gene copy number at the cap locus of Haemophilus influenzae type b. J Bacteriol. 1988;170:859–864. doi: 10.1128/jb.170.2.859-864.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroll J S, Zamze S, Loynds B, Moxon E R. Common organization of chromosomal loci for the production of different capsular polysaccharides in Haemophilus influenzae. J Bacteriol. 1989;171:3343–3347. doi: 10.1128/jb.171.6.3343-3347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musser J M, Barenkamp S J, Granoff D M, Selander R K. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun. 1986;52:183–191. doi: 10.1128/iai.52.1.183-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musser J M, Kroll J S, Granoff D M, Moxon E R, Brodeur B R, Campos J, Dabernat H, Frederiksen W, Hamel J, Hammond G, Hoiby E A, Jonsdottir K E, Kabeer M, Kallings I, Khan W N, Kilian M, Knowles K, Koornhof H J, Law B, Li K I, Montgomery J, Pattison P E, Piffaretti J-C, Takala A K, Thong M L, Wall R A, Ward J I, Selander R K. Global genetic structure and molecular epidemiology of encapsulated Haemophilus influenzae. Rev Infect Dis. 1990;12:75–111. doi: 10.1093/clinids/12.1.75. [DOI] [PubMed] [Google Scholar]
- 10.Musser J M, Kroll J S, Moxon E R, Selander R K. Evolutionary genetics of the encapsulated strains of Haemophilus influenzae. Proc Natl Acad Sci USA. 1988;85:7758–7762. doi: 10.1073/pnas.85.20.7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittman M. Variation and type specificity in the bacterial species Haemophilus influenzae. J Exp Med. 1931;53:471–493. doi: 10.1084/jem.53.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quentin R, Goudeau A, Wallace R J, Jr, Smith A L, Selander R K, Musser J M. Urogenital, maternal, and neonatal isolates of Haemophilus influenzae: identification of unusually virulent serologically non-typable clone families and evidence for a new Haemophilus species. J Gen Microbiol. 1990;136:1203–1209. doi: 10.1099/00221287-136-7-1203. [DOI] [PubMed] [Google Scholar]
- 13.St. Geme J W, III, Cutter D. Evidence that surface fibrils expressed by Haemophilus influenzae type b promote attachment to human epithelial cells. Mol Microbiol. 1995;15:77–85. doi: 10.1111/j.1365-2958.1995.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 14.St. Geme, J. W., III, and D. Cutter. Unpublished data.
- 15.St. Geme J W, III, Cutter D. Influence of pili, fibrils, and capsule on in vitro adherence by Haemophilus influenzae type b. Mol Microbiol. 1996;21:21–31. doi: 10.1046/j.1365-2958.1996.6241331.x. [DOI] [PubMed] [Google Scholar]
- 16.St. Geme J W, III, Cutter D, Barenkamp S J. Characterization of the genetic locus encoding Haemophilus influenzae type b surface fibrils. J Bacteriol. 1996;178:6281–6287. doi: 10.1128/jb.178.21.6281-6287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St. Geme J W, III, Falkow S. Loss of capsule expression by Haemophilus influenzae type b results in enhanced adherence to and invasion of human cells. Infect Immun. 1991;59:1325–1333. doi: 10.1128/iai.59.4.1325-1333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St. Geme J W, III, Falkow S, Barenkamp S J. High-molecular-weight proteins of nontypeable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci USA. 1993;90:2875–2879. doi: 10.1073/pnas.90.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St. Geme J W, III, Takala A, Esko E, Falkow S. Evidence for capsule gene sequences among pharyngeal isolates of nontypeable Haemophilus influenzae. J Infect Dis. 1994;169:337–342. doi: 10.1093/infdis/169.2.337. [DOI] [PubMed] [Google Scholar]