Abstract
| Strategy, Management and Health Policy | ||||
|---|---|---|---|---|
| Venture Capital Enabling Technology | Preclinical Research | Preclinical Development Toxicology, Formulation Drug Delivery, Pharmacokinetics | Clinical Development Phases I-III Regulatory, Quality, Manufacturing | Postmarketing Phase IV |
Binding affinities of purine derivatives at A3 adenosine receptors in different species were compared. Binding was carried out using the novel high affinity agonist ligand [125I]AB-MECA (3-iodo-4-aminobenzyladenosine-5′-N-methyluronamide) in the presence of 1.0 μM XAC (8-[4-[[[[(2-aminoethyl)amino]carbonyl]methyl]oxy]phenyl]-1,3-dipropylxanthine), an A1- and A2a-adenosine antagonist. XAC was added to eliminate binding to non-A3 receptors. In rat brain membranes [125I]AB-MECA exhibited saturable, specific binding with a Kd of 2.28 nM and a Bmax of 43 fmol/mg protein. The affinity of [125I]AB-MECA at the gerbil and rabbit brain A3-receptors was similar to the rat, suggesting that the affinity of this agonist is not highly species dependent. The affinity of various xanthine derivatives was measured in [125I]AB-MECA competition binding assays. Gerbil and rabbit brain A3-receptors were similar in the affinity of antagonists whose potency order in both species was: BWA522 ≥ CPX > XCC, XAC, SPX, BWA1433 > theophylline. The affinities of 8-arylxanthines at the rat, rabbit, and gerbil brain A3 receptors were considerably less than the previously reported affinities at cloned sheep and human A3 receptors. Species differences in agonist affinity were assessed by comparing Ki values at cloned rat brain A3 receptors expressed in CHO cells with cloned sheep and human A3 receptors. Human and rat brain A3 receptors were highly similar in the relative affinities of agonists, and sheep brain A3 receptors were unlike either human or rat A3 receptors in agonist affinity.
Keywords: xanthines, gerbil, rabbit, radioligand binding, adenosine analogues
INTRODUCTION
Four adenosine receptors, A1, A2a, A2b, and A3, have been cloned [Libert et al., 1991; Maenhaut et al., 1990; Stehle et al., 1992; Zhou et al., 1992]. All are present and functional in the CNS, and the molecular structure and the nature of their effector coupling have been studied [Stiles, 1992]. A1 receptors couple to a variety of second messenger systems, including inhibition of adenylate cyclase, inhibition or stimulation of phosphoinositol turnover, and activation of ion channels, and A2 subtypes stimulate adenylate cyclase. The newly cloned rat A3 adenosine receptor shows 56% identity with the rat A1 receptor. Furthermore, rat A3 receptors have been shown to stimulate phosphoinositide metabolism [Ramkumar et al., 1993] and, like A1 receptors, inhibit adenylate cyclase [Zhou et al., 1992; Linden et al., 1993].
Cloned sheep and human A3 receptors [Linden et al., 1993; Salvatore et al., 1993], on the other hand, show major pharmacological differences with the cloned rat receptor, in particular in the effects of xanthines. In rodents, xanthines known to be potent antagonists at A1 and A2a receptors do not antagonize the second messenger effects of A3 receptor activation [Zhou et al., 1992] or block the behavioral depression elicited by an A3 selective agonist [Jacobson et al., 1993]. On the other hand, although xanthines do not bind with high affinity to rat A3 receptors, some xanthines, especially those containing a negative charge, such as 3-(4-amino-3-iodo-benzyl)-8-[4-[[[carboxy]methyl]oxy]phenyl]-l-propylxanthine (BWA-522), show nearly nanomolar affinity at both sheep and human A3 receptors. To explore these differences in greater detail, we have investigated central A3 receptors using the novel radioligand [125I]AB-MECA (N6-4-amino-3-iodo-benzyladenosine-5′-N-methyluronamide) [Olah et al., 1994a]. At the same time, the feasibility of using [125I]AB-MECA (in combination with the appropriate subtype selective additives) for binding to A3 receptors in brain membranes of various species has also been studied.
Characterization of the dramatically large species differences in ligand binding at the newly cloned A3 receptor is essential for the development of both research probes and potential therapeutic agents designed to act via these receptors. Selective antagonism of A3 receptors in the lung and elsewhere has been suggested as potentially useful in the treatment of inflammatory diseases [Beaven et al., 1994; Bai et al., 1994]. Adenosine agonists may also have therapeutic applications, for example in diseases that induce neurodegeneration [von Lubitz et al., 1994].
MATERIALS AND METHODS
Materials
Most of the adenosine receptor ligands were obtained from Research Biochemicals International (Natick, MA). BWA522 and 1,3-dipropyl-8-[4-(carboxyethynyl)phenyl]xanthine (BWA1433) were the kind gifts of Dr. J. Linden (University of Virginia, Charlottesville, VA) and Dr. S. Daluge (Burroughs Wellcome, Research Triangle, NC). [125I]AB-MECA was prepared as described [Olah et al., 1994a]. N6-R-phenylisopropyladenosine ([3H]PIA) was from Amersham (Arlington Heights, IL), and 2-[4-[(2-carboxyethyl)phenyl]ethylamino]-5′-N-ethylcarboxamidoadenosine [3H]CGS21680 was from Dupont NEN (Boston, MA).
F-12 (Ham’s) medium, fetal bovine serum (FBS), and penicillin/streptomycin were from Gibco BRL (Gaithersburg, MD). Adenosine deaminase (ADA) was from Boehringer Mannheim (Indianapolis, IN). The composition of lysis buffer was: 10 mM Tris/5mM EDTA, pH 7.4, at 5°C. All other materials were from standard local sources and of the highest grade commercially available.
Cell Culture and Membrane Preparation
CHO cells stably expressing the rat A3 receptor [Zhou et al., 1992] were grown in F-12 medium containing 10% FBS and penicillin/streptomycin (100 U/mL and 100 μg/mL, respectively) at 37°C in a 5% CO2 atmosphere. For the final passage cells were grown in 150 × 50 mm tissue culture dishes. Cells were washed twice with 10 mL of lysis buffer. After final addition of 5 mL of lysis buffer, cells were mechanically scraped and homogenized in an ice-cold Dounce homogenizer (20 strokes by hand). The suspension was centrifuged at 43,000g for 10 min. The pellet was resuspended in the minimum volume of ice-cold 50 mM Tris(hydroxymethyl)aminomethane/10 mM MgCl2/l mM EDTA buffer (pH 8.26 at 5°C) required for the binding assay and homogenized in a Dounce homogenizer. Typically, 6–8 175 cm2 flasks were used for a 48-tube assay. Adenosine deaminase was added to a final concentration of 3 U/mL, and the suspension was incubated at 37°C for 20 min; the membrane suspension was subsequently kept on ice until use. The preparation was stored at −70°C and retained its [125I]AB-MECA binding properties for at least 1 month.
Rat and rabbit forebrain tissue was isolated by dissection from whole brains, obtained frozen from Pel-Freeze Biologicals Co. (Rogers, AR). Gerbil brains were obtained by decapitation and processed similarly, but without freezing. Membranes were homogenized in 20 volumes of ice cold 50 mM Tris, containing 10 mM MgCl2 and 1 mM EDTA, adjusted to pH 8.26 with hydrochloric acid, using a polytron (Kinematica, Gmbh., Lucerne, Switzerland) at a setting of 2–3 for 10 sec. The membrane suspension was then centrifuged at 37,000g for 20 min at 4°C. The pellet was resuspended in the above buffer solution, and the membranes were again homogenized and centrifuged. Finally the pellet was suspended in buffer (100 mg wet weight per ml) and stored frozen at −70°C until use. Protein was determined using the BCA protein assay reagents (Pierce Chemical Co., Rockford, IL).
Radioligand Binding
Binding of [125I]AB-MECA to CHO cells stably transfected with the A3 receptor clone was performed as described [Olah et al., 1994a]. Assays were performed in 50/10/1 buffer in glass tubes and contained 100 μl of the membrane suspension, 50 μl of [125I]AB-MECA (final concentration 0.4 nM), and 50 μl of inhibitor. Stock solutions of the drugs (5 mM) in dimethylsulfoxide (DMSO) were diluted with DMSO to a concentration of ≤0.1 mM prior to adding to the aqueous medium. The final concentration of DMSO in the assay medium was generally 2%. Incubations were carried out in duplicate for 1 h at 37°C using 0.4 nM [125I]AB-MECA, in the presence of 0.05 M Tris buffer at pH 8.25, 1 mM EDTA, 10 mM MgCl2, and 1 μM XAC. The incubation was terminated by rapid filtration over Whatman GF/B filters, using a Brandell cell harvester (Brandell, Gaithersburg, MD). Tubes were washed three times with 3 mL of buffer. Radioactivity was determined in a Beckman gamma 5500B γ-counter. Non-specific binding was determined in the presence of 200 μM 5′-N-ethylcarboxamidoadenosine (NECA). IC50 values were computer-generated using a non-linear regression formula on InPlot Version-4.00 (GraphPAD, San Diego, CA). Comparison of Ki-values was carried out using regression analysis on InStat Version 2.04 (GraphPAD). Ki-values were calculated [Cheng and Prusoff, 1973] assuming a Kd [125I]AB-MECA of 1.46 nM in CHO cells (rat) and using the experimentally determined values (Table 1) in brain membranes. In brain membranes the Hill coefficients for competition by antagonists, but not agonists, were approximately equal to 1.
TABLE 1.
Summary of Data Obtained From Scatchard Analysis of Binding of [125I]AB-MECA in Brain Membranes
| Species | Kd (nM) | Bmax (tmol/mg protein) | n |
|---|---|---|---|
| Rat | 2.28 ± 0.11 | 43.3 ± 2.6 | 4 |
| Rabbit | 1.83 ± 0.13 | 118 ± 3 | 3 |
| Gerbil | 1.63 ± 0.12 | 28.5 ± 5.5 | 4 |
The binding of N6-[2-(4-aminophenyl)ethyl]adenosine ([125I]APNEA) at A3 receptors in CHO cell membranes was as described [van Galen et al., 1994]. The binding of [3H]PIA and [3H]CGS 21680 at A1 and A2a receptors in cortical and striatal membranes, respectively, from rabbit and gerbil brain was carried out by an adaptation of standard procedures [Jacobson et al., 1992; Jarvis et al., 1989].
RESULTS
Radioligand Binding at A3 Receptors in Brain Membranes
In order to compare the affinity of various purine derivatives at A3 receptors in different species, for which the receptor clones are not yet available, [125I]AB-MECA (3-iodo-4-aminobenzyladenosine-5′-N-methyluronamide) [Olah et al., 1994a] was used as the radioligand. [125I]AB-MECA was shown to bind with nanomolar affinity at A3 receptors in mouse brain membranes [Jacobson et al., 1993]. Since the specific binding of [125I]AB-MECA was shown to occur in the mouse brain at both A1 and A3 receptors, in our previous study an A1 selective antagonist, 1,3-dipropyl-8-cyclopentylxanthine (CPX) (100 nM), was added to eliminate the A1 component of binding. It is possible that some [125I]AB-MECA binding may also occur at brain A2a receptors, since a Kd value of 25 nM was demonstrated for the canine A2a receptor [Olah et al., 1994a]. Thus, in this study, we used the xanthine amine congener, XAC (1 μM; Fig. 1) to eliminate the potential binding of [125I]AB-MECA to either A1 or A2a receptors. In rat, rabbit, and gerbil brain, XAC has both very low affinity at A3 receptors (see below) and relatively high affinity at A1 and A2a receptors. Ki values for XAC in the inhibition of radioligand binding at rat brain A1 and A2a receptors are 1.2 and 63 nM, respectively [Daly and Jacobson, 1989]. In this study, XAC displaced binding of [3H]PIA (1 nM) at rabbit and gerbil A1 receptors with IC50 values of 21.2 ± 1.3 and 14.2 ± 1.2 nM, respectively, whereas the corresponding affinities of XAC at A2a receptors were found to be 7.5 ± 0.5 (Ki vs. [3H]XAC [Ji et al., 1991]) and 13.3 ± 4.1 nM (IC50 vs. [3H]CGS 21680, 5 nM). Experiments also indicated that addition of 1 μM XAC rather than CPX afforded a more satisfactory fraction of specific binding of [125I]AB-MECA to A3 receptors in the gerbil brain. Since our previous results [Jacobson et al., 1993] suggested a widespread distribution of A3 receptors in various regions of the mouse brain, we did not separate brain regions in this study. Binding was performed for 1 h, with non-specific binding defined in the presence of 200 μM NECA. The amount of specific binding at 0.4 nM [125I]AB-MECA was approximately 29% (rat), 57% (rabbit), or 22% (gerbil) of total binding.
Figure 1.
Structures of xanthines used in this study.
Representative isotherms for saturation of specific [125I]AB-MECA binding in the presence of 1.0 μM XAC in rat, rabbit, and gerbil brain membranes are shown in Figure 2. The Scatchard plots of the data were linear, indicating a homogeneous population of non-cooperative binding sites. In rat brain membranes a Kd value of 2.28 nM and a binding capacity (Bmax) of 43.3 fmol/mg protein were obtained. The affinity of [125I]AB-MECA in rat brain membranes was in close agreement with the affinity of this radioligand at A3 receptors in RBL-2H3 rat mast cells (Kd 3.6 nM) and at rat brain A3 receptors in transfected CHO cells (Kd 1.5 nM) [Olah et al., 1994a]. Since similar Kd values were found at A3 receptors in rabbit and in gerbil forebrain membranes (Table 1), the affinity of the agonist radioligand [125I]AB-MECA is not highly species dependent. Moreover, consistent with our previous findings in the mouse brain [Jacobson et al., 1993], the density of binding sites was very low compared to typical A1 receptor densities (0.5–1.0 pmol/mg protein) in rodent brain [Williams and Jacobson, 1990]. Rabbit brain, on the other hand, appeared to contain a higher density of A3 receptors than either rat or gerbil brain.
Figure 2.
Typical saturation of binding isotherms for specific binding of [125I]AB-MECA at A3 receptors in rat (A), rabbit (B), and gerbil (C) brain membranes. Scatchard transformations are also shown in insets (refer to Table 1 for Kd and Bmax values). The binding of [125I]AB-MECA was carried out for 60 min at 37°C, in 0.05 M Tris buffer at pH 8.25, in the presence of 1 mM EDTA, 10 mM MgCl2, and 1 μM XAC. Specific binding, determined in the presence of 200 μM NECA, was approximately 29% (rat), 57% (rabbit), or 22% (gerbil) of total binding.
Affinity of Xanthines
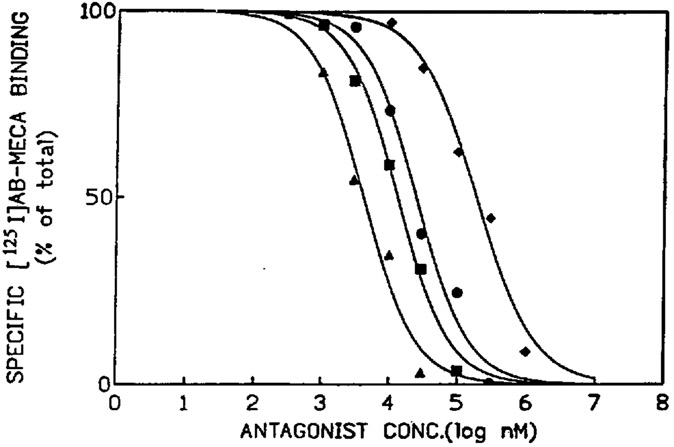
The Ki values of various xanthines (structures shown in Fig. 1) at A3 receptors were determined in competition experiments vs. [125I]AB-MECA. The results are listed in Table 2. At gerbil and rabbit A3 receptors, binding was carried out in brain membranes by the method described above, and at rat A3 receptors in membranes from CHO cells stably transfected with A3 receptor cDNA as described by Olah et al. [1994a]. Typical displacement curves for the rabbit brain, which showed a favorable ratio of specific to non-specific binding, are shown in Figure 3. The affinities of the xanthines at A3 receptors in brain membranes were very similar in the rabbit and gerbil, and the potency order for antagonists in competition for [125I]AB-MECA was BWA522 ≥ CPX > (8-[4-[[[carboxy]methyl]oxy]phenyl]-1,3-dipropylxanthine) (XCC), XAC, 1,3-dipropyl-8-(4-sulfophenyl)xanthine (SPX), BWA1433 > theophylline. There was a moderate correlation with the affinities measured at rat A3 receptors in transfected CHO cells. SPX had a higher affinity at rabbit and gerbil brain A3 receptors than at rat A3 receptors.
TABLE 2.
Affinity, Expressed as Ki Values, of Adenosine Receptor Antagonists at Central A3-Adenosine Receptors in Various Species
| Compound | Ki (μM) at A3-receptors | ||||
|---|---|---|---|---|---|
| Rabbita | Gerbila | Ratb | Sheepc | Humanc | |
| Theophylline | 92.3 ± 5.1 | 116 ± 13 | 85.2 ± 12.7d | 424 | n.d. |
| SPX | 22.5 ± 5.0 | 18.6 ± 1.7 | 90.1 ± 10.9d | 0.183 | n.d. |
| XAC | 20.6 ± 3.2 | 21.6 ± 5.9 | 29.0 ± 7.0d | 0.18 | 0.071 |
| CPX | 5.27 ± 1.45 | 3.17 ± 0.70 | 5.29 ± 0.79d | 49.3 | 0.76 |
| XCC | 17.0 ± 2.3 | 16.4 ± 2.8 | 27.5 ± 2.5 | n.d. | n.d. |
| BWA1433 | 24.6 ± 3.6 | 25.3 ± 2.0 | 15.0 ± 1.7 | 0.021 | 0.055 |
| BWA522 | 1.96 ± 0.23 | 2.76 ± 0.50 | 1.17 ± 0.18 | 0.003 | 0.018 |
In membranes from rabbit or gerbil brain vs. [125I]AB-MECA (0.4 nM) carried out for 60 min at 37°C, in 0.05 M Tris buffer at pH 7.4, in the presence of 1 mM EDTA, 10 mM MgCl2, and 1 μM XAC. Ki values are the mean ± standard deviation of 3–4 experiments done in duplicate.
In membranes prepared from CHO cells stably transfected with rat A3 receptor cDNA [Zhou et al., 1992] vs. [125I]AB-MECA (0.4 nM) carried out for 60 min at 37°C, in 0.05 M Tris buffer at pH 7.4, in the presence of 1 mM EDTA, and 10 mM MgCl2. Ki values are the mean ± standard deviation of 3–4 experiments done in duplicate.
vs. binding of [125I]4-aminobenzyladenosine at cloned receptors expressed in CHO cells [Linden et al., 1993; Salvatore et al., 1992], n.d. = not determined.
van Galen et al. [1994] reported that Ki values determined at rat A3 receptors expressed in CHO cells for theophylline, XAC, and SPX were > 100 μM, and the Ki value for CPX was > 10 μM.
Figure 3.
Typical curves for inhibition of binding of [125I]AB-MECA (0.4 nM) to rabbit forebrain A3-adenosine receptors by various adenosine receptor antagonists: theophylline (diamonds); BWA1433 (circles); XCC (squares); CPX (triangles). Hill coefficients for these curves were 1.0–1.2. The binding was carried out for 60 min at 37°C, in 0.05 M Tris buffer at pH 8.25, in the presence of [125I]AB-MECA (0.4 nM), 1 mM EDTA, 10 mM MgCl2, and 1 μM XAC.
The antagonist Ki values determined in the present study were also compared with the published Ki values at cloned sheep [Linden et al., 1993] and human [Salvatore et al., 1993] A3 receptors, using [125I]ABA (N6-(4-aminobenzyl)adenosine) as radioligand. Similar to the rat, sheep and human receptors have been expressed in CHO cells (Table 2). Based on a comparison of binding of [1251]APNEA (similar in structure to [125I]ABA) and [125I]AB-MECA at cloned rat A3 receptors [Gallo-Rodriguez et al., 1994], the Ki values are not likely to depend on which A3 agonist radioligand is used. In this study, there was a low correlation between xanthine affinities at brain A3 receptors in rabbits and rodents compared to receptors found in the sheep and human brain. Four xanthine derivatives (XAC, CPX, BWA1433, and BWA522) that were examined as competitors in binding to human brain A3 receptors by Salvatore et al. [1993] were all much less potent in the rat, rabbit, and gerbil. On the other hand, at the sheep brain A3 receptors [Linden et al., 1993], compared to the rabbit/gerbil brain A3 receptors, XAC affinity increased by two orders of magnitude, and CPX lost affinity by one order of magnitude. The largest species dependent difference in affinity was seen with an acidic xanthine, BWA1433, which was 1,200-fold less potent in the rabbit and gerbil than in the sheep. Divergence between sheep and human brain A3 receptors in the affinity of xanthines was also present, i.e., CPX was 65-fold more potent at human vs. sheep A3 receptors.
Affinity of Adenosine Derivatives
Agonist affinities were measured at cloned rat brain A3 receptors stably expressed in CHO cells as described [van Galen et al., 1994], Ki values (expressed as nM vs. binding of [125I]APNEA) were determined to be: CV1808, 4,390 ± 1,170; 2-chloroadenosine, 1,890 ± 900; S-PIA, 920 ± 311; CPA, 240 ± 36; R-PIA, 158 ± 52; and NECA, 113 ± 34. These values were compared with competition data for agonists at the sheep [Linden et al., 1993] and human [Salvatore et al., 1993] brain A3 receptors, also expressed in CHO cells. A high degree of correlation shown in Figure 4B vs. a lesser degree in Figure 4A suggests that human and rat brain A3 receptors are highly similar in the relative affinity of agonist, and that sheep brain A3 receptors are unlike either human or rat in the order of agonist affinities.
Figure 4.
Correlation graphs showing affinity measured in binding assays at cloned rat brain A3 receptors (stably transfected CHO cells, using [125I]APNEA as radioligand) vs. cloned A3 receptors from sheep (A, using [125I]ABA as radioligand) or from human (B, using [125I]ABA as radioligand) brain. Values at sheep and human receptors were from Linden et al. [1993] and Salvatore et al. [1993]. Linear regression analysis for each set of data calculated r2 values of < 0.105 (A) and 0.995 (B).
DISCUSSION
Despite the low selectivity of [125I]AB-MECA for A3 receptors [Olah et al., 1994a] and a relatively low density of these receptors in brain membranes, it has been possible to use this radioligand to compare interspecies differences.
The existence of such differences for antagonists at A3 receptors has been indicated by several studies. Zhou et al. [1992] and van Galen et al. [1994] observed that the rat A3 receptor is insensitive to the commonly used xanthine antagonists, such as XAC. On the other hand, cloning of the sheep and human A3 receptors [Linden et al., 1993; Salvatore et al., 1993] has clearly demonstrated that many xanthines are active in those species. Although no more than one A3-like clone has been found in any of the studied species, one cannot exclude a possibility that the cloned receptors are really different subtypes. However, an alternative explanation is that A3 receptors characterized so far comprise a single subtype that has unusually pronounced pharmacological species differences. Substantial species differences have also been noted for xanthines at A1 receptors [Ukena et al., 1986; Ferkany et al., 1986] and at A2a receptors [Stone et al., 1988]. These differences were less pronounced than those at A3 receptors.
Previous studies of species differences in adenosine receptor binding related these differences to specific substituent groups, such as the xanthine 8-aryl group. We have therefore chosen structurally diverse xanthines (Fig. 1) in this study. Structural categories included: a simple xanthine (theophylline); 8-arylxanthines containing carboxylic (XCC, BWA1433, and BWA522), sulfonate (SPX), and amino ⟨XAC) groups; and an 8-cycloalkylxanthine (CPX). Rat, rabbit, and gerbil A3 receptors were similar in affinities of all the xanthines examined, and the potency enhancement by anionic groups noted previously for sheep A3 receptors [Linden et al., 1993] was not apparent. At sheep and human brain A3 receptors the 8-arylxanthines were considerably more potent (≥ two orders of magnitude) than in the other species. This enhancement of affinity was present for a cationic (XAC) as well as anionic xanthines. An 8-cycloalkylxanthine (CPX) distinguished clearly the specificity of human vs. sheep A3 receptors, with the affinity in the rat, rabbit and gerbil being intermediate. The potency enhancement by the 3-[2-(4-amino-3-iodophenyl)ethyl group in BWA522 (compare to XCC) was maintained across species.
Interspecies differences in agonist affinity at A3 receptors were less pronounced than those for xanthines. Among adenosine agonists of varied structure (5′-, 2-, and N6-derivatives), binding at rat A3 receptors is similar in relative order to binding at human, but not sheep A3 receptors. Although the correlation between human and rat receptors for four adenosine derivatives (Fig. 4B, r2 = 0.995) was linear, the Ki values in rat were several-fold higher than those in human. In human and rat, NECA was more potent than 2-chloroadenosine, while in sheep, the order was reversed. In all three species, the stereoselectivity for R- vs. S-PIA, well characterized for A1 receptors, was preserved [van Galen et al., 1994].
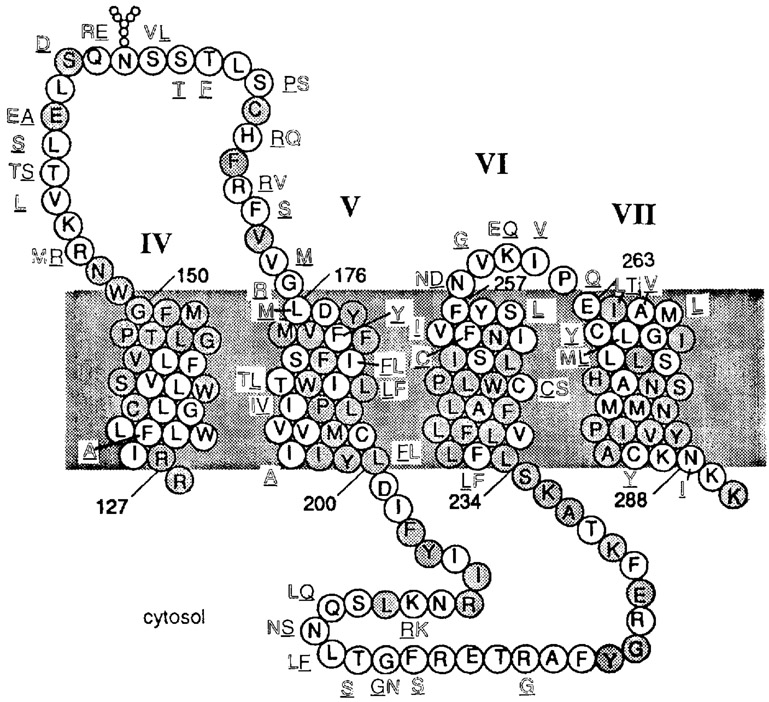
The specific amino acid residues in adenosine receptors responsible for ligand recognition and the interspecies differences in antagonist or agonist affinity are the subject of molecular modeling [van Galen et al., 1994] and site-directed mutagenesis experiments [Olah et al., 1994b; Townsend-Nicholson and Schofield, 1994]. According to our hypothetical molecular model proposed for ligand binding to A3 receptors [van Galen et al., 1994], most of the ligand interaction sites are in the transmembrane region, between the middle of the helix and the extracellular side, particularly in helices IV–VII (Fig. 5). With respect to binding of 8-arylxanthines in that region, the human A3 receptors resemble sheep more closely than rat A3 receptors. This finding appears to be consistent with the 92% homology between human and sheep receptors in the entire transmembrane region vs. 81% for sheep compared to rat and 82% for human compared to rat.
Figure 5.
Sequence of the rat A3 receptor in the region spanning transmembrane helices IV to VII (including residues 126–290), in which ligand binding is likely to occur. Amino add residues that are different in the sheep (underlined) or human (outlined) A3 receptor sequences are indicated. The position occupied by Leu159 of the rat receptor is deleted in the sheep and human receptors. Shaded residues are conserved between rat A1 and A3 receptors.
If species differences for xanthines are indeed related to discrepant homology between the rat, on the one hand, and the sheep and human receptors, on the other, then the similarity between rat and human in agonist binding does not fit with this analysis. However, a detailed examination of the sequence shows that there are six residues in the transmembrane region that are conserved between rat and human A3 receptors and that are absent in sheep receptors. Of these six only two residues fall within the upper half of helix IV–VII, i.e., Thr186 and Ile190 (both within helix V). In addition, both Ile185 and Ile264 are uncharged aliphatic residues in the rat and human receptors but not in the sheep A3 receptors. Hence it is possible that the commonality in agonist binding between rat and human is related to one or more of these four residues. Also, within the extracellular loops the same conservation pattern is found in: Thr158, Glu158, Ser167 (second loop), and Asn258 (third loop).
Fozard and Carruthers [1993] have attributed a xanthine-insensitive component of the hypotensive effects of adenosine agonists in rat to A3 receptor activation, and recently, Fozard and Hannon [1994] reported that BWA522 was a functional antagonist in the presumed A3-mediated hypotensive response in the rat. However, we have found that BWA522 is considerably less potent at rat A3 receptors than indicated by its previously published affinity at sheep A3 receptors [Linden et al., 1993]. Thus, due to the large species difference in affinity, the development of selective antagonists as pharmacological probes and radioligands for A3 receptors remains problematic. None of the conventionally used xanthines have selectivities that can be generalized across species. To avoid this difficulty, cautious use of xanthines to define the physiological actions of A3 receptor activation is essential.
ACKNOWLEDGMENTS
D.v.L. thanks the Cystic Fibrosis Foundation for financial support. We thank Dr. J. Linden for the sample of BWA522 and Dr. Neli Melman and Kamala Bellamkonda for technical assistance.
Contributor Information
Xiao-duo Ji, Molecular Recognition Section, Laboratory of Bioorganic Chemistry, National Institute of Diabetes, and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland.
Dag von Lubitz, Molecular Recognition Section, Laboratory of Bioorganic Chemistry, National Institute of Diabetes, and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland.
Mark E. Olah, Departments of Medicine and Pharmacology, Duke University Medical Center, Durham, North Carolina
Gary L. Stiles, Departments of Medicine and Pharmacology, Duke University Medical Center, Durham, North Carolina
Kenneth A. Jacobson, Molecular Recognition Section, Laboratory of Bioorganic Chemistry, National Institute of Diabetes, and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland
REFERENCES
- Bai TR, Weir T, Walker BAM, Salvatore CA, Johnson RG, Jacobson MA (1994): Comparison and localization of adenosine A3 receptor expression in normal and asthmatic lung. Drug Dev Res 31:244. [Google Scholar]
- Beaven MA, Ramkumar V, Ali H (1994): Adenosine A3 receptors in mast cells. Trends Pharmacol Sci 15:13–14. [DOI] [PubMed] [Google Scholar]
- Cheng YC, Prusoff WH (1973): Relationship between the inhibition constant (Ki) 1 and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzyme reaction. Biochem Pharmacol 22:3099–3108. [DOI] [PubMed] [Google Scholar]
- Daly JW, Jacobson KA (1989): Molecular probes for adenosine receptors. In Ribeiro JA (ed): Adenosine Receptors in the Nervous System. London: Taylor & Francis, pp 41–52. [Google Scholar]
- Ferkany JW, Valentine HL, Stone GA, Williams M (1986): Adenosine A1 receptors in mammalian brain: Species dillerenccs in their interactions with agonists and antagonists. Drug Dev Res 9:85–93. [Google Scholar]
- Fozard JR, Carruthers AM (1993): Adenosine A3 receptors mediate hypotension in the angiotensin II-supported circulation of the pithed rat. Br J Pharmacol 109:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozard JR, Hannon JP (1994): BW-A522 blocks adenosine A3 receptor-mediated hypotensive responses in the rat. Eur J Pharmacol 252:R5–R6. [DOI] [PubMed] [Google Scholar]
- Galio-Rodriguez C, Ji X-D, Melman N, Siegman BD, Sanders LH, Orlina J, Fischer B, Pu Q-L, Olah ME, van Galen PJM, Stiles GL, Jacobson KA (1994): Structure-activity relationships of N6-benzyladenosine-5′-uronamides as A3-selective adenosine agonists. J Med Chem 37:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Stiles GL, Ji X-D (1992): Chemical modification and irreversible inhibition of striatal A2a-adenosine receptors. Mol Pharmacol 42:123–133. [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Nikodijević O, Shi D, Gallo-Rodriguez C, Olah ME, Stiles GL, Daly JW (1993): A role for central A3-adenosine receptors: Mediation of behavioral depressant responses. FEBS Lett 336:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Schutz R, Hutchison JA, Do E, Sills MA, Williams M (1989): [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J Pharmacol Exp Ther 251:888–893. [PubMed] [Google Scholar]
- Ji X-D, Stiles GL, Jacobson KA (1991); [3H]XAC (xanthine amine congener) is a radioligand for A2-adenosine receptors in rabbit striatum. Neurochem Int 18:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert F, Schififmann SN, Lefort A, Parmentier M, Gerard C, Dumont JE, Vanderhaeghen J-J, Vassart G (1991): The orphan receptor cDNA RDC7 encodes an A1 adenosine receptor. EMBO J 10:1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J, Taylor HE, Robeva AS, Tucker AL, Stehle JH, Rivkees SA, Fink JS, Reppert SM (1993): Molecular cloning and functional expression of a sheep A3 adenosine receptor with widespread tissue distribution. Mol Pharmacol 44:524–532. [PubMed] [Google Scholar]
- Maenhaut C, Sande Jv, Libert F, Abramowicz M, Parmentier M, Vanderhaegen JJ, Dumont JE, Vassal G, Schiffinann S (1990): RDC8 codes for an adenosine A2 receptor with physiological constitutive activity. Biochem Biophys Res Commun 173:1169–1178. [DOI] [PubMed] [Google Scholar]
- Olah ME, Gallo-Rodriguez C, Jacobson KA, Stiles GL (1994a): [125I]AB-MECA, a high affinity radioligand for the rat A3 adenosine receptor. Mol Pharmacol 45:978–982. [PMC free article] [PubMed] [Google Scholar]
- Olah ME, Jacobson KA, Stiles GL (1994b): Identification of an adenosine receptor domain specifically involved in binding of 5′-substituted adenosine agonists. J Biol Chem 269:18016–18020. [PMC free article] [PubMed] [Google Scholar]
- Ramkumar V, Stiles GL, Beaven MA, Ali H (1993): The A3AR is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem 268:16887–16890. [PubMed] [Google Scholar]
- Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG (1993): Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci 90:10365–10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle JH, Rivkees SA, Lee JJ, Weaver DR, Deeds JD, Reppert SM (1992): Molecular cloning and expression of the cDNA for a novel A2-adenosine receptor subtype. Mol Endocrinol 6:384–393. [DOI] [PubMed] [Google Scholar]
- Stiles GL (1992): Adenosine receptors. J Biol Chem 267:6451–6454. [PubMed] [Google Scholar]
- Stone GA, Jarvis MF, Sills MA, Weeks B, Snowhill EW, Williams M (1988): Species differences in high-affinity adenosine A2 binding sites in striatal membranes from mammalian brain. Drug Dev Res 15:31–46. [Google Scholar]
- Townsend-Nicholson A, Schofield P (1994): A threonine residue in the seventh transmembrane domain of the human A1 adenosine receptor mediates specific agonist binding. J Biol Chem 269: 2373–2376. [PubMed] [Google Scholar]
- Ukena D, Jacobson KA, Padgett WL, Ayala C, Shamim MT, Kirk KL, Olsson RA, Daly JW (1986): Species differences in structure-activity relationships of adenosine agonists and xanthine antagonists at brain A1 adenosine receptors. FEBS Lett 209: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Galen PJM, van Bergen AH, Gallo-Rodriguez C, Melman N, Olah ME, IJzerman AP, Stiles GL, Jacobson KA (1994): A binding site model and structure-activity relationships for the rat A3 adenosine receptor. Mol Pharmacol 45:1101–1111. [PMC free article] [PubMed] [Google Scholar]
- von Lubitz DKJE, Lin RC-S, Popik P, Carter MF, Jacobson KA (1994): Adenosine A3 receptor stimulation and cerebral ischemia. Eur J Pharmacol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Jacobson KA (1990): Radioligand binding assays for adenosine receptors. In Williams M (ed): The Adenosine Receptors. Clifton, NJ: Humana Press, pp 17–55. [Google Scholar]
- Zhou QY, Li CY, Olah ME, Johnson RA, Stiles GL, Civelli O (1992): Molecular cloning and characterization of an adenosine receptor—the A3 adenosine receptor. Proc Natl Acad Sci USA 89:7432–7436. [DOI] [PMC free article] [PubMed] [Google Scholar]