Abstract
Background
In on-pump cardiac surgery, the albumin priming strategy could maintain colloid osmotic pressure better than crystalloid solutions and reduce excessive perioperative fluid balance. However, a high-quality meta-analysis is required to compare the safety of these approaches in perioperative red blood cell (RBC) transfusions. Owing to limited direct evidence, we conducted a network meta-analysis (NMA) to increase the pool of studies and provide indirect evidence.
Methods
The pre-defined primary outcomes were intraoperative and the first 24 h postoperative RBC transfusion volume in units. The pre-defined secondary outcome was postoperative blood loss (the first 24 h). We reviewed all randomized controlled trials comparing albumin, crystalloid, and artificial colloid priming strategies. Studies that only displayed pre-defined outcomes could be included. A pairwise meta-analysis was performed on studies that directly compared the pre-defined outcomes between albumin and crystalloids. Additionally, a random-effects network meta-analysis (NMA) model was employed to generate indirect evidence for the pre-defined outcomes between albumin and crystalloids.
Results
The literature search identified 830 studies,10 of which were included in the final analysis. Direct meta-analysis indicated that crystalloid priming significantly decreased total perioperative RBC transfusions (MD: -0.68U; 95%CI: -1.26, -0.09U; P = 0.02) and intraoperative RBC transfusions (MD: -0.20U; 95%CI: -0.39, -0.01U; P = 0.03) compared to albumin. Postoperative RBC transfusions showed a decreasing trend in the crystalloid group; however, the difference was not statistically significant. (MD: -0.16U; 95%CI: -0.45, 0.14U; P = 0.30). After including indirect evidence, the NMA results continued to demonstrate a higher RBC receiving with the albumin priming strategy compared to crystalloids, although the differences did not reach statistical significance. For postoperative blood loss, direct evidence showed no significant differences between albumin and crystalloid priming strategies. However, NMA evidence displayed that albumin exist higher probability of reducing postoperative blood loss than crystalloid.
Conclusion
Both direct and NMA evidence indicated that the albumin priming strategy resulted in more perioperative RBC transfusions than crystalloids. Considering the additional blood management burden, the application of an albumin-priming strategy in on-pump cardiac surgery still needs more consideration.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-024-02414-y.
Keywords: Cardiopulmonary bypass, Albumin, Crystalloid solutions, Red blood cell transfusion
Background
The optimal choice of priming fluid for cardiovascular surgery with cardiopulmonary bypass (CPB) remains uncertain. Compared to crystalloid solutions, the colloid CPB priming strategy has a more profound effect of volume expansion, decreases positive fluid balance, and requires less intraoperative fluid replacement biological [1]. As a normal endogenous colloid, albumin has more potential strength advances than other artificial colloids such as gelatines and hydroxyethyl starch (HES):1. Albumin can maintain the structural integrity of the glycocalyx and exert positive effects on several key physiological processes, such as preserving the vascular barrier, hemostasis, anti-inflammation, and restoration of microcirculation perfusion [2, 3]. 2. Albumin can coat the fluid circuit surface, thereby diminishing the contact between blood and nonbiological materials [4]. 3. Albumin could serve as an optimal component for sustaining colloid oncotic pressure to prevent tissue edema [5, 6]. However, there is a general lack of high-quality evidence for the superiority of the albumin priming strategy over crystalloids [7].
Red blood cell (RBC) transfusions are common practice for perioperative anemia in patients undergoing on-pump cardiovascular surgery and associated with adverse outcomes [8]. Applying hemostatic drugs such as tranexamic acid, finding sensitive physiological parameters to guide RBC transfusion, and designing effective blood management measures can all reduce unnecessary red blood cell transfusion, thus contributing to the prognosis of patients undergoing CPB surgery [8–10]. However, it should be noticed that the priming fluid of CPB can cause acute hemodilution, leading to an increase in the probability of perioperative blood transfusion [11]. Therefore, how to select appropriate priming solution to minimize perioperative RBC transfusion in CPB patients is the focus of clinical research. A recent large randomized controlled trial (RCT) has shown that the albumin priming strategy leads to more RBC transfusions and blood loss than crystalloids [12]. This has raised concerns regarding the albumin priming strategy. Therefore, there is a general need for an updated high-quality meta-analysis that focuses on the impact of albumin and crystalloid priming strategies on RBC transfusions.
Owing to the lack of direct RCTs comparing the priming strategy between albumin and crystalloids in RBC transfusions, we used network meta-analysis (NMA) technique to perform direct comparisons, including albumin vs. artificial colloid and artificial colloid vs. crystalloid, thus obtaining indirect evidence for the comparisons between albumin and crystalloid priming strategies. The small amounts of direct result and more indirect results obtained by NMA will further provide evidence for the difference in the effect of albumin and crystal priming strategies on RBC transfusion in cardiovascular surgery.
Methods
Design
The protocol was registered with the PROSPERO International Prospective Register of Systematic Reviews (PROSPERO; CRD42023435153). The explain for any amendments of the protocol was also provided on the PROSPERO register (CRD42023435153).The study adhered to the PRISMA extension statement for NMA [13] and the Meta-Analysis of Observational Studies in Epidemiology Statement [14]. The PRISMA 2020 checklist [15] of this study is included in Supplemental Table 1.
Eligibility criteria
The PICOS criteria table was shown in Supplemental Table 2.
Patients
Adult patients undergoing cardiovascular surgery with CPB.
Intervention/Comparator
Comparing CPB priming including human albumin or crystalloid directly or indirectly.
Outcomes
Primary outcomes: RBC transfusion volume in units at 24 h.
Secondary outcomes: postoperative blood loss (the first 24 h).
Study selection
RCT comparing at least two different classes of priming strategies among albumin, artificial colloids, and crystalloids. Furthermore, studies that only displayed pre-defined outcomes could be included.
Search strategy
A systematic search was performed using MEDLINE, EMBASE, Web of Science, and Cochrane Library. All English articles published before 1 JULY 2023 were selected. The search strategy is shown in Supplemental Fig. 1. The retrieval field is restricted to “topic” (the title, abstract, keywords and medical subject headings). Additionally, we conducted a snowball search by screening reference lists of published systematic reviews and eligible RCTs for potential consideration of further primary RCTs.
The literature search will be conducted by three researchers. Any disagreements regarding the inclusion of studies will be resolved through a process of discussion. In case of unresolved disagreements, a senior reviewer will be consulted for further input.
Data extraction
Two reviewers independently extracted the data. The standard data extraction form included the following general information.
Study characteristics: publication year, title, authors, contact address, country, duplicate publications and sponsoring.
Methods: randomization procedure, allocation, blinding method, duration of study, design, and statistics were used.
Patient characteristics: sex, age and BMI.
Surgery characteristics: surgery types, bypass time, ischemia time, priming volume and types.
RBC transfusion strategy: the RBC transfusion thresholds during CPB and after surgery were recorded.
Postoperative variable: ventilation time, intensive care unit (ICU) days, hospital days, postoperative acute kidney injury (AKI) rate and mortality.
Primary and secondary outcomes. Considering that most studies only presented intraoperative and postoperative RBC transfusions (the first 24 h) separately, both intraoperative and the first 24 h postoperative RBC transfusions were extracted for further analysis. Total perioperative RBC transfusions were combined intraoperatively and postoperatively (first 24 h). Only the data measured in units for RBC were retained. Postoperative blood loss was defined as blood loss or chest tube drainage within the first 24 h postoperatively.
For continuous outcomes, means with standard deviations or medians with interquartile ranges were extracted. Under the assumption of a normal distribution, we transformed the interquartile range into standard deviations according to the Cochrane Handbook for Systematic Reviews of Interventions (Part 2, Chap. 7.7.3.5). If the standard deviation was zero, the lowest standard deviation of another group within the study was used in the meta-analysis.
Risk of bias
The methodological quality of each trial was assessed using the Cochrane Risk of Bias Tool [16]. Three members of our team independently assessed the risk of bias in the trials based on the following six domains: allocation generation, allocation concealment, blinding, completeness of outcome data, possible selected outcome reporting, and any other potential sources of bias. Additionally, funnel plots were produced to assess reporting bias.
Direct meta-analysis
The direct meta-analysis included studies that directly compared the pre-defined outcomes between albumin and crystalloids. Continuous and dichotomous outcomes were presented as mean differences (MD) with 95% confidence intervals (CI) and odds ratios (RR) with 95% CI, respectively. For continuous data, the inverse variance model was used, whereas for dichotomous data, the Mantel-Haenszel model was used. Statistical heterogeneity was tested using the χ² test and I2 statistic. When there was no statistical evidence of heterogeneity (I2 < 30%, p > 0.1), a fixed-effects model was adopted; otherwise, a random-effects model was used.
Network meta-analysis
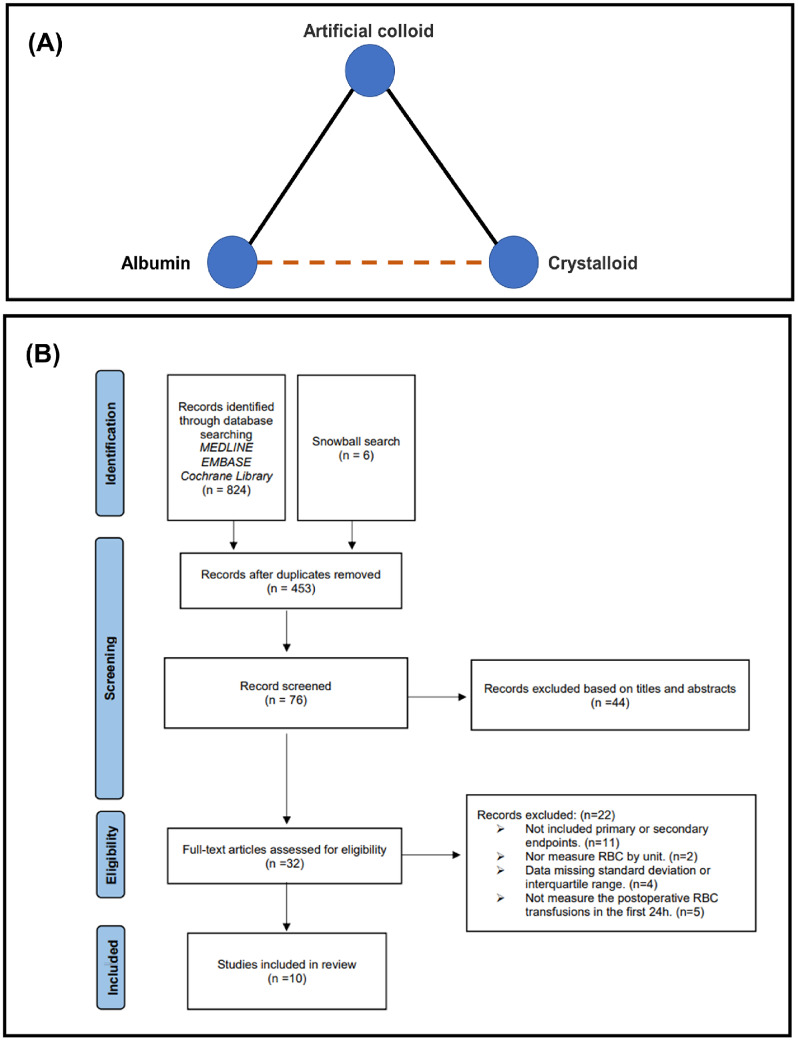
A random-effect NMA model was conducted within a Bayesian framework to combine direct and indirect evidence for the comparison between albumin and crystalloids [17]. The results were reported as MD or OR with a 95% credible interval (Crl). The surface under the cumulative ranking values (SUCRA) was calculated to hierarchically rank each priming strategy based on the probability of being the best for a given outcome, and priming strategies were ranked from best to worst based on progressively lower SUCRA [18]. The entire NMA focuses on whether there is a difference between the pre-defined outcomes of albumin and the crystal priming strategy after indirect inclusion. The network map is shown in Fig. 1A.
Fig. 1.
Management of the whole study. (A) Network map for meta-analytic priming strategies comparison: solid lines represent direct comparisons and dashed lines represent indirect comparisons. (B) Flow diagram for study selection
Transitivity analysis
As an extension of clinical and methodological homogeneity to comparisons across groups of studies, transitivity refers to the validity of indirect comparisons of a treatment network. To meet the transitivity assumption, we evaluated the included studies by comparing the characteristics of the population, intervention, and study design.
Heterogeneity analysis
Estimate the deviation of the heterogeneity variance parameter I2 and conduct a visual display to evaluate the overall heterogeneity of the NMA model [19].
Consistency analysis
We checked the evidence of consistency between direct and indirect analyses using node splitting analysis [20]. If P < 0.05, inconsistency was considered to exist between direct and indirect analyses.
Sensitivity analysis
Sensitivity analysis was conducted using the leave-one-out approach.
Meta-regression
Meta-regression was conducted to analyze the influence of the study-level variables with respect to estimates and variation in the final results.
Statistics
Data processing and direct meta-analysis were conducted using Review Manager (version 5.3). NMA and meta-regression were performed using the package “gemtc” in R (version 4.2.2). The “anohe” and “nodesplit” functions of “gemtc” package were used for heterogeneity and consistency analyses respectively. Funnel plots were generated using the package ‘netmeta’ in R (version 4.2.2).
Certainty assessment
The quality of each direct, indirect, and NMA estimate was rated based on the four-step approach suggested by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group [21]. We rated the certainty of the directed and indirect evidence as high, moderate, low, or very low, based on study limitations, publication bias, inconsistency, indirectness, and imprecision. The final quality of the NMA effect estimates was based on a combination of direct and circumstantial evidence quality ratings.
Results
Search results
A PRISMA flow diagram of the systematic search process is shown in Fig. 1B. Database and snowball searches yielded 830 records. After removing duplicates, 453 records were screened. After screening, 76 abstracts were read and 32 articles were selected for full-text reading. Eleven studies were excluded because they did not access pre-specified outcomes in the study. Additionally, 11 studies were excluded because they did not meet the pre-defined data extraction criteria. Articles excluded after full-text reading are displayed in Supplemental Table 3.
Finally, 10 studies were included in the analysis. For the comparison between albumin and artificial colloid, 4 studies were found [12, 22–24]; for the comparison between artificial colloid and crystalloid, 5 studies were included [23–27]; for the comparison between albumin and crystalloid, 5 studies were found [23, 24, 28–30]. A summary of the included studies is shown in Table 1.
Table 1.
Details of included studies
| Study | Number of patients | Cardiac surgery type | Total prime volume (mL) | Albumin group | Artificial Colloid group | Crystalloid group | Additional priming solutions | Intraoperative RBC transfusion (units) | Postoperative RBC transfusion (the 1st 24 h; units) | Total perioperative red blood cells transfusions (until 1st 24 h after surgery; units) | Postoperative blood loss or chest tube drainage | RBC transfusion thresholds |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ohqvist 1981 [1] | 14 | Valve | 2000 | 20% albumin 200 ml + Ringers 1800 ml | - | Ringers 2000 ml | - | - | - | - | ✔ | - |
| Marelli 1989 [2] | 100 | CABG; Valve | - | Additional 200 ml 25% albumin | - | Ringers | - | ✔ | - | - | - | - |
| Kuitunen 1993 [3] | 30 | CABG | 2000 | - | HES 6% 20 ml/kg + Ringers | Ringers 2000 ml | - | ✔ | - | - | - | During CPB: Hct > 20%; Postoperative criteria: Hct > 30%. |
| Scott 1995 [4] | 93 | CABG | 2000 (1600 for patients < 60 kg) | 4.6% albumin 1000 ml + Plasmalyte 1000 ml | Polygeline 1000 ml + Plasmalyte 1000 ml | Plasmalyte 2000 ml | - | - | - | - | Blood loss | During CPB: Hct > 18%; Postoperative criteria: not applied. |
| Tamayo 2008 [5] | 44 | CABG | 1750 | - | Gelatin 1000 ml + Ringers 500 ml | Ringers 1500 ml | 20% mannitol 100 ml; aprotinin 100 ml; 8.4% sodium bicarbonate 50 ml; heparin 5000IU | - | - | - | Blood loss | During CPB: Hct was maintained at 20–25%; Postoperative criteria: not applied. |
| Cho 2014 [6] | 36 | CABG; Valve; Aortic | - | 5% albumin 500 ml + Plasmalyte 1000 ml | 6% HES 130/0.4 500 ml + Plasmalyte 1000 ml (max 20 ml/kg.day HES) | - | 20% mannitol 5mL/kg; sodium bicarbonate 40mEq; heparin 10 mg/L | ✔ | ✔ | - | Blood loss | During CPB: Hct > 20%; Postoperative criteria: Hct > 25%. |
| Skhirtladze 2014 [7] | 236 | CABG; Valve; Aortic | 1500 | 5% albumin (max 50 ml/kg.day) + Ringers | 6% HES 130/0.4 (max 50 ml/kg.day) + Ringers | Ringers 1500 ml | 20% mannitol 100 ml; heparin 5000 IU | ✔ | ✔ | ✔ | Chest tube drainage | During CPB: Hb > 7 g/dL; Postoperative criteria: Hb > 8-9 g/dL. |
| Yanartas 2015 [8] | 132 | CABG | 1500 | - | 6% HES 130/0.4 10 ml/kg + Ringers 10 ml/kg | Ringers 20 ml/kg | 20% mannitol 0.5 g/kg; 7.5% sodium bicarbonate 1 ml/kg; heparin 150IU/kg | ✔ | ✔ | - | Chest tube drainage | During CPB: Hct was maintained at 22–28%; Postoperative criteria: Hct was maintained at 30%. |
| Maleki 2016 [9] | 60 | CABG | 1500 | 5% albumin 500 ml + 0.9% NaCl 1000 ml | 6% HES 130/0.4 500 ml + 0.9% NaCl 1000 ml | - | 20% Mannitol 5mL/kg; sodium bicarbonate 45mEq; heparin 10 mg/L | - | - | ✔ | Blood loss | During CPB: Hb > 7 g/dL; Postoperative criteria: Hct > 21%. |
| Talvasto 2023 [10] | 1386 | CABG; Valve; Aortic | 1500 | 20% albumin 300 ml + 1200 ml Ringers | - | Ringers 1500 ml | - | ✔ | ✔ | ✔ | - | Based on clinical judgement |
Direct meta-analysis
RBC transfusions
Two studies compared the total perioperative RBC transfusions (up to the first 24 h after surgery) between albumin and crystalloids [12, 24]. Detailed intraoperative RBC transfusions and postoperative RBC transfusions were also reported in these two articles. In addition, Marelli et al. presented the intraoperative RBC receiving comparing albumin and crystalloid solutions [22]. The results are shown in Fig. 2. Overall, total perioperative RBC transfusions decreased in crystalloids compared with albumin (Fig. 2A, MD: -0.68U; 95%CI: -1.26, -0.09U; P = 0.02). Furthermore, intraoperative RBC transfusions were significantly reduced in the crystalloid group (Fig. 2B, MD: -0.20U; 95%CI: -0.39, -0.01U; P = 0.03). Postoperative RBC transfusions showed a decreasing trend in the crystalloid group; however, the difference was not statistically significant. (Fig. 2C, MD: -0.16U; 95%CI: -0.45, 0.14U; P = 0.30).
Fig. 2.
Direct meta-analysis. (A) Total perioperative red blood cells transfusions (until the first 24 h after surgery). (B) Intraoperative red blood cells transfusions. (C) Postoperative red blood cells transfusions during the first 24 h. (D) Postoperative blood loss or chest tube drainage during the first 24 h. CI, Confidence Interval
Postoperative blood loss
Postoperative blood loss or chest tube drainage in the first 24 h after surgery comparing albumin and crystalloid groups was reported in only three studies [23, 24, 30]. Meta-analysis of the pooled results showed no significant difference in postoperative blood loss (Fig. 2D, MD: 12.59 ml; 95%CI: -314.25, 339.43 ml; P = 0.94).
Network meta-analysis
Due to the unavailability of data extracted from the included studies to facilitate an indirect analysis of the overall perioperative RBC transfusions, we conducted NMA for intraoperative and postoperative (the first 24 h) RBC transfusions. Postoperative blood loss was also observed in the NMA group. The details for above outcomes of the included studies on NMA is shown in Supplemental Table 4.
NMA analysis showed decreasing intraoperative and postoperative RBC transfusions and increasing postoperative blood loss trends in the crystalloid group compared with those in the albumin group (Fig. 3A). Because the 95% credible intervals all contain 0, the interpretability of the results is limited.
Fig. 3.
Tables with respect to NMA results. (A) Green color indicates favorable and red unfavorable mean difference with respective 95% credible interval. (B) The Surface Under the Cumulative Ranking (SUCRA) values represent the cumulative probability for each priming to being the best reduce RBC transfusions and blood loss (the closer the value is to 100%, the higher the likelihood that a priming is in the top rank). NMA, network meta-analysis
Furthermore, probability rank analysis was conducted. Based on the SUCRA values, the crystalloid priming strategy had a higher probability of reducing intraoperative and postoperative RBC transfusions but a lower probability of reducing postoperative blood loss compared with the albumin priming strategy (Fig. 3B).
Risk of bias
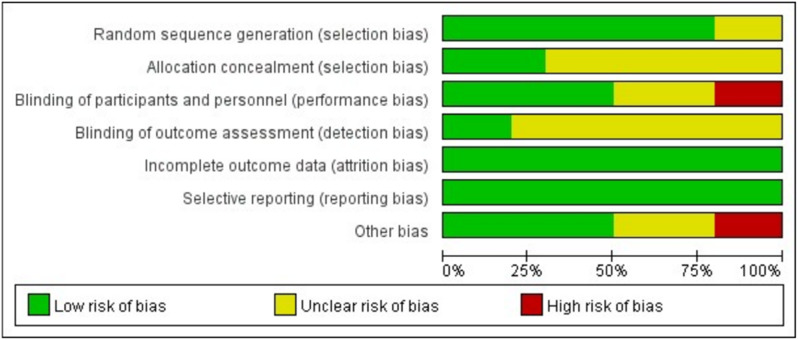
The final risk of bias graph is shown in Fig. 4. The bias in each included study is summarized in Supplemental Fig. 2.
Fig. 4.
Risk of bias graph
Selection bias
The standard randomization method was described in 8 studies. The correct allocation concealment was achieved in 2 studies [12, 24].
Performance bias
Proper double-blinding of the participants and personnel was achieved in 55.6% of the included RCTs. Three studies that not blinded the perfusionist [22, 23, 26], were considered to have unclear risk of bias. Two studies did not blind nurses, anesthesiologists, and surgeons [27, 30], which was considered to have a high risk of bias.
Detection bias
Adequate blinding of the outcome assessment was ensured in only 2 studies [12, 28] and was not mentioned in 7 studies.
Attrition bias
All studies reported reasons for dropouts. Thus, the attrition bias was considered low in all studies.
Other potential bias
Priming volume was not reported in 2 studies [22, 28] and the perioperative RBC transfusion threshold was not mentioned in 2 studies [12, 30]. Therefore, the unclear risk of other potential biases was considered in these 3 studies. In 1 study, tranexamic acid was used, which may affect the pre-specified outcomes (RBC transfusions and blood loss) and can be considered a high risk of bias. Owing to the low number of included studies, a funnel plot to assess publication bias was not conducted.
Reporting bias
Funnel plots for NMA analysis are provided in Supplementary Fig. 3 and did not suggest small study bias.
Transitivity, heterogeneity, consistency and sensitivity analysis
We analyzed the distribution of baseline variables in the included studies between different priming strategies to assess transitivity. In either comparison, the difference between baseline variables was small (Supplemental Table 5). Supplemental Fig. 3 shows the results of the heterogeneity analysis. Supplemental Fig. 4 shows the results of the node-split consistency analysis. The P-values of all direct and indirect comparisons were greater than 0.05, indicating good consistency.
The leave-one-out sensitivity analysis results are displayed in Supplemental Table 6. There was no variation in the NMA analysis of intraoperative and postoperative (the first 24 h) RBC transfusions after changing study inputs. However, excluding Scott et al. changed the final SUCRA rank between albumin and crystalloid in postoperative blood loss (albumin had a lower SUCRA value than crystalloid). This suggests high heterogeneity among the included articles for meta-analysis.
Meta-regression analysis
Supplementary Fig. 6 presents the results of the meta-regression analysis. Six important study-level factors (sample size, age, surgery type, male proportion, CPB time, and aortic cross-clamping time) were included for meta-regression analysis to explore the potential heterogeneity sources of NMA results. The results of meta-regression revealed that the inclusion of the above variables has no significant effect on the final NMA outcomes.
Others
In addition, we analyzed the main postoperative factors in the included studies. There were no significant differences in ventilation time, ICU stay, hospital stay, AKI rate, and mortality among the three priming strategies. However, albumin priming strategy existed lower postoperative Hb counts than crystalloid (Supplemental Table 7, MD: -1.05 g/dl; 95%CI: -1.35, -0.74 g/dl; P < 0.01).
The certainty of evidence assessment according to the GRADE method is presented in Table 2.
Table 2.
Certainty of evidence assessment
| Crystalloid vs. Albumin | |||||||
|---|---|---|---|---|---|---|---|
| Outcome | Direct evidence | Indirect evidence (Back-calculated) | Network meta-analysis | ||||
| Mean Difference (95% confidence interval) | Quality | Mean Difference (95% credible interval) | Quality | Mean Difference (95% credible interval) | Quality | ||
| Intraoperative RBC transfusions | -0.20 [-0.39, -0.01] | Moderate† | 0.11 [-0.90, 1.10] | Low*¶ | -0.14 [-0.70, 0.46] | Low | |
| Postoperative RBC transfusions | -0.16 [-0.45, 0.14] | Moderate† | 0.25 [-1.10, 1.60] | Low*¶ | -0.16 [-0.43, 0.14] | Low | |
| Postoperative blood loss | 12.59 [-314.25, 339.43] | Low†‡¶ | 132 [-290, 560] | Low**‡¶ | 71 [-151, 280] | Low | |
| Total perioperative RBC transfusions | -0.68 [-1.26, -0.09] | High | - | - | - | - | |
¶Limitations (risk of bias). †Inconsistency. ‡Imprecision. *Contributing direct evidence of moderate quality. **Contributing direct evidence of low or very low quality
Discussion
In this study, we aimed to evaluate the safety of albumin and crystalloid priming strategies from a blood management perspective. The direct meta-analysis revealed that albumin priming strategy can increase perioperative RBC transfusions. After conducting NAM incorporating indirect analysis, albumin still displayed an increasing trend in RBC transfusions compared to crystalloid solutions. Additionally, we did not explore any differences in postoperative blood loss between albumin and crystalloid priming strategies in this study.
The main potential strength of albumin is that it can interact with vascular endothelial cells to maintain the integrity of the endothelial glycocalyx layer (EGL) [31]. EGL can be considered a dynamic layer between the vascular endothelial cell and the blood, capable of accommodating a significant amount of non-circulating plasma volume, providing 60% of the intravascular colloid osmotic pressure (COP) in the body [3]. During CPB, mechanical blood perfusion can lead to poor microcirculation and increased shear forces on local blood flow [32, 33]. Complete EGL can improve microcirculation by transmitting shear force signals, and its barrier function can cushion contact between blood cells and the endothelium [34, 35].
Previous clinical evidence suggests that adding albumin to the CPB priming solution can effectively preserve COP and reduce overall perioperative fluid transfusion, avoiding concomitant edema that can compromise organ function [36, 37]. However, the albumin priming strategy did not demonstrate an advantage in hemostasis effectiveness and even showed a detrimental effect. In a previous RCT comparing albumin, hydroxyethyl starch, and crystalloid solutions in 240 patients undergoing cardiac surgery with CPB, patients in the albumin group received RBC transfusions most frequently [24]. A recent randomized, double-blind, single-center clinical trial (The Albumin in Cardiac Surgery trial; ALBICS) involving 1386 patients undergoing on-pump cardiac surgery compared the safety of 4% albumin and Ringer acetate in CPB priming and intravenous volume replacement perioperatively. The results indicated that the albumin group received more RBC transfusions and had clinically significant bleeding [38]. In our study, these two RCTs were also the primary sources of the direct meta-analysis, thus yielding results demonstrating the association between the albumin priming strategy and an increase in RBC transfusions. Considering the limited amount of direct evidence, we conducted a NMA to expand the sample pool. The NMA results still indicated a higher RBC transfusion with albumin priming than with crystalloids, but there was no statistically significant difference. It should be noted that our study exhibited large differences in quality of evidence between the direct and indirect evidence. Although there are few studies that provide direct evidence, the sample size of these studies is large and the research quality is high. In contrast, RCTs that provide indirect evidence are mostly old and have a small sample size, which results in low-quality evidence. Therefore, despite the lack of statistical difference in NMA results, our study still supports that the albumin priming strategy could increase perioperative RBC transfusion compared to crystalloid solutions.
Previous animal experiments have provided evidence that endogenous albumin may exert concentration-dependent anticoagulant effects [39]. Moreover, in previous clinical studies, patients in the albumin priming group had lower Hb concentrations after surgery, which was also observed in this study [12, 23, 24]. The more profound hemodilution effect of albumin, leading to an earlier reduction in Hb, might explain the increased RBC receiving albumin administration [24]. However, excessive hemodilution of albumin may not have serious consequences on postoperative blood loss. In addition, the final concentration of albumin in crystalloid solution is also an important consideration. A recent network meta-analysis revealed that the use of iso-oncotic HA (4-5% final concentration) was associated with the lowest blood loss in the first 24 h after surgery compared with other priming strategies. In contrast, hyper-oncotic HA (25% final concentration) resulted in the longest hospital stay and CPB time compared to other priming strategies, suggesting the poor effect of hyper-oncotic HA [40]. In our meta-analysis, all included albumin priming strategies were isotonic. Our NMA evidence also showed that albumin exist high probability of reducing postoperative blood loss. Even so, it is still imperative to evaluate the suitability of the albumin priming strategies in specific populations. Additional intraoperative albumin supplementation may be necessary in patients with hypoalbuminemia since perioperative hypoalbuminemia has been proven to be independently associated with increased morbidity and mortality after cardiac operations [41, 42]. Furthermore, the infusion volume of the experimental fluid was also a key point. It is interesting that the two RCTs, which provided the most direct evidence in our study, conducted the entire perioperative fluid administration comparison (priming and perioperative volume replacement) [12, 24] thus, we speculate that the impact of albumin on hemostasis is related to the transfusion volume in cardiac surgery. In other words, more studies are needed to evaluate the hemostasis between albumin and crystalloid solutions when considering the priming solution alone or even applying a minimally invasive extracorporeal circulation (MiECC) system to reduce the priming volume.
Limitations
This study has some limitations. First, in the process of article screening and data extraction, to minimize heterogeneity, we defined the postoperative time as within 24 h and standardized the RBC measurements, leading to the exclusion of many studies. However, this ensured that the studies ultimately included in the analysis had a lower potential bias and higher credibility of the obtained results. Second, the varieties of priming strategies among different centers, including the types of crystals or artificial colloids, concentration of albumin, and priming volume, would be uncontrollable factors in this analysis. Third, the included studies were not consistent in perioperative blood management, especially in terms of the different thresholds for perioperative RBC transfusions. This inconsistency may also have lowered the quality of the evidence in this study. Fourth, this study did not differentiate between artificial colloids, such as gelatin and HES, in the analysis. Owing to concerns about the nephrotoxicity of HES, many centers tend to prefer gelatin as a colloid preloading solution [43, 44]. However, there is currently no clear evidence indicating a significant difference in hemostatic effectiveness between gelatin and HES preloading strategies [45, 46], but combining different types of artificial colloids could potentially affect research evidence.
Strength
Despite above limitations, the whole report adhered rigorously to the protocol registered with PROSPERO, and all pre-defined outcomes were carefully analyzed. As the first NMA study to compare the impact of RBC transfusions between albumin and crystalloid priming strategies, the results of this study would be of significant value in optimizing blood preservation measures in patients undergoing cardiovascular surgery with CPB.
Conclusion
Direct evidence has revealed that the albumin priming strategy has a significant effect on perioperative RBC transfusion. After the inclusion of indirect evidence, the NMA results further indicated that the albumin priming strategy resulted in more perioperative RBC receiving than crystalloids, although the statistical significance was limited. More consideration should be given to the application of the albumin priming strategy in on-pump cardiac surgery. Future research should focus on the safety and effectiveness of albumin and crystalloid priming strategies in specific populations (such as patients with hypoalbuminemia) or in the MiECC system.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Supplemental Figure 1. Search strategy.
Supplementary Material 2: Supplemental Figure 2. Summary of the risk of bias among the randomized controlled trials using the Cochrane Risk of Bias tool.
Supplementary Material 3: Supplemental Figure 3. Reporting bias funnel plots for network meta-analysis.
Supplementary Material 4: Supplemental Figure 4. Analysis of heterogeneity.
Supplementary Material 5: Supplemental Figure 5. Node-splitting analysis of inconsistency.
Supplementary Material 6: Supplemental Figure 6. Meta-regression for network meta-analysis.
Supplementary Material 7: Supplemental Table 1. PRISMA (Preferred Reporting Items for Systematic review and Meta-Analysis) 2020 checklist: an updated guideline for reporting systematic reviews.
Supplementary Material 8: Supplemental Table 2. PICOS criteria for the network meta-analysis.
Supplementary Material 9: Supplemental Table 3. Articles excluded after full-text reading.
Supplementary Material 10: Supplemental Table 4. Details for pre-defined outcomes of studies included for network meta-analysis.
Supplementary Material 11: Supplemental Table 5. Baseline variables of the included studies.
Supplementary Material 12: Supplemental Table 6. Leave-one-out sensitivity analysis for network meta-analysis.
Supplementary Material 13: Supplemental Table 7. Main postoperative variables of the included studies.
Abbreviations
- CPB
cardiopulmonary bypass
- HES
hydroxyethyl starch
- RBC
red blood cell
- RCT
randomized controlled trial
- NMA
network meta-analysis
- ICU
intensive care unit
- AKI
acute kidney injury
- MD
mean differences
- CI
confidence intervals
- Crl
credible interval
- SUCRA
surface under the cumulative ranking values
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- EGL
glycocalyx layer
- COP
colloid osmotic pressure
- MiECC
minimally invasive extracorporeal circulation
Author contributions
T.W., J.W. and H.Z. systematic search; M.Z and Q.Z. data extraction; T.W. writing—original draft preparation; Y.W. writing—review and editing; T.W. and W.D. prepared figures; T.W. and J.W. prepared tables; T.W., M.Z. and Q.Z. prepared supplemental materials; G.L. software; T.W. and G.L. PROSPERO registration; B.J. conceptualization and funding acquisition. All authors reviewed the manuscript.
Funding
This study was supported by the National High Level Hospital Clinical Research Funding (Grant number: 2023-GSP-GG-7).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Obradovic M, Kurz A, Kabon B, Roth G, Kimberger O, Zotti O, Bayoumi A, Reiterer C, Stift A, Fleischmann E. The effect of intraoperative goal-directed crystalloid versus colloid administration on perioperative inflammatory markers - a substudy of a randomized controlled trial. BMC Anesthesiol. 2020;20(1):210. doi: 10.1186/s12871-020-01126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang G, Zhang H, Liu D, Wang X, Chinese Critical Ultrasound Study G. Resuscitation fluids as drugs: targeting the endothelial glycocalyx. Chin Med J (Engl) 2022;135(2):137–44. doi: 10.1097/CM9.0000000000001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belinskaia DA, Voronina PA, Goncharov NV. Integrative role of Albumin: evolutionary, biochemical and pathophysiological aspects. J Evol Biochem Physiol. 2021;57(6):1419–48. doi: 10.1134/S002209302106020X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doguet F, Tamion F, Le Guillou V, Bubenheim M, Thuillez C, Richard V, Bessou JP. Albumin limits mesenteric endothelial dysfunction and inflammatory response in cardiopulmonary bypass. Artif Organs. 2012;36(11):962–71. doi: 10.1111/j.1525-1594.2012.01492.x. [DOI] [PubMed] [Google Scholar]
- 5.Beukers AM, de Ruijter JAC, Loer SA, Vonk A, Bulte CSE. Effects of crystalloid and colloid priming strategies for cardiopulmonary bypass on colloid oncotic pressure and haemostasis: a meta-analysis. Interact Cardiovasc Thorac Surg 2022, 35(3). [DOI] [PMC free article] [PubMed]
- 6.Patel J, Prajapati M, Solanki A, Pandya H. Comparison of Albumin, Hydroxyethyl Starch and Ringer Lactate Solution as Priming Fluid for cardiopulmonary bypass in paediatric cardiac surgery. J Clin Diagn Res. 2016;10(6):UC01–04. doi: 10.7860/JCDR/2016/18465.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanley C, Callum J, Karkouti K, Bartoszko J. Albumin in adult cardiac surgery: a narrative review. Can J Anaesth. 2021;68(8):1197–213. doi: 10.1007/s12630-021-01991-7. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Zhao W, Gao S, Yan S, Diao X, Wang Y, Xu X, Tian Y, Ji B. Quality Management of a Comprehensive Blood Conservation Program during Cardiopulmonary Bypass. Ann Thorac Surg. 2022;114(1):142–50. doi: 10.1016/j.athoracsur.2021.07.069. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, Zhou C, Pan W, Sun H, Liu S, Feng W, Wang W, Cheng Z, Wang Y, Zheng Z, et al. Effect of high- vs low-dose tranexamic acid infusion on need for red blood cell transfusion and adverse events in patients undergoing cardiac surgery: the OPTIMAL randomized clinical trial. JAMA. 2022;328(4):336–47. doi: 10.1001/jama.2022.10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putaggio A, Tigano S, Caruso A, La Via L, Sanfilippo F. Red blood cell transfusion guided by Hemoglobin only or integrating perfusion markers in patients undergoing cardiac surgery: a systematic review and Meta-analysis with Trial Sequential Analysis. J Cardiothorac Vasc Anesth. 2023;37(11):2252–60. doi: 10.1053/j.jvca.2023.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Rosenthal T, Kunselman AR, Undar A. Evaluation of different diameter arterial tubing and arterial cannulae in simulated neonatal/pediatric cardiopulmonary bypass circuits. Artif Organs. 2015;39(1):43–52. doi: 10.1111/aor.12446. [DOI] [PubMed] [Google Scholar]
- 12.Talvasto A, Ilmakunnas M, Raivio P, Vlasov H, Hiippala S, Suojaranta R, Wilkman E, Petaja L, Helve O, Juvonen T, et al. Albumin infusion and blood loss after cardiac surgery. Ann Thorac Surg. 2023;116(2):392–9. doi: 10.1016/j.athoracsur.2023.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Hutton B, Catala-Lopez F, Moher D. [The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA] Med Clin (Barc) 2016;147(6):262–6. doi: 10.1016/j.medcli.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Manchikanti L, Datta S, Smith HS, Hirsch JA. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: part 6. Systematic reviews and meta-analyses of observational studies. Pain Physician. 2009;12(5):819–50. doi: 10.36076/ppj.2009/12/819. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teirlinck CH, Verhagen AP, Reijneveld EAE, Runhaar J, van Middelkoop M, van Ravesteyn LM, Hermsen L, de Groot IB, Bierma-Zeinstra SMA. Responders to Exercise Therapy in patients with osteoarthritis of the hip: a systematic review and Meta-analysis. Int J Environ Res Public Health 2020, 17(20). [DOI] [PMC free article] [PubMed]
- 17.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–24. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 18.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–71. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 19.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285–99. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 20.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–44. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 21.Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, Kessels AG, Guyatt GH, Group GW. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 22.Marelli D, Paul A, Samson R, Edgell D, Angood P, Chiu RC. Does the addition of albumin to the prime solution in cardiopulmonary bypass affect clinical outcome? A prospective randomized study. J Thorac Cardiovasc Surg. 1989;98(5 Pt 1):751–6. doi: 10.1016/S0022-5223(19)34297-7. [DOI] [PubMed] [Google Scholar]
- 23.Scott DA, Hore PJ, Cannata J, Masson K, Treagus B, Mullaly J. A comparison of albumin, polygeline and crystalloid priming solutions for cardiopulmonary bypass in patients having coronary artery bypass graft surgery. Perfusion. 1995;10(6):415–24. doi: 10.1177/026765919501000605. [DOI] [PubMed] [Google Scholar]
- 24.Skhirtladze K, Base EM, Lassnigg A, Kaider A, Linke S, Dworschak M, Hiesmayr MJ. Comparison of the effects of albumin 5%, hydroxyethyl starch 130/0.4 6%, and Ringer’s lactate on blood loss and coagulation after cardiac surgery. Br J Anaesth. 2014;112(2):255–64. doi: 10.1093/bja/aet348. [DOI] [PubMed] [Google Scholar]
- 25.Kuitunen A, Hynynen M, Salmenperä M, Heinonen J, Vahtera E, Verkkala K, Myllylä G. Hydroxyethyl starch as a prime for cardiopulmonary bypass: effects of two different solutions on haemostasis. Acta Anaesthesiol Scand. 1993;37(7):652–8. doi: 10.1111/j.1399-6576.1993.tb03783.x. [DOI] [PubMed] [Google Scholar]
- 26.Tamayo E, Alvarez FJ, Alonso O, Castrodeza J, Bustamante R, Gómez-Herreras JI, Florez S, Rodríguez R. The inflammatory response to colloids and crystalloids used for pump priming during cardiopulmonary bypass. Acta Anaesthesiol Scand. 2008;52(9):1204–12. doi: 10.1111/j.1399-6576.2008.01758.x. [DOI] [PubMed] [Google Scholar]
- 27.Yanartas M, Baysal A, Aydın C, Ay Y, Kara I, Aydın E, Cevirme D, Köksal C, Sunar H. The effects of tranexamic acid and 6% hydroxyethyl starch (HES) solution (130/0.4) on postoperative bleeding in coronary artery bypass graft (CABG) surgery. Int J Clin Exp Med. 2015;8(4):5959–71. [PMC free article] [PubMed] [Google Scholar]
- 28.Cho JE, Shim JK, Song JW, Lee HW, Kim DH, Kwak YL. Effect of 6% hydroxyethyl starch 130/0.4 as a priming solution on coagulation and inflammation following complex heart surgery. Yonsei Med J. 2014;55(3):625–34. doi: 10.3349/ymj.2014.55.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosseinzadeh Maleki M, Derakhshan P, Rahmanian Sharifabad A, Amouzeshi A. Comparing the effects of 5% albumin and 6% hydroxyethyl starch 130/0.4 (Voluven) on renal function as Priming solutions for cardiopulmonary bypass: a Randomized double blind clinical trial. Anesth Pain Med. 2016;6(1):e30326. doi: 10.5812/aapm.30326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohqvist G, Settergren G, Bergstrom K, Lundberg S. Plasma colloid osmotic pressure during open-heart surgery using non-colloid or colloid priming solution in the extracorporeal circuit. Scand J Thorac Cardiovasc Surg. 1981;15(3):251–5. doi: 10.3109/14017438109100582. [DOI] [PubMed] [Google Scholar]
- 31.Torres Filho IP, Torres LN, Salgado C, Dubick MA. Plasma syndecan-1 and heparan sulfate correlate with microvascular glycocalyx degradation in hemorrhaged rats after different resuscitation fluids. Am J Physiol Heart Circ Physiol. 2016;310(11):H1468–1478. doi: 10.1152/ajpheart.00006.2016. [DOI] [PubMed] [Google Scholar]
- 32.den Os MM, van den Brom CE, van Leeuwen ALI, Dekker NAM. Microcirculatory perfusion disturbances following cardiopulmonary bypass: a systematic review. Crit Care. 2020;24(1):218. doi: 10.1186/s13054-020-02948-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayes K, Fernando MC, Jordan V. Prothrombin complex concentrate in cardiac surgery for the treatment of coagulopathic bleeding. Cochrane Database Syst Rev. 2022;11(11):CD013551. doi: 10.1002/14651858.CD013551.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers GJ, Wegner J. Endothelial glycocalyx and cardiopulmonary bypass. J Extra Corpor Technol. 2017;49(3):174–81. doi: 10.1051/ject/201749174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. 2019;23(1):16. doi: 10.1186/s13054-018-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell JA, Navickis RJ, Wilkes MM. Albumin versus crystalloid for pump priming in cardiac surgery: meta-analysis of controlled trials. J Cardiothorac Vasc Anesth. 2004;18(4):429–37. doi: 10.1053/j.jvca.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 37.Rex S, Scholz M, Weyland A, Busch T, Schorn B, Buhre W. Intra- and extravascular volume status in patients undergoing mitral valve replacement: crystalloid vs. colloid priming of cardiopulmonary bypass. Eur J Anaesthesiol. 2006;23(1):1–9. doi: 10.1017/S0265021505001687. [DOI] [PubMed] [Google Scholar]
- 38.Pesonen E, Vlasov H, Suojaranta R, Hiippala S, Schramko A, Wilkman E, Eränen T, Arvonen K, Mazanikov M, Salminen US, et al. Effect of 4% albumin solution vs Ringer acetate on major adverse events in patients undergoing cardiac surgery with cardiopulmonary bypass: a Randomized Clinical Trial. JAMA. 2022;328(3):251–8. doi: 10.1001/jama.2022.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paar M, Rossmann C, Nusshold C, Wagner T, Schlagenhauf A, Leschnik B, Oettl K, Koestenberger M, Cvirn G, Hallstrom S. Anticoagulant action of low, physiologic, and high albumin levels in whole blood. PLoS ONE. 2017;12(8):e0182997. doi: 10.1371/journal.pone.0182997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xian-Yu CY, Xu JB, Ma YT, Deng NJ, Tao YT, Li HJ, Gao TY, Yang JY, Zhang C. Management of priming fluids in cardiopulmonary bypass for adult cardiac surgery: network meta-analysis. Ann Med. 2023;55(2):2246996. doi: 10.1080/07853890.2023.2246996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berbel-Franco D, Lopez-Delgado JC, Putzu A, Esteve F, Torrado H, Farrero E, Rodriguez-Castro D, Carrio ML, Landoni G. The influence of postoperative albumin levels on the outcome of cardiac surgery. J Cardiothorac Surg. 2020;15(1):78. doi: 10.1186/s13019-020-01133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padkins M, Breen T, Anavekar N, Barsness G, Kashani K, Jentzer JC. Association between Albumin Level and Mortality among Cardiac Intensive Care Unit patients. J Intensive Care Med. 2021;36(12):1475–82. doi: 10.1177/0885066620963875. [DOI] [PubMed] [Google Scholar]
- 43.Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, Madsen KR, Moller MH, Elkjaer JM, Poulsen LM, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124–34. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 44.Schramko A, Suojaranta-Ylinen R, Niemi T, Pesonen E, Kuitunen A, Raivio P, Salmenperä M. The use of balanced HES 130/0.42 during complex cardiac surgery; effect on blood coagulation and fluid balance: a randomized controlled trial. Perfusion. 2015;30(3):224–32. doi: 10.1177/0267659114540022. [DOI] [PubMed] [Google Scholar]
- 45.Bethlehem I, Wierda K, Visser C, Jekel L, Koopmans M, Kuiper MA. Influence of two colloidal extracorporeal primes on Coagulation of Cardiac Surgical patients: a prospectively randomized open-label pilot trial. J Extra Corpor Technol. 2014;46(4):293–9. doi: 10.1051/ject/201446293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghijselings I, Himpe D, Rex S. Safety of gelatin solutions for the priming of cardiopulmonary bypass in cardiac surgery: a systematic review and meta-analysis. Perfusion. 2017;32(5):350–62. doi: 10.1177/0267659116685418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplemental Figure 1. Search strategy.
Supplementary Material 2: Supplemental Figure 2. Summary of the risk of bias among the randomized controlled trials using the Cochrane Risk of Bias tool.
Supplementary Material 3: Supplemental Figure 3. Reporting bias funnel plots for network meta-analysis.
Supplementary Material 4: Supplemental Figure 4. Analysis of heterogeneity.
Supplementary Material 5: Supplemental Figure 5. Node-splitting analysis of inconsistency.
Supplementary Material 6: Supplemental Figure 6. Meta-regression for network meta-analysis.
Supplementary Material 7: Supplemental Table 1. PRISMA (Preferred Reporting Items for Systematic review and Meta-Analysis) 2020 checklist: an updated guideline for reporting systematic reviews.
Supplementary Material 8: Supplemental Table 2. PICOS criteria for the network meta-analysis.
Supplementary Material 9: Supplemental Table 3. Articles excluded after full-text reading.
Supplementary Material 10: Supplemental Table 4. Details for pre-defined outcomes of studies included for network meta-analysis.
Supplementary Material 11: Supplemental Table 5. Baseline variables of the included studies.
Supplementary Material 12: Supplemental Table 6. Leave-one-out sensitivity analysis for network meta-analysis.
Supplementary Material 13: Supplemental Table 7. Main postoperative variables of the included studies.
Data Availability Statement
No datasets were generated or analysed during the current study.