Abstract
Background:
Pediatric patients with advanced cancer and their caregivers have unique psychosocial needs. Anxiety often worsens throughout treatment for both patients and parents, and, if undertreated, can cause suffering. Animal-assisted interaction (AAI) incorporates animals into patient care in a structured manner for the purpose of therapeutic benefit.
Objective:
To evaluate feasibility of incorporating AAI into patient care and to assess AAI effectiveness in decreasing patient and caregiver anxiety in pediatric patients with advanced cancer, defined by relapsed or refractory disease.
Design:
Randomized controlled study.
Setting/Subjects:
Participants were children (n = 19) and parents (n = 21) who were randomized to AAI group or usual care (UC) group.
Measures:
Participants completed weekly measures to assess anxiety, including the 20-question State-Trait Anxiety Inventory (STAI).
Results:
Our results demonstrated feasibility of the use of AAI in children with advanced cancer. While they did not reveal a significant difference in anxiety scores over the four sessions in either group, parents randomized to the AAI group had lower STAI State subscores at initial visit in comparison to the UC group. The difference in initial STAI State anxiety scores for caregivers may indicate a positive effect of AAI in reducing anxiety surrounding appointments through anticipation of seeing a therapy dog.
Conclusion:
Further research is needed to determine the effectiveness of AAI in pediatric patients with advanced cancer and their caregivers, but results are promising that participation in AAI may lessen caregiver anxiety.
Clinical Trial Registration Number is: NCT03765099
Keywords: animal-assisted interaction, pediatric cancer, pediatric palliative care
Background
Approximately 10,470 children in the United States ages 0 to 14 years were diagnosed with cancer in 2022. Of these, 1050 are expected to die related to their disease.1 While outcomes for children and adolescents with cancer have improved over the last 50 years, it remains a leading cause of death in the pediatric population. Historically, pediatric cancer research focused on survival rates and treatment-related toxicities. However, with improvement in outcomes, research focus has shifted to include not only evaluation of physical functioning but also, importantly, psychosocial and quality of life measures as well.2
Children and adolescents undergoing cancer treatment experience a variety of complex psychosocial symptoms including anxiety, depression, worry, sadness, difficulty with communication, loss of independence, and fear of being alone.3 These symptoms are exacerbated by limited opportunities to spend time with peers, physical symptoms from chemotherapy treatments, and changes in appearance. Patients with advanced disease, defined in our study as relapsed or refractory cancer, may face a higher burden of stress due to fear and anxiety surrounding prognosis. If untreated, this anxiety can lead to diminished quality of life for both patients and families.4–6
Parents of children with advanced cancer diagnoses likewise undergo significant emotional distress due to their child's vulnerability and new limitations to daily living. Tension exists to preserve the parent–child relationship and to communicate effectively with health care providers.7 Parental distress has been correlated with parents' perception of their child's suffering.8 Interventions to alleviate patient suffering may decrease parental psychological distress. Mitigation of parental distress may also enhance children's well-being.
Given that children with advanced cancer and their caregivers have unique psychosocial needs, providers should determine effective methods to address these symptoms. Animal-assisted interactions (AAIs) have been shown to help with communication and socialization and alleviate many symptoms including anxiety, pain, and feelings of worry, sadness, or anger.9,10 Many prior studies have focused on AAI's acute effect on anxiety during hospitalization or surrounding procedures in both oncologic and nononcologic populations and have demonstrated its efficacy.11,12
While those data are encouraging, AAI and its effect on anxiety have been less well studied in children with advanced cancer and their caregivers. Silva and Osorio found that AAI improved patient-reported pain and overall stress in children with cancer and showed improvement in caregiver anxiety and mental confusion, characterized by feelings of worry or uncertainty.13 McCullough et al. conducted a multisite randomized controlled trial in children with newly diagnosed cancer and found similar results in caregivers with decreased stress levels in parents in the AAI intervention compared to a control.14
There have been promising studies evaluating AAI's effect on psychosocial symptoms in adult patients with advanced cancers and terminal illness. In a German qualitative study, researchers observed several positive responses in their patients, including promotion of communication and relaxation.15 Another study of AAI in terminally ill adults found a statistically significant reduction in anxiety, which correlated with prior pet ownership.16
This study evaluated the feasibility of conducting AAI in children with advanced cancer and assessed potential benefit for mitigating anxiety in patients with advanced disease and their caregivers.
Methods
Enrollment
After Institutional Review Board approval, the research team consulted with each patient's primary oncologist before approaching the family for consent to ensure there were no barriers to study participation. All patients were receiving cancer treatments at a pediatric hematology/oncology outpatient clinic in the Southeast United States. Informed consent was obtained from the child's caregiver(s) and assent was obtained from child participants. Inclusion criteria for patients were as follows: age between 3 and 17 years, diagnosis of advanced (relapsed or refractory) cancer, and English speaking (to participate in consents, surveys, and AAI). Inclusion criteria for caregivers were caregiver or parent coming to the majority of their child's appointments and English speaking for consents and surveys. If either child or caregiver did not meet inclusion criteria, then the pair was not deemed eligible for study. Children and parents with cognitive impairment or self-reported fear of or allergy to canines were excluded from study enrollment as well. Of 31 child–parent dyads approached from February 2019 to May 2021, 30 (96.8%) agreed to participate, with one family excluded due to caregiver fear of dogs.
Randomization
Following enrollment, participants were randomly assigned to either the AAI intervention group (AAI group) or usual care group (UC group). Randomization was done using a computer-generated, permuted block program. Those assigned to the AAI group had visits with a therapy dog and its handler during oncology clinic appointments or hospitalizations; those assigned to UC group underwent standard clinic visits. An incentive in the form of a $10 gift card was added weekly for the UC group, as well as a $50 gift card after completion of the study as compensation for time invested to complete surveys.
Procedures
Once randomized, all patients in both groups completed trait measures at baseline and state stress/anxiety measures during each clinic visit for up to 12 weeks. The AAI group participated in up to 12 weekly sessions with a therapy dog and its handler during their clinic appointments or hospitalization. Visits were primarily outpatient (92.5%); however, AAI visits were conducted in the inpatient setting if patients were hospitalized and unable to come to clinic. AAI sessions consisted of ∼15 minutes of activities with the dog such as petting the dog, talking to the dog, teaching the dog tricks, or brushing the dog. Sessions were video-recorded. Researchers reviewed 20% of sessions to confirm consistency and fidelity of implementation.
The registered canine and handler received training and certification from the animal-assisted therapy organization, Pet Partners. To become certified, the handler passed a written online test and practical exam. Mandatory handler education included topics such as confidentiality, infection control, and effective communication. Pet Partners requires handler–canine dyads to be re-evaluated every two years to ensure suitability of the animal as it matures. Certified canines must be at least one year old. While there is no specific training, the dog must be deemed extremely obedient, of the right temperament, and welcoming to all.
The registered canine and handler team from our study remained in good standing with their animal-assisted therapy organization. All visits included in analysis were with the same canine and handler dyad.
Measures
Demographics
Families completed a 14-item family demographic form detailing age, gender, diagnosis, family members, and pets. Handlers also filled out a nine-item Animal Handler Demographic Form addressing qualifications of canine and handler.
Anxiety
The State-Trait Anxiety Inventory-Child (STAI-CH) and STAI were used to assess anxiety. The 20-item STAI-CH is a validated scale of anxiety for patients aged 5 to 17 years; the 20-item STAI is validated to assess anxiety in adult participants.17,18 Specifically, the STAI-CH has been used in multiple childhood cancer studies.19 Child participants in both the AAI and UC groups completed the STAI-CH Trait subscale at baseline before randomization and the STAI-CH State subscale weekly. AAI group participants completed the State subscale each week after AAI intervention. Caregiver participants in both AAI and UC groups completed the STAI Trait subscale at baseline before randomization and the STAI State subscale weekly during clinic visits. For the AAI group, completion of the State subscale was timed for after each AAI intervention (Table 1). The Trait subscale is intended as a general measure of a subject's tendency to have anxiety during activities of daily living and includes questions that assess general measures of calmness, security, and confidence. The State subscale measures a subject's anxiety at the moment they take the survey and includes questions that assess subjective feelings of apprehension, nervousness, worry, and tension. The possible range of scores on each subscale (State and Trait) from the STAI-CH is 20 to 60, while the possible range from the STAI (adults) is 20 to 80. STAI scores are interpreted as “no or low anxiety” (20–37), “moderate anxiety” (38–44), and “high anxiety” (45–80).20 Similarly, higher STAI-CH scores indicated higher levels of anxiety.
Table 1.
Measures and Schedule
| Variable | Measure | Time | Schedule |
|---|---|---|---|
| AIM 1 | Minute | ||
| Safety/feasibility | AAT activity log | 5 | After AAI |
| Feasibility | Recruitment rate and attrition | During consent/assent. After final AAI | |
| Intervention | Videotaping of all sessions | During AAI | |
| AIM 2 | Child | ||
| Anxiety—Trait | STAIC trait form | 5 | Baseline |
| Anxiety—State | STAIC state form | 5 | Baseline and weekly |
| Perceptions of AAI | Open-Ended Interview Questions (intervention group only) | 15 | End of study |
| Parent | |||
| Anxiety—Trait | STAI—Trait form (Adult) | 5 | Baseline |
| Anxiety—State | STAI—State form (Adult) | 5 | Baseline and weekly |
| Perceptions of AAI | Open ended questions (intervention group only) | 15 | End of study |
| Animal–Handler (intervention group only) | |||
| Personal characteristics | Demographic and information form | 10 | Baseline |
| Canine assessment | Canine behavioral assessment (CBARQ) | 10 | Baseline |
AAI, animal-assisted interaction; STAIC, State-Trait Anxiety Inventory for Children.
Data analysis
For inclusion in data analysis, patients and caregivers must have completed a minimum of four weeks of assessments. Of the initial 30 randomized families, 19 patients and 21 parents met the criteria and were included in the analysis (Fig. 1). Data analyses were conducted using IBM SPSS Statistics (Version 28). Frequency distributions were used to summarize nominal and ordinal categorical variables. Due to the small sample size and skewness, medians, interquartile ranges (IQRs), and range were used for summarizing the continuous age and STAI score distributions. Mann–Whitney and chi-square tests of Independence were conducted to compare the demographic and clinical characteristics of the participants in the two study data analysis groups at baseline. Assessments of the effects of AAI on the State anxiety scores for both children and parents were conducted using generalized linear models that included the respective scores after the first clinic visit as a covariate. Interpretations of statistical significance maintained a maximum alpha of 0.05 (p < 0.05).
FIG. 1.
Consort diagram. Thirty-one patients and their caregivers were approached to either receive AAI intervention or UC. One family was excluded due to reported fear of dogs. In the AAI group, one family was lost to follow up for unknown reason. Of the remaining 14 participating families, 9 children and 10 caregivers met analysis criteria with completion of at minimum four STAI surveys. In the UC group, four families were lost to follow up; one due to insufficient time to complete surveys, one due to moving away, and two for unknown reasons. Of the remaining 11 participating families, 10 children and 11 caregivers met analysis criteria with completion of at minimum 4 STAI surveys. AAI, animal-assisted interaction; STAI, State-Trait Anxiety Inventory; UC, usual care.
Results
The 19 children in the analysis sample ranged in age from 5 to 17 years with a median age of 9. Slightly more than half (53%) were female, and approximately two-thirds self-reported as white or Caucasian. The children had a variety of diagnoses, including 6 patients (32%) with leukemia/lymphoma, 10 patients (52%) with solid tumors, and 3 patients (16%) with cranial nervous system (CNS) tumors. Parental participants were mostly mothers (75%) with fathers representing the other 25%. No statistically significant differences between the study groups in any of the characteristics were observed (p > 0.20). Trait anxiety scores at baseline were also very similar in both groups for both the children and adults. Both median values tended to be in the lower-to-middle portion of the possible range of scores for their respective measures (children: median = 32; adults: median = 40) (Table 2).
Table 2.
Demographic Characteristics by Study Group
| Overall (N = 19) | Usual care (N = 10) | AAI (N = 9) | p | |
|---|---|---|---|---|
| Child with cancer | ||||
| Median (IQR) min, max | Median (IQR) min, max | Median (IQR) min, max | ||
|---|---|---|---|---|
| Age (years) |
9 (7, 12) 5, 17 |
10 (7, 12) 5, 13 |
8 (5, 12) 5, 17 |
0.511 |
| STAI trait score | 32 (27, 40) 22, 48 |
30 (27, 41) 22, 46 |
35 (27, 40) 25, 48 |
0.596 |
| n (%) | n (%) | n (%) | ||
|---|---|---|---|---|
| Gender |
|
|
|
0.809 |
| Male |
9 (47) |
5 (50) |
4 (44) |
|
| Female |
10 (53) |
5 (50) |
5 (56) |
|
| Race |
N = 18 |
N = 9 |
|
0.343 |
| White/Caucasian |
12 (67) |
6 (67) |
6 (67) |
|
| Black/African American |
1 (6) |
0 (0.0) |
1 (11) |
|
| Othera |
3 (16) |
1 (11) |
2 (22) |
|
| Multiple |
2 (11) |
2 (22) |
0 (0) |
|
| Diagnosis group |
|
|
|
0.279 |
| Leuk/lymp |
6 (32) |
2 (20) |
4 (45) |
|
| Solid |
10 (52) |
7 (70) |
3 (33) |
|
| CNS |
3 (16) |
1 (10) |
2 (23) |
|
| Family currently has pets |
|
|
|
0.845 |
| No |
8 (42) |
4 (40) |
4 (44) |
|
| Yes |
11 (58) |
6 (60) |
5 (56) |
|
| Adult participant | Overall (N = 21) | Usual care (N = 11) | AAI (N = 10) | |
|---|---|---|---|---|
| Relationship to patient |
N = 20 |
|
N = 9 |
0.795 |
| Mother |
15 (75) |
8 (73) |
7 (78) |
|
| Father |
5 (25) |
3 (27) |
2 (22) |
|
| Age group (years) |
N = 20 |
N = 11 |
N = 9 |
0.201 |
| 18–25 |
2 (10) |
1 (9) |
1 (11) |
|
| 26–35 |
8 (40) |
5 (46) |
3 (33) |
|
| 36–45 |
7 (35) |
2 (18) |
5 (56) |
|
| 46 or older |
3 (15) |
3 (27) |
0 (0) |
|
| Race |
N = 19 |
N = 11 |
N = 8 |
0.517 |
| White/Caucasian |
13 (69) |
8 (73) |
5 (63) |
|
| Black/African American |
1 (5) |
1 (9) |
0 (0) |
|
| Hispanic/Latino |
1 (5) |
0 (0) |
1 (12) |
|
| Otherb | 4 (21) | 2 (18) | 2 (25) |
| |
Median (IQR) min, max |
Median (IQR) min, max |
Median (IQR) min, max |
|
|---|---|---|---|---|
| N = 18 | N = 9 | N = 9 | ||
| STAI trait score | 40 (31, 44) 26, 68 |
39 (34, 48) 30, 68 |
40 (30, 43) 26, 56 |
0.690 |
Include 1 Asian, 1 Hawaiian, 1 Unknown.
Include 1 Asian, 1 Hawaiian, 2 Unknown.
CNS, central nervous system; IQR, inter-quartile range.
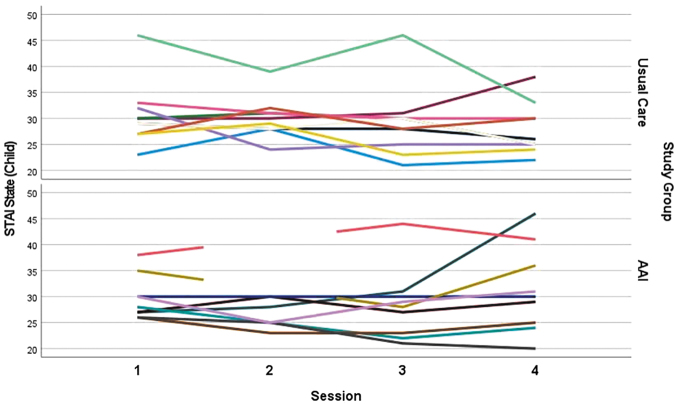
Summaries of the state anxiety scores for both the child and parental participants are shown in Table 3. No statistically significant difference was observed between the State STAI-CH scores during their first clinic visit (UC) and after their first session with the dog (AAI, p = 0.621). Median scores for both groups 30 (UC) and 28 (AAI) are in the lower end of the range of possible scores for that measure (20–60). After controlling for first session scores, no statistically significant difference between the groups was observed in the change in STAI-CH scores from first to fourth session (p = 0.170). Median change was −3 points for children in the UC group (range: −13 to +8) while the median change was +1 for the children in the AAI group (range: −6 to 19, Fig. 2 and Table 3).
Table 3.
Summaries of State State-Trait Anxiety Inventory Scores Initial Sessions and Fourth Sessions
| |
Overall |
Usual care |
AAI |
p |
|---|---|---|---|---|
| Median (IQR) min, max |
Median (IQR) min, max |
Median (IQR) min, max |
||
| Child with cancer | (N = 19) | (N = 10) | (N = 9) | |
| First session | 29 (27, 32) 23, 46 |
30 (27, 33) 23, 46 |
28 (26, 33) 26, 38 |
0.621a |
| Fourth session | 29 (25, 33) 20, 46 |
26 (24, 31) 22, 38 |
30 (24, 39) 20, 46 |
0.411b |
| Change from first (state) | −1 (−4, 2) −13, 19 |
−3 (−6, 0) −13, 8 |
1 (−3, 3) −6, 19 |
0.170c |
| Adult | (N = 21) | (N = 11) | (N = 10) | |
|---|---|---|---|---|
| First session | 34 (25, 48) 20, 71 |
48 (34, 59) 23, 71 |
27 (21, 33) 20, 47 |
0.003a |
| Fourth session | 30 (25, 44) 20, 58 |
33 (30, 44) 28, 58 |
25 (22, 41) 20, 52 |
0.035b |
| Change from first (state) | −2 (−9, 2) −35, 21 |
−7 (−18, −2) −35, 21 |
−1 (−3, 7) −4, 14 |
0.706c |
Mann–Whitney test.
Mann–Whitney test, does not control for study group difference in first session scores.
Regression: Study group difference after controlling for first session scores.
FIG. 2.
STAI-CH State subscores of child participants across four sessions in UC groups and AAI groups.
Contrary to the children, there was a statistically significant difference between the first week State anxiety scores for the parents (p = 0.003). Median parental score at the first clinic visit (UC) was 48 (IQR = 34, 59), while the median score just after the first session with the dog for the parents in the AAI group was 27 (IQR = 21, 33). After controlling for those initial scores, there was no statistically significant difference between the two groups in the amount of change in the state scores from the first to fourth session (p = 0.706). Given the dramatically higher anxiety scores during the first clinic visit for the UC group, the scores for those parents tended to decrease toward the levels observed for the parents in the AAI group after the first session with the dog yet still remained higher after four visits (median = 33 vs. 25 respectively, p = 0.037) (Table 3 and Fig. 3).
FIG. 3.
STAI State subscores of adult participants across four sessions in UC groups and AAI groups.
Discussion
Children with advanced cancer and their caregivers experience significant stress and anxiety. These psychological symptoms worsen toward the end of life and, if undertreated, lead to further patient and caregiver suffering. Our study evaluated AAI as a means to reduce anxiety experienced by patients and families.
We found no significant difference in STAI-CH State subscale scores in the first week in child participants between our two study groups. However, there was a statistically significant difference in STAI State subscale scores in the first week in caregiver participants, with lower anxiety in the AAI-intervention group. Interestingly, there was no statistically significant difference between caregiver groups in baseline STAI Trait subscale scores, indicating that proneness to anxiety in both groups was similar. Therefore, the lower initial STAI State scores among AAI group caregivers may suggest that awareness of continued participation in AAI may have been impactful enough to decrease caregiver anxiety scores. This finding is consistent with prior qualitative studies that have explored caregiver perceptions of AAI, noting a common theme for parents is that knowledge of seeing a dog in the hospital or clinic lessens anxiety for children, which in turn may lessen parental anxiety.21,22
While we found a significant difference in initial caregiver STAI State scores, we did not see a statistically significant difference in anxiety scores over time in either the UC or AAI groups in children or caregivers. There are several confounding factors and limitations to consider. First, we did not obtain a blinded initial State anxiety assessment due to survey implementation after randomization to study groups. Therefore, we cannot know whether State anxiety scores were dissimilar in caregiver groups at baseline before randomization or if the difference is attributable to the AAI intervention. Additionally, only the UC group received a financial incentive, which may have had some effect on anxiety in the UC group alone. The relatively low baseline Trait and first visit State anxiety scores in our patient populations were also a limitation. While we know from prior research that our targeted population is at risk for high anxiety, our cohort of patients and caregivers had relatively low initial anxiety scores. Trait anxiety scores in both children and adults were toward the lower end of the scale, as were most State anxiety scores. With lower initial anxiety scores, it is more challenging to demonstrate statistically significant change over time. Furthermore, any change in anxiety scores may have been impacted by changes to patient medical condition over the course of study. Lastly, the small sample size may have had an impact on our results. We may have failed to detect a statistically significant difference between the UC and AAI groups due to insufficient statistical power. Recruitment was impacted by the COVID-19 pandemic, as recruitment and data collection were paused for approximately five months when hospital visitation was restricted.
Importantly, a strength of our study is that it is one of only a few longitudinal randomized controlled trials in the literature focusing on AAI-based interventions in pediatric patients with advanced cancer. Our results demonstrate the feasibility of conducting research in this patient population, which we hope will further the impetus for study in this area. While we did not demonstrate a statistically significant difference in change in anxiety scores between groups, the difference in initial STAI State anxiety scores for caregivers may indicate a positive effect of AAI in reducing anxiety surrounding appointments through anticipation of seeing a therapy dog. Addition of qualitative data will be important in the future to further our understanding of the impact of AAI on caregivers.
For this study, we focused specifically on anxiety in a pediatric oncology population. However, as prior literature has shown, AAI can be an effective therapy for patients with many different chronic illnesses and can affect not only anxiety but communication, quality of life, and even physical symptoms such as pain. Future studies should continue to explore utilizing AAI as supportive care for all vulnerable patient populations. Evaluating AAI and anxiety in siblings or other family members may illuminate areas of need as well. Additionally, we did not explore whether underlying cultural or socioeconomic factors have any bearing on AAI-intervention and its effectiveness. Another interesting area of future study would be to evaluate whether pet ownership has any effect on AAI's impact on anxiety in a pediatric population, as it has been shown to have a significant effect in adults.16
Conclusion
Our results demonstrate the feasibility of conducting AAI-based research in this patient population and the potential for AAI to reduce anxiety in pediatric patients with relapsed or refractory cancer and their caregivers. Further research is needed to continue to evaluate AAI's effectiveness and role in the care of these patients.
Acknowledgments
We thank the children and their parents who kindly participated in this study.
Authors' Contributions
A.B.M.: Writing—original draft preparation, M.J.G.: Conceptualization, Writing—Reviewing and Editing, T.F.A.: Writing—Reviewing and Editing, B.A.C.: Writing—Reviewing and Editing, M.S.D.: Data analysis, Writing—Reviewing and Editing, J.L.N.: Writing—Reviewing and Editing.
Funding Information
This study, in part, was funded by the Human-Animal Bond Research Institute (HABRI, grant number HAB18-010). This study also utilized REDCap for data collection, and thus benefitted from National Center for Advancing Translational Science/National Institutes of Health Support (grant number UL1 TR000445).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. National Cancer Institute. Childhood Cancers. 2022. Available from: https://www.cancer.gov/types/childhood-cancers [Last accessed: January 30, 2023].
- 2. Collins JJ, Byrnes M, Dunkel I, et al. The measurement of symptoms in children with cancer. J Pain Symptom Manage 2000;19(5):363–377; doi: 10.1016/s0885-3924(00)00127-5 [DOI] [PubMed] [Google Scholar]
- 3. Weaver MS, Heinze KE, Kelly KP, et al. Palliative care as a standard of care in pediatric oncology. Pediatr Blood Cancer 2015;62 Suppl 5(Suppl 5):S829–S833; doi: 10.1002/pbc.25695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badr H. Psychosocial interventions for patients with advanced cancer and their families. Am J Lifestyle Med 2016;10(1):53–63; doi: 10.1177/1559827614530966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hechler T, Blankenburg M, Friedrichsdorf SJ, et al. Parents' perspective on symptoms, quality of life, characteristics of death and end-of-life decisions for children dying from cancer. Klin Padiatr 2008;220(3):166–174; doi: 10.1055/s-2008-1065347 [DOI] [PubMed] [Google Scholar]
- 6. Mack JW, Currie ER, Martello V, et al. Barriers to optimal end-of-life care for adolescents and young adults with cancer: Bereaved caregiver perspectives. J Natl Compr Canc Netw 2021;19(5):528–533; doi: 10.6004/jnccn.2020.7645 [DOI] [PubMed] [Google Scholar]
- 7. Verberne LM, Kars MC, Schouten-van Meeteren AYN, et al. Parental experiences and coping strategies when caring for a child receiving paediatric palliative care: A qualitative study. Eur J Pediatr 2019;178(7):1075–1085; doi: 10.1007/s00431-019-03393-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenberg AR, Dussel V, Kang T, et al. Psychological distress in parents of children with advanced cancer. JAMA Pediatr 2013;167(6):537–543; doi: 10.1001/jamapediatrics.2013.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chubak J, Hawkes R, Dudzik C, et al. Pilot study of therapy dog visits for inpatient youth with cancer. J Pediatr Oncol Nurs 2017;34(5):331–341; doi: 10.1177/1043454217712983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gagnon J, Bouchard F, Landry M, et al. Implementing a hospital-based animal therapy program for children with cancer: A descriptive study. Can Oncol Nurs J 2004;14(4):217–222; doi: 10.5737/1181912x144217222 [DOI] [PubMed] [Google Scholar]
- 11. Jennings ML, Granger DA, Bryce CI, et al. Effect of animal assisted interactions on activity and stress response in children in acute care settings. Compr Psychoneuroendocrinol 2021;8:100076; doi: 10.1016/j.cpnec.2021.100076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perez M, Cuscaden C, Somers JF, et al. Easing anxiety in preparation for pediatric magnetic resonance imaging: A pilot study using animal-assisted therapy. Pediatr Radiol 2019;49(8):1000–1009; doi: 10.1007/s00247-019-04407-3 [DOI] [PubMed] [Google Scholar]
- 13. Silva NB, Osorio FL. Impact of an animal-assisted therapy programme on physiological and psychosocial variables of paediatric oncology patients. PLoS One 2018;13(4):e0194731; doi: 10.1371/journal.pone.0194731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCullough A, Ruehrdanz A, Jenkins MA, et al. Measuring the effects of an animal-assisted intervention for pediatric oncology patients and their parents: A multisite randomized controlled trial [formula: see text]. J Pediatr Oncol Nurs 2018;35(3):159–177; doi: 10.1177/1043454217748586 [DOI] [PubMed] [Google Scholar]
- 15. Schmitz A, Beermann M, MacKenzie CR, et al. Animal-assisted therapy at a University Centre for Palliative Medicine—A qualitative content analysis of patient records. BMC Palliat Care 2017;16(1):50; doi: 10.1186/s12904-017-0230-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scagnetto F, Poles G, Guadagno C, et al. Animal-assisted intervention to improve end-of-life care: The moderating effect of gender and pet ownership on anxiety and depression. J Altern Complement Med 2020;26(9):841–842; doi: 10.1089/acm.2019.0342 [DOI] [PubMed] [Google Scholar]
- 17. Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken) 2011;63 Suppl 11(0 11):S467–S472; doi: 10.1002/acr.20561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spielberger CD, Gorsuch RL, Lushene V, et al. State-Trait Anxiety Inventory for Adults. Mind Garden Inc.: Menlo Park, CA; 1983; pp. 1–75. [Google Scholar]
- 19. Mahakwe G, Johnson E, Karlsson K, et al. A systematic review of self-report instruments for the measurement of anxiety in hospitalized children with cancer. Int J Environ Res Public Health 2021;18(4):1911; 10.3390/ijerph18041911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kayikcioglu O, Bilgin S, Seymenoglu G, et al. State and trait anxiety scores of patients receiving intravitreal injections. Biomed Hub 2017;2(2):1–5; doi: 10.1159/000478993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cowfer BA, Akard TF, Gilmer MJ. Animal-assisted interventions for children with advanced cancer: Child and parent perceptions. Palliat Med Rep 2021;2(1):328–334; doi: 10.1089/pmr.2021.0039;PMID:34927159;PMCID:PMC8675225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moreira RL, Gubert FD, Sabino LM, et al. Assisted therapy with dogs in pediatric oncology: Relatives' and nurses' perceptions. Rev Bras Enferm 2016;69(6):1188–1194; doi: 10.1590/0034-7167-2016-0243;PMID:27925097 [DOI] [PubMed] [Google Scholar]