Abstract
Background and Purpose
The purpose of this study was to assess the incidence of seizures in patients with spontaneous intracerebral hemorrhage (ICH) who received prophylactic levetiracetam.
Methods
This was a retrospective cohort study evaluating the use of levetiracetam in patients without a history of seizures who experienced a spontaneous intracerebral hemorrhage. Patients were excluded if they were younger than 18 years of age, had a documented history of a seizure disorder, or had an antiseizure drug documented on their home medication list. Patients were based on their exposure to levetiracetam. The primary outcome was incidence of seizure during hospital admission. Secondary outcomes included occurrence of adverse events, intensive care unit (ICU) length of stay (LOS), and hospital LOS.
Results
Of the 229 patients included in the final analysis, 21 were in the levetiracetam group (LEV) and 208 were in the no levetiracetam group (no LEV). No statistical difference in seizure incidence was observed when comparing the LEV and no LEV groups (1 [4.8%] LEV vs 3 [1.4%] no LEV; P = .32). There was also no statistical difference in the median ICU LOS (2 days [1 day, 5 days] LEV vs 2 days [1 day, 3 days] no LEV; P = .27), median hospital LOS (6 days [2 days, 8 days] LEV vs 6 days [3 days, 9 days] no LEV; P = .27), or adverse events.
Conclusions
This study does not support the use of levetiracetam prophylaxis in patients who have experienced an ICH.
Keywords: levetiracetam, intracerebral hemorrhage, seizure prophylaxis
Introduction
Seizures are one of many detrimental sequelae that can follow spontaneous intracerebral hemorrhages (ICH), with as many as 16% of patients who suffer from ICH experiencing clinical seizures within one week of hemorrhage. 1 Patients whose bleed involves cortical tissue are at higher risk of experiencing seizures, and roughly a third of patients may experience a seizure despite prophylactic anticonvulsant therapy. 2 The majority of seizures occur prior to or near the time of presentation to the hospital. 2
The most recent ICH guidelines recommend treating both clinical and subclinical seizures but recommend against prophylactic use of antiseizure drugs (ASD). 2 In addition, current literature is conflicting regarding the benefit of prophylactic ASD in these patients. 2 Current recommendations against the use of prophylactic ASD are based on studies that largely focused on the use of phenytoin. In 2009, Messe and colleagues published one of the first studies assessing seizure prophylaxis in ICH. They found that use of phenytoin was independently associated with a poor outcome, defined as a Modified Rankin Score (mRS) of 5-6. 3 Naidech and colleagues published a study in the same year also assessing prophylactic ASD use in ICH, where most patients were treated with phenytoin. The investigators found that ASD use was not associated with decreased incidence of seizure but was associated with increased risk of fever and worse National Institutes of Health (NIH) Stroke Scale scores at 14 days. 1 Finally, in 2012, Battey et al. performed a study including 1182 patients with ICH and investigated the incidence of seizures with and without seizure prophylaxis. 4 Again, the majority of these patients received phenytoin. When the analysis was restricted to patients who survived past day 5, no difference was seen in 90-day mortality or 90-day functional outcome (assessed using mRS) between patients who received prophylactic ASDs vs those who did not. From these studies, one could conclude that phenytoin has not been associated with a decrease in seizures after ICH and is associated with poor outcomes and adverse effects.1,3
As compared to phenytoin, levetiracetam has a favorable side effect profile. Thus, it is an attractive alternative to phenytoin when considering an ASD for prophylaxis. However, it has not been widely studied for its efficacy and safety as seizure prophylaxis in ICH. The purpose of this study is to assess the efficacy and safety of levetiracetam use for seizure prophylaxis following ICH.
Methods
This was an Institutional Review Board approved, single-center, retrospective cohort study including patients treated for spontaneous ICH at a large, academic medical center and Comprehensive Stroke Center from October 2018 to August 2020. Research was conducted in accordance with the Declaration of Helsinki. A waiver of informed consent was granted by the Institutional Review Board. Patients were excluded if they were less than 18 years of age, had a history of seizure disorder documented on the history and physical during the admission for ICH, had an ASD on the admission home medication list, or if they received levetiracetam for the treatment of seizures that developed prior to admission and after ICH. Groups were dichotomized based on administration of levetiracetam for seizure prophylaxis as opposed to no levetiracetam administration during admission. The decision to utilize and duration of levetiracetam as seizure prophylaxis was left to the discretion of the provider.
The primary outcome of this study was the incidence of seizures after admission. A new seizure was defined as witnessed seizure-like activity documented in the medical record or seizure detected by electroencephalography (EEG) during the hospitalization. Secondary outcomes included median time to development of seizures, intensive care unit (ICU) length of stay (LOS) and hospital LOS. Safety outcomes included development of adverse events during admission. The 2 primary adverse events assessed in this study included infection during hospitalization and fever within the first 7 days of admission. A new infection was included if documented in the discharge summary of the medical record that was not present on admission. A fever was defined as any one-time temperature greater than or equal to 100.4 degrees Fahrenheit. We assessed incidence of fever due to this being the main adverse effect seen in the previous phenytoin studies and assessed incidence of infection to account for confounding factors that could increase incidence of fevers.
Data Analysis
All statistical analysis was conducted with SAS (SAS Institute, Inc; Cary, NC; Version 9.4 [TS1M5]). Continuous variables were evaluated for normality with the Shapiro-Wilk test, Kolmogorov-Smirnov test, and QQ Plots. Variables found to have normal distribution were presented as mean and standard deviation. Between groups comparisons were made with a t-test. If a continuous variable violated the assumption of normality, a Mann Whitney U test was utilized, and results presented as median and interquartile range. Between group comparisons of dichotomous variables were made with a Chi Square or Fisher’s Exact test as appropriate. Results of such comparisons were presented as count and group proportion.
Results
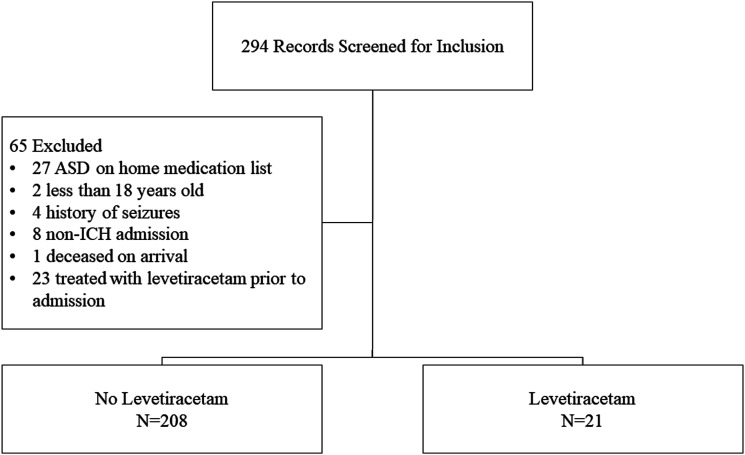
A total of 294 patients met inclusion criteria and were screened for eligibility; 65 were excluded for the reasons listed in Figure 1. Of the 229 patients included in the final analysis, 21 received levetiracetam (LEV) and 208 were in the no levetiracetam group (no LEV). The mean age was 69 years (SD 15.1 years) in the LEV group and 66 years (SD 15.8 years) in the no LEV group (P = .357). The majority of the patients were male (13 [61.9%] LEV vs114 [54.8%] no LEV; P = .533), white (18 [85.7%] LEV vs 188 [90.4%] no LEV; P = .451) and presented with a median ICH score of 3 (3 [IQR 2,4] LEV vs 3 [IQR 1,5]; P = .839). The median Charlson Comorbidity Score was 4 (IQR 2, 5) in the LEV group and 3 (IQR 2, 5) in the no LEV group (P = .324). Baseline characteristics, as seen in Table 1, were similar between groups. However, the initial median Glasgow Coma Scale (GCS) was lower in the LEV group (7 [5, 10] vs 13 [7, 15], P = .001). In addition, more patients in the LEV group were on an anticoagulant, while more patients in the no LEV group were on an antiplatelet; however, these differences were not found to be statistically significant.
Figure 1.
Study participant inclusion and exclusion.
Table 1.
Baseline Demographics.
| Variable | LEV (n = 21) | No LEV (n = 208) | P-value |
|---|---|---|---|
| Age, years, mean (SD) | 69.2 (15.1) | 66.0 (15.8) | .357 |
| Weight, kg, mean (SD) | 88.7 (32.0) | 84.2 (24.4) | .441 |
| Height, inches, mean (SD) | 172.5 (10.8) | 171 (11.0) | .552 |
| ICH a score, median (IQR), (n = 18, n = 182) | 3 (2, 4) | 3 (1, 5) | .839 |
| Initial GCS b , median (IQR) | 7 (5, 10) | 13 (7, 15) | .001 |
| Levetiracetam duration, days, median (IQR) | 2 (1, 5) | — | — |
| External ventricular drain duration, days, median (IQR) | 0 (0, 0) | 0 (0, 0) | .919 |
| Charlson comorbidity score, median (IQR) | 4 (2, 5) | 3 (2,5) | .324 |
| Total levetiracetam dose (mg), median (IQR) | 2000 (1000, 5000) | — | — |
| Daily levetiracetam dose (mg), median (IQR) | 1500 (1000, 2000) | — | — |
| Female, n (%) | 8 (38.1) | 94 (45.2) | .533 |
| Race, n (%) | |||
| White | 18 (85.7) | 188 (90.4) | .451 |
| Black | 0 (0) | 9 (4.3) | >.999 |
| Hispanic | 0 (0) | 1 (.5) | >.999 |
| Unknown | 3 (14.3) | 10 (4.8) | .104 |
| Intracerebral location, n (%) | |||
| Basal ganglia | 6 (28.6) | 42 (20.2) | .400 |
| Thalamus | 4 (19.1) | 29 (13.9) | .517 |
| Pons/Midbrain | 1 (4.8) | 17 (8.2) | >.999 |
| Cerebellum | 2 (9.5) | 22 (10.6) | >.999 |
| Other | 16 (76.2) | 125 (60.1) | .148 |
| Tobacco history, n (%) | .588 | ||
| Present smoker | 3 (21.4) | 53 (33.5) | |
| Former smoker | 2 (14.3) | 25 (15.8) | |
| Never smoker | 9 (64.3) | 80 (50.6) | |
| History of alcohol use, n (%) | 0 (0) | 23 (14.7) | .374 |
| Past medical history, n (%) | |||
| Myocardial infarction | 2 (9.5) | 8 (3.9) | .2304 |
| Congestive heart failure | 2 (9.5) | 8 (3.9) | .230 |
| Peripheral vascular disease | 0 (0) | 4 (1.9) | >.999 |
| Cerebrovascular disease | 5 (23.8) | 39 (18.8) | .565 |
| Dementia | 1 (4.8) | 11 (5.3) | >.999 |
| Connective tissue disease | 0 (0) | 0 (0) | — |
| Ulcer disease | 3 (14.3) | 29 (13.9) | >.999 |
| Mild liver disease | 0 (0) | 2 (1.0) | >.999 |
| Diabetes without complications | 3 (14.3) | 23 (11.1) | .715 |
| Diabetes with end organ damage | 2 (9.5) | 16 (7.7) | .674 |
| Hemiplegia | 0 (0) | 0 (0) | — |
| Moderate or severe renal disease | 2 (9.5) | 11 (5.3) | .339 |
| Solid tumor | 1 (4.8) | 13 (6.3) | >.999 |
| Leukemia | 0 (0) | 4 (1.9) | >.999 |
| Lymphoma or multiple myeloma | 0 (0) | 0 (0) | — |
| Moderate or severe liver disease | 0 (0) | 3 (1.4) | >.999 |
| Metastatic solid tumor | 1 (4.8) | 11 (5.3) | >.999 |
| AIDS c | 0 (0) | 0 (0) | — |
| Presence of external ventricular drain | 2 (9.5) | 18 (18.7) | >.999 |
| Selective serotonin reuptake inhibitor | 3 (14.3) | 29 (13.9) | >.999 |
| Anticoagulant | 6 (28.6) | 39 (18.8) | .263 |
| Antiplatelet | 6 (28.6) | 69 (33.2) | .669 |
| NSAID d | 1 (4.8) | 16 (7.7) | >.999 |
aIntracerebral hemorrhage.
bGlasgow Coma Score.
cAcquired immunodeficiency syndrome.
dNon-steroidal anti-inflammatory drug.
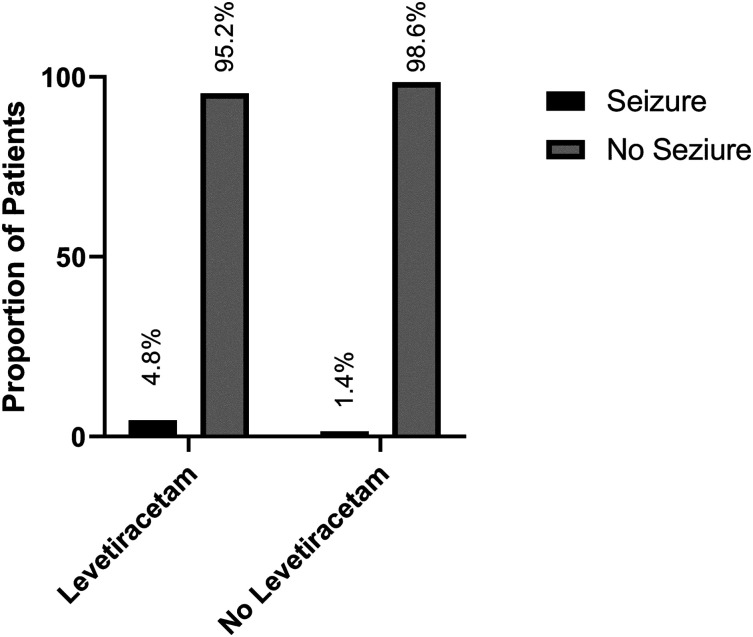
For the primary outcome seizure during admission, no difference was seen between groups (1 [4.8%] LEV vs 3 [1.4%] no LEV, P = .320) (Figure 2). In addition, when comparing the LEV group to the no LEV group, there was no difference in the median number of days to seizure development (2 days [2 days, 2 days] vs 2 days [1 day, 7 days]; P > .999).
Figure 2.
Proportion of patients experiencing a seizure in the levetiracetam and no levetiracetam groups.
When comparing the LEV group and no LEV group, there was no statistical difference in the number of days in the ICU (2 days [1 day, 5 days] vs 2 days [1 day, 3 days]; P = .267) or in hospital length of stay (6 days [2 days, 8 days] vs 6 days [3 days, 9 days]; P = .269).
Overall, the occurrence of adverse events was relatively common. The most commonly occurring adverse event was fever within the first 7 days of admission. As compared to the no LEV group, the proportion of patients with fever within the first 7 days of admission was numerically higher in the LEV group (11 [53.4%] vs 73 [35.1%]; P = .117). In addition, there was no statistical difference in the incidence of new infection (2 [9.5%] LEV vs 27 [13%] no LEV; P > .999).
Discussion
In this study assessing the efficacy and safety of levetiracetam as seizure prophylaxis in patients following ICH, levetiracetam was not found to reduce the incidence of seizures. These findings contrast with a retrospective study recently published by Christie et al, which assessed the safety and efficacy of seizure prophylaxis following ICH. 5 The study by Christie and colleagues included a total of 360 patients, 258 of whom were receiving levetiracetam for prophylaxis. Those investigators found a statistically significant reduction in incidence of seizures when prophylaxis was used compared to when it was not (6% prophylaxis vs 16% no prophylaxis, P = .003). Baseline characteristics between the Christie study and our present study were similar with respect to age and sex, however the studies differed with a higher percentage of patients included in the prophylaxis group in the Christie study vs the current study (75.8% vs 9.2%). Overall incidence of seizures was also higher than the current study (8.3% vs 1.7%). This is possibly related to the investigators’ inclusion of patients with a history of seizure diagnosis prior to admission, though this was only 7.1% of the total population. Although the Christie study found a decreased incidence of seizures with the use of prophylaxis, they also found significantly decreased incidence of good outcome at discharge (mRS ≤3) in the prophylaxis group (P = .002), as well as longer length of stay (P = .003), higher NIHSS (P = .002), and higher mRS (P < .001). However, there was no difference in good outcome (mRS ≤3) at 90 days between groups, and fewer patients in the prophylaxis group had a good outcome at 90 days, though this number was not statistically significant (57.7% vs 67.7%, P = .160).
The results of the study performed by Christie and colleagues were included in a meta-analysis that evaluated 8 studies involving 2852 patients. The meta-analysis found that the use of seizure prophylaxis did not reduce the incidence of seizures following ICH. 6 It is important to note that only 345 patients who received levetiracetam for seizure prophylaxis were included in this meta-analysis. The findings of our study support this conclusion and suggest that seizure prophylaxis following ICH is likely unnecessary.
The results of the PEACH trial were recently released. 7 This trial was a randomized, placebo-controlled trail that evaluated the use of levetiracetam (500 mg every 12 hours) or matching placebo for seizure prophylaxis in adult patients with spontaneous ICH. Seizures were defined clinically or electrographically via a monitored continuous electroencephalogram. A total of 50 patients were included in the study; however, only 19 in the levetiracetam group and 23 in the placebo group were included in the modified intention-to-treat population. In contrast to our study, the PEACH trial had the benefit of being prospective in design and utilized continuous EEG monitoring to detect subclinical seizures. Unlike in our study, the PEACH trial observed a larger number of electrographic seizures in the placebo group (6 seizures vs 158 seizures; P = .002). However, much like our study, there was no difference in observed clinical seizures (0 vs 0; P > .990). 7 This study highlights the potential problem with under reporting seizures in this population when only evaluating clinical seizures. However, the increase in electrographic seizures did not translate to a difference in short-term or long-term functional outcomes or quality of life. Therefore, the question remains about the clinical utility of using levetiracetam in this population.
Regarding safety outcomes, our study suggests that the use of levetiracetam is not associated with harm, which is in contrast to previous trials that evaluated the use of phenytoin. A trial published by Mackey et. al. in 2017 assessed the safety of levetiracetam after ICH and found no harm with its use. 8 Specifically, the study reported that the use of levetiracetam did not increase the risk of a poor functional outcome at hospital discharge, defined as a mRS of 4-6. However, despite the lack of harm, the lack of efficacy in preventing seizures after ICH means that levetiracetam should still not be recommended for patients without evidence of seizures. In addition, the PEACH trial did not report any difference in the number of adverse events. 7
The strengths of this study include that it is one of only a few studies assessing levetiracetam use in ICH. It adds to a growing body of evidence that seizure prophylaxis is likely not effective in these patients to prevent clinical seizure activity. We excluded patients who were previously on ASD and who had a history of a seizure disorder prior to admission to decrease confounding variables. Additionally, even though this was a retrospective study, in which prescriptive intent of medications can often be lost, our study included the intent of levetiracetam use, whether for prophylaxis or treatment of seizures.
This study is not without limitations. It was retrospective in nature and dependent on review of the electronic medical record for the primary outcome of seizure occurrence. This is dependent upon the accuracy of the documentation at the time of admission and is further confounded by the subjective nature of seizure-like activity without EEG confirmation, which was the basis of seizure diagnosis and treatment for many patients included. To account for subjectivity, the investigators sought to be as objective as possible in definitions of outcomes, such as infection development and fever during admission. Additionally, few patients included received levetiracetam for prophylaxis and the duration of therapy was relatively short. This is due to the current practice at this institution, which does not routinely include seizure prophylaxis in ICH treatment and is at the provider’s discretion. To account for this, we collected a large total number of consecutive patients for analysis. Finally, due to the sample size of patients who received levetiracetam, we cannot rule out the possibility of a type II error. However, it should be noted that the observed difference in seizure incidence between groups was low and would require a very large sample size to detect if it was true.
In conclusion, does not support the use of levetiracetam for the prevention of seizures following ICH. Although the use of levetiracetam is likely safe, the results of this trial do not support the routine use of levetiracetam prophylaxis in patients who have experienced an ICH.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
A. Shaun Rowe https://orcid.org/0000-0002-2533-9233
References
- 1.Naidech AM, Garg RK, Liebling S, et al. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke; a journal of cerebral circulation. Dec 2009;40(12):3810-3815. doi: 10.1161/STROKEAHA.109.559948 [DOI] [PubMed] [Google Scholar]
- 2.Greenberg SM, Ziai WC, Cordonnier C, et al. 2022. Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American heart association/American Stroke association. Stroke; a journal of cerebral circulation. Jul 2022;53(7):e282-e361. doi: 10.1161/STR.0000000000000407 [DOI] [PubMed] [Google Scholar]
- 3.Messe SR, Sansing LH, Cucchiara BL, et al. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocritical Care. 2009;11(1):38-44. doi: 10.1007/s12028-009-9207-y. [DOI] [PubMed] [Google Scholar]
- 4.Battey TW, Falcone GJ, Ayres AM, et al. Confounding by indication in retrospective studies of intracerebral hemorrhage: antiepileptic treatment and mortality. Neurocritical Care. Dec 2012;17(3):361-366. doi: 10.1007/s12028-012-9776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christie C, Daggubati L, Patel N, Matthews N, Lehman EB, Cockroft KM. Effect of newer generation anticonvulsant prophylaxis on seizure incidence after spontaneous intracerebral hemorrhage. World neurosurgery. Sep. 2020;141:e461-e465. doi: 10.1016/j.wneu.2020.05.197. [DOI] [PubMed] [Google Scholar]
- 6.Tran QK, Bzhilyanskaya V, Afridi LZ, et al. Preventing seizure occurrence following spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of seizure prophylaxis. Seizure. Apr 2021;87:46-55. doi: 10.1016/j.seizure.2021.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Peter-Derex L, Philippeau F, Garnier P, et al. Safety and efficacy of prophylactic levetiracetam for prevention of epileptic seizures in the acute phase of intracerebral haemorrhage (PEACH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. Sep 2022;21(9):781-791. doi: 10.1016/S1474-4422(22)00235-6. [DOI] [PubMed] [Google Scholar]
- 8.Mackey J, Blatsioris AD, Moser EAS, et al. Prophylactic anticonvulsants in intracerebral hemorrhage. Neurocritical Care. Oct 2017;27(2):220-228. doi: 10.1007/s12028-017-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]