Abstract
Background: Tick-Borne Encephalitis virus (TBEV) is a positive-sense single-stranded RNA virus belonging to the Flaviviridae family. TBEV transmission typically occurs through infected Ixodes tick bite or by consumption of unpasteurised milk from infected cattle. Case report: We report the clinical, neuroimaging, electroencephalogram (EEG), and laboratory (microbiological tests and spinal tap) data of a 6- year-old boy with Tick-borne encephalitis. Our patient presented with a biphasic course, initially with a myositis-like picture on his first admission to the emergency department, and after a few days with an encephalitic picture, resulting in a second hospitalization. EEG showed focal slow activity, while his brain magnetic resonance imaging (MRI) showed a signal abnormality, which completely resolved on repeat MRI after 3 months. Conclusion: To our knowledge, this is the youngest patient presenting with myositis in the first phase of Tick-borne encephalitis (TBE). In the presence of a biphasic clinical course, with previous myositis, aspecific MRI changes in the thalamic and midbrain regions and an EEG documenting slowed bioelectrical activity should prompt suspicion of TBEV infection.
Keywords: encephalitis < central nervous system infections, tick-borne, virus, biphasic
Background
Tick-Borne Encephalitis virus (TBEV) is a positive-sense single-stranded RNA virus that belongs to the Flaviviridae family.1,2 Three TBEV subtypes differing in their virulence have been identified: European (TBEV-Eur), Siberian (TBEV-Sib), and Far Eastern (TBEV-FE). Additionally, other tick-borne flaviviruses are found worldwide, such as the Powassan virus, which causes encephalitis in the United States.1,3 Most TBEV infections are asymptomatic, but symptomatic cases typically have neurological manifestations, such as meningitis, encephalitis, and meningoencephalitis, which are collectively referred to as tick-borne encephalitis (TBE).4-6 The initial phase usually occurs with non-specific symptoms such as fever, headache, and muscular pain. The clinical spectrum of the second phase of the disease typically ranges from mild to severe meningoencephalitis. 7 TBEV transmission typically occurs through the bite of an infected Ixodes tick.1,8
Case Presentation
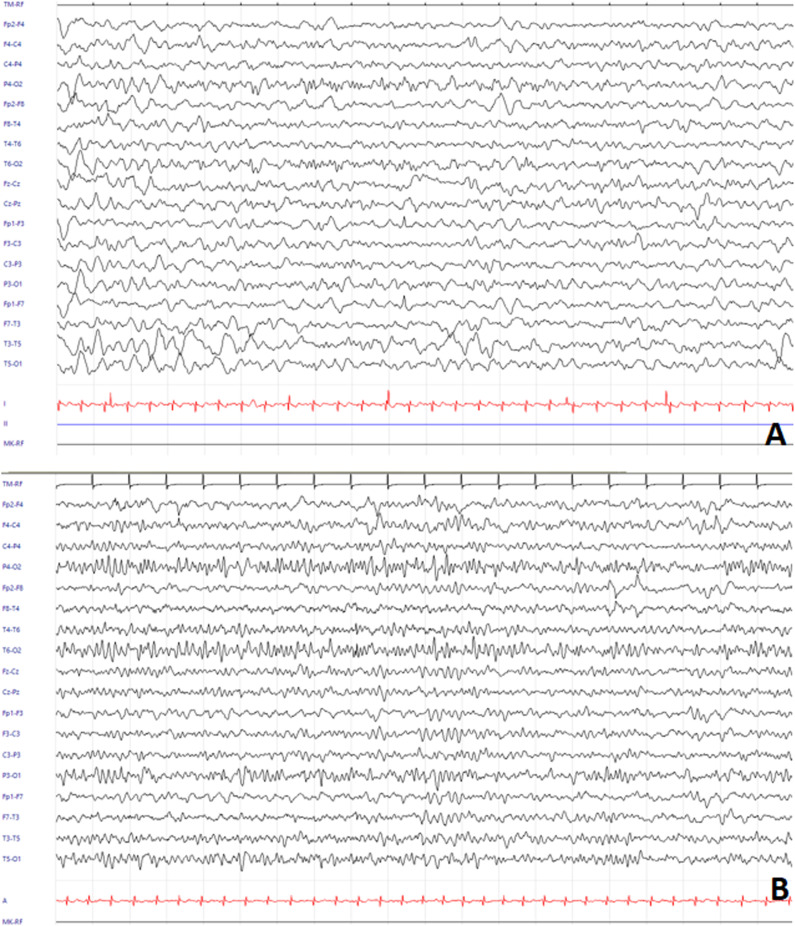
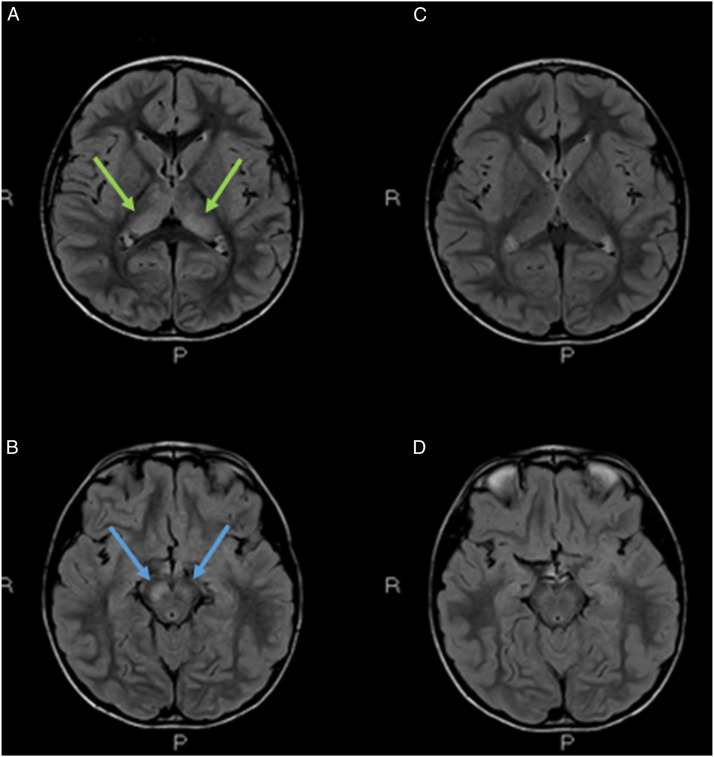
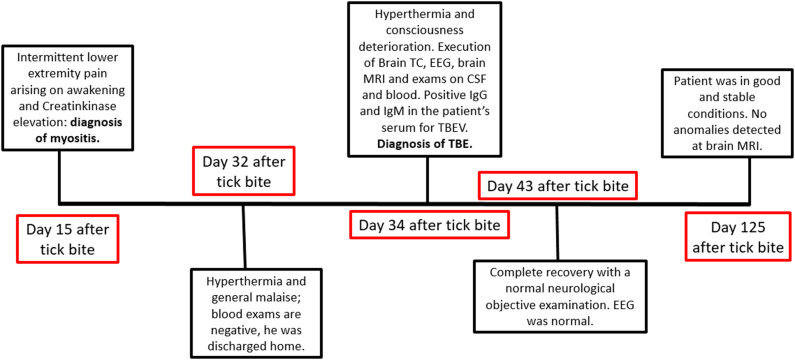
We present the case of a 6-year-old, previously healthy boy. The family history was negative for neurological disorders. The patient initially visited the pediatric emergency department for intermittent lower extremity pain arising on awakening in the absence of fever. Blood tests showed elevation of Creatine Kinase (CK) (8331 U/L, normal values 32 - 294 U/L); serological tests for adenovirus, cytomegalovirus (CMV), mycoplasma, parvovirus, and Epstein Barr virus (EBV) were negative. After 4 days of hospitalization in the pediatric ward, he was discharged in the absence of fever and in the absence of lower extremity pain, with normalization of CK values, with the diagnosis of myositis. 13 days later, he presented to the emergency department again with fever and general malaise. Blood tests showed leukocytosis (21 690/mm3, normal values 4000-10000) with neutrophil predominance (neutrophils 85% in leukocyte formula) and C-reactive protein elevation (5.47 mg/dL, normal values .0-.5); liver enzymes, lactate dehydrogenase and CK were in normal range. In view of the clinical stability and the absence of current signs of alarm he was discharged home. After 2 days, the child was taken again by his mother to the emergency room for fever and consciousness deterioration. Evaluating the patient’s medical history, a tick bite was reported 15 days before onset of the myositis-like symptoms, during an holiday in the city of Trento (Trentino-Alto Adige region, Northern Italy). Urgent blood tests were ordered (decreasing inflammatory indexes, total white cells count of 16,350/mmc, normal values 4,00 - 10,00; C-reactive protein: 2.45 mg/dl, normal values 0,00 - 0,50; procalcitonin:0.09 ng/mL, normal values 0,01 - 0,50; CPK:46 U/l, normal values 32-294), blood culture, complete urine analysis, chest X-ray and ECG were unremarkable. EEG showed focal slowing of the background, which was consistent with focal brain disruption (Figure 1A)). Brain CT scan was negative and spinal tap showed inflammation with pleiocytosis (clear and colorless CSF, total protein count 49.6 mg/dl, glucose 76 mg/dl, lactate 11.5 mg/dl, leukocytes 87/μL, neutrophils 18%, lymphocytes 70%, monocytes 12%, red blood cells:1/μL). Brain MRI was performed to document signal changes in the thalamic structures on both sides and in the midbrain, with hyperintensity suggesting inflammation visible in FLAIR sequences, a picture compatible with arbovirus encephalitis (Figure 2A-B). The child was also supported by intravenous hydrosaline hydration and broad-spectrum antibiotic therapy with ceftriaxone and intravenous acyclovir (discontinued following CSF negativity for Herpes Simplex virus 1, Herpes Simplex virus 2, Varicella Zoster virus, Human Herpesvirus 6, CMV, EBV, Enterovirus and for Borrelia DNA). On blood tests, it was then found to be positive for IgG and IgM for TBEV; indirect immunofluorescence technique was used for the identification of TBEV IgG and IgM antibodies. The serological finding, together with the history, clinical course of the little patient, EEG and brain MRI lead us to formulate the diagnosis of TBE. During the course of his hospital stay, his condition gradually improved with progressive clinical and EEG normalization (Figure 1(B)). The patient's recovery was complete and rapid; he manifested walking difficulties with balance deficits until the fourth day of hospitalization and then gradually regained fluidity of movement and coordination; he was discharged in good general clinical condition after nine days of hospitalization. At 3 months follow-up, we noticed complete resolution of signal anomalies at brain MRI (Figure 2C-D).
Figure 1.
A EEG showing background asymmetry due to slowing of background activity in the left posterior regions (20s/pg, 14 μV/mm, 0.1 s, 15 Hz, Notch 50 Hz). B EEG on the sixth day since hospitalization, devoid of frank abnormalities (20s/pg, 14 μV/mm, 0.1 s, 15 Hz, Notch 50 Hz).
Figure 2.
(A-B) Brain MRI, axial FLAIR images, showed signal anomalies in the thalami (A) on both sides (green arrows) and midbrain (B) with main involvement of cerebral peduncles/black matter bilaterally, predominantly on the right (blue arrows). (C-D). 3-months follow-up. Complete resolution of signal anomalies.
Discussion
The case presented in this article showed the same clinical course as that previously described in the literature. Infectious myositis may be due to a direct pathogenic effect on the muscle or by a pathogen-mediated immune response. 7 Innate and adaptive immune responses, particularly the induction of early antibody responses, are essential for neutralizing flavivirus infections. The surface envelope glycoprotein (E-protein) is the main target for the antibody response to flaviviruses, which may result in the production of cross-reactive, non-neutralizing antibodies, particularly within the same serocomplex, owing to the protein structure containing virus-specific and cross-reactive epitopes.9-12 In particular, the classic biphasic course 7 with an initial myositis (unaccompanied by hyperpyrexia in our patient) and an encephalitic manifestation occurred approximately 15 days later. Montvydaite et al. 7 found that the presence of myositis predicted a severe disease course. Instead, in this case report, we demonstrated complete recovery even in patients experiencing a biphasic course. Figure 3. Evaluating the patient’s medical history, a tick bite 15 days before the onset of myositis-like symptoms was reported. The fact that the tick bite occurred near the city of Trento is more than a coincidence, as cases have been increasing in this region since 2012 despite the availability of an appropriate vaccine. 13 Acute myositis in the first phase of the disease is extremely rare in children, with only one report to date. 14 To our knowledge, this is the youngest patient presenting with myositis in the first phase of TBE. In our case, follow-up EEG, brain MRI, and neurological examination were all normal. Some authors suggest that in adults, the presence of myositis in tick-borne encephalitis heralds a more severe disease course. 7 This is difficult to judge in children, because cases are very limited in the literature. However, neither our case nor that reported by Arnez et al. 14 developed neurological sequelae after the second stage of the disease, although CNS (Central Nervous System) involvement was evident. 14 The involvement of different brain areas on MRI is unusual. Indeed, both the thalami and midbrain appeared hyperintense in T2 sequences. In children and adolescents, MRI often shows preferential involvement of the thalamic and caudate nuclei as well as the putamen, which is in accordance with MRI findings in adults and children with TBE. 15
Figure 3.
Timeline.
Conclusion
In the presence of a biphasic clinical course, with initial myositis, pathognomonic MRI changes in the thalamic and midbrain regions, and an electroencephalogram showing slowing of bioelectrical activity, the possibility of TBE infection needs to be suspected. Further studies are required to fully understand the pathophysiology underlying the clinical manifestations of the disease and its biphasic course.
Acknowledgements
The authors would like to thank all those who participated in the study.
Appendix.
List of abbreviations
- CNS
Central nervous system
- EEG
Electroencephalogram
- MRI
Magnetic resonance imaging
- TBEV
Tick-borne encephalitis virus
Footnotes
Author Contributions: CAC, DF, ML, FB, CS, DB, SR, MN, RP and ADF drafted the manuscript. CF reviewed the manuscript critically. All authors approved the final manuscript and agreed to be accountable for all aspects of this work.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Statement
Ethics Approval
No local ethical committee approval number is associated with this publication as no formal approval is required for this type of publication.
Consent for Publication
Written informed consent was obtained from a parent of the patient.
ORCID iD
Carlo A. Cesaroni https://orcid.org/0000-0003-0092-5604
Data Availability Statement
The data used in the writing of this article are available from the corresponding author on reasonable request.
References
- 1.Chiffi G, Grandgirard D, Sendi P, Dietmann A, Bassetti CLA, Leib SL. Sleep-wake and circadian disorders after tick-borne encephalitis. Microorganisms. 2022;10(2):304. doi: 10.3390/microorganisms10020304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarmer J, Zlatkovic J, Tsouchnikas G, et al. Variation of the specificity of the human antibody responses after tick-borne encephalitis virus infection and vaccination. J Virol. 2014;88(23):13845-13857. doi: 10.1128/JVI.02086-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloch EM, Tobian AAR, Katz LM. Powassan virus: what is the risk to the blood supply? Transfusion. 2021;61(12):3286-3288. doi: 10.1111/trf.16725 [DOI] [PubMed] [Google Scholar]
- 4.Bogovic P, Strle F. Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. World J Clin Cases. 2015;3(5):430-441. doi: 10.12998/wjcc.v3.i5.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taba P, Schmutzhard E, Forsberg P, et al. EAN consensus review on prevention, diagnosis and management of tick-borne encephalitis. Eur J Neurol. 2017;24(10):1214e61. doi: 10.1111/ene.13356 [DOI] [PubMed] [Google Scholar]
- 6.Grard G, Moureau G, Charrel RN, et al. Genetic characterization of tick-borne flaviviruses: new insights into evolution, pathogenetic determinants and taxonomy. Virology. 2007;361(1):80-92. doi: 10.1016/j.virol.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 7.Montvydaite M, Seskute G, Minseviciute G, et al. The manifestation of myositis in tick-borne encephalitis as a prophet of severe disease course: a rare case report. Clin Rheumatol. 2022;41(4):1241-1245. doi: 10.1007/s10067-022-06058-6 [DOI] [PubMed] [Google Scholar]
- 8.Sumilo D, Asokliene L, Bormane A, Vasilenko V, Golovljova I, Randolph SE. Climate change cannot explain the upsurge of tick-borne encephalitis in the Baltics. PLoS One. 2007;2(6):e500. doi: 10.1371/journal.pone.0000500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papa A, Karabaxoglou D, Kansouzidou A. Acute West Nile virus neuroinvasive infections: cross-reactivity with dengue virus and tick-borne encephalitis virus. J Med Virol. 2011;83(10):1861-1865. doi: 10.1002/jmv.22180 [DOI] [PubMed] [Google Scholar]
- 10.Rathore APS, St John AL. Cross-reactive immunity among flaviviruses. Front Immunol. 2020;11:334. doi: 10.3389/fimmu.2020.00334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansfield KL, Horton DL, Johnson N, et al. Flavivirus-induced antibody cross-reactivity. J Gen Virol. 2011;92(Pt 12):2821-2829. doi: 10.1099/vir.0.031641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilibic-Cavlek T, Ferenc T, Vujica Ferenc M, et al. Cross-reactive antibodies in tick-borne encephalitis: case report and literature review. Antibodies. 2022;11(4):72. doi: 10.3390/antib11040072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alfano N, Tagliapietra V, Rosso F, Ziegler U, Arnoldi D, Rizzoli A. Tick-borne encephalitis foci in northeast Italy revealed by combined virus detection in ticks, serosurvey on goats and human cases. Emerg Microbes Infect. 2020;9(1):474-484. doi: 10.1080/22221751.2020.1730246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnež M, Avšič-Županc T, Ružić-Sabljić E. Acute myositis associated with the initial phase of tick-borne encephalitis. J Clin Virol. 2011;51(4):276-278. doi: 10.1016/j.jcv.2011.05.021 [DOI] [PubMed] [Google Scholar]
- 15.von Stülpnagel C, Winkler P, Koch J, et al. MRI-imaging and clinical findings of eleven children with tick-borne encephalitis and review of the literature. Eur J Paediatr Neurol. 2016;20(1):45-52. doi: 10.1016/j.ejpn.2015.10.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the writing of this article are available from the corresponding author on reasonable request.