Abstract
Background:
The introduction of illicitly made fentanyl in the United States has slowly replaced heroin. New illicit drugs are often associated with changes in frequency and modes of administration. We assessed changes in injection frequency and smoking fentanyl in the new era of fentanyl availability in San Francisco.
Methods:
We used targeted sampling to recruit 395 people who inject drugs (PWID) into an observational cohort study in San Francisco 2018–2020. We assessed changes in injection frequency, opioid injection frequency and fentanyl smoking frequency in four six-month periods. We also conducted qualitative interviews with PWID asking about motivations for injecting and smoking opioids.
Results:
The median number of past-month injections steadily decreased by semi-annual calendar year from 92 injections in July to December 2018 to 17 injections in January to June 2020. The rate of opioid injections reduced by half (Adjusted Incidence Rate Ratio=0.41; 95% Confidence Interval=0.25, 0.70; p<0.01). The number of days smoking fentanyl was associated with fewer number of injections (X2(2) = 11.0; p<0.01). Qualitative interviews revealed that PWID’s motivation for switching from injecting tar heroin to smoking fentanyl was related to difficulties accessing veins. After switching to smoking fentanyl, they noticed many benefits including how the drug felt, improved health, fewer financial constraints, and reduced stigma.
Conclusion:
Between 2018 and 2020, there was a shift from injecting tar heroin to smoking fentanyl in San Francisco. Reductions in injection of illicit drugs may offer public health benefit if it reduces risk of blood-borne viruses, abscesses and soft-tissue infections, and infective endocarditis.
Keywords: Fentanyl, Epidemiology, Qualitative, Drugs, Smoke, Inject
1. Introduction
There are an estimated six million people who have injected drugs (PWID) in the United States (Bradley et al., 2020; Lansky et al., 2014; Tempalski et al., 2013) and 15.6 million PWID globally (Degenhardt et al., 2017). Injection drug use is associated with transmission of blood borne infectious diseases such as HIV (Des Jarlais et al., 1985; Des Jarlais et al., 2020) and viral hepatitis (Girardi et al., 1990; Rashti et al., 2020; Sharhani et al., 2021), as well as abscesses and other soft tissue infections (Binswanger et al., 2000; See et al., 2020), and infective endocarditis (Kadri et al., 2019). Reducing the number of people who inject drugs and the number of times people inject drugs are public health goals, given the health complications associated with injection drug use. Public health campaigns in Scotland, Spain, and Australia in the 1990s aimed to convince PWID to switch to non-injectable routes of administration (Bridge, 2010; Dolan et al., 2004; Wodak, 1997). One study in Germany in 2012 showed it was possible to have people switch to smoking if safe consumption sites provided free sterile aluminum foil (Stover and Schaffer, 2014). However, these recommendations have been difficult for people to heed, as there are many reasons why people inject their drugs as opposed to other modes of administration.
Modes of administering drugs have long been dependent on the motivations for using drugs, types of drugs used, and drug market availability (Harris et al., 2015; Syvertsen et al., 2016) and cost (Swift et al., 1999). For most substances, bioavailability is highest when injected, making injection the most economical choice, an important factor when drugs are scarce or expensive relative to income. Others value the fast onset of injected drugs, which can be particularly important in situations where people are in drug withdrawal (Bluthenthal et al., 2020; Valente et al., 2020). The type of drug solution can also dictate modes of administration. For example, for many decades, heroin in the Western United States has consisted of a tar-like substance that is hard to administer in any way other than injection (Ciccarone, 2009; Roth et al., 2017).
The frequency with which people inject drugs can also be dependent upon the types of drugs they use. For example, people who inject cocaine tend to inject more times per day than people who inject methamphetamine or heroin (Ciccarone and Bourgois, 2016; Hyshka et al., 2012). People who use fentanyl appear to use it more frequently than people who use heroin because it has a shorter duration of action (Buresh et al., 2019; Geddes et al., 2018; Kim et al., 2020; Lambdin et al., 2019; Zibbell et al., 2021). The types of drugs used also confer risk for infectious diseases, overdoses, and soft tissue infections (Hyshka et al., 2012; Ivsins et al., 2020; Lambdin et al., 2019; Rhodes et al., 2007).
Given that types of drugs can dictate modes and frequency of administration, shifts in drug markets can be an important contributing factor to the prevalence of injection. The transition from smoking opium to injecting heroin in southeast Asia in the 1970s was driven by policing practice and price changes (Westermeyer, 1976). The shift in the drug market from powder cocaine to crack cocaine reduced drug injection significantly in the United Kingdom and Spain in the 1990s (Barrio et al., 1998; Hunter et al., 1995), and Montreal, Canada in the early 2010s (Roy et al., 2012). In the US in the 2010s, the tightening regulation of opiate pills led to a surge in heroin use, which increased the number of PWID (McCabe et al., 2020).
With the US illicit opioid market having made a momentous shift towards illicitly made fentanyl in the mid-2010s, there have been devastating increases in related overdose mortality (Mattson et al., 2021). We reported recently that fentanyl use was increasing among PWID in Los Angeles and San Francisco and found that nonfatal overdose increased among those using fentanyl (Lambdin et al., 2019). During this period, San Francisco California has had a 270 percent increase in opioid-related overdose mortality from 2018 to 2020 (259 opioid overdose fatalities in 2018, 442 in 2019, and 699 in 2020)(Thadani, 2021). The proportion of overdose deaths that involved fentanyl in San Francisco increased from 16% in 2017 to 34% in 2018, 54% in 2019, and 72% in 2020 (Rodda, 2021). It is important to learn whether these recent changes in the drug market have impacted the mode of administration, including injection. We conducted a mixed methods observational study to assess changes in the prevalence of injections and smoking of fentanyl among PWID in the context of growing fentanyl availability during 2018–2020.
2. Methods
Data analyzed in this study were collected using an exploratory sequential mixed method study design, which included quantitative and qualitative community-based methods. Using targeted sampling, we recruited 395 PWID in San Francisco, California (Kral et al., 2010; Watters and Biernacki, 1989). Participants completed quantitative interviews at baseline, 6 and 12 months from 2018 to 2020. Qualitative data collection (N=21) took place before, during, and following quantitative data collection to help with quantitative instrument design, provide rich description of social dynamics relating to domains being explored quantitatively, and to provide context for quantitative findings. Fentanyl smoking was discussed in qualitative interviews throughout the study. Qualitative interview participants included PWID and key informants involved in providing services to PWID. All methods were approved by an Institutional Review Board at the University of California San Diego.
2.1. Quantitative Research Procedures
Study participants were recruited using targeted sampling methods in San Francisco (Watters and Biernacki, 1989). These methods provide similar sample composition to respondent-driven sampling (Kral et al., 2010). Study eligibility at baseline included (1) being 18 years or older, (2) having injected illicit drugs in past 30 days, as verified by visual inspection of injection stigmata (Cagle et al., 2002), and (3) providing competent informed consent. All participants provided informed consent prior to data collection. There were no refusals by anyone who presented at the field site and was eligible. To help track study participants during the study, they were asked to provide name, address, phone numbers, social media handles, and other ways to be contacted, along with consent for us to contact them.
Baseline, 6-month, and 12-month interviews were conducted face-to-face by trained interviewers who read questions aloud and entered answers directly into a computer-assisted personal interviewing program (Blaise, Statistics Netherlands, The Hague, Netherlands). After shelter-in-place ordinance was instituted due to COVID-19 pandemic in March 2020, the remaining 12-month interviews were conducted by phone. The surveys included questions about demographics, drug use, and social and medical services utilization. Participants were remunerated $20 for completing the baseline, $30 for completing the 6-month and $30 for completing the 12-month follow-up surveys. Participants were able to complete their follow-up surveys up to three months after their scheduled appointment. In order to facilitate retention, we also had participants stop by the field office once every month during the study in order to update their contact information and collect an additional $10 for taking the time to do so. Baseline data collection occurred between July 2018 and June 2019.
2.2. Quantitative Study Measures
We collected socio-demographic variables including age, gender (female, male, transgender), race/ethnicity (white, Latinx, Black, Asian/Pacific Islander, Native American, and mixed race), sexual identity (heterosexual, bisexual, gay), and homeless (yes or no). We collected extensive drug use data for past 30 days including injection frequency, types of drugs injected, and sharing injection equipment. For each of the following drugs or drug combinations, we asked how many days in the past 30 days did they use the drug without injecting and how many times in the past 30 days did they inject the drug: heroin, heroin mixed with fentanyl, fentanyl and other fentanyl analogs, speedball (admixture of heroin and cocaine), goofball (admixture of heroin and methamphetamine), powder cocaine, crack cocaine, methamphetamine, prescription opiates, prescription stimulants, sedatives, benzodiazepines, methadone, and buprenorphine. Fentanyl-specific questions were not added until January 2019, which means we do not have data on those variables during the first 6-month period. While we did not ask the specific mode of administration when people were asked about non-injected fentanyl use, our qualitative research indicated that people either inject or smoke fentanyl in San Francisco. As such, our operational definition of smoking fentanyl is “non-injected use of fentanyl.”
2.3. Quantitative Analysis
Interview data were used to address three main research questions: (1) During the time-period 2018 to 2020, did PWID significantly reduce their monthly frequency of drug injections and frequency of opioid injections? (2) During the time-period 2019 to 2020, did PWID who use opioids increase the number of days they smoke fentanyl, and (3) Was injection frequency associated with number of days smoking fentanyl?
To assess changes over time, we divided the two years of data collection into four six-month time-periods: July to December 2018, January to June 2019, July to December 2019, and January to June 2020. We summarized the number of injections and days of fentanyl use in past 30 days by time-period, regardless of whether the observation was a baseline, 6-month, or 12-month interview. To assess the potential for retention bias, supplemental analysis involved assessing whether the injection frequency distribution differed within each time-period by type of interview (baseline, 6-month, and 12-month) as well as comparing the distribution of injection frequency at baseline between participants who did and did not complete a follow-up visit using Wilcoxon rank-sum tests.
To answer Research Question 1, we compared the rate of injections and rate of opioid injections between calendar time periods using negative binomial generalized estimating equation (GEE) models, given the skewed distribution in reported number of injections. To answer Research Question 2, we used a Poisson GEE model with a log link built to compare the proportion of people using opioids who smoked fentanyl over the time-periods (Chen et al., 2018; Zou, 2004). We employed a negative binomial GEE model to compare the rate of smoking fentanyl (days in past month) over time-periods. To answer Research Question 3, we assess whether the number of days smoking fentanyl was associated with the number of times injecting drugs using a negative binomial GEE model with a log link, including an interaction between time-period and number of injections. All GEE models were estimated using robust standard errors and an unstructured working correlation structure, and adjusted for interview type (baseline, 6-month, or 12-month), age, and current enrollment in a substance use treatment program. Statistical significance was pre-determined at p<0.05 for all analyses. All analyses were carried out using Stata 16.1 (StataCorp, College Station, Texas, USA).
2.4. Qualitative data collection
Key informants were recruited either via social service agencies providing services to PWID, by being referred from the quantitative arm of the study, or (for syringe services program workers) via ‘dear colleague’ email recruitment. Interviews took place before, during, and after quantitative analysis, with the final five interviews conducted in December 2020-January 2021 specifically addressing issues emerging from our quantitative analysis. We used purposeful sampling methods to select information-rich cases to contextualize the quantitative analysis. Information rich cases are those from which one can learn a great deal about issues of central importance to the purpose of the research (Patton, 1990). Qualitative interviews were conducted with PWID and service providers. Inclusion criteria therefore were having injected drugs in the last 30 days, being 18 years of age or older and willingness to provide informed consent for PWID. For key informant interviews with service providers, inclusion criteria were working for a harm reduction service provider and willingness to provide consent. Interviews were between 20–60 minutes long. Most were conducted in person (with social distancing for interviews conducted post-COVID-19) with two conducted via video. Key informants were remunerated $20 for their contribution to the research. Interviews were audio-recorded and transcribed verbatim.
2.5. Qualitative analysis
Interviews utilized a brief ‘probe sheet’ listing topics of particular interest. The probe sheet was iteratively modified throughout the data collection period to allow follow-up on issues emerging from earlier interviews and to incorporate new topics of interest which emerged from quantitative findings. Among related topics, we asked questions about motivations for switching from injecting tar heroin to smoking fentanyl, the benefit and disadvantages of smoking fentanyl, and the types of fentanyl respondents were buying and using. All transcripts were read in their entirety and analyzed using an inductive analysis approach (Thomas, 2006). As themes emerged theoretical memos were written that included direct quotes from transcripts that contextualize participants’ drug use experiences.
3. Results
3.1. Quantitative Findings
There were 395 participants enrolled. Retention in the study was 81% (n=322) at 6-months (January 2019 to February 2020) and 77% (n=305) at 12-months (July 2019 to June 2020). The sample consisted of a quarter ciswomen, 19% African American, 14% Latinx, the vast majority considered themselves to be homeless (86%), and the median age was 39 years old (interquartile range (IQR) 32–50, Table 1). Almost all (88%) had injected opioids at least once in the 30 days prior to enrollment. The distribution of injection frequency at enrollment did not differ by loss to follow-up (z = ‒0.44; p=0.66).
Table 1.
Demographics of people who inject drugs in San Francisco at baseline, 2018–2020 (N=395)
| N | (%) | |
|---|---|---|
| Total | 395 | (100) |
| Age – mean, median (range) | 41, 39 | (20–72) |
| Female | 97 | (25) |
| Latinx | 55 | (14) |
| Race 1 | ||
| White | 275 | (70) |
| Black | 82 | (21) |
| Asian/Pacific Islander | 9 | (2) |
| Native American or Alaskan Native | 34 | (9) |
| People experiencing homelessness or who are marginally housed | 340 | (86) |
| Years of injection drug use - mean, median (IQR) | 19, 16 | (7–30) |
| Ever participated in substance use treatment | 277 | (70) |
| Currently enrolled in substance use treatment program | 108 | (27) |
| Ever sentenced to jail or prison | 336 | (85) |
| Substance use in the past 30 days | ||
| Injected opioids | 349 | (88) |
| Heroin or heroin mixed with other drugs | 267 | (68) |
| Cocaine (powder or crack) | 218 | (55) |
| Methamphetamine | 288 | (73) |
| Opiate pills | 102 | (26) |
| Tranquilizers or sedatives | 149 | (38) |
| Stimulant pills | 21 | (5) |
| Enrollment period | ||
| Jul-Dec 2018 | 190 | (48) |
| Jan-Jun 2019 | 205 | (52) |
Multiple responses allowed
3.1.1. Research Question 1.
The median number of injections steadily decreased by semi-annual calendar year from 92 in July to December 2018 (IQR 45–120) to 65 in January to June 2019 (IQR 18–100), 25 in July to December 2019 (IQR 4–90) and 13 in January to June 2020 (IQR 0–90, Table 2). The distribution of injection frequency in each time-period was similar when we stratified by visit (January to June 2019: z =1.659, p=0.10; July to December 2019: z = 0.341; p=0.73). The rate of past-month injection frequency in January to June 2020 was estimated to be half the rate of injecting drugs in July to December 2018 (adjusted incidence rate ratio [Adj IRR]=0.46; 95% CI= 0.30, 0.72; p<0.01). Likewise, the rate of opioid injections reduced by half (Adj IRR=0.41; 95% CI=0.25, 0.70; p<0.01).
Table 2.
Number of times people injected drugs in last 30 days in San Francisco 2018–2020
| Period | Obs* | Median | Interquartile Range | Mean | SD | Adj IRR1 | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|
| July–December 2018 | 190 | 90 | (45 – 120) | 95.6 | 74.7 | Ref | - | - |
| January–June 2019 | 353 | 60 | (18 – 100) | 71.5 | 69.8 | 0.74 | (0.62, 0.87) | <0.01 |
| July–December 2019 | 297 | 25 | (4 – 90) | 49.4 | 63.3 | 0.53 | (0.39, 0.72) | <0.01 |
| January–June 2020 | 178 | 12.5 | (0 – 90) | 46.0 | 67.2 | 0.46 | (0.30, 0.72) | 0.01 |
Obs = number of observations; Includes baseline, 6-month, and 12-month observations. Data were missing for two observations (participant didn’t know)
Adj IRR = adjusted incident rate ratio; estimated using negative binomial GEE with unstructured correlation, adjusting for visit type (baseline, 6-month, 12-month), participant’s age, and currently enrolled in substance use treatment program. Ref= referent
3.1.2. Research Question 2.
Among participants who used opioids, the proportion reporting smoking fentanyl did not change appreciably from January 2019 – June 2020 (X2(2)=0.01; p=0.99); approximately 50% smoked fentanyl within each 6-month time period. However, among those who reported any smoking of fentanyl, the adjusted incident rate of days of non-injected fentanyl use during the past month in January to June 2020 was 1.7 times the rate in January to June 2019 (Adj IRR=1.71; 95% CI 1.09, 2.68; p=0.02; Table 3). In January to June 2019, 14% of people using opioids reported smoking fentanyl every day of the past month. During the same period one year later, 28% of people using opioids smoked fentanyl every day.
Table 3.
Among participants who used opioids, the proportion who reported smoking fentanyl and reported number of days smoked fentanyl in last 30 days
| Proportion smoking fentanyl | Days smoking fentanyl (among those who smoke) | |||||
|---|---|---|---|---|---|---|
| Period | Adj RR | 95% CI | p-value | Adj IRR2 | 95% CI | p-value |
| January–June 2019 | Ref | - | - | Ref | ||
| July–December 2019 | 0.99 | (0.76, 1.28) | 0.92 | 1.40 | (1.03, 1.90) | 0.03 |
| January–June 2020 | 0.96 | (0.65, 1.43) | 0.84 | 1.71 | (1.09, 2.68) | 0.02 |
RR = relative risk (i.e. prevalence of fentanyl smoking); estimated using Poisson GEE with unstructured correlation, adjusting for visit type (baseline, 6-month, 12-month), participant’s age, and currently enrolled in substance use treatment program. Ref = referent
Adj IRR = adjusted incident rate ratio; estimated using negative binomial GEE with unstructured correlation, adjusting for visit type (baseline, 6-month, 12-month), participant’s age, and currently enrolled in substance use treatment program. Ref = referent
3.1.3. Research Question 3.
The number of days smoking fentanyl was significantly associated with fewer number of injections over time (test for interaction between time period and number of injections: X2(2) = 11.0; p<0.01). Figure 1 depicts the change in median number of opioid injections and days smoking fentanyl over time among those who reported smoking fentanyl.
Figure 1.
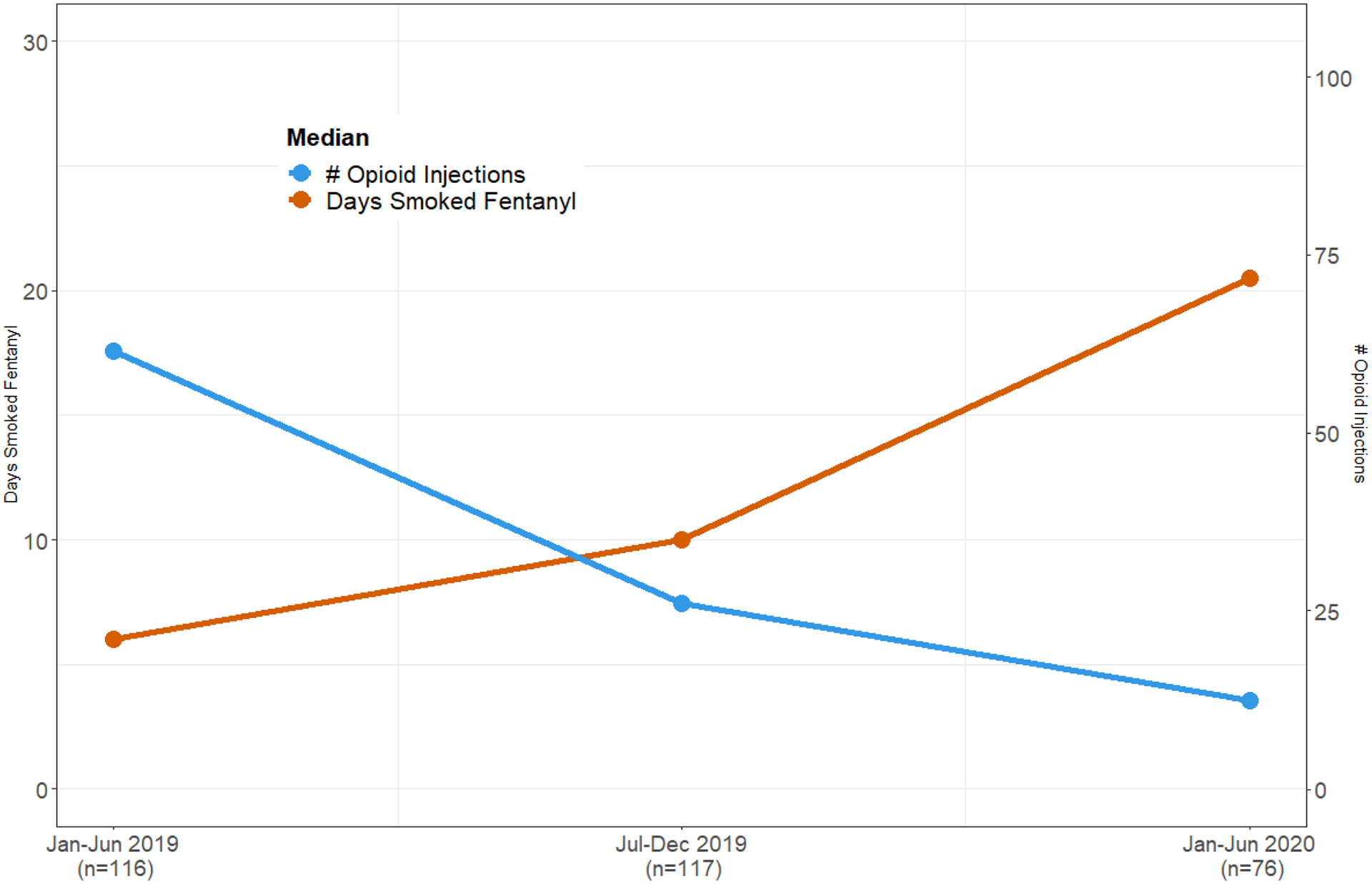
Median number of opioid injections and median days smoked fentanyl in the past 30 days among people who inject drugs in San Francisco who reported smoking fentanyl
3.2. Qualitative Findings
During qualitative interviews with PWID, they noted that their motivation for switching from injecting tar heroin to smoking fentanyl was related to their difficulties finding easily accessible veins and needing injection assistance.
“What happened was it was getting harder and harder for me to [inject] myself and I was having to rely on other people to [inject] me, having to pay them, and people get irritated when I’d ask them to do that. And it was just easier for me to go and buy some fentanyl and smoke it and get high as f___ and not bother anybody to [inject] or have to pay anybody to [inject] me or whatnot… I was doing heroin and fentanyl, heroin when I was around somebody that could [inject] me and fentanyl when I wasn’t, and then gradually I went all over to fentanyl.”
After switching from injecting heroin to smoking fentanyl, they noticed many benefits ranging from how the drug felt to improved health, fewer financial constraints, and reduction in stigma. In terms of how the drug felt, they reported that when injecting tar heroin, they would inject the whole drug solution at once, making for a bolus of drug effect immediately that would slowly dissipate with time. By switching to fentanyl, they could smoke a little bit all day long, making for a more even drug effect. One participant described it as follows:
“It’s when you smoke it, it’s more of a controlled way of getting--you’re not just taking one, like big like amount and just having it hit you all at once. When smoking it, it’s paced. When you shoot it, there’s no way to be able to like, you can’t just like hold your arm and let some of the shot go, you know what I mean? Like when smoking it, you can pace yourself, you know when to not--I don’t know. It’s just a more controlled way to do it.”
Participants spoke at length about how they felt healthier after transitioning from injecting tar heroin to smoking fentanyl. The most noticeable positive health effect was the reduction in abscesses and soft tissue infections.
“But I feel it’s really gotten better for as far as my veins, taking care of my veins and my body. I [no longer] get abscesses. No more abscesses. And also no more scarring, like I don’t have the track marks. I don’t have to hide those or anything.”
They also spoke about the dangers of overdosing when injecting fentanyl.
“You can still OD [when smoking], for sure. But it’s almost like guaranteed that you’re going to OD when you shoot it. If you don’t have a tolerance to it, or it’s not something like if I shot it, even though I smoke it, if I tried to shoot it, I would probably OD because I don’t have that tolerance of that, like, all-at-once hit that it is.”
Another reported benefit of switching from injecting tar heroin to smoking fentanyl is financial. One woman described in detail the economic relief she has experienced since switching from injecting heroin to smoking fentanyl:
“See, this is like I think turning to fentanyl was probably the most fiscally responsible decision I’ve made as a drug user because if you have $10 and you buy $10 worth of heroin, that heroin will only get me high one time and then I would have to buy more. But the $10 for the fentanyl, I can smoke on that for the rest of the night. So it lasts a lot longer and I can use it a lot more frequently and share it also with my friends more because the heroin I could only use it for just one time and that’s it. It’s definitely drawn out. I guess it makes it so I’m able to stay well a lot more than when I was on heroin especially, it was a lot more of a struggle to stay on top of that before. But now on fentanyl, my money goes a lot further.”
Participants spoke about how transitioning from injecting tar heroin to smoking fentanyl also helped reduce the stigma associated with the act of injection and the visible scars on limbs associated with injection. One person talked about how reducing this potential stigma helps with job opportunities.
“Question: Anything else you can think of in terms of benefits of smoking over injecting?
Answer: The way people look at you. I feel like that, that stigma is a little less harsh when it comes to smokers. To whip out a foil on the street and take a hit is not so frowned upon like, [taking out] a needle and taking a shot. And I’m definitely not the type of person that would normally let that bother me, but I’m 31 years old and I’ve got this amazing opportunity to, like, work. Stuff like that, which I didn’t have. And like it’s making me realize that I’m capable of doing more than just being a junkie.”
Participants clearly stated they currently prefer smoking fentanyl to injecting tar heroin. Beyond the aforementioned benefits, now that they have started smoking fentanyl, injecting tar heroin was no longer desirable because it no longer produced the same physiological effects as it had prior. We were told by one man that “I do like heroin better, but the problem with that is, because of the fentanyl habit, when I do a shot of heroin, I don’t feel it anymore. It blocks the entire feeling, kind of like methadone.” They spoke about negative physiological effects of using tar heroin after having switched to smoking fentanyl:
“Question: And how come you don’t inject heroin anymore?
Answer: At first, I would just buy a little fentanyl with my heroin. And then I started just buying a little heroin with my fentanyl, and then I just didn’t even like the heroin anymore. I started having a reaction to it. The last time I did shoot heroin, my face got all puffy and swelled up. And the pins and needles felt horrible. I felt like, ‘ew, I can’t believe I did this for so long.’”
We interviewed a syringe services program provider that provides sterile aluminum foil for people to use for smoking drugs. She said “Even with foil, it’s hard to [provide enough aluminum] foil, that’s how fast it’s going out the door. So it’s that issue. So we’re looking at how we can make it happen at the [syringe services program] … so as to not overwhelm the staff.”
To assess whether the transition from injecting heroin to smoking fentanyl is occurring throughout the United States, we asked drug ethnographers, researchers, and syringe service providers in 17 cities around the US in December 2020 (See acknowledgments section for personal communications). While our informants in five California cities all acknowledged that many people have started smoking fentanyl in their city, none of the informants in other states indicated that smoking fentanyl is widespread. One of the potential reasons for the stark difference by geographical area could be that the fentanyl drug solution may be different in California. As such, we asked the people who smoked fentanyl about the fentanyl they were purchasing and using. In San Francisco, our participants informed us that there are four different colors of fentanyl powder: purple, pink, yellow and blue. They reported that some colored fentanyl powders are better for smoking while others are better for injecting.
“The blue is the most potent, then it would be the purple, pink, and then the yellow. The yellow is the best for smoking because of the way it burns. It’s more of an oily based substance. And then the purples and the blues burn up a lot faster but they’re a lot--they’re not cut so much because it’s the cut that makes it oily.”
Discussion
Our quantitative results suggest the number of injections has been decreasing precipitously among PWID in San Francisco from 2018 to 2020. During the same period, we found that among PWID who use fentanyl, the number of days they smoke fentanyl has increased. We received data on the number of syringes provided by the largest syringe services program in San Francisco during our study period. They provided fewer syringes in January to June 2020 (n=1,479,976) than in the previous three semi-annual periods (1,727,777 in July to December 2018, 1,903,005 in January to June 2019, and 1,717,482 in July to December 2019; Personal Communication Ro Giuliano, San Francisco AIDS Foundation, January 27, 2021). Because decreasing injection drug use trends does not necessarily mean that smoking increased, we conducted several qualitative interviews with PWID to learn more about this. Our qualitative data showed that PWID have switched from injecting tar heroin to smoking fentanyl because it is hard for them to find veins in which to inject after many years of injecting tar heroin. Once they switched to smoking fentanyl, they observed many benefits from an even high to less abscesses, less stigma, and less cost. We learned that health and cost are bigger drivers of the switch from injecting tar heroin to smoking fentanyl than the effects of the drug.
Key informants mentioned that a benefit of switching from injecting heroin to smoking fentanyl was less abscesses. Abscesses/soft tissue infections are highly prevalent among PWID in tar heroin markets (Binswanger et al., 2000; Mars et al., 2016). Public health practitioners have unsuccessfully advocated for PWID to switch to non-injected routes of administration to reduce infectious disease risk (Bridge, 2010; Dolan et al., 2004; Wodak, 1997). Drug markets are often the most powerful agents of health change for PWID, both negative and positive. Switching from injection of heroin to smoking fentanyl in California may inadvertently stem infectious disease transmission and abscesses (Centers for Disease and Prevention, 2001). Local syringe services programs started providing aluminum foil and glass bubble pipes in 2019. The two programs we interviewed both reported it has been hard to keep smoking equipment stocked as many people are requesting them. Smoking drugs presents a potential risk for exacerbating COPD and transmitting respiratory illnesses such as COVID-19 if pipes are shared (Burhan et al., 2019; Harris, 2020; Hulin et al., 2020; Mehta et al., 2020; Nightingale et al., 2020). By providing sterile smoking paraphernalia, harm reduction programs help attenuate the risk of viral transmission (eg. COVID-19 and hepatitis C).
Overdose mortality increased by 270 percent in San Francisco during the study period(Thadani, 2021). While our data cannot distinguish whether opioid overdose mortality increases are related to increases in smoking fentanyl, this steep increase in opioid overdose mortality in San Francisco requires urgent attention. To assess whether mode of fentanyl administration is associated with fentanyl overdose mortality would require a large prospective cohort study of people who use fentanyl. The overdose education and naloxone distribution program in San Francisco (the DOPE Project) has increased its volume of trainings and naloxone distribution. California should also implement numerous safe consumption sites, which have been proven to eliminate overdose deaths on site internationally and in the U.S. (Kral et al., 2020; Marshall et al., 2011). These sites should include safe smoking rooms, given the increase in fentanyl smoking. Passage of California Senate Bill 57 would allow San Francisco, Los Angeles, and Oakland to implement safe consumption sites (Wiener et al., 2020).
Interviews with drug researchers and harm reduction service providers in numerous cities throughout the United States indicate that the switch from injection to smoking fentanyl is mainly occurring in California. It is not known whether this is due to a different fentanyl product in California that is easier to smoke, although it is worth noting that heroin smoking does not appear widespread in the US (Ciccarone, 2019). Heroin in California is sold as tar heroin, which is a low-soluble, gummy substance (Ciccarone, 2009). Fentanyl is sold as a powder product distinct from tar heroin in San Francisco. Key informants explained that there are four types of powder fentanyl in San Francisco, distinguished by color, but also by whether it was oily when heated, which they felt made it easier to insufflate. It is possible that fentanyl in the California illicit drug market is uniquely cut with powders that make it easier to burn and insufflate. On the East Coast, there have been reports that fentanyl is adulterated with the veterinary tranquilizer xylazine (Johnson et al., 2021). There needs to be continual surveillance of the substances that are part of drug solutions in all geographic regions, something that can be done through testing using Fourier-transform infrared spectroscopy (Ti et al., 2020).
These findings support a more nuanced approach to drug use epidemiology. To adequately understand how drug form/type influences routes of administration and frequency of use, we need to begin asking new questions. Items worth considering include asking about form (powder, fluid, lump), color of powder, and reported potency of drugs (Mars et al., 2018). Further, we need to address the availability of smoking supplies for people who use drugs, and to assess the potential negative consequences of non-injection routes of administration. For instance, there a several case reports that associate consumption of heroin by heating on foil and inhaling with negative clinical outcomes (Alambyan et al., 2018; Cordova et al., 2014; Gossop et al., 2004; Griffiths et al., 1994; Long et al., 2003; Pizzey and Hunt, 2008; Yalamanoglu and Schuurmans, 2018).
Limitations which need to be considered when interpreting the data in this study are as follows. First, the cohort study is subject to selection bias as participants were not randomly selected. PWID comprise a population that cannot be randomly sampled in community-based studies due to drug use being illegal and stigmatized. Targeted sampling methods are commonly used to sample PWID and attempt to be as representative as possible (Kral et al., 2010). Second, there is potential for retention bias, though retention was around 80% at both six and 12 months. We found no differences in injection frequency by study visit within each time-period, and baseline frequency of injections among those lost to follow-up was comparable to those who completed follow-up. Third, the data is self-reported, which means it is subject to biases from recall and social desirability. However, there is no reason to believe such bias would be differential by time-period. Fourth, we did not specifically ask about smoking or insufflating fentanyl, but rather assessed non-injected fentanyl use. In our qualitative interviews with people who smoke fentanyl and people who work at syringe service programs confirmed that fentanyl pills or patches are virtually non-existent in the black market in San Francisco, leading us to believe that all non-injected fentanyl was smoked. Fifth, the qualitative data from people who use drugs is from a small number of people and is by no means deemed representative or generalizable. It is meant to provide context to our quantitative data. Lastly, this study was only conducted in one city, limiting its generalizability. Studies in other cities should consider conducting similar analyses.
Our study suggests there has been a significant reduction in the number of injections among PWID in San Francisco between 2018 and 2020 with PWID transitioning from injecting heroin to smoking fentanyl as a potential explanation. Our qualitative data indicates that this transition from injecting to insufflation is driven by health and economic concerns more than a desire for the unique drug effects smoking fentanyl provides. Shifting away from injection of illicit drugs may offer potential public health benefit as it could reduce the risks of transmitting blood-borne viruses and morbidity related to abscesses and soft-tissue infections. While reductions in injection and uptake of smoking fentanyl appear to be local to California, it is worth investigating the extent to which this is occurring through the U.S. and in other countries. It will also be important to learn why these new trends in drug use appear to be local to California. We need to investigate risks associated with smoking fentanyl. Harm reduction programs should start to provide clean safer smoking supplies (Leonard et al., 2008; Prangnell et al., 2017). Safe consumption sites should be implemented and need to include ventilated smoking areas.
Highlights.
The number of injections decreased precipitously among people who inject drugs in San Francisco from 2018 to 2020.
Concomitantly, we found that among people who inject drugs that use fentanyl, the number of days they smoke fentanyl increased.
Qualitative interviews revealed that people who inject drugs’ main motivation for switching from injecting tar heroin to smoking fentanyl was related to their difficulties finding easily accessible veins.
After switching to smoking fentanyl, people noticed many benefits including how the drug felt, improved health, fewer financial constraints, and reduced stigma.
Acknowledgments
This work was supported by Arnold Ventures and the United States National Institute on Drug Abuse grants R01DA046444 and R01DA046049.
We would like to thank the following people for important contributions: Finn Black, Veronica Majano, Terry Morris, Veronica Majano, and Rachel Robinson. We would like to thank the following ethnographers, drug use researchers and syringe service providers for providing us context on whether smoking is a predominant mode of administrating fentanyl in their cities: Shannon Knox (Los Angeles), Braunz Courtney and David Showalter (Oakland), Tara Buesig (San Diego), Roxanne Butterfield (San Jose), Susan Sherman (Baltimore), Mary Wheeler (Boston), Kat Humphries (Denver), Louise Vincent (Greensboro), Hansel Tookes (Miami), Tino Fuentes (New York City, Christopher Moraff (Philadelphia), Haven Wheelock (Portland OR), Karla Wagner (Reno), Ryan McNeil (Vancouver Canada), Kris Nyrop (Seattle), Andrea Lopez (Washington DC), Jess Tilley (Western Massachusetts).
Role of Funding Source:
This research was funded by Arnold Ventures and United States National Institute on Drug Abuse, neither of which had any role in study design, data collection, analysis and interpretation of data, the writing of the report or the decision to submit the article for publication.
Footnotes
Conflict of Interest: No author has any conflicts to declare
References
- Alambyan V, Pace J, Miller B, Cohen ML, Gokhale S, Singh G, Shun MC, Hammond A, Ramos-Estebanez C, 2018. The Emerging Role of Inhaled Heroin in the Opioid Epidemic: A Review. JAMA Neurol 75, 1423–1434. 10.1001/jamaneurol.2018.1693. [DOI] [PubMed] [Google Scholar]
- Barrio G, De la Fuente L, Royuela L, Diaz A, Rodriguez-Artalejo F, 1998. Cocaine use among heroin users in Spain: the diffusion of crack and cocaine smoking. Spanish Group for the Study on the Route of Administration of Drugs. J Epidemiol Community Health 52, 172–180. 10.1136/jech.52.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Kral AH, Bluthenthal RN, Rybold DJ, Edlin BR, 2000. High prevalence of abscesses and cellulitis among community-recruited injection drug users in San Francisco. Clin Infect Dis 30, 579–581. 10.1086/313703. [DOI] [PubMed] [Google Scholar]
- Bluthenthal RN, Simpson K, Ceasar RC, Zhao J, Wenger L, Kral AH, 2020. Opioid withdrawal symptoms, frequency, and pain characteristics as correlates of health risk among people who inject drugs. Drug Alcohol Depend 211, 107932. 10.1016/j.drugalcdep.2020.107932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley H, Rosenthal EM, Barranco MA, Udo T, Sullivan PS, Rosenberg ES, 2020. Use of Population-Based Surveys for Estimating the Population Size of Persons Who Inject Drugs in the United States. J Infect Dis 222, S218–S229. 10.1093/infdis/jiaa318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge J, 2010. Route transition interventions: potential public health gains from reducing or preventing injecting. Int J Drug Policy 21, 125–128. 10.1016/j.drugpo.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Buresh M, Genberg BL, Astemborski J, Kirk GD, Mehta SH, 2019. Recent fentanyl use among people who inject drugs: Results from a rapid assessment in Baltimore, Maryland. Int J Drug Policy 74, 41–46. 10.1016/j.drugpo.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhan H, Young R, Byrne T, Peat R, Furlong J, Renwick S, Elkin T, Oelbaum S, Walker PP, 2019. Screening Heroin Smokers Attending Community Drug Services for COPD. Chest 155, 279–287. 10.1016/j.chest.2018.08.1049. [DOI] [PubMed] [Google Scholar]
- Cagle H, Fisher d., Senter T, Thurmond R, Kastar A, 2002. Classifying skin lesions of injection drug users: A method for corroborating disease risk. . DHHS Pub No (SMA)02. Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration., Rockville, MD. [Google Scholar]
- Centers for Disease, C., Prevention, 2001. Soft tissue infections among injection drug users--San Francisco, California, 1996–2000. MMWR Morb Mortal Wkly Rep 50, 381–384 [PubMed] [Google Scholar]
- Chen W, Qian L, Shi J, Franklin M, 2018. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol 18, 63. 10.1186/s12874-018-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone D, 2009. Heroin in brown, black and white: structural factors and medical consequences in the US heroin market. Int J Drug Policy 20, 277–282. 10.1016/j.drugpo.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone D, 2019. Heroin Smoking Is Not Common in the United States. JAMA Neurol 76, 508. 10.1001/jamaneurol.2019.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone D, Bourgois P, 2016. Injecting drugs in tight spaces: HIV, cocaine and collinearity in the Downtown Eastside, Vancouver, Canada. Int J Drug Policy 33, 36–43. 10.1016/j.drugpo.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova JP, Balan S, Romero J, Korniyenko A, Alviar CL, Paniz-Mondolfi A, Jean R, 2014. ‘Chasing the dragon’: new knowledge for an old practice. Am J Ther 21, 52–55. 10.1097/MJT.0b013e31820b8856. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, Stone J, Cunningham EB, Trickey A, Dumchev K, Lynskey M, Griffiths P, Mattick RP, Hickman M, Larney S, 2017. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 5, e1192–e1207. 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Friedman SR, Hopkins W, 1985. Risk reduction for the acquired immunodeficiency syndrome among intravenous drug users. Ann Intern Med 103, 755–759. 10.7326/0003-4819-103-5-755. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Sypsa V, Feelemyer J, Abagiu AO, Arendt V, Broz D, Chemtob D, Seguin-Devaux C, Duwve JM, Fitzgerald M, Goldberg DJ, Hatzakis A, Jipa RE, Katchman E, Keenan E, Khan I, Konrad S, McAuley A, Skinner S, Wiessing L, 2020. HIV outbreaks among people who inject drugs in Europe, North America, and Israel. Lancet HIV 7, e434–e442. 10.1016/S2352-3018(20)30082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan K, Clement N, Rouen D, Rees V, Shearer J, Wodak A, 2004. Can drug injectors be encouraged to adopt non-injecting routes of administration (NIROA) for drugs? Drug Alcohol Rev 23, 281–286. 10.1080/09595230412331289437. [DOI] [PubMed] [Google Scholar]
- Geddes L, Iversen J, Memedovic S, Maher L, 2018. Intravenous fentanyl use among people who inject drugs in Australia. Drug Alcohol Rev 37 Suppl 1, S314–S322. 10.1111/dar.12668. [DOI] [PubMed] [Google Scholar]
- Girardi E, Zaccarelli M, Tossini G, Puro V, Narciso P, Visco G, 1990. Hepatitis C virus infection in intravenous drug users: prevalence and risk factors. Scand J Infect Dis 22, 751–752. 10.3109/00365549009027133. [DOI] [PubMed] [Google Scholar]
- Gossop M, Stewart D, Marsden J, Kidd T, Strang J, 2004. Changes in route of drug administration among continuing heroin users: outcomes 1 year after intake to treatment. Addict Behav 29, 1085–1094. 10.1016/j.addbeh.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Griffiths P, Gossop M, Powis B, Strang J, 1994. Transitions in patterns of heroin administration: a study of heroin chasers and heroin injectors. Addiction 89, 301–309. 10.1111/j.1360-0443.1994.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Harris M, 2020. An urgent impetus for action: safe inhalation interventions to reduce COVID-19 transmission and fatality risk among people who smoke crack cocaine in the United Kingdom. Int J Drug Policy 83, 102829. 10.1016/j.drugpo.2020.102829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Forseth K, Rhodes T, 2015. “It’s Russian roulette”: adulteration, adverse effects and drug use transitions during the 2010/2011 United Kingdom heroin shortage. Int J Drug Policy 26, 51–58.doi: 10.1016/j.drugpo.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Hulin J, Brodie A, Stevens J, Mitchell C, 2020. Prevalence of respiratory conditions among people who use illicit opioids: a systematic review. Addiction 115, 832–849. 10.1111/add.14870. [DOI] [PubMed] [Google Scholar]
- Hunter GM, Donoghoe MC, Stimson GV, 1995. Crack use and injection on the increase among injecting drug users in London. Addiction 90, 1397–1400. 10.1046/j.1360-0443.1995.9010139711.x. [DOI] [PubMed] [Google Scholar]
- Hyshka E, Strathdee S, Wood E, Kerr T, 2012. Needle exchange and the HIV epidemic in Vancouver: lessons learned from 15 years of research. Int J Drug Policy 23, 261–270. 10.1016/j.drugpo.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivsins A, Boyd J, Beletsky L, McNeil R, 2020. Tackling the overdose crisis: The role of safe supply. Int J Drug Policy 80, 102769. 10.1016/j.drugpo.2020.102769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Pizzicato L, Johnson C, Viner K, 2021. Increasing presence of xylazine in heroin and/or fentanyl deaths, Philadelphia, Pennsylvania, 2010–2019. Inj Prev. 10.1136/injuryprev-2020-043968. [DOI] [PubMed] [Google Scholar]
- Kadri AN, Wilner B, Hernandez AV, Nakhoul G, chahine J, Griffin B, Pettersson G, Grimm R, Navia J, Gordon S, Kapadia SR, Harb SC, 2019. Geographic Trends, Patient Characteristics, and Outcomes of Infective Endocarditis Associated With Drug Abuse in the United States From 2002 to 2016. Journal of the American Heart Association 8.doi: 10.1161/jaha.119.012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MM, Conyngham SC, Smith C, Higgins D, Nassau T, Terrell C, Brady KA, 2020. Understanding the Intersection of Behavioral Risk and Social Determinants of Health and the Impact on an Outbreak of Human Immunodeficiency Virus Among Persons Who Inject Drugs in Philadelphia. J Infect Dis 222, S250–S258. 10.1093/infdis/jiaa128. [DOI] [PubMed] [Google Scholar]
- Kral AH, Lambdin BH, Wenger LD, Davidson PJ, 2020. Evaluation of an Unsanctioned Safe Consumption Site in the United States. N Engl J Med 383, 589–590. 10.1056/NEJMc2015435. [DOI] [PubMed] [Google Scholar]
- Kral AH, Malekinejad M, Vaudrey J, Martinez AN, Lorvick J, McFarland W, Raymond HF, 2010. Comparing respondent-driven sampling and targeted sampling methods of recruiting injection drug users in San Francisco. J Urban Health 87, 839–850. 10.1007/s11524-010-9486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambdin BH, Bluthenthal RN, Zibbell JE, Wenger L, Simpson K, Kral AH, 2019. Associations between perceived illicit fentanyl use and infectious disease risks among people who inject drugs. Int J Drug Policy 74, 299–304. 10.1016/j.drugpo.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansky A, Finlayson T, Johnson C, Holtzman D, Wejnert C, Mitsch A, Gust D, Chen R, Mizuno Y, Crepaz N, 2014. Estimating the number of persons who inject drugs in the united states by meta-analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS One 9, e97596. 10.1371/journal.pone.0097596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard L, DeRubeis E, Pelude L, Medd E, Birkett N, Seto J, 2008. “I inject less as I have easier access to pipes”: injecting, and sharing of crack-smoking materials, decline as safer crack-smoking resources are distributed. Int J Drug Policy 19, 255–264. 10.1016/j.drugpo.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Long H, Deore K, Hoffman RS, Nelson LS, 2003. A fatal case of spongiform leukoencephalopathy linked to “chasing the dragon”. J Toxicol Clin Toxicol 41, 887–891. 10.1081/clt-120025358. [DOI] [PubMed] [Google Scholar]
- Mars SG, Bourgois P, Karandinos G, Montero F, Ciccarone D, 2016. The Textures of Heroin: User Perspectives on “Black Tar” and Powder Heroin in Two U.S. Cities. J Psychoactive Drugs 48, 270–278. 10.1080/02791072.2016.1207826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars SG, Ondocsin J, Ciccarone D, 2018. Toots, tastes and tester shots: user accounts of drug sampling methods for gauging heroin potency. Harm Reduct J 15, 26. 10.1186/s12954-018-0232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BD, Milloy MJ, Wood E, Montaner JS, Kerr T, 2011. Reduction in overdose mortality after the opening of North America’s first medically supervised safer injecting facility: a retrospective population-based study. Lancet 377, 1429–1437. 10.1016/S0140-6736(10)62353-7. [DOI] [PubMed] [Google Scholar]
- Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL, 2021. Trends and Geographic Patterns in Drug and Synthetic Opioid Overdose Deaths - United States, 2013–2019. MMWR Morb Mortal Wkly Rep 70, 202–207. 10.15585/mmwr.mm7006a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Boyd CJ, Evans-Polce RJ, McCabe VV, Schulenberg JE, Veliz PT, 2020. Pills to Powder: A 17-Year Transition From Prescription Opioids to Heroin Among US Adolescents Followed Into Adulthood. J Addict Med. 10.1097/ADM.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S, Parmar N, Kelleher M, Jolley CJ, White P, Durbaba S, Ashworth M, 2020. COPD and asthma in patients with opioid dependency: a cross-sectional study in primary care. NPJ Prim Care Respir Med 30, 4. 10.1038/s41533-0190-161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale R, Griffiths P, Mortimer K, Walker P, Byrne T, Marwood K, Morrison-Griffiths S, Renwick S, Rylance J, Burhan H, 2020. Exploring perspectives on chronic obstructive pulmonary disease in people who smoke heroin: a qualitative study. BJGP Open 4. 10.3399/bjgpopen20X101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton MQ, 1990. Qualitative Evaluation and Research Methods. Sage, Newbury Park, CA. [Google Scholar]
- Pizzey R, Hunt N, 2008. Distributing foil from needle and syringe programmes (NSPs) to promote transitions from heroin injecting to chasing: an evaluation. Harm Reduct J 5, 24. 10.1186/1477-7517-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prangnell A, Dong H, Daly P, Milloy MJ, Kerr T, Hayashi K, 2017. Declining rates of health problems associated with crack smoking during the expansion of crack pipe distribution in Vancouver, Canada. BMC Public Health 17, 163. 10.1186/s12889-017-4099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashti R, Sharafi H, Alavian SM, Moradi Y, Mohamadi Bolbanabad A, Moradi G, 2020. Systematic Review and Meta-Analysis of Global Prevalence of HBsAg and HIV and HCV Antibodies among People Who Inject Drugs and Female Sex Workers. Pathogens 9. 10.3390/pathogens9060432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes T, Briggs D, Kimber J, Jones S, Holloway G, 2007. Crack-heroin speedball injection and its implications for vein care: qualitative study. Addiction 102, 1782–1790. 10.1111/j.1360-0443.2007.01969.x. [DOI] [PubMed] [Google Scholar]
- Rodda LN, 2021. OCME Accidental Overdose Reports: Recent information and preliminary data on accidental overdose deaths in San Francisco. https://sf.gov/sites/default/files/2021-05/2021%2005_OCME%20Overdose%20Report.pdf accessed on May 27, 2021.
- Roth AM, Armenta RF, Wagner KD, Strathdee SA, Goldshear JL, Cuevas-Mota J, Garfein RS, 2017. Cold Preparation of Heroin in a Black Tar Market. Subst Use Misuse 52, 1202–1206. 10.1080/10826084.2017.1302956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy E, Arruda N, Vaillancourt E, Boivin JF, Morissette C, Leclerc P, Alary M, Bourgois P, 2012. Drug use patterns in the presence of crack in downtown Montreal. Drug Alcohol Rev 31, 72–80. 10.1111/j.1465-3362.2011.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See I, Gokhale RH, Geller A, Lovegrove M, Schranz A, Fleischauer A, McCarthy N, Baggs J, Fiore A, 2020. National Public Health Burden Estimates of Endocarditis and Skin and Soft-Tissue Infections Related to Injection Drug Use: A Review. J Infect Dis 222, S429–S436. 10.1093/infdis/jiaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharhani A, Jorjoran Shushtari Z, Rahmani A, Armoon B, Noroozi M, Ahounbar E, Karimi SE, Higgs P, 2021. Incidence of HIV and HCV in people who inject drugs: a systematic and meta-analysis review protocol. BMJ Open 11, e041482. 10.1136/bmjopen-2020-041482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover HJ, Schaffer D, 2014. SMOKE IT! Promoting a change of opiate consumption pattern - from injecting to inhaling. Harm Reduct J 11, 18. 10.1186/1477-7517-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift W, Maher L, Sunjic S, 1999. Transitions between routes of heroin administration: a study of Caucasian and Indochinese heroin users in south-western Sydney, Australia. Addiction 94, 71–82. 10.1046/j.1360-0443.1999.941714.x. [DOI] [PubMed] [Google Scholar]
- Syvertsen JL, Ohaga S, Agot K, Dimova M, Guise A, Rhodes T, Wagner KD, 2016. An ethnographic exploration of drug markets in Kisumu, Kenya. Int J Drug Policy 30, 82–90. 10.1016/j.drugpo.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempalski B, Pouget ER, Cleland CM, Brady JE, Cooper HL, Hall HI, Lansky A, West BS, Friedman SR, 2013. Trends in the population prevalence of people who inject drugs in US metropolitan areas 1992–2007. PLoS One 8, e64789. 10.1371/journal.pone.0064789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thadani T, 2021. 2020 was SF’s deadliest year for overdoses, by far., San Francisco Chronicle. [Google Scholar]
- Thomas DR, 2006. A General Inductive Approach for Analyzing Qualitatie Evaluation Data American journal of Evlauation 36, 416–430 [Google Scholar]
- Ti L, Tobias S, Lysyshyn M, Laing R, Nosova E, Choi J, Arredondo J, McCrae K, Tupper K, Wood E, 2020. Detecting fentanyl using point-of-care drug checking technologies: A validation study. Drug Alcohol Depend 212, 108006. 10.1016/j.drugalcdep.2020.108006. [DOI] [PubMed] [Google Scholar]
- Valente PK, Bazzi AR, Childs E, Salhaney P, Earlywine J, Olson J, Biancarelli DL, Marshall BDL, Biello KB, 2020. Patterns, contexts, and motivations for polysubstance use among people who inject drugs in non-urban settings in the U.S. Northeast. Int J Drug Policy 85, 102934. 10.1016/j.drugpo.2020.102934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters JK, Biernacki P, 1989. Targeted Sampling: Options for the Study of Hidden Populations. Soc Probl 36, 416–430 [Google Scholar]
- Westermeyer J, 1976. The pro-heroin effects of anti-opium laws in Asia. Arch Gen Psychiatry 33, 1135–1139. 10.1001/archpsyc.1976.01770090125014. [DOI] [PubMed] [Google Scholar]
- Wiener S, Chiu D, Friedman C, Kamlager S, Eggman S, Skinner N, Bonta R, Carillo W, Ting P, Wicks B, 2020. Senate Bill No. 57. California Legislature--2020–2022 Regular Session. An act to add and repeal Section 11376.6 of the Health and Safety Code, relating to controlled substances. https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=202120220SB57 accessed on 3/25/2021. [Google Scholar]
- Wodak A, 1997. Injecting nation: achieving control of hepatitis C in Australia. Drug Alcohol Rev 16, 275–284. 10.1080/09595239800187451. [DOI] [PubMed] [Google Scholar]
- Yalamanoglu A, Schuurmans MM, 2018. [Foil Smoking/Heroin Inhalation]. Praxis (Bern 1994) 107, 1393–1398. 10.1024/1661-8157/a003128. [DOI] [PubMed] [Google Scholar]
- Zibbell JE, Peiper NC, Duhart Clarke SE, Salazar ZR, Vincent LB, Kral AH, Feinberg J, 2021. Consumer discernment of fentanyl in illicit opioids confirmed by fentanyl test strips: Lessons from a syringe services program in North Carolina. Int J Drug Policy, 103128. 10.1016/j.drugpo.2021.103128. [DOI] [PubMed] [Google Scholar]
- Zou G, 2004. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159, 702–706. 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]


