ABSTRACT
The Anopheles symbiont, Microsporidia MB, is maternally inherited and has a strong malaria transmission-blocking phenotype in Anopheles arabiensis. Microsporidia MB is also vertically transmitted, sexually transmitted, and avirulent. These characteristics are expected to promote its spread through mosquito populations, enhancing the potential of Microsporidia MB as a candidate for the development of a symbiont-mediated malaria transmission-blocking strategy. We found that the patterns of Microsporidia MB localization over the development of An. arabiensis indicate accumulation in tissues linked to its transmission, specifically the male and female gonadal tissues. Transovarial vertical transmission of Microsporidia MB occurs in the female An. arabiensis ovary when Microsporidia MB becomes localized to the cytoplasm of the developing oocyte. In male An. arabiensis, Microsporidia MB is localized in the testis and vas deferens. Notably, a high intensity of Microsporidia MB can also be observed in the An. arabiensis adult but not larval gut. The levels of Microsporidia MB found in the female ovary are linked to the progression of oogenesis, increasing after blood feeding initiates the development of eggs. There was a significant change in Microsporidia MB levels in female and male An. arabiensis gonads, where intensity tended to decrease as mosquitoes aged. However, the intensities did not significantly change in the male or female guts. Altogether, the high specificity of Microsporidia MB tissue localization patterns and changes in infection prevalence and intensity suggest adaptation to maximize transmission and avirulence in Anopheles arabiensis.
IMPORTANCE
Microsporidia MB is a symbiont with a strong malaria transmission-blocking phenotype in Anopheles arabiensis. It spreads in mosquito populations through mother-to-offspring and sexual transmission. The ability of Microsporidia MB to block Plasmodium transmission, together with its ability to spread within Anopheles populations and its avirulence to the host, makes it a very attractive candidate for developing a key strategy to stop malaria transmissions. Here, we report tissue tropism and localization patterns of Microsporidia MB, which are relevant to its transmission. We find that Microsporidia MB accumulates in Anopheles arabiensis tissues, linked to its sexual and vertical transmission. Its prevalence and intensity in the tissues over the mosquito life cycle suggest adaptation to maximize transmission and avirulence in Anopheles arabiensis. These findings provide the foundation for understanding the factors that may affect Microsporidia MB transmission efficiency. This will contribute to the development of strategies to maximize Microsporidia MB transmission to establish and sustain a high prevalence of the symbiont in Anopheles mosquito populations for malaria transmission blocking.
KEYWORDS: Microsporidia MB, malaria vectors, symbiotic microbes
INTRODUCTION
The malaria disease burden remains a major impediment to good health and economic development in many regions of sub-Saharan Africa. In 2021, a total of 247 million cases were reported, which resulted in 619,000 deaths, a strong indication that current control measures and their deployment levels are insufficient (1). Large-scale insecticide-treated net (ITN) distribution campaigns have had a significant impact on reducing the number of malaria cases (2). However, many malaria vectors have now developed resistance to the insecticides used in ITNs (3), and they are increasingly biting outdoors, where nets offer no protection (4). In addition, many malaria control efforts were significantly disrupted by the coronavirus disease 2019 pandemic (5), and the recent invasion of Anopheles stephensi across Africa is leading to a significant rise in malaria transmission (6–8). Altogether, these factors threaten to reverse the gains achieved for malaria reduction and indicate an urgent need for new strategies to control Anopheles mosquito populations or their capacity to transmit Plasmodium parasites.
A novel and potentially transformative method of controlling vector-borne disease involves the use of transmission-blocking symbionts. For dengue, a control strategy based on the transmission-blocking symbiont Wolbachia has been highly effective, and controlled field trials are currently implemented in over 13 countries (9). A similar strategy, based on a Plasmodium transmission-blocking symbiont in Anopheles mosquitoes, could be transformative for controlling malaria. The microsporidian symbiont, Microsporidia MB, is naturally found in anopheline mosquito populations and has been shown to block Plasmodium development (10). In addition, Microsporidia MB is both vertically and sexually transmitted (11). In conjunction with Plasmodium blocking, the high efficiency of vertical and sexual transmission of Microsporidia MB, which could facilitate the spread and maintenance of Microsporidia MB in Anopheles mosquito populations, has led to the suggestion that this symbiont could be deployed as a new tool for malaria control (12).
The success of a Microsporidia MB-based malaria control strategy will depend on the efficient vertical and horizontal transmission of the symbiont, which would enable the spread and maintenance of Microsporidia MB in Anopheles vector populations. For symbionts that are vertically and sexually transmitted, avirulence toward the host can be expected (13). In addition, vertically and sexually transmitted symbionts can be selected for their ability to enhance host fitness (14, 15). The tissue-level localization pattern and intensity of infection have been shown to play an important role in symbiont transmission and host fitness effects. In Anopheles arabiensis, Microsporidia MB is transmitted vertically from females to offspring and horizontally between males and females during mating, suggesting an important interaction with reproductive tissues (10, 11). Here, we investigated the tissue-level localization and changes in Microsporidia MB infection intensity across the development of An. arabiensis. We found that Microsporidia MB was predominately found in the reproductive organs of An. arabiensis males and females. Additionally, we observed Microsporidia MB inside developing oocytes in the An. arabiensis ovaries, indicating transovarial vertical transmission. Interestingly, the intensity of the Microsporidia MB infection in the female ovaries increased after blood feeding. The prevalence and intensity of Microsporidia MB infection in the female ovaries were found to decrease as An. arabiensis mosquitoes aged. In male An. arabiensis, Microsporidia MB is found in the testis and vas deferens, offering further confirmation of the basis of male-to-female sexual transmission as previously reported (11). Microsporidia MB is also found in the An. arabiensis adult gut at moderate prevalences. Notably, Microsporidia MB is always absent from the larval gut but is present in the larval body.
RESULTS
The An. arabiensis male and female gonads are the main sites of Microsporidia MB infection and localization in An. arabiensis
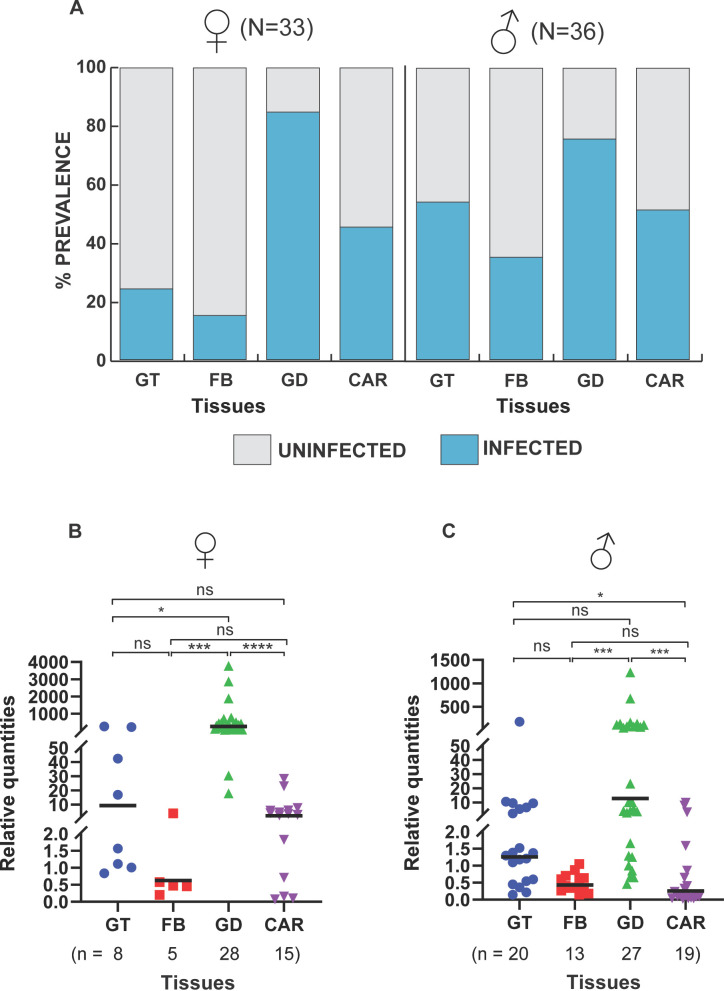
The prevalence and intensity of Microsporidia MB were investigated in the gonads, gut, fat body, and carcass of 7-day-old adult An. arabiensis by quantitative PCR (qPCR) (Fig. 1). We show that An. arabiensis male and female gonads had the highest Microsporidia MB prevalence, followed by the carcass, the gut, and the fat body (Fig. 1A). In females, Microsporidia MB infection prevalence was 24% in the guts, 15% in the fat body, 85% in the gonads, and 45% in the carcass, while for males, it was 56% in the guts, 36% in the fat body, 75% in the gonads, and 53% in the carcass. Notably, the prevalence in the guts of males was double that of female guts. Additionally, we measured and compared the relative intensity of Microsporidia MB (Microsporidia MB 18S RNA copies relative to the geometric mean of three host gene copy numbers) across all four tissues investigated (Table 1). We found that there was a significantly higher relative intensity of Microsporidia MB in the female gonads than in all other female tissues investigated (Fig. 1B). In males, the relative intensity of Microsporidia MB was significantly higher in the gonads than in the fat bodies and carcasses but not guts (Fig. 1C). In addition, the mean relative Microsporidia MB intensity in the male gut was significantly higher than that in the fat body and carcass, indicating that Microsporidia MB intensity can reach high levels in this tissue. In both sexes, the intensity of Microsporidia MB was lowest in the fat body (Fig. 1B and C). These findings indicate that the main site of Microsporidia MB infection in both male and female adult An. arabiensis is the gonadal tissue, and the gut and carcass are likely to be the minor sites of infection. This is in line with a study that investigated Microsporidia MB in only male An. arabiensis (11).
FIG 1.
Microsporidia MB infection prevalence and intensity in adult An. arabiensis tissues. Prevalence rates and relative intensities of Microsporidia MB were measured for the guts (GT), fat bodies (FB), gonads (GD), and carcasses (CAR) of 7-day-old mosquitoes using qPCR. (A) The gonads were the tissues with the highest prevalence of Microsporidia MB in the dissected tissues, indicating that the gonads are the main site of infection. (B and C) Relative quantification of Microsporidia MB intensity in the tissues that turned out positive showed high infection intensities in the gonadal tissues in both females and males. The variations in the intensity of Microsporidia MB between the tissues were determined using the Kruskal-Wallis H test. A significant variation in the intensity was observed in both female tissues [H(3, n = 56) = 37.50, P < 0.0001] and male tissues [H(3, n = 79) = 36.70, P < 0.0001]. Post-hoc pairwise comparisons indicating the significance between the different groups were conducted and further affirmed gonads as the main sites of infection. Asterisks show the level of significance (*P < 0.05, ***P < 0.001, and ****P < 0.0001), while ns indicates no significance. The bars in the plots indicate the median for each tissue group. The number of independent replicates is 7.
TABLE 1.
Sequences of primers used for Microsporidia MB detection, Anopheles species identification, and amplification of reference genes for relative quantification of Microsporidia MB
| Target | Primer name | Primer sequence (5′–3′) | Annealing temp. |
|---|---|---|---|
| Microsporidia MB 18S gene | MB18S 181F (10) | CGCCGGCCGTGAAAAATTTA | 62°C |
| MB18S 650R (10) | CCTTGGACGTGGGAGCTATC | ||
| An. gambiae s.s. short interspersed elements (SINEs) 200 | SINE 200F (16) | TCGCCTTAGACCTTGCGTTA | 56°C |
| SINE 200R (16) | CGCTTCAAGAATTCGAGATAC | ||
| Anopheles ribosomal S7 | S7UniF (10) | TCCTGGAGCTGGAGATGAAC | 62°C |
| S7UniR (10) | GACGGGTCTGTACCTTCTGG | ||
| An. gambiae s.s. actin gene | Actin-F (this study) | AAGATGACGTGTTGTGCCCT | 64°C |
| Actin-R (this study) | ATTCCCGTTGCTGGAAGTGT | ||
| An. gambiae s.s. GAPDH gene | An. ga. GAPDH-Aste-QPCR-F (this study) | GCGGTGGGCAAGGTCATCCC | 64°C |
| GAPDH-Aste-QPCR-R (17) | TTCATCGGTCCGTTGGCGGC |
Microsporidia MB cells are identified by microscopy in oocytes in female and in the testis and gut in male An. arabiensis
To better understand where Microsporidia MB is located inside the male and female An. arabiensis gonads and guts, we visualized these tissues using fluorescent in situ hybridization (FISH) and confocal microscopy. The visualization of Microsporidia MB confirmed that male and female An. arabiensis gonads were the main sites of infection (Fig. 2A and B). In the An. arabiensis female gonad, Microsporidia MB is observed in all stages of egg chamber development (Fig. 2A). As the oocytes enter vitellogenesis, higher intensities of Microsporidia MB are observed. In the male gonad, Microsporidia MB is observed in the testis and vas deferens, in close proximity to developing sperm cells (Fig. 2B). In the An. arabiensis male gut, Microsporidia MB is occasionally observed at high infection levels in a small number of cells in the midgut and hindgut regions of males (Fig. 2C). We did not observe Microsporidia MB in the female guts, most likely because of the low prevalence rates in females and generally low-intensity infections in the guts.
FIG 2.
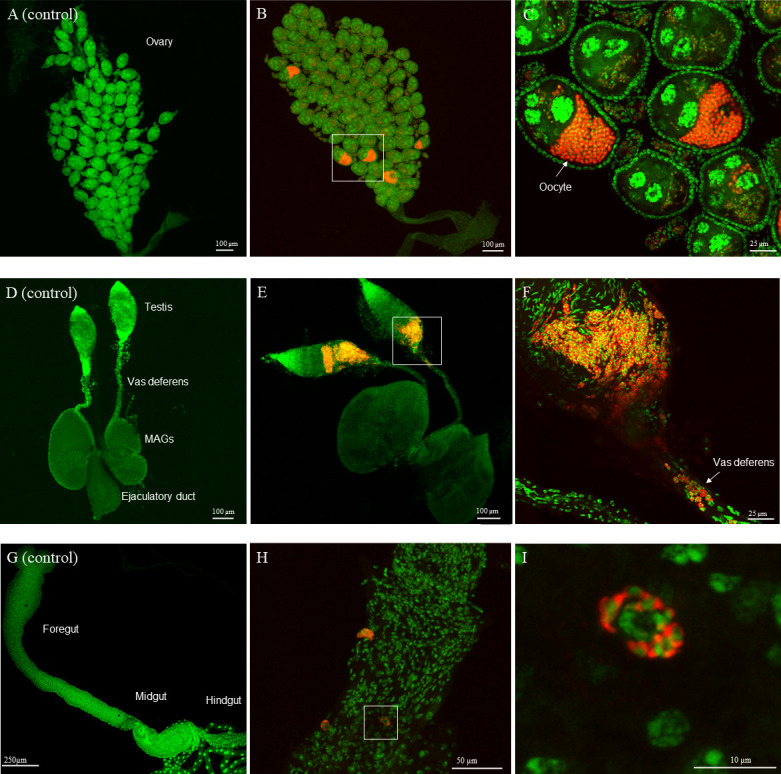
Microsporidia MB localization to cells in the male and female gonads and male gut. Localization of Microsporidia MB in the gonads and gut of 7-day-old An. arabiensis mosquitoes was conducted using FISH and confocal fluorescence microscopy. (A) Control Microsporidia MB-uninfected An. arabiensis female gonad. (B) Microsporidia MB-infected An. arabiensis female gonad. (C) A high intensity of Microsporidia MB is observed in vitellogenic oocytes. (D) Control Microsporidia MB-uninfected An. arabiensis male gonad. (E) Microsporidia MB-infected An. arabiensis male gonad. (F) A high intensity of Microsporidia MB is observed in parts of the testis and vas deferens that connect the testis to the ejaculatory duct. (G) Control Microsporidia MB-uninfected An. arabiensis male gut. (H) Microsporidia MB-infected An. arabiensis male gut (I) Microsporidia MB is observed in the An. arabiensis male midgut cells. The left panel in each row is a Microsporidia MB uninfected control tissue. The red signal in the images is the Microsporidia MB probe-CY5 FISH probe targeting Microsporidia MB while the green signal is the general DNA stain Sytox-Green. The number of independent replicates = 54 (ovaries), 11 (testis and vas deferens), and 7 (male guts).
Microsporidia MB is not found in the larval gut and is higher in female adult mosquitoes
To better understand where Microsporidia MB is located during the larval stage of An arabiensis, L4 larval stages were dissected to separate the gut from the carcass, with carcasses containing all the remaining larval tissue, including the hemolymph. We observed that none of the gut samples were infected with Microsporidia MB (Fig. 3A). However, larval carcasses were found to have high Microsporidia MB infections, with a prevalence rate of 100% for all infected larvae investigated. The relative intensities of Microsporidia MB infection in An. arabiensis larvae were significantly less than those observed in adult female but not male mosquitoes (Fig. 3B). FISH and confocal microscopy analysis of the localization of Microsporidia MB in the larvae revealed that the symbiont is housed in a distinct structure that is located next to the gut epithelial cells (Fig. 3C).
FIG 3.
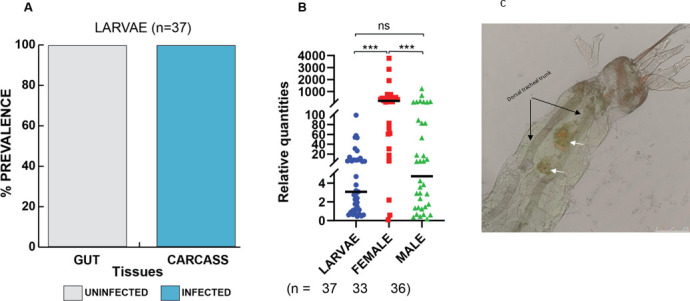
Localization of Microsporidia MB in larval tissues and comparison of its intensity between larvae and adult mosquito stages. L4 larvae and 1-week-old mosquitoes were used to compare Microsporidia MB infections in larvae and adult mosquitoes. (A) Using PCR, the presence and absence of Microsporidia MB were determined, and counts were used to calculate prevalence. Whereas there were no infected larval gut tissues, the larval carcass tissues had high prevalences. (B) A comparison of Microsporidia MB levels between the larvae, female, and male adult mosquitoes using qPCR revealed significant differences in the infection levels between these groups [H(3, n = 106) = 34.48, P < 0.0001]. Furthermore, post-hoc analysis with Dunn’s multiple comparisons test revealed that females have high levels of Microsporidia MB. The asterisks show the level of significance (***P < 0.001), while ns represent no significance. The bars represent the median. The number of independent replicates is 13 (larvae) and 7 (adult mosquitoes). (C) Localization of Microsporidia MB in An. arabiensis larvae using FISH and confocal microscopy. FISH image of whole larvae merged with brightfield signal to show localization of Microsporidia MB (white arrows) in a distinct structure at the posterior end of the larvae. The red signal is the MB probe-CY5 FISH probe targeting Microsporidia MB, while the green signal is the general DNA stain Sytox-Green. The microscopy image shown is representative of five independent observations.
Blood meal affects the intensity of Microsporidia MB in the ovaries
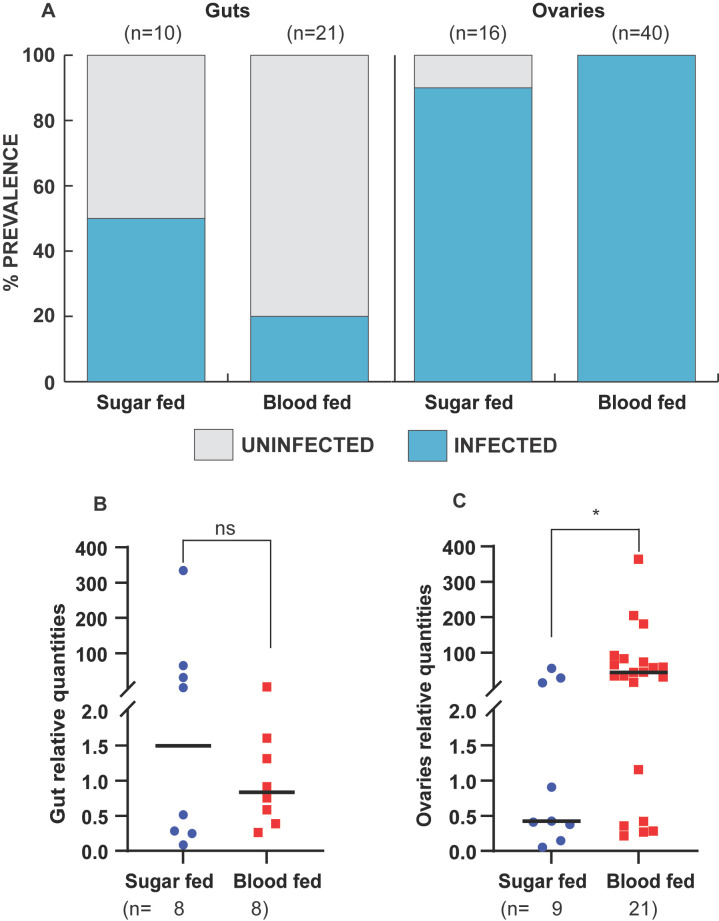
In An. arabiensis, oogenesis begins after female mosquitoes eclose but is arrested until females take a blood meal. To determine if the intensity of Microsporidia MB in An. arabiensis is affected by the onset of egg development and other physiological changes associated with taking a blood meal, we compared the prevalence and intensity of Microsporidia MB infection in the guts and gonads of An. arabiensis females that had fed on a blood meal with those that fed on sugar. In the An. arabiensis female gut, the prevalence of Microsporidia MB in sugar-fed mosquitoes was 53%, while for blood-fed mosquitoes, it was 20% (Fig. 4A). However, there was no change in the relative intensity of Microsporidia MB in the gut after blood feeding (Fig. 4B). The prevalence of Microsporidia MB infection was high in ovaries regardless of feeding status (Fig. 4A). However, we observed significantly higher relative intensities of Microsporidia MB in the gonads of blood-fed An. arabiensis than of sugar-fed controls (Fig. 4C).
FIG 4.
Effect of blood feeding on Microsporidia MB prevalence and intensity in female An. arabiensis gonads and gut tissues. Two- to three-day-old mosquitoes were fed on blood, and Microsporidia MB prevalence rates and infection levels were checked 5 days post-blood feeding and compared to sugar-fed controls using conventional PCR and qPCR, respectively. (A) The prevalence of Microsporidia MB in the guts and ovaries is expressed as a percentage of the tissues infected with Microsporidia MB compared to those uninfected. Blood feeding decreases prevalence in the gut but not in the gonads. (B) Microsporidia MB levels in the guts of the blood-fed group and the control group (fed on 10% glucose only) female mosquitoes were not different (two-tailed Mann–Whitney test, P = 0.879, bars reflect the median). (C) In contrast to sugar-fed female mosquitoes, fed female mosquitoes have significantly higher intensities of Microsporidia MB in their ovaries (two-tailed Mann–Whitney test, P = 0.019, bars reflect the median). The asterisk shows the level of significance (*P < 0.05), while ns represents non-significance. The number of independent replicates is 4 (guts) and 2 (ovaries).
Age affects the intensity of Microsporidia MB in An. arabiensis gonads and guts
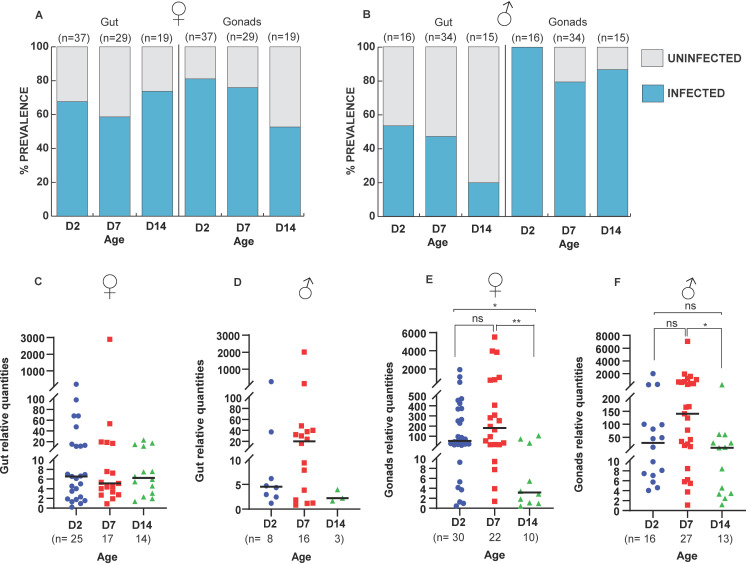
To investigate the effect of aging on the prevalence and intensity of Microsporidia MB in An. arabiensis gonadal and gut tissue, male and female An. arabiensis were aged for 2, 7, and 14 days prior to dissection of tissues and quantification of Microsporidia MB by qPCR. The prevalence of Microsporidia MB in the guts of females remained relatively stable, while the prevalence in the gonads had a decreasing trend as mosquitoes aged (Fig. 5A). The prevalence of Microsporidia MB in the guts of male An. arabiensis decreased as mosquitoes aged, with 14-day-old males having the lowest infection rate (Fig. 5B). Similarly, the prevalence of male gonads decreased with age (Fig. 5B). In both male and female guts, there was no significant change in Microsporidia MB intensity across the different age groups (Fig. 5C and D). There was a significant change in the relative intensity of Microsporidia MB in female and male An. arabiensis gonads, where intensity tended to decrease as mosquitoes aged (Fig. 5E and F). Pairwise comparisons post-hoc analysis with Dunn’s multiple comparisons test revealed a significant decrease between days 2 and 14 (P = 0.032) and days 7 and 14 (P = 0.003) in females (Fig. 5E), while in males, a significant decrease was only observed between days 7 and 14 (P = 0.011) (Fig. 5F). These results suggest that age affects Microsporidia MB infection levels only in the gonads but not the guts in both males and females.
FIG 5.
Effect of age on Microsporidia MB in An. arabiensis gonad and gut. The prevalence and intensity of Microsporidia MB infections were measured on 2-, 7-, and 14-day-old mosquitoes to determine how age affects Microsporidia MB. (A and B) In females, the prevalence of Microsporidia MB in the guts is higher at day 14 (74%) relative to day 2 (68%) and day 7 (59%), while in the gonads, the prevalence drops as mosquitoes age, with day 14 having the lowest (53%) compared to days 7 (76%) and 2 (81%). In males, the prevalence of Microsporidia MB in the guts drops as mosquitoes age over days 2 (53%), 7 (47%), and 14 (20%). However, the prevalence in the male gonads is relatively constant as the mosquitoes age (100%, 79%, and 87% for days 2, 7, and 14, respectively). (C and D) Intensities of Microsporidia MB in the guts of both female and male An. arabiensis were not affected by the age of the mosquitoes [female guts: H(2, n = 56) = 0.0098, P = 0.9951, male guts: H(2, n = 27) = 2.602, P = 0.2723]. (E) In the female gonads, age significantly affected the relative intensities of Microsporidia MB [H(2, n = 62) = 10.96, P = 0.0042]. Upon conducting post-hoc analysis with Dunn’s multiple comparisons test, there was a significant decrease at day 14 compared to days 2 and 7. (F) Similarly, the relative intensities in the male gonads were significantly affected by mosquito age [H(2, n = 56) = 8.782, P = 0.0124]. Upon conducting post-hoc analysis with Dunn’s multiple comparisons test, there was a significant decrease only at day 14 compared to day 7. Asterisks show the level of significance (*P < 0.05, **P < 0.01), while ns indicates no significance. The number of independent replicates is 19.
DISCUSSION
Our findings demonstrate that the gonads of male and female An. arabiensis are the main sites of Microsporidia MB infection. This suggests that maternal and sexual transmission are likely to be the most important Microsporidia MB infection routes. Both maternal and sexual transmission routes require that An. arabiensis hosts do not incur significant fitness costs when infected with Microsporidia MB (13). In line with this, Microsporidia MB does not have any known major effect on key metrics of fitness, including larval developmental time and pupation rate, fecundity of female mosquitoes, and survival rate of infected adult mosquitoes (10).
Other insect endosymbionts, including Wolbachia, have intensity-dependent effects on host fitness (18). It is therefore possible that very high-intensity Microsporidia MB infections do have a fitness cost, but this would need further investigation. The link between Wolbachia intensity and host fitness is complex, and, in many cases, fitness is not affected by high endosymbiont intensities (19). It is likely that the pattern of endosymbiont localization in host tissues has important fitness consequences for endosymbionts such as Wolbachia and Microsporidia MB. A strain of Wolbachia, wMelPop, that overproliferates and decreases host fitness has been found at high intensities in the central nervous system and muscles of its insect hosts (20, 21). It is possible that endosymbionts mitigate effects on host fitness by limiting most of the infection and proliferation to certain tissues.
Our findings indicate that Microsporidia MB is primarily found in gonadal tissue, and this is a suggestive adaptation to minimize host fitness costs without compromising transmission rates. Fluorescence confocal microscopy revealed that Microsporidia MB was present in oocytes and nurse cells across all stages of oogenesis. These findings clearly demonstrate that Microsporidia MB maternal transmission is transovarial, which explains the need for higher Microsporidia MB intensities to be maintained in the female gonads.
In the male germline, Microsporidia MB is primarily localized to the testis and vas deferens. The testis is the site of sperm production, comprising a proximal end with stem cell divisions, spermatocysts in different stages of development, and a distal spermatozoa reservoir (22). In An. arabiensis, we observed Microsporidia MB cells as clusters in the spermatocyst and spermatozoa reservoir regions of the testis and, more rarely, in the stem cell region. These findings suggest that Microsporidia MB is packaged with spermatozoa at their early development stages.
We observed very low levels of Microsporidia MB in the An. arabiensis adult fat body. Pathogenic Microsporidians are known to infect and proliferate in the insect’s fat body (22). It is likely that, as the primary energy storage tissue, fat bodies provide the nutrients required for this proliferation. The low levels of Microsporidia MB in the adult fat body of An. arabiensis could be part of the explanation for its avirulence in An. arabiensis. The moderate levels of Microsporidia MB in the carcass samples, which contained all the tissues apart from the gut, gonads, and fat body, suggest that we cannot exclude there being another Microsporidia MB infection reservoir in adult An. arabiensis.
In the adult An. arabiensis gut, there were a small proportion of samples that had very high intensities of Microsporidia MB. The significance of these high-intensity gut infections is yet to be resolved. Fluorescence confocal microscopy revealed that Microsporidia MB was found at very high intensities in a small subset of cells in the adult male mid and hindgut. The shape and positioning of these cells suggest that they could be progenitor cells (intestinal stem cells and enteroblasts) (23). Progenitor cells are important for cell replenishment in the gut in response to intrinsic factors that bring about gut epithelium turnover (23). It has been shown that there are low levels of progenitor cells in Anopheles gambiae mosquitoes (24). This could explain the overall low prevalence in the gut tissues in the various experiments.
It is notable that, in contrast to adults, Microsporidia MB was never observed in the larval gut. A possible explanation for this is that its presence in the larval gut could have prohibitive fitness costs since larvae need to develop rapidly and do so by feeding continuously (25). Furthermore, larval guts are made up of regenerative endoreplicating cells that account for a greater portion of the midgut epithelial cells. This differs from adult guts, where midgut epithelial cells are derived from progenitor cells (26, 27). The absence of Microsporidia MB in the gut contradicts our previous report on its presence in the larval epithelial tissues (10). In the current study, we have conducted exhaustive additional FISH and confocal microscopy experiments as well as qPCR (which was not used in the original experiments). Based on this new data, we are no longer able to observe Microsporidia MB in the larval gut. We do, however, find that Microsporidia MB is localized in a distinct structure that is proximal to the larval gut, which likely explains our previous characterization. The high Microsporidia MB prevalence rates in the larval carcass suggest that Microsporidia MB establishes itself in the host tissues at a very early stage of An. arabiensis development cycle. The higher overall intensity of Microsporidia MB in adult females compared to adult males and larvae suggests a higher replication rate of the symbiont in females and may suggest that vertical transmission is the primary mode of transmission. We noted variations in prevalence rates and intensities in the F1s used across experiments. This is likely due to variations in transmission rates and seasonal and environmental influences on Microsporidia MB prevalences in the field-collected mothers (10).
We also investigated how localization and Microsporidia MB intensity change as mosquitoes age and upon blood feeding. In An. arabiensis, oogenesis starts when mosquitoes eclose but is then paused until females take a blood meal (28). Once females take a blood meal, several physiological changes occur that enable the blood to be digested and ultimately meet the nutritional demands of egg production (29). Since there are major changes occurring in the gut and gonads of females post-blood feeding, we investigated the changes in the levels of Microsporidia MB in these tissues.
We observed a moderate decrease in the prevalence of Microsporidia MB in female An. arabiensis guts after blood feeding. However, the levels of Microsporidia MB in the gut did not decrease significantly in response to blood feeding. It is possible that the decrease in prevalence could be linked to the process of gut epithelium renewal, which occurs after blood feeding (23). In contrast, we observed a marked increase in the intensity of Microsporidia MB in the female gonad after blood feeding. Notably, Microsporidia MB is also observed by microscopic examination at high intensities in the later (vitellogenic) stages of oogenic development, which develop after the blood meal-induced initiation of oogenesis (26). Therefore, the proliferation of Microsporidia MB in vitellogenic oocytes is a possible explanation for the increase in the intensity of Microsporidia MB after blood feeding.
We did not observe Microsporidia MB levels increasing in male or female guts and gonads as An. arabiensis mosquitoes aged. This pattern of growth is consistent with symbionts that regulate proliferation to mitigate fitness costs (28). Notably, there was a significant decrease in the prevalence and intensity of Microsporidia MB in female gonads between 7 and 14 days of aging. This may indicate a loss of Microsporidia MB from germline cells after numerous rounds of division and an inability for these cells to be re-infected. However, the aged An. arabiensis female mosquitoes had not been blood-fed and therefore had not completed any gonotrophic cycles, which could be expected to lower the rate of stem cell division (29). In addition, we observed a decrease in the prevalence and intensity of Microsporidia MB in male gonads between 7 and 14 days of aging. This could be linked to the loss of Microsporidia MB from stem cells after multiple rounds of division and the shedding of Microsporidia MB in the male ejaculate, or perhaps it reflects that Microsporidia MB can only survive for a limited time in the male ejaculatory duct.
Overall, our findings suggest that Microsporidia MB localization patterns may serve to maximize transmission success while minimizing negative effects on host fitness. Microsporidia MB has proliferation and localization patterns that are consistent with a co-evolved symbiont that exploits sexual and vertical transmission routes. A better understanding of changes in localization and symbiont intensity across the An. arabiensis lifecycle will contribute to improving Microsporidia MB-infected mosquito rearing and aid in the formulation of strategies to disseminate Microsporidia MB.
MATERIALS AND METHODS
Mosquito sample collection
Wild-caught gravid adult female Anopheles gambiae s.l. were collected using mouth aspiration. Collected mosquitoes were identified morphologically to the species level, and sub-species identification of Anopheles gambiae s.l. was carried out by PCR (16). Sampling was conducted from one site in Kenya, the Ahero irrigation scheme (−34.9190W, −0.1661N). The mosquitoes used in this study were collected between September 2021 and October 2021. Sampled mosquitoes were transported to the ICIPE—Duduville campus in Nairobi and maintained on 10% glucose and water.
Mosquito processing and development of isofemale lines
Wild collected mosquitoes were maintained in an insectary at 27 ± 2.5°C, humidity 60%–80%, and 12-h day and 12-h night cycles and induced to oviposit in individual microcentrifuge tubes containing a wet 1 cm × 1 cm Whatman filter paper. Eggs from each female were counted under a compound microscope using a paintbrush and then dispensed into water troughs for larval development at 30.5°C and 30%–40% humidity. Tetramin baby fish food was used to feed developing larvae. Upon laying eggs, the G0 females were screened for the presence of Microsporidia MB by PCR. The larval offspring of Microsporidia MB-positive field-caught female mosquitoes were transferred into larval rearing troughs for further development. Upon reaching the L4 larval stage, representative samples from each positive isofemale line were screened to determine the Microsporidia MB infection status of the line. Mosquitoes were maintained as adults; this was done in an insectary at 30°C with a relative humidity of 75%, 12-h day and 12-h night cycles, and a feeder with a 10% glucose solution.
Larval and adult dissection
An. arabiensis G1 larvae and adult mosquitoes were dissected using forceps under the Zeiss Stemi 2000-C stereomicroscope to obtain G1 L4 larval and adult Anopheles tissues. During the dissection of the L4 larvae, alive samples were placed on a drop of 1× phosphate buffered saline (PBS). The larvae were restrained at the junction between the head and thorax with a pair of forceps. A dissecting needle pin was then used to probe the intersection between the second and last abdomen segments. The siphon and saddle of the larvae were held and pulled gently to obtain the gut of the larvae using a new pair of forceps. The carcass and the gut were placed in separate 1.5 microcentrifuge tubes containing 20 µL of 1× PBS and 0.5 mm zirconium beads for DNA extractions. For adults, G1 mosquito samples were first anesthetized for a few minutes until immobilized by aspirating them into 1.5 microcentrifuge tubes and placing them in ice. Upon immobilization, the mosquitoes were placed in a drop of 1× PBS on a glass slide. The junction between the last and second-last abdomens was probed using a dissecting needle. The last segment was gently pulled to release the gonads and the gut. The gut and gonads were separated and placed in separate 1.5 microcentrifuge tubes containing 20 µL of 1× PBS and 0.5 mm zirconium beads ready for nucleic acid extractions. Similarly, the fat bodies and carcasses (all remaining tissue) were also separated and placed in separate 1.5 microcentrifuge tubes, as described above. Forceps were sterilized after every dissection to prevent contamination.
Individual adult mosquitoes and larvae used in all experiments were pre-screened, and those with at least one tissue(s) positive for Microsporidia MB were then considered the final sample sizes for prevalence and intensity determination in all the data generated. The prevalence of Microsporidia MB in the dissected tissues was obtained by counting tissue samples that turned out positive after PCR screening and expressed as a percentage of the final sample size. To compare the intensities of Microsporidia MB between mosquito adults and larvae, summations of the relative intensities from the dissected adult tissues (used in tissue localization experiments) and those from larvae carcasses were compared.
Mosquito blood feeding
Two- to three-day-old Microsporidia MB-positive and -negative An. arabiensis mosquitoes maintained at a temperature of 30°C and a relative humidity of 75% were starved for 2 h without water or glucose in preparation for blood feeding. Membrane feeding was conducted according to reference (30). Briefly, bovine blood was transferred into a Hemotek feeding apparatus (Hemotek, UK) with a Parafilm-A membrane set at 37°C. The Hemotek feeding apparatus was placed on top of a cage of starved An. arabiensis mosquitoes, and feeding was allowed to take place for 1 h. Fully engorged An. arabiensis were transferred to new cages, maintained for 5 days post-blood feeding, and later dissected to obtain the gut and ovaries, which were screened for Microsporidia MB presence and levels.
Flourescent in situ hybridisation localization
Dissected tissues were fixed overnight in 4% paraformaldehyde at 4°C. After fixation, the samples were rehydrated in 50% ethanol for 30 minutes and transferred to 1× PBS for another 30 minutes. FISH was then conducted to localize Microsporia MB within the tissues. Hybridization was done by incubating the tissues in 100 µL of hybridization buffer overnight at 50°C. The FISH hybridization mix contained hybridization buffer (dH2O, 5 M NaCl, 1 M Tris/HCl [pH = 8, and 10% SDS), a Microsporidia MB-specific CY5 probe (10), a 0.5 µM final concentration, and SYTOX Green general DNA staining. After staining, the samples were washed twice with 100 µL of prewarmed wash buffer (dH2O, 5 M NaCl, 1 M Tris/HCl [pH = 8], 0.5 M EDTA, and 10% SDS). The tissues were then placed on a slide and visualized immediately using a Leica SP5 confocal microscope (Leica Microsystems, USA). The images were analyzed with the ImageJ 1.50i software package (31).
DNA extraction and molecular detection and quantification of Microsporidia MB
DNA was extracted using the ammonium acetate protein precipitation method (10). Extracted DNA samples were screened to determine their Microsporidia MB infection status using the Microsporidia MB-specific primers (Table 1) (10). The PCR reaction used in detection comprised a 10 µL reaction consisting of 2 µL HOTFirepol Blend Master mix Ready-To-Load (Solis Biodyne, Estonia), 0.5 µL of 5 pmol/µL forward and reverse primers, 2 µL of the DNA template, and 5 µL nuclease-free PCR water. The thermocycling conditions employed were initial denaturation at 95°C for 15 min, followed by 35 cycles of denaturation at 95°C for 1 min, annealing at 62°C for 90 s, and extension at 72°C for a further 60 s. Final elongation was done at 72°C for 5 min. Samples positive for Microsporidia MB were subjected to relative qPCR analysis to quantify infection levels and species ID (10, 16). The qPCR analysis involved the MB18SF and MB18SR primers, normalized with the geometric means of cycle threshold values for the primers targeting the Anopheles ribosomal S7 gene, Actin gene, and GAPDH gene (Table 1) as the reference host genes. The 10 µL PCR reaction consisted of 2 µL HOT FIREPol EvaGreen HRM no ROX Mix Solis qPCR Master mix (Solis Biodyne, Estonia), 0.5 µL of 5 pmol/µL forward and reverse primers, 2 µL of the DNA template from Microsporidia MB-positive samples, and 5 µL nuclease-free PCR. The thermocycling conditions employed were an initial denaturation at 95°C for 15 min, followed by 35 cycles of denaturation at 95°C for 1 min, annealing at 62°C for 90 s, and extension at 72°C for a further 60 s. Finally, melting curves were generated by melting analysis with temperature ranges from 65°C to 95°C. The PCR and qPCR were carried out on a MIC qPCR cycler (BioMolecular Systems, Australia). Each sample was confirmed to have the characteristic melt curve associated with the Microsporidia MB MB18SF/MB18SR primers.
Data analysis
All the data were tested for normality using the Shapiro-Wilk test. For non-normal unpaired data, a two-tailed Mann–Whitney U test was used to determine the difference between the groups. In cases with more than two data groups, we used the Kruskal-Wallis H test to determine the significance of differences between the groups. If significant, we carried out Dunn’s post-hoc test. All statistical analyses were performed using Graphpad Prism version 6.0 c software or R (version 8.0.2). P-values of *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 were deemed to be statistically significant. The percentage of tissues with Microsporidia MB infection compared to those without infection was used to express the prevalence data using Adobe Illustrator (AI) (version 23.0.1). Figures were created and/or edited using AI.
ACKNOWLEDGMENTS
We thank the support provided by the ICIPE insectary team (Milcah Gitau, Jeniffer Thiong’o, Peris Wambui, David Alila, and Charles Amara), the fieldwork team (Gerald Ronoh and Robinson Kisero), and the project administrator (Faith Kyengo).
The authors gratefully acknowledge financial support for this research from the following organizations and agencies: Open Philanthropy (SYMBIOVECTOR Track A), the Bill and Melinda Gates Foundation (INV0225840), the Children’s Investment Fund Foundation (SMBV-FFT), the Swedish International Development Cooperation Agency (Sida), the Swiss Agency for Development and Cooperation (SDC), the Australian Centre for International Agricultural Research (ACIAR), the Norwegian Agency for Development Cooperation (Norad), the Federal Democratic Republic of Ethiopia, and the Government of the Republic of Kenya. The views expressed herein do not necessarily reflect the official opinion of the donors.
E.E.M.—conceptualization, data curation, validation, visualization, formal analysis, investigation, methodology, writing—original draft, writing—review & editing. T.O.O.—data curation, validation, visualization, formal analysis, investigation, methodology, supervision, writing—original draft, writing—review & editing. J.G., F.G.O., A.W.W., J.N.M.—investigation, writing—review & editing. L.K.—conceptualization, supervision, writing—review & editing. J.K.H.—conceptualization, data curation, formal analysis, funding acquisition, methodology, supervision, validation, visualization, writing—original draft, writing—review & editing.
AFTER EPUB
[This article was published on 8 December 2023 with missing material in Acknowledgments. The Acknowledgments were corrected in the current version, posted on 14 December 2023.]
Contributor Information
Jeremy K. Herren, Email: jherren@icipe.org.
Edward G. Ruby, University of Hawaii at Manoa, Honolulu, Hawaii
Ewa Chrostek, Uniwersytet Jagielloński w Krakowie, Cracow, Poland.
DATA AVAILABILITY
The PCR and qPCR data are available in the Dryad Digital Repository at https://doi.org/10.6084/m9.figshare.24637809.v1.
REFERENCES
- 1. World Health Organization . 2022. The world malaria report 2022
- 2. Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, et al. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526:207–211. doi: 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . 2018. Global report on insecticide resistance in malaria vectors: 2010–2016. World Health Organization [Google Scholar]
- 4. Kabbale FG, Akol AM, Kaddu JB, Onapa AW. 2013. Biting patterns and seasonality of Anopheles gambiae sensu lato and Anopheles funestus mosquitoes in Kamuli district, Uganda. Parasit Vectors 6:340. doi: 10.1186/1756-3305-6-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . 2021. The world malaria report 2021
- 6. Tadesse FG, Ashine T, Teka H, Esayas E, Messenger LA, Chali W, Meerstein-Kessel L, Walker T, Wolde Behaksra S, Lanke K, et al. 2021. Anopheles stephensi mosquitoes as vectors of Plasmodium vivax and falciparum, horn of Africa, 2019. Emerg Infect Dis 27:603–607. doi: 10.3201/eid2702.200019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ochomo EO, Milanoi S, Abong’o B, Onyango B, Muchoki M, Omoke D, Olanga E, Njoroge L, Juma E, Otieno JD, Matoke D, Kamau L, Rafferty C, Gimnig JE, Shieshia M, Wacira D, Mwangangi J, Maia M, Chege C, Omar A, Mbogo C, Kariuki L. 2023. Molecular surveillance leads to the first detection of Anopheles stephensi in kenya. In review. doi: 10.21203/rs.3.rs-2498485/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mnzava A, Monroe AC, Okumu F. 2022. Anopheles stephensi in Africa requires a more integrated response. Malar J 21:156. doi: 10.1186/s12936-022-04197-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Utarini A, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Ansari MR, Supriyati E, Wardana DS, Meitika Y, Ernesia I, Nurhayati I, Prabowo E, Andari B, Green BR, Hodgson L, Cutcher Z, Rancès E, Ryan PA, O’Neill SL, Dufault SM, Tanamas SK, Jewell NP, Anders KL, Simmons CP, AWED Study Group . 2021. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N Engl J Med 384:2177–2186. doi: 10.1056/NEJMoa2030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herren JK, Mbaisi L, Mararo E, Makhulu EE, Mobegi VA, Butungi H, Mancini MV, Oundo JW, Teal ET, Pinaud S, Lawniczak MKN, Jabara J, Nattoh G, Sinkins SP. 2020. A microsporidian impairs Plasmodium falciparum transmission in Anopheles arabiensis mosquitoes. Nat Commun 11:2187. doi: 10.1038/s41467-020-16121-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nattoh G, Maina T, Makhulu EE, Mbaisi L, Mararo E, Otieno FG, Bukhari T, Onchuru TO, Teal E, Paredes J, Bargul JL, Mburu DM, Onyango EA, Magoma G, Sinkins SP, Herren JK. 2021. Horizontal transmission of the symbiont Microsporidia MB in Anopheles arabiensis. Front. Microbiol 12:647183. doi: 10.3389/fmicb.2021.647183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bukhari T, Pevsner R, Herren JK. 2022. Microsporidia: a promising vector control tool for residual malaria transmission. Front Trop Dis 3:66. doi: 10.3389/fitd.2022.957109 [DOI] [Google Scholar]
- 13. Lipsitch M, Siller S, Nowak MA. 1996. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution 50:1729–1741. doi: 10.1111/j.1558-5646.1996.tb03560.x [DOI] [PubMed] [Google Scholar]
- 14. Knell RJ, Webberley KM. 2004. Sexually transmitted diseases of insects: distribution, evolution, ecology and host behaviour. Biol Rev Camb Philos Soc 79:557–581. doi: 10.1017/s1464793103006365 [DOI] [PubMed] [Google Scholar]
- 15. Moran NA. 2006. Symbiosis. Curr Biol 16:R866–71. doi: 10.1016/j.cub.2006.09.019 [DOI] [PubMed] [Google Scholar]
- 16. Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, della Torre A. 2008. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J 7:1–10. doi: 10.1186/1475-2875-7-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto DS, Sumitani M, Kasashima K, Sezutsu H, Matsuoka H. 2016. Inhibition of malaria infection in transgenic anopheline mosquitoes lacking salivary gland cells. PLoS Pathog 12:e1005872. doi: 10.1371/journal.ppat.1005872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, Jiggins FM, Teixeira L. 2013. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet 9:e1003896. doi: 10.1371/journal.pgen.1003896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. López-Madrigal S, Duarte EH. 2019. Titer regulation in arthropod-Wolbachia symbioses. FEMS Microbiol Lett 366:fnz232. doi: 10.1093/femsle/fnz232 [DOI] [PubMed] [Google Scholar]
- 20. Min KT, Benzer S. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci U S A 94:10792–10796. doi: 10.1073/pnas.94.20.10792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albertson R, Casper-Lindley C, Cao J, Tram U, Sullivan W. 2009. Symmetric and asymmetric mitotic segregation patterns influence Wolbachia distribution in host somatic tissue. J Cell Sci 122:4570–4583. doi: 10.1242/jcs.054981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huho BJ, Ng’habi KR, Killeen GF, Nkwengulila G, Knols BGJ, Ferguson HM. 2006. A reliable morphological method to assess the age of male Anopheles gambiae. Malar J 5:1–11. doi: 10.1186/1475-2875-5-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hixson B, Taracena ML, Buchon N. 2021. Midgut epithelial dynamics are central to mosquitoes' physiology and fitness, and to the transmission of vector-borne disease. Front Cell Infect Microbiol 11:653156. doi: 10.3389/fcimb.2021.653156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janeh M, Osman D, Kambris Z. 2019. Comparative analysis of midgut regeneration capacity and resistance to oral infection in three disease-vector mosquitoes. Sci Rep 9:14556. doi: 10.1038/s41598-019-50994-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones JC. 1978. The feeding behavior of mosquitoes. Sci Am 238:138–148. doi: 10.1038/scientificamerican0678-138 [DOI] [Google Scholar]
- 26. Jayakrishnan L, Sudhikumar AV, Aneesh EM. 2018. Role of gut inhabitants on vectorial capacity of mosquitoes. J Vector Borne Dis 55:69–78. doi: 10.4103/0972-9062.242567 [DOI] [PubMed] [Google Scholar]
- 27. Ray K, Mercedes M, Chan D, Choi CY, Nishiura JT. 2009. Growth and differentiation of the larval mosquito Midgut. J Insect Sci 9:1–13. doi: 10.1673/031.009.5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Becnel JJ, Andreadis TG. 2014. Microsporidia in insects, p 521–570. In Microsporidia pathog oppor first Ed [Google Scholar]
- 29. Mitchell SN, Catteruccia F. 2017. Anopheline reproductive biology: impacts on vectorial capacity and potential avenues for malaria control. Cold Spring Harb Perspect Med 7:a025593. doi: 10.1101/cshperspect.a025593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kajla MK, Barrett-Wilt GA, Paskewitz SM. 2019. Bacteria: a novel source for potent mosquito feeding-deterrents. Sci Adv 5:eaau6141. doi: 10.1126/sciadv.aau6141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to Imagej: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The PCR and qPCR data are available in the Dryad Digital Repository at https://doi.org/10.6084/m9.figshare.24637809.v1.