United States organ allocation has struggled with how to share deceased donor organs across the country since its inception. In the 1990s, organs were controlled and distributed locally, creating severe geographic disparities in access to transplantation. The Department of Health and Human Services responded by issuing the Final Rule in 1998, which nationalized organ transplantation and mandated that organs should be allocated to candidates “in order of decreasing medical urgency status” and “neither place of residence nor place of listing shall be a major determinant of access to a transplant.”1 Despite this clear mandate and improvements in organ preservation technology, progress in geographic equity was slow over the subsequent 20 years. Indeed, access to transplantation depended heavily on which donation service area (DSA) a candidate happened to live in. The Centers for Medicare and Medicaid Services divides the country into 57 DSAs, each served by an organ procurement organization responsible for recovering organs for transplantation in its DSA. The DSA map was not optimized for organ allocation, because DSA boundaries were determined by the location of donor hospitals affiliated with the organ procurement organization, rather than by the geographic distribution of patients requiring access to transplantation.
The impetus for change came from an external shock to the system. In November 2017, a New York City lung transplantation candidate sued the Department of Health and Human Services, arguing correctly that DSA-based allocation violated the Final Rule.2 The OPTN (Organ Procurement and Transplantation Network)/United Network for Organ Sharing Executive Committee immediately conceded the obvious and eliminated DSAs from lung allocation, replacing them with concentric rings around the donor hospital called “acuity circles.” The OPTN acuity circles, which were implemented urgently, ignored the distribution of population density across the United States. Unsurprisingly, they had only marginal effects on geographic equity.
A revamped donor heart allocation system was implemented in 2018 that better stratified candidates by waitlist mortality, shortened waiting times for high priority candidates (without apparent adverse effects on recipient outcomes), and broadened geographic sharing of donor hearts with acuity circles.3 Whereas the revised system represents a significant improvement toward meeting the Final Rule mandate and making organs available to the sickest patients in a timely fashion, disparities in access to transplantation remain and in some cases may have been exacerbated. For example, defining the highest urgency statuses by use of temporary mechanical circulatory support has relegated patients on durable left ventricular assist devices (LVADs) to a lower priority status and has dramatically reduced use of this life-saving therapy as a bridge to transplantation.4 Similarly, the increasing use of temporary mechanical circulatory support5 has disproportionately benefitted large hospital systems with the extensive resources and intensive care unit capacity required to care for these highly prioritized patients. Finally, the revised allocation system has resulted in an unprecedented number of exception requests submitted to prioritize patients who do not meet the standard listing criteria—a practice pattern that may undermine the ability of the new system to identify the patients with the greatest survival benefit from transplantation.6,7 For these reasons, among others, donor heart allocation continues to evolve.
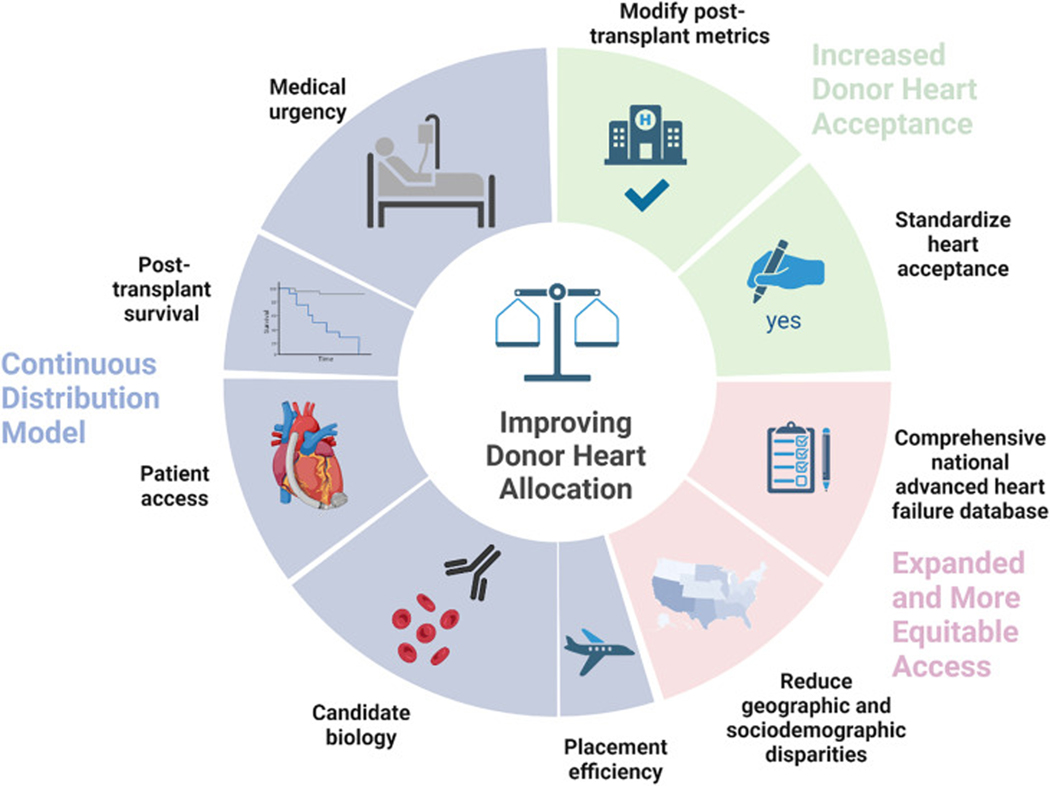
The OPTN’s latest solution to the problem of equitable national organ sharing is continuous distribution.8 Aiming to “dissolve hard boundaries that exist in the current system,” continuous distribution will rank-order candidates for a particular donor based on an overall composite allocation score (CAS). The CAS will be composed of points earned in 5 areas: 1) medical urgency; 2) post-transplantation survival; 3) candidate biology; 4) patient access; and 5) placement efficiency, with a higher score leading to a higher ranking. A candidate’s CAS will be specific to a given match run, because a candidate’s biology and placement efficiency (distance to donor hospital) points will change with each donor.
Spurred by a recent recommendation to accelerate the continuous distribution process by the National Academy of Medicine,9 the OPTN Heart Committee will begin developing the heart CAS in January 2023.10 Each score component in the CAS poses a major challenge for heart allocation (Table 1). Most glaring is the construction of the “medical urgency” score component. Should the OPTN convert the current treatment-based categorical status levels into numerical values, or should a novel multivariable model be developed? Currently medical urgency is heavily based on therapy, which creates challenges related to local resources and potential overtreatment. Simultaneously, many objective markers of medical urgency are ignored. The U.S. heart transplantation community should seize the opportunity presented by continuous distribution to develop a novel multivariable model that is “smart” enough to more objectively capture urgency and prevent manipulation.
TABLE 1.
Continuous Distribution for Organ Allocation: Challenges and Proposed Solutions
| Continuous Distribution Score Component | Challenge | Proposed Solutions |
|---|---|---|
|
| ||
| Medical urgency | Unclear how to convert 6 therapy-based categories into continuous score | Develop novel multivariable model using post-2018 data and ensure that it is less susceptible to manipulation |
| Post-transplantation survival | Difficult to predict post-transplantation outcomes using only candidate waitlist data | Update post-transplantation models and use donor-candidate interactions. Assign a small weight to this score component |
| Patient access | Candidates with durable LVADs will have substantially lower medical urgency score | Add points for waiting time for patients with durable LVAD and those ineligible for LVAD therapy |
| Candidate biology | Patients with blood type O and who are highly sensitized are at a disadvantage | Add points for candidates with blood type O and who are highly sensitized |
| Placement efficiency | Evolving organ transport and preservation technologies make ischemic time effects difficult to estimate | Assign a very small weight to this score component |
LVAD = left ventricular assist device.
Each of the other score components represents a unique problem. “Post-transplantation survival” is difficult to predict using only candidate waitlist data, so we argue that donor-candidate interactions (such as size-matching) should be considered. If the post-transplantation model accuracy remains poor, even after adding donor factors, a small weight should be assigned to this category to avoid unfairly deprioritizing candidates. Any novel multivariable medical urgency score will deprioritize patients with LVADs; we therefore propose that these candidates should accumulate waiting time points in the “patient access” category to encourage use of bridge-to-transplantation LVAD support. Finally, the “candidate biology” score should account for the disadvantages faced by blood type O and highly sensitized candidates, whereas the “placement efficiency” weight should be very small, given improved organ transport and preservation technologies.
Ideally, organ allocation systems will also provide incentives to make transplantation available to a larger patient population. Currently, the United States continues to suffer from a shortage of available donor hearts for transplantation; despite this, only 30%−40% of available hearts are accepted for transplantation.11 Improving donor heart use will go a long way toward alleviating the organ shortage and improving transplantation rates. In this respect, the United States could learn from European countries that tend to be much more liberal in donor heart acceptance, given their pool of older donors with more medical comorbidities. However, unlike European centers, U.S. transplantation programs are closely scrutinized by national regulatory bodies and are held to very high standards in terms of 1-year post-transplantation mortality and other metrics. These metrics may therefore disincentivize use of higher-risk donor hearts. One could therefore envision a system in which transplantations using higher-risk donor hearts are excluded from calculations of post-transplantation survival, or the bar for 1-year survival is lowered to allow centers to take more risk in organ acceptance, in order to increase transplantation access for more patients with advanced heart failure (HF). In parallel, perhaps donor heart acceptance should be standardized across centers, such that acceptance of appropriately matched hearts is mandatory, and maintenance of program certification is contingent on maintaining a certain level of organ acceptance. Such measures would certainly be controversial and would require extensive modeling of their impact on the transplantation system, as well as buy-in from transplantation centers, patient advocacy groups, and other affected parties.
Finally, the central goal of the heart allocation system should be to reduce “overall heart failure morbidity,” not only the outcomes of patients listed for transplantation. There are multiple implications of this distinction, as discussed in this commentary. First, the allocation system should incentivize increased organ use and bridge-to-transplantation LVAD implantation. Whereas prioritizing waitlist patients with an LVAD will not improve waitlist survival, incentivizing increased use of LVADs will likely have an overall positive impact on HF morbidity. Second, equitable access to transplantation across geographic regions and sociodemographic groups is necessary, both ethically and to reduce HF morbidity. Our fragmented health system has vast economic and geographic barriers that prohibit many patients from having access to a large, high-volume transplantation center. In that context, revisions to the allocation system must preserve access to transplantation via smaller programs. Moreover, if the goal of transplantation is reducing overall HF morbidity, should monitoring— on the institutional and national level—be based on overall HF morbidity, rather than the morbidity of the select few listed for transplantation? Data submission for all patients who undergo evaluation would shift the focus to how programs manage their entire advanced HF population, not only those listed for transplantation. This broader perspective is important because listing thresholds vary across programs, and historically disadvantaged groups have been less likely to be listed for transplantation. To meet this overarching goal, the National Heart, Lung, and Blood Institute should fund the construction of a comprehensive national database of advanced HF analogous to the United States Renal Data System.
Regardless of the initial design of the continuous distribution system, it should be the first of multiple iterations. Any allocation system will quickly become outdated and in need of revision; the past cannot perfectly predict the future because clinical practice will change, partly in response to evolving allocation rules. For example, the increased use of temporary mechanical circulatory support following the 2018 changes impacted the prognostic significance of temporary support. Instead of ignoring this reality, the allocation system should be intentionally adaptive. To enable such adaptation, the United Network for Organ Sharing should continue to collect granular patient data and prospectively plan updates to the continuous distribution model. If a specific patient group consistently has higher waitlist mortality, the score should be revised to increase the medical urgency for that cohort. Forward-thinking and flexible adaptations will move us ever closer to an allocation system that finally achieves equitable access to transplantation for the large and diverse U.S. population of patients suffering from end-stage HF (Figure 1).
FIGURE 1. Suggested Strategies to Improve the Donor Heart Allocation System.
Adapted with permission from “Risk Factors of Dementia” by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates. Created with BioRender.com.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
Drs Khush, Sandhu, and Parker have received research support from the National Institutes of Health/National Heart Lung and Blood Institute (R01HL125303 to Dr Khush, K23HL151672 to Dr Sandhu, and K08HL150291 to Dr Parker).
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Health Resources and Services Administration, Department of Health and, Human Services. Organ procurement and transplantation network. Final rule. Fed Regist. 2013;78(128):40033–40042. [PubMed] [Google Scholar]
- 2.Miriam Holman v United States Department of Health and Human Services. United States District Court Southern District of New York; 2017. [Google Scholar]
- 3.Goff RR, Uccellini K, Lindblad K, et al. A change of heart: preliminary results of the US 2018 adult heart allocation revision. Am J Transplant. 2020;20(10):2781–2790. [DOI] [PubMed] [Google Scholar]
- 4.Colvin M, Smith JM, Ahn Y, et al. OPTN/SRTR 2020 annual data report: heart. Am J Transplant. 2022;22(suppl 2):350–437. [DOI] [PubMed] [Google Scholar]
- 5.Varshney AS, Berg DD, Katz JN, et al. Critical Care Cardiology Trials Network Investigators. Use of temporary mechanical circulatory support for management of cardiogenic shock before and after the United Network for Organ Sharing donor heart allocation system changes. JAMA Cardiol. 2020;5(6):703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker WF, Anderson AS, Gibbons RD, et al. Association of transplant center with survival benefit among adults undergoing heart transplant in the United States. JAMA. 2019;322(18):1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker WF, Chung K, Anderson AS, Siegler M, Huang ES, Churpek MM. Practice changes at U.S. transplant centers after the new adult heart allocation policy. J Am Coll Cardiol. 2020;75(23): 2906–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasiske BL, Pyke J, Snyder JJ. Continuous distribution as an organ allocation framework. Curr Opin Organ Transplant. 2020;25(2):115–121. [DOI] [PubMed] [Google Scholar]
- 9.National Academies of Sciences, Engineering and Medicine; Health and Medicine Division; Board on Health Care Services, et al. Realizing the Promise of Equity in the Organ Transplantation System. National Academies Press (US). 2022. Accessed October 5, 2022. https://www.ncbi.nlm.nih.gov/books/NBK578320/) [PubMed] [Google Scholar]
- 10.UNOS. Organ distribution: making allocation more fair and flexible. Accessed November 18, 2022. https://unos.org/policy/organ-distribution/
- 11.Khush KK, Zaroff JG, Nguyen J, Menza R, Goldstein BA. National decline in donor heart utilization with regional variability: 1995–2010. Am J Transplant. 2015;15(3):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]