ABSTRACT
Background:
Epilepsy is a neurological disorder characterized by anomalous brain activity, convulsions, and odd behavior. Several substituted-(naphthalen-2-yl)-3-(1H-indol-3-yl) allyl)-1,4-dihydropyridine-4-carboxylic acid derivatives (5a-j) were intended to be produced in the current research effort to reduce convulsions and seizures.
Materials and Methods:
The newly developed compounds were produced by the prescribed process. Numerous methods (infrared spectroscopy (IR), nuclear magnetic resonance (NMR), mass, elemental analysis, etc.) were used to characterize these substances. Several models were used to test each of these molecules for anticonvulsant activity. By using the rotarod and ethanol potentiation techniques, neurotoxicity was also evaluated. The study meticulously examined each parameter and showed absorption, distribution, metabolism, and excretion (ADME) predictions for each of the 10 congeners that were produced. In addition, studies on molecular docking employed the gamma amino butyric acid (GABA)-A target protein.
Results:
Anticonvulsant screening results identified compounds 5f, 5h, 5d, and 5b as the most efficacious of the series. All synthesized equivalents largely passed the neurotoxicity test. The results of molecular docking revealed significant interactions at the active site of GABA-A with LEU B: 99, TYR A: 62, Ala A: 174, and THR B: 202, and the outcomes were good and in agreement with in vivo findings.
Conclusions:
The study’s findings showed that some substances had promising anticonvulsant properties that were comparable to those of the standard drug. The highly active novel anticonvulsant analogs may therefore represent a possible lead, and additional studies may result in a potential new drug candidate.
KEYWORDS: Anticonvulsant activity, GABA-A indole, in-silico, molecular docking, pyridine
INTRODUCTION
Globally, almost 50 million people are living with epilepsy. Epilepsy is a neurological disorder causing abnormal brain activity, seizures, and unusual behavior. Seizure types are classified into generalized, focal, and unknown onsets, as per the International League Against Epilepsy (ILAE).[1,2] Limited treatments include medicines, surgery, electrical stimulation, and dietary interventions. Despite advancements, managing seizures remains challenging. Medicinal chemists continue to search for new antiepileptic molecules with specificity and minimal central nervous system (CNS) toxicity.[3-5] According to the World Health Organization, there are around 50 million epileptics across the globe, with almost 80 percent of them living in countries with low or middle incomes. Studies show that with the right diagnosis and treatment, up to 70% of people with epilepsy can enjoy seizure-free lives.[6]
Finding a suitable and efficient treatment, particularly for pharmaco-resistant epilepsy, is therefore an important unmet therapeutic need. Selectivity and toxicity among the antiepileptic drugs (AEDs) that are already on the market are still issues that need to be addressed. Therefore, there is constantly a need for better anticonvulsant medications with lower hazards. Our limited understanding of the intricate mechanisms underlying epilepsy (such as the alteration of voltage-dependent Na+ and/or Ca2+ channels, the enhancement of inhibition mediated by gamma amino butyric acid (GABA) or the other impact on the GABA system, the reduction of synaptic excitement mediated by ionotropic glutamate receptors, and the alteration of synaptic release, for example) hinders the development of new anticonvulsant medications. This class of compounds is crucial since numerous synthetic nitrogen heterocycles have been demonstrated to have beneficial pharmacological effects. The heterocyclic moieties like pyridine, indole, pyrrole, pyrrolidine, thiadiazole, triazole, oxadiazole, triazines, quinazolines, etc., are nitrogen-bearing heterocycles. The structure of many anticonvulsant drugs was once widely assumed to be made up of nitrogen-bearing heterocycles, primarily lactam or imides connected by phenyl or alkyl groups.[7,8]
Some novel pyridine derivatives have shown good anticonvulsant activity which are Food and Drug Administration (FDA) approved and some natural molecules have pyridine ring with hypnotic action. These drugs are used clinically for their anxiolytic, hypnotic, muscle-relaxant, and anticonvulsant actions. They act allosterically to influence central ϒ-aminobutyric acid (GABA)-mediated neurotransmission.[9,10] Nicotine stimulates the ion exchange channels to activate the discharge of neurotransmitters including serotonin (5-HT) and GABA into the mesolimbic area, the corpus striatum, and the frontal cortex which contains the pyridine scaffold.[11]
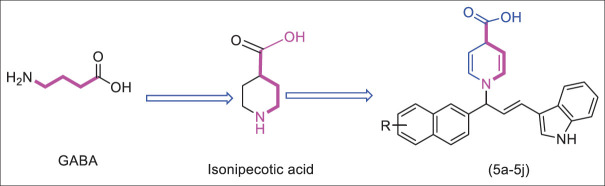
Heterocyclic amino acid derivatives based on isonipecotic acid (a previously reported lead molecule against epilepsy[12]) like structure, having a nitrogen-containing ring in which the nitrogen atom of heterocyclic moiety serves as amino function and attached carboxylic group to the ring meet the criteria of amino acid are envisioned as the basic structural skeleton of this study [Figure 1]. Structural similarities of these hypothetical compounds with GABA and the inherent compatibility of the designed molecules with the human physiological system are the central ideas of this research.
Figure 1.
Isonipecotic acid is structurally similar to 4-aminobutyric acid (GABA)
The current work aims to find compounds that are potent in typical electroshock seizure or chemo-shock seizure assays based on the information discussed previously. We have attempted to develop a series of 10 compounds with indole and pyridine system as a critical scaffold. To qualitatively assess their anticonvulsant capabilities, all synthesized substances were submitted to a molecular docking analysis to ascertain their affinity to bind with ligand-gated ion channel (GABA-A) receptors. The data obtained from molecular docking were correlated with those obtained from biological screening.
EXPERIMENTAL
Chemistry
General
Most of the chemicals and reagents used in the investigation came from Merck Chemicals in Bangalore, India. Compound melting points measured with the Hicon melting point device (New Delhi, India) were found to be inaccurate and to have exposed capillary tubes. Thin-layer chromatography (TLC) plates fixed with silica gel G were employed to assess how pure the newly produced chemicals were. As a detection toolkit, UV light and iodine fumes were employed. A Fourier-transform infrared spectroscopy (FT-IR) spectrophotometer from Shimadzu was utilized to get the FT-IR spectra. DRX400 series of Nuclear Magnetic Resonance (NMR) spectrometer (Bruker, US) was used to acquire the IH spectra (at 400 MHz) and 13C spectra (at 100 MHz). Tetrmethylsilane (TMS) was used as a comparative internal standard, and chemical changes (δ) are expressed in parts per million (ppm), while J values (coupling constants) are expressed in hertz (Hz). The mass of the developed chemical was measured using a mass spectrometer (Applied Biosystems; API-3000), and the result is given in daltons. C, H, and N elemental analysis of synthetic derivatives was performed using a Perkin-Elmer (240C analyzer) (PerkinElmer, US).
Synthesis of 3-(1H-indol-3-yl)-1-(naphthalen-2-yl)prop-2-en-1-one derivatives (2a-j)
A quantity of equimolar 1H-indole-3-carbaldehyde (0.1 M) and 1-(naphthalen-2-yl)ethan-1-one derivatives (1a-j) was solubilized in ethanol, and 1 ml of 40% NaOH solution was mixed to the mixture. The mixture was agitated at room temperature for half a day. The resultant reaction mixture was kept on a floor of crushed ice, and the pH was adjusted to 7 using diluted HCl. The final solid underwent filtering, rinsing, and drying. The crude product was crystallized in ethanol to produce pure chalcones. By using toluene, ethyl acetate, and formic acid (5:4:1) v/v in TLC, the purity and pace of the reaction were examined.
Synthesis of 3-(1H-indol-3-yl)-1-(naphthalen-2-yl)prop-2-en-1-ol derivatives (3a-j) from (2a-j)
In a solution of product derivatives (2a-2j) containing 0.01 mol of absolute methanol, 0.46 g (0.012 mol) of solid sodium borohydride was added over 30 min with steady stirring at room temperature. The solvent was vaporized under reduced pressure, the residue was triturated with water, and the crystalline product was filtered, cleaned, and dried. The freshly made compounds were recrystallized in methanol. To appraise the speed of reaction and purity of the compound, TLC was utilized with a mobile phase consisting of benzene: acetone (8:2) v/v.
Synthesis of 3-(3-chloro-3-(naphthalen-2-yl)prop-1-en-1-yl)-1H-indole derivatives (4a-j) from (3a-j)
A solution of chemical compounds (3a-j) in dry toluene containing 0.01 mol was combined with 1.54 g (0.013 mol) of SOCl2, and the mixture was then refluxed for 4 h. At a lower pressure, the solvent was vaporized and the residue was dissolved in ether before being twice washed with water and 10% NaHCO3. A methanol-crystallized residue was created when it was concentrated under a vacuum and dried over Na2SO4. The purity of the compounds was examined using TLC with an acetone: benzene (2:8) v/v mobile phase.
Synthesis of target derivatives of 1-(3-(1H-indol-3-yl)-1-(naphthalen-2-yl)allyl)-1,4-dihydropyridine-4-carboxylic acid (5a-j) (Pyridine derivatives)
In 20 ml of pure ethanol, 0.003 mol of compounds (4a-j) and 0.003 mol of pyridine-4-carboxylic acid were mixed with 1 ml of triethylamine (TEA), and the mixture was refluxed for 12–15 h. After the reaction was finished, the flask’s contents were reduced to half and left overnight. The resultant crystalline material was separated, rinsed with water, dried, and recrystallized from ethanol to produce the finished product. The characterization data of the titled compounds are available in Supplementary Text 1.
Pharmacology
Anticonvulsant action assessment
The protocol laid down by the Institutional Animal Care and Use Committee (IACUC), with permission number VUSC-23-1-23, was followed by the Faculty of Veterinary Medicine at the University of Sadat City (Egypt) in handling the animals. The protocol also included the administration of samples and their disposal. The test subjects were male albino mice weighing 25–30 g. The test compounds were suspended in PEG 200 for maximal electroshock seizure (MES) and subcutaneous pentylenetetrazole (scPTZ) screening. Six animals were kept in each cage, at room temperature, with free access to food and water, as is customary in lab settings.
MES test
Intraperitoneal (i.p.) injections of test substances at dose levels of 30, 100, and 300 mg/kg were used to assess the anticonvulsant effect at intervals of 0.5 and 4 h. The most severe electroshock seizures in mice were generated using ear clip electrodes and 0.2 s of 60 Hz, 50 mA electrical shocks. The lack of the tonic extensor component of seizures in 50% or more of the animals is classified as protection.[13]
scPTZ seizure test
The scPTZ is used to assess which substances enhance the threshold for seizures. The scPTZ test employs pentylenetetrazole at a dosage of 85 mg/kg. This causes at least 5-s clonic convulsions in almost 97% of the examined mice. The complete procedure can be found in the previously reported literature.[14] The capacity of synthesized analogs to offset pentylenetetrazole’s influence on the seizure threshold was determined by the absence of jerking movement in half of the group or more of the mice over the designated period.[14]
Neurotoxicity study
Rotarod test
Rodent motor function was evaluated using the rotarod test. During the study, mice were taught to remain on a rotating shaft with an outer diameter of 3.2 cm and a rotational speed of 10 revolutions per minute. In all three trials, the degree of neurotoxicity was assessed by measuring the mice’s inability to uphold equilibrium on the revolving shaft for 1 min. The amount of drug at which 50% of the animals lost their ability to be stable on the rotating rod was determined as the toxic dose.[15]
Ethanol potentiation test
Mice were administered test substances at three graded doses (i.e., 30, 100, 300 mg/kg). A dose of 2.5 g kg-1 of ethanol was given to all test animals after an hour, excluding the control animals. There will not be any lateral positioning caused by alcohol in control mice. After ethanol treatment, the number of mice in each batch that had been found in the lateral position was counted.[16]
Molecular docking studies and ADME prediction
Pre-ADMET software (https://preadmet.webservice.bmdrc.org/) was used to evaluate the absorption, distribution, metabolism, and excretion (ADME) properties of synthesized drugs. Human intestinal absorption (HIA), log P, plasma protein binding (PPB), blood-brain barrier penetration (BBB), and skin permeability (SP) were among the traits that were investigated.
The AutoDock Vina program was employed to carry out a docking study to ascertain the ligand’s predicted positioning and confirm its presence at the binding site. The structures of the synthesized pyrrolidine analogs (5a-j) were shown using Cambridge Soft’s Chem Draw program. Chem3D Ultra 8.0 software was employed to transform the two-dimensional (2D) to three-dimensional (3D) structures.
The Protein Data Bank (PDB) provided the X-ray crystal organization of the GABA-A receptor, the GABA(A)R-beta3 homopentamer (code 4COF). UCSF Chimera 1.15 was employed to prepare the receptor with the aid of the receptor preparation wizard. A PDBQT file comprising the receptor’s structure and the hydrogen atoms in each of its polar residues was created by the PyRx program after the protein was entered into it. The Lamarckian Genetic Algorithm approach of calculation was employed. After the conclusion of the docking findings, the finest alignment possessing the least docked power was chosen. Ten AutoDock Vina scans were performed for each compound, and the finest position from each run was kept. Discovery Studio 3.5 looked into interactions between protein and ligand structure. The docking score, pi-pi interactions, and hydrogen bonds were used to determine the affinities of the chemical at the receptor’s dynamic position.
RESULTS AND DISCUSSION
Chemistry
In this paper, the synthesis and characterization of substituted-(naphthalen-2-yl)-3-(1H-indol-3-yl)allyl)-1,4-dihydropyridine-4-carboxylic acid analogs are described as shown in Figure 2. These compounds have been designed keeping in mind the structure of the indigenous neurotransmitter GABA which plays a vital role in controlling seizure generation and spread.
Figure 2.
Synthesis of novel heterocyclic amino-acid derivatives. Reagents and parameters include (i) Methanol, agitation; (ii) NaOH, EtOH; reflux; (iii) neutralizing processes by diluted HCl; and (iv) EDC-HCl, HOBT, DMF, triethylamine, stirring for about 12–15h, water, brine, sodium sulfate, ethyl acetate used for recrystallization
Figure 2. Synthesis of novel heterocyclic amino acid derivatives. Reagents and parameters include (i) Methanol, agitation; (ii) NaOH, EtOH; reflux; (iii) neutralizing processes by diluted HCl; and (iv) EDC-HCl, HOBT, DMF, triethylamine, stirring for about 12–15 h, water, brine, sodium sulfate, ethyl acetate used for recrystallization.
These newly produced substances were tested for their ability to treat convulsions while molecular docking experiments were carried out. Ten different molecules were created overall using the synthetic process outlined in Figure 2. The findings of investigations into various physicochemical parameters are shown in Supplementary Table 1. These new analogs have been identified using NMR and IR spectroscopic data for the substances that had been crystallized and purified using the appropriate solvent. The analyses of elements for the new molecules were also carried out. In IR evaluation, amino, carbonyl, and C-N stretching bands were visible in the range of 3376-3270 cm-1, 1730-1720 cm-1, and 1243-1239 cm-1, correspondingly. With various electron-donating group (EDG) and electron-withdrawing group (EWG) replacements, these recently produced products detected Ar-H in the range of 7.08–8.65 ppm, a singlet for COOH in the range of 11.76–12.64 ppm, and a singlet for N-H in the range of 11.49–11.98 ppm. The carbonyl carbon at pyridine attained its maximum in 13C evaluation between 171.7 and 183.8 ppm. The measured data for C, H, and N in analyses were found to be within 0.4% of the theoretic values.
Supplementary Table 1.
Physicochemical properties of prepared analogs (5a- j)
| Analog | - R | Analog formulae | Mol. Wt. of analog | Percent yield | M.P. (C) | Rf* | Physical state |
|---|---|---|---|---|---|---|---|
| 5a | 7- Cl | C27H21ClN2O2 | 440.93 | 48 | 185 | 0.51 | Yellow (Solid) |
| 5b | 6- Cl | C31H23ClN2O2 | 440.93 | 53 | 136 | 0.55 | Off white (Powder) |
| 5c | 7- Br | C27H21BrN2O2 | 485.38 | 41 | 141 | 0.39 | White (Solid) |
| 5d | 6- Br | C27H21BrN2O2 | 485.38 | 55 | 112 | 0.41 | White (Powder) |
| 5e | 7- NO2 | C27H21N3O4 | 451.48 | 50 | 130 | 0.38 | White (Solid) |
| 5f | 6- NO2 | C27H21N3O4 | 451.48 | 52 | 123 | 0.426ty | White (Solid) |
| 5g | 7- OCH3 | C28H24N2O3 | 436.51 | 43 | 114 | 0.62 | Cream (Solid) |
| 5h | 6- OCH3 | C28H24N2O3 | 420.51 | 59 | 122 | 0.65 | Off white (Powder) |
| 5i | 7- CH3 | C28H24N2O3 | 420.51 | 56 | 101 | 0.79 | Yellow (Solid) |
| 5j | 6- CH3 | C32H26N2O3 | 470.57 | 47 | 112 | 0.83 | Off white (Crystal) |
*Toluene:ethyl acetate:formic acid (5:4:1)-solvent system was used
Pharmacological assessment
According to the Anticonvulsant Drug Development (ADD) program’s standard operating procedure, numerous pharmacological research was conducted. There have also been investigations of the neurotoxicity of the substance. The analysis methods included neurotoxicity (Tox), scPTZ, and MES. The synthesized analogs’ anticonvulsant action was assessed between 0.5 and 4 h after intraperitoneal injections at the graded dosages of 30, 100, and 300 mg/kg. The rotarod test and ethanol potentiation were used to assess neurotoxicity. Table 1 displays the findings of the anticonvulsant and neurotoxicity data.
Table 1.
Data on analogs (5a-j)’s neurotoxicity and anticonvulsant screening
| Compound Number | Mice receiving an intraperitoneal/subcutaneous injectiona | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| MES assessment | scPTZ assessment | Neurotoxicity assessment | Ethanol potentiation test | ||||
|
|
|
|
|||||
| Half hour | 4 h | Half hour | 4 h | Half hour | 4 h | ||
| 5a | 300 | 300 | 300 | 300 | X | X | X |
| 5b | 100 | 100 | 100 | 100 | - | - | - |
| 5c | 300 | 300 | 300 | 300 | X | X | X |
| 5d | 100 | 100 | 100 | 100 | - | - | - |
| 5e | 100 | 300 | 100 | 300 | - | - | - |
| 5f | 30 | 100 | 100 | 100 | - | - | - |
| 5 g | 300 | 300 | 300 | 300 | + | + | X |
| 5h | 100 | 100 | 100 | 100 | - | - | - |
| 5i | - | - | X | X | - | + | X |
| 5j | 100 | 300 | 100 | 300 | - | - | - |
| Phenytoin | 30 | 30 | - | - | 100 | 100 | - |
| Carbamazepine | 30 | 100 | 100 | 300 | 100 | 300 | - |
a30 mg/kg, 100 mg/kg, and 300 mg/kg of the drug were administered. After 0.5 and 4 h, the protective effect and neurotoxicity were studied. The results showed how much of a minimum dose of lead was needed to protect 50% or more of the animals or cause neurotoxicity in at least 50% of them. The dash (—) meant that neither neurotoxicity nor anticonvulsant action was present in these compounds. X— means that it was not analyzed. bEthanol potentiation test: (+) means that at least half of the animals had positive results; [–]means that at least half of the animals had negative results. X— stands for “not tested”
After 0.5 h, all molecules in the MES model demonstrated electroshock protection, however 5f showed activity at a dose of 30 mg/kg, indicating a quick onset of action and being very potent. Molecules 5b, 5d, 5e, 5h, and 5j demonstrated positive results at 100 mg/kg for 0.5 h. At a dose of 100 mg/kg at a time interval of 4 h, molecules 5b, 5d, 5f, and 5h displayed significant action, demonstrating that the analogs have a prolonged half-life at a judicious dose. The two most often used drugs carbamazepine and phenytoin were used as standard.
In the scPTZ model, it was discovered that analogs 5b, 5d, 5e, 5f, 5h, and 5r showed activity at 100 mg/kg at 0.5 h. The compounds 5b, 5d, 5f, and 5h were protective at 100 mg/kg after 4.0 h. When put through their various testing, most of the substances failed to exhibit any neurotoxic effects. At 0.5 and 4 h, only analogs 5g and 5i were discovered to be neurotoxic.
Out of all the produced compounds, compounds 5f, 5h, and 5d were determined to be the most effective and to function as specific GABA mediators. The other compounds used in the MES and scPTZ studies either displayed no action or lacked any distinguishing characteristics. The EWG substitutions at position 6 of the aryl ring appeared to be the most powerful compounds among the series, according to the structure-based activity (SAR) profiles. A powerful EWG (-NO2) is located at position 6 of the potent compound 5f in the scPTZ and MES screening. The activity was modest for compounds 5h and 5d containing EWG/EDG (-Br and -OCH3) in position 6. It was discovered that compounds containing EWG at position 7 were less active. In general, it was found that compounds having the EDG (CH3) at position 6 were likewise less active.
ADME prediction
The results of the ADME prediction on the designed compounds are displayed in Supplementary Table 2. By looking at BBB penetration, it was possible to assess if a specific drug would be able to pass through the BBB. The values discovered also assisted in reducing side effects and pollutants, and they may have improved the effectiveness of medications that had a psychological impact on the brain. Most of the targets examined had positive values, indicating that they could easily cross the BBB. The value for 5d is the highest, 5.57043, which suggests that it is the most active. Human intestinal absorption (HIA) refers to the method by which oral drugs left the gastrointestinal tract (GIT) and entered the bloodstream. The produced compounds performed better than 95%, indicating that they are well-absorbed substances that can also be absorbed by the human intestine. The efficiency of a drug as well as how prolonged a chemical halts in the body can be affected by PPB. How well a medicine binds to plasma proteins has a significant impact on how well it functions and travels through the body. A percent bound value below 90 was considered low, and a value exceeding 90 was considered high, as previously reported.
Supplementary Table 2.
ADME prediction of synthesized derivatives
| Compd | Substitution (R) | LogP | aBBB | bBS (mg/l) | CYP- inhibition | cHIA | dPPB | eSP |
|---|---|---|---|---|---|---|---|---|
| 5a | 7- Cl | 6.44184 | 5.18895 | 21.9441 | 2D6 noninhibitor | 95.24178 | 100 | - 2.5663 6 |
| 5b | 6- Cl | 6.441840 | 5.30167 | 21.9441 | 2D6 noninhibitor | 95.241779 | 100 | - 2.5673 2 |
| 5c | 7- Br | 6.559240 | 5.46725 | 10.0069 | 2D6 noninhibitor | 95.304661 | 100 | - 2.5795 9 |
| 5d | 6- Br | 6.559240 | 5.57043 | 10.0069 | 2D6 noninhibitor | 95.304661 | 100 | - 2.5805 |
| 5e | 7- NO2 | 5.777560 | 0.119399 | 7.19746 | 2D6 noninhibitor | 95.585284 | 97.427919 | - 2.8583 |
| 5f | 6- NO2 | 5.777560 | 0.116783 | 7.19746 | 2D6 noninhibitor | 95.585284 | 97.427471 | 7 - 2.8587 |
| 5g | 7- OCH3 | 5.726640 | 1.87162 | 34.7371 | 2D6 noninhibitor | 95.499720 | 94.588646 | 9 - 2.5546 |
| 5h | 6- OCH3 | 5.726640 | 1.9574 | 34.7371 | 2D6 noninhibitor | 95.499720 | 95.407789 | 7 -2.55552 |
| 5i | 7- CH3 3 | 6.256750 | 4.71166 | 26.3261 | 2D6 noninhibitor | 95.138593 | 99.484142 | - 2.3635 |
| 5j | 6- CH3 | 6.256750 | 4.71756 | 26.3261 | 2D6 noninhibitor | 95.138593 | 99.358944 | 8 - 2.3642 8 |
aBlood-brain barrier, bBuffer solubility, cHuman Intestinal Absorption, dPlasma Protein Binding, e Skin Permeabili ty
Every chemical that was produced demonstrated robust bonding for a plasmatic protein having an intensity greater than 90%, while compounds 5a, 5b, 5c, and 5d had values of 100%, as was shown. Additionally, how effectively a drug interacts with plasma proteins has a significant impact on how the drug is absorbed. One of the most crucial aspects of medications administered via the skin is the SP rate. It is well recognized that a key factor in the way medicine is absorbed via the skin is how it enters the lipid tissue between cells. The SP check was unsuccessful for every substance that was tested, proving that it cannot be directed via the skin. The log P values represent the probability that the substance will reach the intended CNS target tissues. The attempted analogs were lipid-loving because Log P > 0 (or P > 1). Analog 5f has the highest log P and is the most lipophilic.
Molecular docking
To determine how the synthesized analogs (5a-j) may bind and how much energy they would require to do so, molecular docking tests were conducted. Moreover, finding the potential leads was an added advantage of molecular docking. The potential compounds’ docking scores against the GABA-A receptor varied from -10.8 to -9. The docking images of all the produced compounds 5a-j are shown in Supplementary Figure 1 (257KB, tif) . The docking findings are presented in Table 2 as derivative scores (5a-r) with GABA-A (4 COF).
Table 2.
Results of docking in pyrrolidine derivatives (5a-j) with the GABA-A receptor, 4COF
| Derivative | Ligand | Binding affinity |
|---|---|---|
| 5a | 4cof__uff_E=478.91 | -9.4 |
| 5b | 4cof__uff_E=494.06 | -10.3 |
| 5c | 4cof__uff_E=478.36 | -9.4 |
| 5d | 4cof__uff_E=492.38 | -10.5 |
| 5e | 4cof__uff_E=510.40 | -10.3 |
| 5f | 4cof__uff_E=521.26 | -10.8 |
| 5 g | 4cof__uff_E=534.27 | -9 |
| 5h | 4cof__uff_E=535.95 | -10.5 |
| 5i | 4cof__uff_E=483.60 | -9.4 |
| 5j | 4cof__uff_E=514.02 | -10.1 |
With a score of - 10.8 in the docking studies, 5f was discovered to be the most active. At distances of 5.42 and 3.80 Å, respectively, the residues Leu B: 99 and Ser A: 46 are involved in hydrogen bonding with the compound’s carboxyl group. Additionally, the nitro group formed hydrogen bonds with the ASN B: 100 residues and the THR B: 151 residues at distances of 5.15 and 3.17 Å. The N-H of imidazole displayed a second hydrogen bond with ASN B: 54 at a distance of 4.81 Å. PRO A: 184, ALA B: 135, and MET B: 55 exhibit some Pi-alkyl interactions, while MET B: 137, CYS B: 150, and CYS B: 136 exhibit van der Waals interactions. The second-most active molecule was discovered to be 5h, which had a docking score of -10.5. Pi-pi interactions with TYR A: 62 and PHE B: 200, Pi-alkyl contacts with ALA B: 201, and hydrogen bond interactions of compounds containing carboxyl groups with residue LYS A: 173 were all strongly noted.
Van der Waals interactions and C-H bond interactions were discovered with the residue LEU B: 99. A similar docking score of -10.5 indicated that 5d was likewise active. The carboxyl in this molecule formed a hydrogen bond with residues LYS A: 173 and ALA A: 174 at distances of 6.10 and 3.78 Å, respectively. At a distance of 4 Å, another hydrogen bond interaction of the NH bond with THR B: 202 was seen. The residues TYR A: 62 and PHE B: 200 showed Pi-Pi interactions, while LEU B: 99 showed van der Waals interactions.
The hydrogen bond contact of the NH group with THR B: 202 and the carboxyl bond interaction with ALA A: 174, both residues and common interactions with 5d, were also discovered for compound 5b with a docking score of -10.3. Alkyl bond interaction was noted with distances of 5.15 and 4.92 Å between ALA B: 201 and VAL B: 199, respectively. Van der Waals interactions with LEU B: 99 and TYR A: 62 and 200 Pi-Pi bond interactions were also noted.
The analysis of the docking patterns in the synthetic molecules 5a-j showed that the main binding mechanism for the synthetic compound’s receptor is hydrogen bonding. Additionally, compounds 5f, 5h, 5d, and 5b had the highest observed docking scores. All four of these compounds have alterations at position 6, indicating that position 6 is more active. LEU B: 99, TYR A: 62, Ala A: 174, and THR B: 202 are the key amino acid residues identified to be involved in the binding interactions. These residues acted as an active site for the ligand to access the GABA-A receptor.
According to evidence from in-silico experiments, adding an electron-withdrawing substituent to an aryl ring at position 6 can considerably boost GABA levels and, consequently, anticonvulsant action. The results correspond to those from the animal model. This virtual screening and in vivo activity result demonstrated activity in the following order: NO2>OCH3>Br>Cl.
CONCLUSION
The present investigation involved the synthesis and anticonvulsant activity evaluation of substituted-(naphthalen-2-yl)-3-(1H-indol-3-yl)allyl-1,4-dihydropyridine-4-carboxylic acid derivatives (5a-j) employing the MES and scPTZ models. It was discovered that the series of fused derivatives exhibited anticonvulsant activity. Throughout the series, it was discovered that compounds 5f, 5h, 5d, and 5b were very efficient against both models and had a quick action onset. Therefore, it is postulated that these four substances are highly potent and selective GABA-only facilitators. These compounds have also cleared the neurotoxicity test excluding compound 5g, which research has shown to be motor impairing. The article also provides predictions for all analog ADMEs and molecular interactions. Each ADME parameter is thoroughly explored in the manuscript. The protein GABA-A served as the target and Autodock Vina was employed to carry out docking screening. Significant interactions occur at the active site of GABA-A between LEU B: 99, TYR A: 62, Ala A: 174, and THR B: 202. A comprehensive analysis of van der Waals interactions, pi-pi, pi-alkyl, and substantial hydrogen bonding is provided. The docking outcomes are consistent with the animal model. The virtual screening and in vivo activity studies have shown that the EWG can greatly increase GABA concentration and have an anticonvulsant effect by replacing at the sixth position of the aryl ring. This group of synthesized analogs can be viewed as intriguing potential research subjects.
Study limitations
Although isonipecotic acid acts as a GABA-A receptor agonist, its potential as an antiepileptic agent remains unconfirmed. It is necessary to conduct further research to verify the findings of this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This project was supported by the Scientific Research Center at Buraydah Private Colleges under the research project (BPC-SRC/2022-003).
Supplementary Files
Supplementary Text 1:
(E)-1-(1-(7-chloronaphthalen-2-yl)-3-(1H-indol-3-yl) allyl)-1,4-dihydropyridine-4-carboxylic acid (5a).
IR (KBr) (cm-1): 3376 (NH),1720 (C = O acid), 1239 (C-N), 860 (C-Cl); 1H-NMR (400 MHz, CDCl3) δ (ppm): 12.12 (s, 1H, COOH), 11.96 (s, 1H, NH indole), 7.08-7.80 (m, 11H, CH aromatic), 6.66 (dd, 1H, CH), 5.83 (d, 1H, CH), 4.42 (d, 1H, CH), 4.62-5.60 (m, 4H, CH pyridine) 13C-NMR (100 MHz, CDCl3) δ(ppm): 183.8, 136.3, 131.6, 129.3, 128.9, 126.1, 110.6, 102.7, 71.5, 42.3; ESI MS (m/z): 441 [M+H]; Anal. Calculated for C27H21ClN2O2: C, 73.55; H, 4.80; Cl, 8.04: N, 6.35; O, 7.26; Found: C, 73.48; H, 4.81; Cl, 8.00; N, 6.37; O, 7.28%.
(E)-1-(1-(6-chloronaphthalen-2-yl)-3-(1H-indol-3-yl) allyl)-1,4-dihydropyridine-4-carboxylic acid (5b).
Yield: 53%; mp 36°C; IR (KBr) (cm-1): 3384 (NH),1729 (C = O acid), 1233 (C-N), 856 (C-Cl); 1H-NMR (400 MHz, CDCl3) δ (ppm): 12.70 (s, 1H, COOH), 11.92 (s, 1H, NH indole), 7.08-7.79 (m, 11H, CH aromatic), 6.5 (dd, 1H, CH), 5.91 (d, 1H, CH), 4.45 (d, 1H, CH), 4.59-5.60 (m, 4H, CH pyridine) 13C-NMR (100 MHz, CDCl3) δ(ppm): 183.6, 135.3, 130.7, 129.5, 128.2, 125.0, 110.1, 102.9, 71.2, 42.3; ESI MS (m/z): 441 [M+H]; Anal. Calculated for C27H21ClN2O2: C, 73.55; H, 4.80; Cl, 8.04: N, 6.35; O, 7.26; Found: C, 73.50; H, 4.78; Cl, 8.10; N, 6.39; O, 7.22%.
(E)-1-(1-(7-bromonaphthalen-2-yl)-3-(1H-indol-3-yl) allyl)-1,4-dihydropyridine-4-carboxylic acid (5c).
Yield: 41%; mp 141°C; IR (KBr) (cm-1): 3250 (NH), 1724 (C = O acid), 1241 (C-N), 746 (C-Br); 1H-NMR (400 MHz, CDCl3) δ (ppm): 11.76 (s, 1H, COOH), 11.96 (s, 1H, NH indole), 7.22-8.62 (m, 11H, CH aromatic), 6.62 (dd, 1H, CH), 5.87 (d, 1H, CH), 4.38 (d, 1H, CH), 4.95-5.80 (m, 4H, CH pyridine) 13C-NMR (100 MHz, CDCl3) δ(ppm): 183.6, 135.3, 130.6, 129.5, 128.2, 125.0, 119.7, 110.1, 102.9, 71.2, 42.3; ESI MS (m/z): 486 [M+H]; Anal. Calculated for C27H21BrN2O2: C, 66.81; H, 4.36; Br, 16.46: N, 5.77; O, 6.59; Found: C, 66.78; H, 4.38; Br, 16.49; N, 5.72; O, 6.52%.
(E)-1-(1-(6-bromonaphthalen-2-yl)-3-(1H-indol-3-yl) allyl)-1,4-dihydropyridine-4-carboxylic acid (5d).
Yield: 55%; mp 112°C; IR (KBr) (cm-1): 3284 (NH), 1722 (C = O acid), 1244 (C-N), 742 (C-Br); 1H-NMR (400 MHz, CDCl3) δ (ppm): 12.32 (s, 1H, COOH), 11.98 (s, 1H, NH indole), 7.26-8.65 (m, 11H, CH aromatic), 6.59 (dd, 1H, CH), 5.91 (d, 1H, CH), 4.38 (d, 1H, CH), 4.90-5.86 (m, 4H, CH pyridine) 13C-NMR (100 MHz, CDCl3) δ(ppm): 181.6, 135.1, 130.4, 129.1, 128.2, 124.7, 119.5, 108.1, 103.2, 72.4, 43.3; ESI MS (m/z): 486 [M+H]; Anal. Calculated for C27H21BrN2O2: C, 66.81; H, 4.36; Br, 16.46: N, 5.77; O, 6.59; Found: C, 66.84; H, 4.42; Br, 16.42; N, 5.79; O, 6.54%.
(E)-1-(3-(1H-indol-3-yl)-1-(7-nitronaphthalen-2-yl) allyl)-1,4-dihydropyridine-4-carboxylic acid (5e).
Yield: 50%; mp 130°C; IR (KBr) (cm-1): 3278 (NH), 1726 (C = O acid), 1241 (C-N), 1480, 1312 (NO stretching asymmetric and symmetric); 1H-NMR (400 MHz, CDCl3) δ (ppm): 12.64 (s, 1H, COOH), 11.96 (s, 1H, NH indole), 7.80-8.17 (m, 11H, CH aromatic), 6.66 (dd, 1H, CH), 5.83 (d, 1H, CH), 4.42 (d, 1H, CH), 4.62-5.60 (m, 4H, CH pyridine) 13C-NMR (100 MHz, CDCl3) δ(ppm): 183.8, 145, 137.11, 134.2, 132, 129.3, 127.4, 117.8, 110.6, 71.5, 42.3; ESI MS (m/z): 452 [M+H]; Anal. Calculated for C27H21N3O4: C, 71.83; H, 4.69; N, 9.31; O, 14.17; Found: C, 71.95; H, 4.64; N, 31 9.39; O, 14.28%.
(E)-1-(3-(1H-indol-3-yl)-1-(6-nitronaphthalen-2-yl) allyl)-1,4-dihydropyridine4-carboxylic acid (5f).
Yield: 52%; mp 123°C; IR (KBr) (cm-1): 3264 (NH), 1730 (C = O acid), 1244 (C-N), 1492, 1315 (NO stretching asymmetric and symmetric); 1H-NMR (400 MHz, CDCl3) δ (ppm): 12.2 (s, 1H, COOH), 11.94 (s, 1H, NH indole), 7.56-8.23 (m, 11H, CH aromatic), 6.98 (dd, 1H, CH), 5.89 (d, 1H, CH), 4.56 (d, 1H, CH), 4.58-5.61 (m, 4H, CH pyridine) 13C-NMR (100 MHz, CDCl3) δ(ppm): 183.5, 144.2, 136.5, 134.0, 129.1, 127.5, 118.0, 110.2, 70.3, 41,1; ESI MS (m/z): 452 [M+H]; Anal. Calculated for C27H21N3O4: C, 71.83; H, 4.69; N, 9.31; O, 14.17; Found: C, 71.87; H, 4.73; N, 9.33; O, 14.43%.
(E)-1-(3-(1H-indol-3-yl)-1-(7-methoxynaphthalen-2-yl) allyl)-1,4-dihydropyridine-4-carboxylic acid (5g).
Yield: 43%; mp 114°C; IR (KBr) (cm-1): 3362 (NH),1724 (C = O acid), 1239 (C-N), 1203 (C-O); 1H-NMR (400 MHz, CDCl3) δ (ppm): 12.42 (s, 1H, COOH), 11.53 (s, 1H, NH indole), 7.12-7.93 (m, 11H, CH aromatic), 6.34 (dd, 1H, CH), 5.82 (d, 1H, CH), 4.39 (d, 1H, CH), 4.70-5.95 (m, 4H, CH pyridine), 3.81 (s, 3H, CH3) 13C-NMR (100 MHz, CDCl3) δ(ppm): 173.0, 157.1, 135.7, 132.0, 129.1, 127.5, 72.7, 55.8, 43.7; ESI MS (m/z): 437 [M+H]; Anal. Calculated for C28H24N2O3: C, 77.04; H, 5.54; N, 6.42; O, 11.00; Found: C, 77.23.48; H, 5.32; N, 6.37; O, 10.28%.
(E)-1-(3-(1H-indol-3-yl)-1-(6-methoxynaphthalen-2-yl) allyl)-1,4-dihydropyridine-4-carboxylic acid (5h).
Yield: 59%; mp 122°C; IR (KBr) (cm-1): 3370 (NH),1719 (C = O acid), 1243 (C-N), 1210 (C-O); 1H-NMR (400 MHz, CDCl3) δ (ppm): 12.38 (s, 1H, COOH), 11.49 (s, 1H, NH indole), 7.32-8.00 (m, 11H, CH aromatic), 6.28 (dd, 1H, CH), 5.62 (d, 1H, CH), 4.44 (d, 1H, CH), 4.73-5.99 (m, 4H, CH pyridine), 3.83 (s, 3H, CH3) 13C-NMR (100 MHz, CDCl3) δ(ppm): 173.2, 157.2, 135.9, 132.2, 129.5, 128.1, 72.5, 55.3, 43.5; ESI MS (m/z): 437 [M+H]; Anal. Calculated for C28H24N2O3: C, 77.04; H, 5.54; N, 6.42; O, 11.00; Found: C, 77.01; H, 5.61; N, 6.47; O, 11.08%.
(E)-1-(3-(1H-indol-3-yl)-1-(7-methylnaphthalen-2-yl) allyl)-1,4-dihydropyridine-4-carboxylic acid (5i).
Yield: 56%; mp 101°C; IR (KBr) (cm-1): 3376 (NH), 3062 (CH stretch), 1720 (C = O acid), 1239 (C-N); 1H-NMR (400 MHz, CDCl3) δ (ppm): 12.32 (s, 1H, COOH), 11.89 (s, 1H, NH indole), 7.22-7.80 (m, 11H, CH aromatic), 6.58 (dd, 1H, CH), 5.72 (d, 1H, CH), 4.52 (d, 1H, CH), 4.72-5.60 (m, 4H, CH pyridine), 2.66 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ(ppm):172.9, 142.0, 134.8, 132.3, 126.8, 121.7, 118.6, 112.0, 72.8, 44.5, 21.3; ESI MS (m/z): 421 [M+H]; Anal. Calculated for C28H24N2O2: C, 79.98; H, 5.75; N, 6.66; O, 7.61; Found: C, 79.48; H, 5.81; N, 6.62; O, 7.64%.
(E)-1-(3-(1H-indol-3-yl)-1-(6-methylnaphthalen-2-yl) allyl)-1,4-dihydropyridine-4-carboxylic acid (5j).
Yield: 47%; mp 112°C; IR (KBr) (cm-1): 3369 (NH), 3111 (CH stretch), 1718 (C = O acid), 1242 (C-N); 1H-NMR (400 MHz, CDCl3) δ (ppm): 12.39 (s, 1H, COOH), 11.72 (s, 1H, NH indole), 7.02-7.71 (m, 11H, CH aromatic), 6.63 (dd, 1H, CH), 5.71 (d, 1H, CH), 4.49 (d, 1H, CH), 4.82-5.70 (m, 4H, CH pyridine), 2.68 (s, 3H, CH3); 13C-NMR (100 MHz, CDCl3) δ(ppm):171.7, 142.1, 133.3, 126.9, 121.5, 118.8, 112.1, 72.6, 44.3, 21.5; ESI MS (m/z): 421 [M+H]; Anal. Calculated for C28H24N2O2: C, 79.98; H, 5.75; N, 6.66; O, 7.61; Found: C, 79.91; H, 5.72; N, 6.72; O, 7.73%.
2D Ligand interaction of compound (a-j)
REFERENCES
- 1.Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the international league against epilepsy: Position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:522–30. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 2.Stevens CE, Stafstrom CE. Pharmacotherapy for focal seizures in children and adolescents. Drugs. 2018;78:1321–37. doi: 10.1007/s40265-018-0959-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: A 30-year longitudinal cohort study. JAMA Neurol. 2018;75:279–86. doi: 10.1001/jamaneurol.2017.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilcox KS, West PJ, Metcalf CS. The current approach of the epilepsy therapy screening program contract site for identifying improved therapies for the treatment of pharmacoresistant seizures in epilepsy. Neuropharmacology. 2020;166:107811. doi: 10.1016/j.neuropharm.2019.107811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Góra M, Czopek A, Rapacz A, Gębska A, Wójcik-Pszczoła K, Pękala E, et al. Synthesis, anticonvulsant, and antinociceptive activity of new 3-(2-Chlorophenyl)- and 3-(3-Chlorophenyl)-2,5-dioxo-pyrrolidin-1-yl-acetamides. Molecules. 2021;26:1564. doi: 10.3390/molecules26061564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon CS, Wagner RG, Carpio A, Jetté N, Newton CR, Thurman DJ. The worldwide epilepsy treatment gap: A systematic review and recommendations for revised definitions - A report from the ILAE epidemiology commission. Epilepsia. 2022;63:551–64. doi: 10.1111/epi.17112. [DOI] [PubMed] [Google Scholar]
- 7.Kaushik D, Khan SA, Chawla G, Kumar S. N'-[(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene] 2/4-substituted hydrazides: Synthesis and anticonvulsant activity. Eur J Med Chem. 2010;45:3943–9. doi: 10.1016/j.ejmech.2010.05.049. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui N, Ahsan W. Triazole incorporated thiazoles as a new class of anticonvulsants: Design, synthesis and in vivo screening. Eur J Med Chem. 2010;45:1536–43. doi: 10.1016/j.ejmech.2009.12.062. [DOI] [PubMed] [Google Scholar]
- 9.De S, Kumar S K A, Shah SK, Kazi S, Sarkar N, Banerjee S, et al. Pyridine: The scaffolds with significant clinical diversity. RSC Adv. 2022;12:15385–406. doi: 10.1039/d2ra01571d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin SX, Curtis MA, Sperry J. Pyridine alkaloids with activity in the central nervous system. Bioorg Med Chem. 2020;28:115820. doi: 10.1016/j.bmc.2020.115820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pal S, editor. Exploring chemistry with pyridine derivatives. BoD–Books on Demand; 2023. Jan 4, [Google Scholar]
- 12.Zhao LX, Park JG, Moon YS, Basnet A, Choi J, Kim EK, et al. Design, synthesis and anticonvulsive activity of analogs of gamma-vinyl GABA. Farmaco. 2004;59:381–8. doi: 10.1016/j.farmac.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Song M, Zhao W, Zhu Y, Liu W, Deng X, Huang Y. Design, synthesis, and evaluation of anticonvulsant activities of new triazolopyrimidine derivatives. Front Chem. 2022;10:925281. doi: 10.3389/fchem.2022.925281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S. A brief review of the biological potential of indole derivatives. Future J Pharm Sci. 2020;6:1–9. [Google Scholar]
- 15.Shan HM, Maurer MA, Schwab ME. Four-parameter analysis in modified rotarod test for detecting minor motor deficits in mice. BMC Biol. 2023;21:177. doi: 10.1186/s12915-023-01679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arshad MF, Siddiqui N, Elkerdasy A, Al Rohaimi AH, Khan SA. Anticonvulsant and neurotoxicity evaluation of some newly synthesized thiazolyl coumarin derivatives. Am J Pharmacol Toxicol. 2014;9:132. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
2D Ligand interaction of compound (a-j)