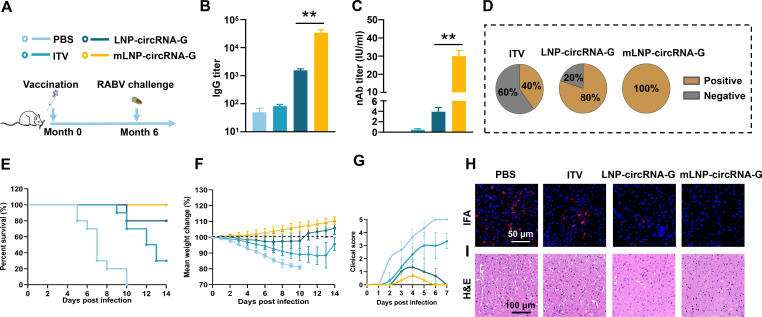
Fig 6.
Long-term protective efficacy of mLNP-circRNA-G against RABV in mice. (A) Scheme of mLNP-circRNA-G immunization and RABV challenge. Each mouse received intramuscular injections of 2 µg of mLNP-circRNA-G or LNP-circRNA-G followed by intracranial injection of 50 LD50 of the RABV strain CVS-24 6 months after inoculation. Mice receiving a 0.1 dose of commercial inactivated vaccine were used as positive controls (n = 10). (B) Measurement of the IgG antibody titer elicited by the vaccine. (C) Measurement of the nAb titer elicited by the vaccine. (D) The proportion of mice with seroconversion induced by vaccination. nAb values greater than 0.5 IU/mL were considered positive. (E) Survival rates. A log-rank test was used to evaluate intergroup differences in survival rates. (F) Mouse body weight changes. (G) The clinical scores of mice. (H and I) Histological and viral level analysis of brain sections. Representative images of RABV detected using P protein monoclonal antibodies (H). Brains were sectioned and stained with H&E (I).