ABSTRACT
Legionella is a common intracellular parasitic bacterium that infects humans via the respiratory tract, causing Legionnaires’ disease, with fever and pneumonia as the main symptoms. The emergence of highly virulent and azithromycin-resistant Legionella pneumophila is a major challenge in clinical anti-infective therapy. The CRISPR-Cas acquired immune system provides immune defense against foreign nucleic acids and regulates strain biological functions. However, the distribution of the CRISPR-Cas system in Legionella and how it regulates gene expression in L. pneumophila remain unclear. Herein, we assessed 915 Legionella whole-genome sequences to determine the distribution characteristics of the CRISPR-Cas system and constructed gene deletion mutants to explore the regulation of the system based on growth ability in vitro, antibiotic sensitivity, and intracellular proliferation of L. pneumophila. The CRISPR-Cas system in Legionella was predominantly Type II-B and was mainly concentrated in the genome of L. pneumophila ST1 strains. The Type II-B CRISPR-Cas system showed no effect on the strain’s growth ability in vitro but significantly reduced resistance to azithromycin and decreased proliferation ability due to regulation of the lpeAB efflux pump and the Dot/Icm type IV secretion system. Thus, the Type II-B CRISPR-Cas system plays a crucial role in regulating the virulence of L. pneumophila. This expands our understanding of drug resistance and pathogenicity in Legionella, provides a scientific basis for the prevention of Legionnaires’ disease outbreaks and the rational use of clinical drugs, and facilitates effective treatment of Legionnaires’ disease.
KEYWORDS: Legionella pneumophila, CRISPR-Cas, antibiotic susceptibility, pathogenesis, regulation mechanism
INTRODUCTION
Legionella microorganisms can spread through aerosols and enter human alveolar macrophages to cause Legionnaires’ disease, and they are widespread in natural and artificial aquatic environments (1, 2). Seventy species of Legionella have been identified (www.bacterio.net/legionella.html), of which >25 species can cause human diseases and >90% of cases are caused by Legionella pneumophila (3, 4). Following the multiple outbreaks of novel coronavirus in recent years, bacterial infection has been detected in COVID-19 patients, mainly involving L. pneumophila, Streptococcus pneumoniae, and Klebsiella pneumoniae (5, 6). The increasing bacterial infection rate in COVID-19 patients infected in intensive care units may be due to repeated infection with antibiotic-resistant bacteria.
Clinical legionnaires’ disease is mainly treated using macrolides and fluoroquinolones (7, 8), among which erythromycin and azithromycin are the main macrolide drugs (9, 10). However, some L. pneumophila strains are resistant to azithromycin, which makes clinical treatment much more challenging. To date, 17% of environmental and clinical Legionella bacteria in China were found to display azithromycin resistance, and the lpeAB efflux pump that transports azithromycin is present in the genome (11, 12).
The clusters of regularly interspaced short palindromic repeats-associated genes (CRISPR-Cas) system is an acquired immune system encoded in the genomes of bacteria and archaea (13). CRISPR loci are usually composed of short repeats and unique spacer sequences (homologous to the invading DNA sequence), and a set of genes encoding nucleases (Cas genes) are usually located upstream of the CRISPR array (14). This system can target and cleave the protospacers of plasmids, bacteriophages, and other foreign DNA elements through spacers, endowing the host with the ability to defend against foreign nucleic acid invasion (15). This mainly involves three processes: adaptation, expression/maturation, and targeted interference (16–18). In addition, the CRISPR-Cas system is involved in regulating group behavior and endogenous gene expression in some species, especially the growth metabolism and pathogenicity of bacteria. Cas3 of the Type I-E CRISPR-Cas system in Salmonella modifies bacterial virulence, enhances biofilm formation and intracellular parasitism, and increases pathogenicity (19). In Campylobacter jejuni, cas9 is essential for adhering and invading colorectal epithelial cells, and it regulates bacterial susceptibility to antibiotic drugs (20). Pseudomonas aeruginosa uses a type I CRISPR-Cas system, similar to that present in Porphyromonas gingivalis, to target the mRNA of the lasR quorum-sensing gene to dampen recognition by the host (21).
Previous research on the CRISPR-Cas system of Legionella has mainly focused on the adaptive immune system that fights against invading foreign DNA elements (e.g., plasmids, bacteria, phages, and viruses) through the synergistic action of spacer arrays and Cas genes, and the CRISPR-Cas system can be used to type new outbreak strains (22–24). The Legionella CRISPR-Cas system was reported to be important in virulence because the cas2 of L. pneumophila is required for intracellular infection of Amoebae (25, 26), but the Legionella CRISPR-Cas system remains poorly understood.
In this study, we explored the presence and functions of CRISPR-Cas in Legionella. We performed drug sensitivity tests, cytology experiments, and quantitative polymerase chain reaction (PCR) analyses to explore the functions of CRISPR in the mechanisms of drug resistance and pathogenesis.
RESULTS
The presence and distribution of CRISPR-Cas systems in Legionella
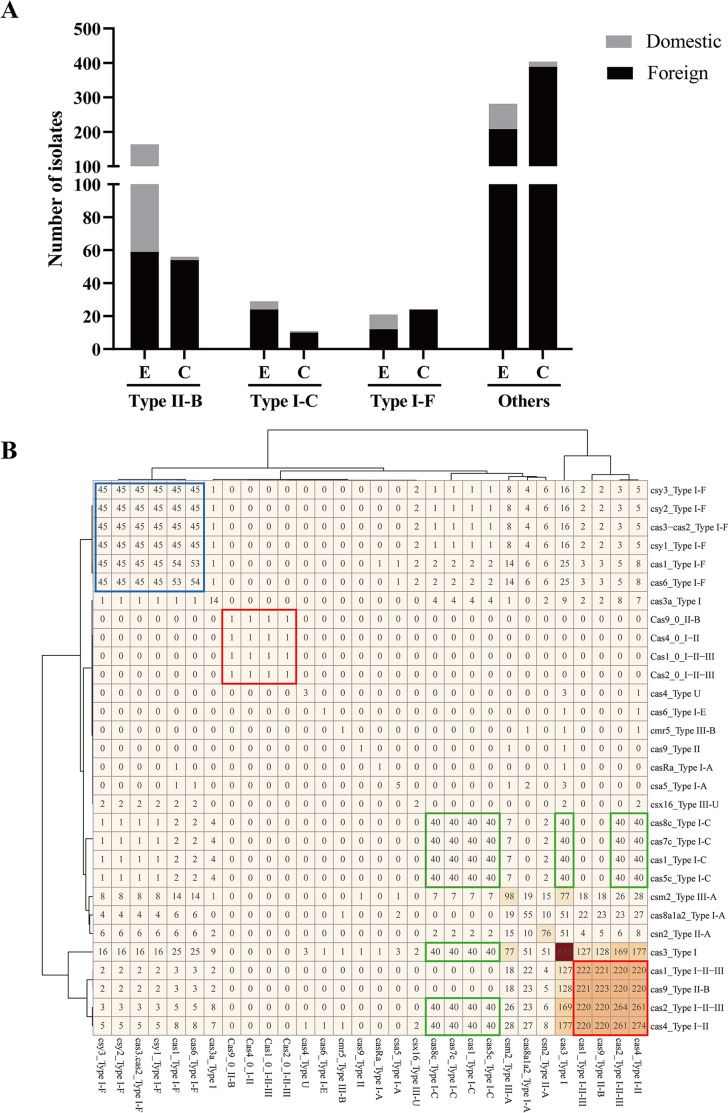
The whole-genome sequences of 915 Legionella isolates were analyzed to determine the distribution of the CRISPR-Cas locus. A total of 302 strains possessed complete Type I-C, I-F, and II-B CRISPR-Cas systems, of which Type II-B (220/302) accounted for the largest proportion, followed by I-F (45/302) and I-C (40/302). Source analysis showed that the CRISPR-Cas system was mainly distributed in environmental strains (213/302, 70.53%), and to a lesser extent in clinical strains (89/302, 29.47%).
For the Type II-B system, environmental strains (164/220, 74.55%) were still dominant and were mainly isolated from China (105/164, 64.02%). Among clinical strains, those isolated from foreign countries (54/56, 96.43%) were dominant, and those isolated from China (2/56, 3.57%) were fewer (Fig. 1A). Among the 302 Legionella strains with a complete CRISPR-Cas system, 296 were L. pneumophila, including 219 strains of Type II-B, 40 strains of Type I-C, and 40 strains of Type I-F. Two strains of L. pneumophila co-existed with Type II-B and Type I-F, and one strain co-existed with Type I-C and Type I-F. Of the remaining six non-L. pneumophila strains, two L. drozanskii isolates were classified as Type I-F and four L. sp. isolates were classified as Type II-B and Type I-F (Table S1).
Fig 1.
Characterization of Legionella isolates. (A) CRISPR-Cas system distribution of 915 Legionella strains. E represents the environmental strain, and C represents the clinical strain. (B) Cas gene distribution based on the whole-genome sequences of 915 Legionella strains. Numbers represent the results of pairwise alignment of Cas genes in the genomes of all strains, and the shades of color represent the number of occurrences of Cas genes. The blue box represents the Type I-F system, the green box represents the Type I-C system, and the red box represents the Type II-B system.
Whole-genome analysis of 915 Legionella strains revealed that the dominant Type II-B system consisted of four Cas genes (cas9, cas1, cas2, and cas4), and cas1, cas2, and cas4 genes were more closely related to Type I system homologs. The I-F system consisted of six Cas genes (cas1, cas3- cas2, csy1, csy2, csy3, and cas6), with cas3 fused to cas2 to form cas3–cas2. The I-C system consisted of seven Cas genes (cas3, cas5c, cas8c, cas7c, cas4, cas1, and cas2). In addition, there were a large number of orphan Cas genes in the genome (Fig. 1B).
Correlations between CRISPR-Cas systems and SBT of Legionella in China
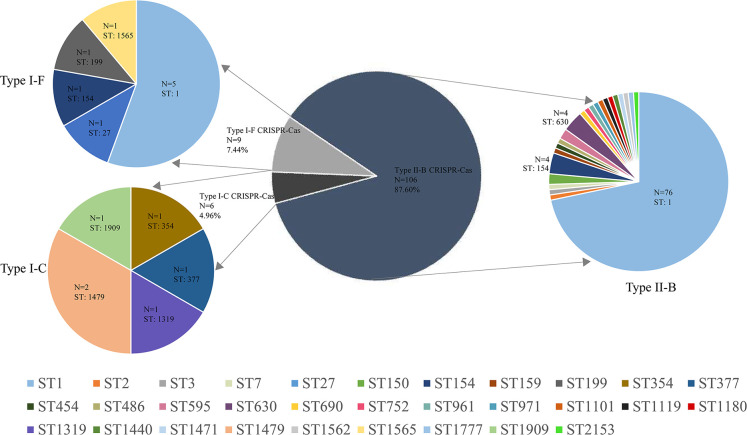
We also explored the correlations between SBT types and the CRISPR-Cas system by analyzing 121 L. pneumophila isolates from China. The results revealed 31 different SBT types in the 121 Legionella strains carrying the CRISPR-Cas system. The dominant SBT type was ST1 (81/121, 66.94%), followed by ST154 (5/121, 4.13%), ST630 (4/121, 3.31%), and ST1479 (2/121, 1.65%). Only ST1 and ST154 were found in both the II-B and I-F systems, and no cross-distribution was found in other SBT types. Further study on the SBT typing of CRISPR-Cas systems showed that the Type II-B system accounted for 87.60%, containing 23 ST types, mainly ST1 (76/106, 71.70%); the Type I-F system accounted for 7.44%, containing five ST types, including ST1 (5/9, 55.56%), ST1565 (1/9, 11.11%), ST199 (1/9, 11.11%), ST154 (1/9, 11.11%), and ST27 (1/9, 11.11%); and Type I-C system accounted for 4.96%, including ST354 (1/6, 16.67%), ST377 (1/6, 16.67%), ST1319 (1/6, 16.67%), ST1479 (2/6, 33.33%), and ST1909 (1/6, 16.67%). ST1 was dominant in both II-B and I-F systems, while it was not found in the Type I-C system (Fig. 2).
Fig 2.
Distribution of SBT types in CRISPR-Cas system-positive L. pneumophila strains in China.
CRISPR-Cas loci in L. pneumophila strain SH137
Using CRISPRCasFinder, we identified a single Type II-B CRISPR-Cas locus in the genome of the virulent L. pneumophila SH137 strain. The Type II-B system consists of four Cas genes (cas9, cas1, cas2, and cas4) and the downstream CRISPR array (the repeat sequence is conserved, sequence composition 5′-CCAATAATCCCTCATCTAAAAATCCAACCACTGAAAC-3′), and the Cas gene constitutes an operon headed by cas9, a representative gene of the Type II system (27) (Fig. 3).
Fig 3.
The Type II-B CRISPR-Cas locus of L. pneumophila strain SH137.
Bacterial growth
Legionella pneumophila wild type (WT) and Δcas9, Δcas1, Δcas2, Δcas4 and ΔCRISPR strains were grown in buffered yeast extract (BYE broth) for determination of standard growth curves. Comparing the proliferation of deletion mutants with WT after 48 h incubation at 37℃ showed that growth trends were consistent, implying that the CRISPR-Cas system has no effect on bacterial growth (Fig. 4).
Fig 4.
Growth curves of L. pneumophila Type II-B CRISPR-Cas system deletion mutants determined in vitro.
Antimicrobial susceptibility
The antimicrobial susceptibility of L. pneumophila WT, deletion mutant, and complementation mutant strains was evaluated using nine routine clinical therapeutic antibiotics (ciprofloxacin, levofloxacin, moxifloxacin, erythromycin, azithromycin, clarithromycin, rifampicin, tigecycline, and doxycycline). The results showed that the sensitivity of Δcas9, Δcas1, Δcas2, Δcas4, and ΔCRISPR mutants to azithromycin and erythromycin was lower than that of WT and complement strains; the minimum inhibitory concentration (MIC) values of strains for erythromycin decreased from 0.75 to 0.38 or 0.032, and the MIC values for azithromycin decreased from 2.0 to 1.0, 0.064 or 0.047. The MICs of deletion mutants for azithromycin and erythromycin were significantly reduced by the absence of cas9, cas1, cas2, and CRISPR, but slightly reduced by the absence of cas4. For other antibiotics, the MIC values for all gene deletion mutant and complementation mutant strains were not significantly different from those of WT (Table 1).
TABLE 1.
Minimum inhibitory concentration of WT SH137, deletion mutants, and complementation mutants to different types of drugsb
| Drug | MIC range (mg/L) | ECOFF (mg/L) | ATCC 33152 | WT (SH137) | cas9 | cas1 | cas2 | cas4 | CRISPR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δcas9 | cas9-CM | Δcas1 | cas1-CM | Δcas2 | cas2-CM | Δcas4 | cas4-CM | ΔCRISPR | CRISPR-CM | |||||
| Ciprofloxacin | 0.25–2 | 1.0 | 0.38a | 0.38a | 0.38a | 0.38a | 0.38a | 0.38a | 0.38a | 0.38a | 0.38a | 0.38a | 0.38a | 0.38a |
| Levofloxacin | 0.064–1 | 0.50 | 0.125a | 0.094a | 0.094a | 0.094a | 0.094a | 0.094a | 0.094a | 0.094a | 0.094a | 0.094a | 0.094a | 0.094a |
| Moxifloxacin | 0.25–1 | 1.0 | 0.75a | 0.50a | 0.50a | 0.50a | 0.50a | 0.50a | 0.50a | 0.50a | 0.50a | 0.50a | 0.50a | 0.50a |
| Erythromycin | 0.032–2 | 1.0 | 0.50a | 0.75a | 0.032a | 0.75a | 0.032a | 0.75a | 0.032a | 0.75a | 0.38a | 0.75a | 0.032a | 0.75a |
| Azithromycin | 0.038–8 | 1.0 | 0.25a | 2.0 | 0.047a | 2.0 | 0.047a | 2.0 | 0.064a | 2.0 | 1.0 | 2.0 | 0.064a | 2.0 |
| Clarithromycin | 0.064–1 | 0.50 | 0.19a | 0.19a | 0.19a | 0.19a | 0.19a | 0.19a | 0.19a | 0.19a | 0.19a | 0.19a | 0.19a | 0.19a |
| Rifampicin | 0.004–0.032 | 0.032 | <0.016a | <0.016a | <0.016a | <0.016a | <0.016a | <0.016a | <0.016a | <0.016a | <0.016a | <0.016a | <0.016a | <0.016a |
| Tigecycline | 1–16 | 16 | 0.5a | 0.75a | 0.75a | 0.75a | 0.75a | 0.75a | 0.75a | 0.75a | 0.75a | 0.75a | 0.75a | 0.75a |
| Doxycycline | 1–8 | 8 | 4a | 1.5a | 1.5a | 1.5a | 1.5a | 1.5a | 1.5a | 1.5a | 1.5a | 1.5a | 1.5a | 1.5a |
MIC, MIC values within the range of susceptibility.
MIC, minimum inhibitory concentration. ECOFF, epidemiological cut-off value.
Intracellular growth assay
To evaluate the effect of the CRISPR-Cas system on the proliferation of L. pneumophila in human alveolar macrophages, mouse J774 macrophages were infected with L. pneumophila in vitro and the proliferation ability of the strain in human alveolar macrophages was simulated. The results showed that the number of intracellular strains of Δcas9, Δcas1, Δcas2, and ΔCRISPR increased rapidly after 24 h of cell infection and leveled off at 48 h, while the number of intracellular cells decreased slightly after 72 h due to massive host cell death. Moreover, the intracellular growth trends of Δcas9, Δcas1, Δcas2, and ΔCRISPR mutants were consistent with those of WT, and the proliferation ability in macrophages was not altered significantly. However, the proliferation ability of the Δcas4 mutant was significantly lower in macrophages than that of WT; at 24 h after infection, the number of intracellular proliferating strains in Δcas4 mutant-infected cells was significantly lower than that in WT, and the number of intracellular Δcas4 mutant strains remained low after 48 h and 72 h of infection, but the intracellular proliferation phenotype of bacteria returned to the WT state after the introduction of the complete cas4 gene in the Δcas4 mutant (Fig. 5).
Fig 5.
Proliferation ability of L. pneumophila CRISPR-Cas system deletion mutants in J774 mouse macrophages.
mRNA expression levels of genes related to drug resistance and intracellular proliferation
To verify the up- or down-regulation of azithromycin resistance- and intra-cellular proliferation-related genes, we measured the relative expression levels of the lpeAB efflux pump gene, CRISPR-Cas-related genes, and Dot/Icm secretion system-related genes by qRT-PCR. The results showed that CRISPR-Cas system genes of L. pneumophila were mutually regulated; deletion of any gene affected the expression of the other genes. Specifically, after deleting cas9, cas1, cas2, and CRISPR alone, the expression of the remaining genes showed a statistically significant decrease. After deleting the cas4 gene, the expression of the remaining four genes was also decreased, but the decrease was lower (Fig. 6A).
Fig 6.
Comparison of the expression levels of some key genes in deletion mutants of L. pneumophila. (A) Expression of CRISPR-Cas system-related genes. (B) Expression of the lpeAB efflux pump gene. (C–G) Expression of Dot/Icm secretory system-related structural protein genes in deletion mutants. (H) Expression of the flaA flagellin gene.
Expression analysis of drug resistance genes showed that the expression of the lpeAB efflux pump gene was decreased to different degrees in each deletion mutant compared with the WT strain, with Δcas9, Δcas1, Δcas2, and ΔCRISPR deletion mutants showing a more pronounced decrease in lpeAB expression than the Δcas4 deletion mutant, based on L. pneumophila ATCC 33152 as a negative control (Fig. 6B).
The growth and proliferation of L. pneumophila in macrophages is dependent on the Dot/Icm transporter, a type IV secretion system (28). Due to the significantly reduced proliferation ability of the Δcas4 deletion mutant in macrophages, we analyzed the structural gene expression of the Dot/Icm system in the Δcas4 deletion mutant, it was observed that most gene expression levels were significantly decreased compared with those of WT, and differences were statistically significant. This indicates that cas4 gene deletion leads to a decrease in the expression of most Dot/Icm type IV secretion system structural genes, which in turn leads to a significant decrease in the intracellular proliferation ability of the Δcas4 deletion mutant. By analyzing the type IV secretion system of Δcas9, Δcas1, Δcas2, and ΔCRISPR deletion mutants, it was found that the structural genes of the Dot/Icm system also had a slight downward trend, presumably due to the inter-regulation of the Cas genes in the CRISPR-Cas system (Fig. 6C through G). The J774 cells can express several inflammasomes, including NLRC4, which responds to Legionella flagellin. We therefore examined the expression levels of flagellin in each strain to determine whether ∆cas4 triggers a stronger host inflammasome response due to increased flagellin expression, thereby limiting its intracellular growth. The results showed that the expression level of flagellin in each deletion strain showed a decreasing trend compared to that in the wild-type strain, suggesting that the CRISPR-Cas system can affect the expression level of flagellin in strains and that the reduced intracellular proliferative capacity of the ∆cas4 deletion mutant may have little to do with the host inflammasome response (Fig. 6H).
DISCUSSION
The widespread L. pneumophila is an important human pathogen known to cause respiratory diseases, leading to legionnaires’ disease. Several studies have shown that the CRISPR-Cas system is widely distributed and can regulate expression processes in a variety of microorganisms, but its distribution and genetic evolution in Legionella and regulation of gene expression in L. pneumophila remain unclear. Our results indicated that the dominant Type II-B system affects the antibiotic sensitivity and intracellular proliferation of L. pneumophila and regulates the lpeAB efflux pump gene and Dot/Icm type IV secretion system structural genes, thereby altering the biological characteristics of the strain.
The CRISPR-Cas system in Legionella was dominated by Type I-C and Type I-F in the Class I system and by Type II-B in the Class II system, with the Type II-B system dominant in Legionella. The CRISPR-Cas system was mainly clustered in Legionella strains of environmental origin and less so in strains of clinical origin, probably because the external environment is more complex and variable, environmental pressure forces strains to evolve, and the presence of the CRISPR-Cas system helps strains to resist invasion by exogenous phages and viruses and enhances autoimmune defense functions (29, 30). It was also found that the CRISPR-Cas system was not unique in the genome of Legionella and that different classes (Type I and Type II systems) or different subclasses (Type I-F and Type I-C) can co-exist in the genome of the same strain, suggesting that the strain might have evolved multiple CRISPR-Cas systems to enhance its immune defense mechanism under complex external environments. Furthermore, there are many orphan CRISPR arrays or Cas genes that do not cluster with the rest of the CRISPR-Cas genes, implying that the CRISPR-Cas system is rich and diverse. The deleted CRISPR arrays also reflect, to some extent, the conserved and inclusive nature of bacteria during evolution (31, 32). They also suggest there may be many undiscovered Cas genes or CRISPR arrays that constitute a new undiscovered complete CRISPR-Cas system with currently known orphan genes and that orphan genes still perform important phenotypic functions and exert biological effects in the genome, besides their immune defense functions. Interestingly, the Type II CRISPR-Cas system effector complex consists of the signature protein cas9 and the cas1 and cas2 that exert the effect during the adaptation phase. While its signature protein cas9 cannot be used as a marker gene of the Type II system, it can be identified as a Type II-B CRISPR-Cas system only when cas1 and cas2 genes are present near the cas9 gene due to the high sequence similarity between cas9 and cas9 gene homologs (not related to the CRISPR-Cas system) (33). However, the Type II-B CRISPR-Cas system of Legionella contains the cas4 gene originally found in the Type I system, and its cas1, cas2, and cas4 in the Type II-B system are more closely related to the corresponding homologs in the Type I system, suggesting that the Type II-B system may be derived from evolutionary recombination of the Type I system (34).
Sequence-based typing (SBT) is a method for multi-genotyping strains by amplifying internal gene fragments, which is important for studying the genetic evolution and epidemiological distribution of strains. In previous studies, ST1 was the dominant type among L. pneumophila (27, 35). In the present work, we found that among the strains positive for the CRISPR-Cas system, ST1 was still the predominant type, mainly distributed in the Type II-B system, followed by Type I-F, while it was not found in the Type I-C system. Thus, we speculate that ST1 strains are driven to acquire the Type II-B CRISPR-Cas system to resist the invasion of foreign nucleic acids and that ST1 strains and Type II-B strains may have similar genetic evolutionary pathways.
The emergence of antibiotic resistance helps Legionella to survive in hostile environments, but this makes clinical treatment of Legionnaires’ disease increasingly challenging. Previous studies have shown that the CRISPR system identifies and cleaves resistance genes acquired by horizontal gene transfer (HGT), mainly through its spacer sequence, to prevent strains from acquiring drug resistance (36, 37). However, upregulation of Campylobacter jejuni cj0309c efflux pump expression can affect the growth of the strain under high levels of oxygen, and the cas9 gene in the CRISPR-Cas system can alter cj0309c efflux pump expression in relation to antibiotic resistance in this strain (38). Herein, we found that deletion of cas9, cas1, cas2, cas4, and CRISPR in the Type II-B CRISPR-Cas system of L. pneumophila decreased the resistance of the strain to azithromycin and erythromycin compared with WT, revealing that the Cas and CRISPR in the Type II-B system of L. pneumophila may be involved in regulating azithromycin and erythromycin resistance. The cas9, cas1, cas2, and CRISPR play a crucial role in regulating azithromycin and erythromycin resistance, while cas4 may play an auxiliary role in this regulation, and deletion of the cas4 gene had little effect on the antibiotic susceptibility of the strain. Further investigation of the regulatory mechanism revealed that although deletion of each Cas gene did not eliminate the expression of upstream and downstream Cas genes, it significantly reduced the relative expression levels of upstream and downstream Cas genes, suggesting that in the CRISPR-Cas system, as an operon structure, deletion of one may affect the expression levels of other genes and affect the biological traits of the strain. The high expression of the lpeAB efflux pump gene confers resistance to azithromycin in Legionella and affects the susceptibility to macrolide antibiotics. The lpeAB is a member of the RND efflux pump family, and it shares functional homology with the E. coli MacAB-TolC efflux pump (39). However, unlike the MacAB-TolC efflux pump, lpeAB does not affect the susceptibility of Legionella to clarithromycin (39, 40). The present study further confirmed that the expression level of the lpeAB gene in each deletion mutant was decreased to varying degrees, leading to changes in the sensitivity of each deletion mutant to azithromycin and erythromycin, but not to clarithromycin. It was revealed that the CRISPR-Cas system of L. pneumophila regulates the expression level of the lpeAB efflux pump, thereby affecting the antibiotic susceptibility of the strain.
The Dot/Icm type IV secretory system (T4BSS) is thought to form a protein complex that transfers effector proteins from the bacterial cytoplasm to the host cell, and it is closely associated with the pathogenicity of the strain, which is essential for the growth and intracellular transport of human macrophages and Amoebae (2). In the present study, we found that the intracellular proliferation ability of ∆cas9, ∆cas1, ∆cas2, and ∆CRISPR mutants was not significantly altered compared with that of WT, indicating that they are not necessary for the proliferation of strains in macrophages. By contrast, the proliferative ability of the ∆cas4 mutant was significantly decreased in macrophages, and the intracellular survival rate of L. pneumophila containing the intact Type II-B CRISPR-Cas system was significantly higher than that of L. pneumophila lacking the cas4 gene, suggesting that the cas4 gene, as the terminal gene of the Cas operon, may affect the expression level of the T4BSS secretion system. Previous studies have shown that cas4 assists directional spacer acquisition in CRISPR-Cas (41) and affects the specific integration of CRISPR spacers (42). Additionally, cas4 has a specific motif for RecB exonuclease, suggesting that cas4 may be involved in DNA metabolism or gene expression (43, 44). Our qRT-PCR analysis revealed that deletion of the cas4 gene reduced the expression levels of most structural genes of the T4BSS associated with intracellular proliferation in Legionella, thus impairing the intracellular proliferation ability of the strain. Therefore, cas4 may be involved in regulating the T4BSS, and thereby regulating the intracellular proliferation ability of Legionella and affecting the pathogenicity of ∆cas4 mutant.
MATERIALS AND METHODS
Bacterial strains, media, and bioinformatics analysis
A total of 915 non-duplicated Legionella strains were analyzed in this study. Among these, 212 Legionella isolates were randomly isolated from environmental and clinical samples in several provinces of China, of which 173 strains were collected and stored by the Laboratory of Ecological Medicine at the Chinese Center for Disease Control and Prevention (Table S2), and 39 strains were obtained from the GenBank public database (http://www.ncbi.nlm.nih.gov/genbank). Additionally, 703 foreign Legionella genome sequences were also obtained from the GenBank public database. The bacterial strains and plasmids used in gene editing studies are described in Table S3.
All Legionella complete genome sequences (915 in total) were uploaded to CRISPRCasFinder software (https://crisprcas.i2bc.paris-saclay.fr/CrisprCasFinder/Index) to identify CRISPR-CAS loci. SBT of 121 strains of L. pneumophila from China possessing a complete CRISPR-Cas system was performed based on the method recommended by the European Working Group on Legionella Infections (EWGLI) (http://www.Ewgli.org).
The Type II-B ST1 environmental strain represented by Legionella pneumophila SH137 (resistant to azithromycin) was used as the WT strain. Legionella strains were cultured on buffered charcoal-yeast extract (BCYE) agar plates (OXOID) at 37°C in 5% CO2 or in N-(2-acetamido)−2-aminoethanesulfonic acid-BYE broth at 37°C. Escherichia coli DH5α cells were used for cloning and grown in Luria–Bertani medium, and ampicillin 100 µg/mL and chloramphenicol 25 µg/mL were required.
Construction of gene-disrupted mutants
To generate the deletion mutants, we used the lambda RED recombination method as described previously (45, 46). Upstream and downstream fragments of cas9, cas1, cas2, cas4, and CRISPR array with homologous arms were separately amplified by PCR from SH137 DNA using primers (Table S4). Upstream and downstream fragments were fused by overlap PCR, and products were ligated into the pGEM-T Easy vector (Promega, Madison, WI) via the TA cloning method to generate pT-Cas/CRISPR constructs, which were digested with the restriction enzyme SmaI. The kanamycin resistance (Kmr) cassette generated from pUT-mini-Tn5 Km was inserted into the pT-Cas/CRISPR plasmid to form pT-Cas/CRISPR-Km using the enzymatic digestion and ligation method. pT-Cas/CRISPR-Km constructs were then digested with NotI, and fragments carrying the disrupted Cas/CRISPR region were cloned into the NotI site of allelic exchange vector pLAW344 to generate pLAW344-Cas/CRISPR-Km. Recombinant plasmids carrying Cas and CRISPR array were introduced into competent cells of the original strain by electroporation, and transformants were selected on BCYE agar plates supplemented with kanamycin (30 µg/mL) and ampicillin (100 ug/mL). Finally, transformants were cultured on BCYE plates containing kanamycin and 5% sucrose, and deletion strains of Δcas9, Δcas1, Δcas2, Δcas4, and ΔCRISPR were screened out.
Construction of plasmids for complementation
The target Cas genes and CRISPR array were amplified from genomic DNA extracted from the WT strain. PCR products were digested with the corresponding restriction enzymes and ligated into the PMMB207 plasmid using the same restriction site to generate recombinant PMMB207-Cas/CRISPR plasmids. These were introduced into the deletion strain, and transformants were selected on BCYE agar plates containing chloramphenicol (5 µg/mL) and kanamycin (30 µg/mL). Positive colonies were confirmed by PCR.
Determination of standard growth curves
WT and deletion strains were inoculated on BCYE plates and cultured at 37°C with 5% CO2. A single colony was selected and inoculated in 5 mL BYE medium and incubated overnight at 37°C with shaking at 200 rpm. The optical density at 600 nm (OD600) of the medium used for overnight incubation was measured and quantitatively transferred to 50 mL of fresh liquid medium to an initial OD600 of 0.2. Cultures were incubated at 37°C with shaking at 200 rpm, and OD600 was measured every 6 h until reaching a stable period. OD600 data were used to plot a curve. This experiment was repeated three times for each group.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed according to EUCAST recommendations and manufacturer’s instructions (https://www.eucast.org/eucastguidancedocuments/). The E test strip method was used to assess the MIC of azithromycin, erythromycin, rifampicin, moxifloxacin, levofloxacin, ciprofloxacin, tigecycline, clindamycin, and doxycycline (Liofilchem, Teramo, Italy) against complement, gene-disrupted, and WT strains. Fresh colonies were selected and resuspended in phosphate-buffered saline (PBS), and the suspension concentration was adjusted to 0.5 McFarland standard. The surface of a BCYE-α plate was evenly swabbed with a sterile cotton swab dipped in bacterial solution, and the E test strip was placed in the center. After incubation at 35°C for 48 h, the MIC value of the E test strip was determined. L. pneumophila ATCC 33152 served as a control for drug sensitivity tests. The epidemiological cut-off (ECOFF) of resistance breakpoints as determined by EUCAST guidelines was used to interpret the results. This experiment was repeated three times for each group.
Intracellular growth assay
J774 mouse macrophages were cultured in DMEM-H medium (Gibco, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) at 37°C with 5% CO2. Bacterial suspensions at ~ 1 × 108 colony-forming units (CFU)/mL in the logarithmic growth stage were diluted 10-fold in DMEM-H medium and used to infect J774 cells (~1 × 105 cells/mL) at a multiplicity of infection (MOI) of 100. Infected cells were incubated at 37°C with 5% CO2 for 1.5 h. The bacteria solution was discarded, and cells were washed three times with 500 µL of PBS to remove extracellular bacteria. A 0.5 mL volume of DMEM-H medium containing 10% FBS was then added to each well, the number of proliferating bacteria in J774 cells in each well was determined at 24-h intervals, and total CFU was calculated by plating the cell suspension onto BCYE plates. This experiment was repeated three times for each group.
Quantitative real-time PCR (qRT-PCR)
A single colony of each strain was placed into 5 mL of BYE liquid medium and adjusted to 2.0 McFarland standard. A 1 mL volume of adjusted bacterial suspension was placed into 4 mL of BYE and incubated at 37°C with shaking at 200 rpm for 18 h. Bacteria were centrifuged (5,000 rpm, 20 min, 4°C), and RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA). Extracted RNA was treated with RNase-free DNaseI (Qiagen, Hilden, Germany) to remove DNA. A cDNA synthesis kit (Invitrogen) was used according to the manufacturer’s instructions for cDNA synthesis. Measurement of the expression levels of related genes was performed in a one-step reaction using SYBR Green I and a CFX 96 real-time system (Bio-Rad) with a 25 µL reaction volume containing 2 µL of cDNA purified from each sample. The amplification reaction consisted of 40 cycles of denaturation at 95°C for 30 s, followed by denaturation at 95°C for 5 s, and annealing/extension at 60°C for 20 s. The gyrB gene served as an internal control. Relative changes in gene expression were calculated using the 2-ΔΔCT method. The primer sequences are shown in S4 Table. Three independent experiments of each group were performed.
Statistical analysis
Statistical significance was assessed using Student’s t-test and one-way analysis of variance (ANOVA), and P < 0.05 was considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001).
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (No. 81671985), the Science Foundation for the State Key Laboratory for Infectious Disease Prevention and Control of China (No. 2022SKLID209 and No. 2019SKLID403), and the Public Health Service Capability Improvement Project of the National Health Commission of the People’s Republic of China (No. 2100409034 and No. 0104205).
Contributor Information
Tian Qin, Email: qintian@icdc.cn.
Igor E. Brodsky, University of Pennsylvania, Philadelphia, Pennsylvania, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/iai.00229-23.
Legionella strains of CRISPR-Cas system in this study.
Information on 173 strains of Legionella in China.
The bacterial strains and plasmids used in gene editing studies.
Primers used in this study.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Blatt SP, Parkinson MD, Pace E, Hoffman P, Dolan D, Lauderdale P, Zajac RA, Melcher GP. 1993. Nosocomial legionnaires' disease: aspiration as a primary mode of disease acquisition. Am J Med 95:16–22. doi: 10.1016/0002-9343(93)90227-g [DOI] [PubMed] [Google Scholar]
- 2. Mondino S, Schmidt S, Rolando M, Escoll P, Gomez-Valero L, Buchrieser C. 2020. Legionnaires' disease: state of the art knowledge of pathogenesis mechanisms of Legionella. Annu Rev Pathol 15:439–466. doi: 10.1146/annurev-pathmechdis-012419-032742 [DOI] [PubMed] [Google Scholar]
- 3. Yu VL, Plouffe JF, Pastoris MC, Stout JE, Schousboe M, Widmer A, Summersgill J, File T, Heath CM, Paterson DL, Chereshsky A. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J Infect Dis 186:127–128. doi: 10.1086/341087 [DOI] [PubMed] [Google Scholar]
- 4. Doleans A, Aurell H, Reyrolle M, Lina G, Freney J, Vandenesch F, Etienne J, Jarraud S. 2004. Clinical and environmental distributions of Legionella strains in France are different. J Clin Microbiol 42:458–460. doi: 10.1128/JCM.42.1.458-460.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fattorini L, Creti R, Palma C, Pantosti A, Unit of Antibiotic Resistance and Special Pathogens, Unit of Antibiotic Resistance and Special Pathogens of the Department of Infectious Diseases, Istituto Superiore di Sanità, Rome . 2020. Bacterial coinfections in COVID-19: an underestimated adversary. Ann Ist Super Sanita 56:359–364. doi: 10.4415/ANN_20_03_14 [DOI] [PubMed] [Google Scholar]
- 6. Lai C-C, Wang C-Y, Hsueh P-R. 2020. Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect 53:505–512. doi: 10.1016/j.jmii.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pedro-Botet L, Yu VL. 2006. Legionella: macrolides or quinolones? Clin Microbiol Infect 12 Suppl 3:25–30. doi: 10.1111/j.1469-0691.2006.01394.x [DOI] [PubMed] [Google Scholar]
- 8. Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, Ortqvist A, Schaberg T, Torres A, van der Heijden G, Read R, Verheij TJM, Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases . 2011. Guidelines for the management of adult lower respiratory tract infections--full version. Clin Microbiol Infect 17 Suppl 6:E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jonas D, Engels I, Daschner FD, Frank U. 2000. The effect of azithromycin on intracellular Legionella pneumophila in the Mono Mac 6 cell line at serum concentrations attainable in vivo. J Antimicrob Chemother 46:385–390. doi: 10.1093/jac/46.3.385 [DOI] [PubMed] [Google Scholar]
- 10. Stout JE, Arnold B, Yu VL. 1998. Activity of azithromycin, clarithromycin, roxithromycin, dirithromycin, quinupristin/dalfopristin and erythromycin against Legionella species by intracellular susceptibility testing in HL-60 cells. J Antimicrob Chemother 41:289–291. doi: 10.1093/jac/41.2.289 [DOI] [PubMed] [Google Scholar]
- 11. Jia X, Ren H, Nie X, Li Y, Li J, Qin T. 2019. Antibiotic resistance and azithromycin resistance mechanism of Legionella pneumophila serogroup 1 in China. Antimicrob Agents Chemother 63:e00768-19. doi: 10.1128/AAC.00768-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Natås OB, Brekken AL, Bernhoff E, Hetland MAK, Löhr IH, Lindemann PC. 2019. Susceptibility of Legionella pneumophila to antimicrobial agents and the presence of the efflux pump LpeAB. J Antimicrob Chemother 74:1545–1550. doi: 10.1093/jac/dkz081 [DOI] [PubMed] [Google Scholar]
- 13. Bhaya D, Davison M, Barrangou R. 2011. CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu Rev Genet 45:273–297. doi: 10.1146/annurev-genet-110410-132430 [DOI] [PubMed] [Google Scholar]
- 14. Hille F, Richter H, Wong SP, Bratovič M, Ressel S, Charpentier E. 2018. The biology of CRISPR-Cas: backward and forward. Cell 172:1239–1259. doi: 10.1016/j.cell.2017.11.032 [DOI] [PubMed] [Google Scholar]
- 15. Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712. doi: 10.1126/science.1138140 [DOI] [PubMed] [Google Scholar]
- 16. Makarova KS, Haft DH, Barrangou R, Brouns SJJ, Charpentier E, Horvath P, Moineau S, Mojica FJM, Wolf YI, Yakunin AF, van der Oost J, Koonin EV. 2011. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol 9:467–477. doi: 10.1038/nrmicro2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marraffini LA, Sontheimer EJ. 2010. CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat Rev Genet 11:181–190. doi: 10.1038/nrg2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al-Attar S, Westra ER, van der Oost J, Brouns SJJ. 2011. Clustered regularly interspaced short palindromic repeats (CRISPRs): the hallmark of an ingenious antiviral defense mechanism in prokaryotes. Biol Chem 392:277–289. doi: 10.1515/BC.2011.042 [DOI] [PubMed] [Google Scholar]
- 19. Cui L, Wang X, Huang D, Zhao Y, Feng J, Lu Q, Pu Q, Wang Y, Cheng G, Wu M, Dai M. 2020. CRISPR-cas3 of Salmonella upregulates bacterial biofilm formation and virulence to host cells by targeting quorum-sensing systems. Pathogens 9:53. doi: 10.3390/pathogens9010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Louwen R, Horst-Kreft D, de Boer AG, van der Graaf L, de Knegt G, Hamersma M, Heikema AP, Timms AR, Jacobs BC, Wagenaar JA, Endtz HP, van der Oost J, Wells JM, Nieuwenhuis EES, van Vliet AHM, Willemsen PTJ, van Baarlen P, van Belkum A. 2013. A novel link between Campylobacter jejuni bacteriophage defence, virulence and Guillain-Barré syndrome. Eur J Clin Microbiol Infect Dis 32:207–226. doi: 10.1007/s10096-012-1733-4 [DOI] [PubMed] [Google Scholar]
- 21. Li R, Fang L, Tan S, Yu M, Li X, He S, Wei Y, Li G, Jiang J, Wu M. 2016. Type I CRISPR-Cas targets endogenous genes and regulates virulence to evade mammalian host immunity. Cell Res 26:1273–1287. doi: 10.1038/cr.2016.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deecker SR, Ensminger AW. 2020. Type I-F CRISPR-CAS distribution and array dynamics in Legionella pneumophila. G3 (Bethesda) 10:1039–1050. doi: 10.1534/g3.119.400813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rao C, Chin D, Ensminger AW. 2017. Priming in a permissive type I-C CRISPR-Cas system reveals distinct dynamics of spacer acquisition and loss. RNA 23:1525–1538. doi: 10.1261/rna.062083.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lück C, Brzuszkiewicz E, Rydzewski K, Koshkolda T, Sarnow K, Essig A, Heuner K. 2015. Subtyping of the Legionella pneumophila "Ulm" outbreak strain using the CRISPR-Cas system. Int J Med Microbiol 305:828–837. doi: 10.1016/j.ijmm.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 25. Gunderson FF, Mallama CA, Fairbairn SG, Cianciotto NP. 2015. Nuclease activity of Legionella pneumophila Cas2 promotes intracellular infection of amoebal host cells. Infect Immun 83:1008–1018. doi: 10.1128/IAI.03102-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gunderson FF, Cianciotto NP. 2013. The CRISPR-associated gene cas2 of Legionella pneumophila is required for intracellular infection of amoebae. mBio 4:e00074–13. doi: 10.1128/mBio.00074-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mercante JW, Caravas JA, Ishaq MK, Kozak-Muiznieks NA, Raphael BH, Winchell JM. 2018. Genomic heterogeneity differentiates clinical and environmental subgroups of Legionella pneumophila sequence type 1. PLoS One 13:e0206110. doi: 10.1371/journal.pone.0206110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ge J, Shao F. 2011. Manipulation of host vesicular trafficking and innate immune defence by Legionella Dot/Icm effectors. Cell Microbiol 13:1870–1880. doi: 10.1111/j.1462-5822.2011.01710.x [DOI] [PubMed] [Google Scholar]
- 29. Watson BNJ, Staals RHJ, Fineran PC. 2018. CRISPR-Cas-mediated phage resistance enhances horizontal gene transfer by transduction. mBio 9:e02406-17. doi: 10.1128/mBio.02406-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du Toit A. 2018. Viral infection: CRISPR-Cas enhances HGT by transduction. Nat Rev Microbiol 16:186. doi: 10.1038/nrmicro.2018.28 [DOI] [PubMed] [Google Scholar]
- 31. Touchon M, Rocha EPC. 2010. The small, slow and specialized CRISPR and anti-CRISPR of Escherichia and Salmonella. PLoS One 5:e11126. doi: 10.1371/journal.pone.0011126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kunin V, Sorek R, Hugenholtz P. 2007. Evolutionary conservation of sequence and secondary structures in CRISPR repeats. Genome Biol 8:R61. doi: 10.1186/gb-2007-8-4-r61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJJ, Charpentier E, Haft DH, Horvath P, Moineau S, Mojica FJM, Terns RM, Terns MP, White MF, Yakunin AF, Garrett RA, van der Oost J, Backofen R, Koonin EV. 2015. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol 13:722–736. doi: 10.1038/nrmicro3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chylinski K, Makarova KS, Charpentier E, Koonin EV. 2014. Classification and evolution of type II CRISPR-Cas systems. Nucleic Acids Res 42:6091–6105. doi: 10.1093/nar/gku241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qin T, Zhou H, Ren H, Guan H, Li M, Zhu B, Shao Z. 2014. Distribution of sequence-based types of Legionella pneumophila serogroup 1 strains isolated from cooling towers, hot springs, and potable water systems in China. Appl Environ Microbiol 80:2150–2157. doi: 10.1128/AEM.03844-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou Y, Tang Y, Fu P, Tian D, Yu L, Huang Y, Li G, Li M, Wang Y, Yang Z, Xu X, Yin Z, Zhou D, Poirel L, Jiang X. 2020. The type I-E CRISPR-Cas system influences the acquisition of blaKPC-IncF plasmid in Klebsiella pneumonia. Emerging Microbes & Infections 9:1011–1022. doi: 10.1080/22221751.2020.1763209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wheatley RM, MacLean RC. 2021. CRISPR-Cas systems restrict horizontal gene transfer in Pseudomonas aeruginosa. ISME J 15:1420–1433. doi: 10.1038/s41396-020-00860-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shabbir MAB, Wu Q, Shabbir MZ, Sajid A, Ahmed S, Sattar A, Tang Y, Li J, Maan MK, Hao H, Yuan Z. 2018. The CRISPR-cas system promotes antimicrobial resistance in Campylobacter jejuni. Future Microbiology 13:1757–1774. doi: 10.2217/fmb-2018-0234 [DOI] [PubMed] [Google Scholar]
- 39. Vandewalle-Capo M, Massip C, Descours G, Charavit J, Chastang J, Billy PA, Boisset S, Lina G, Gilbert C, Maurin M, Jarraud S, Ginevra C. 2017. Minimum inhibitory concentration (MIC) distribution among wild-type strains of Legionella pneumophila identifies a subpopulation with reduced susceptibility to macrolides owing to efflux pump genes. Int J Antimicrob Agents 50:684–689. doi: 10.1016/j.ijantimicag.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 40. Massip C, Descours G, Ginevra C, Doublet P, Jarraud S, Gilbert C. 2017. Macrolide resistance in Legionella pneumophila: the role of LpeAB efflux pump. J Antimicrob Chemother 72:1327–1333. doi: 10.1093/jac/dkw594 [DOI] [PubMed] [Google Scholar]
- 41. Kobayashi N, Nishino K, Yamaguchi A. 2001. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J Bacteriol 183:5639–5644. doi: 10.1128/JB.183.19.5639-5644.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hu C, Almendros C, Nam KH, Costa AR, Vink JNA, Haagsma AC, Bagde SR, Brouns SJJ, Ke A. 2021. Mechanism for Cas4-assisted directional spacer acquisition in CRISPR-Cas. Nature 598:515–520. doi: 10.1038/s41586-021-03951-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Z, Pan S, Liu T, Li Y, Peng N. 2019. Cas4 nucleases can effect specific integration of CRISPR spacers. J Bacteriol 201:e00747-18. doi: 10.1128/JB.00747-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dixit B, Anand V, Hussain MS, Kumar M. 2021. The CRISPR-associated Cas4 protein from Leptospira interrogans demonstrate versatile nuclease activity. Curr Res Microb Sci 2:100040. doi: 10.1016/j.crmicr.2021.100040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stewart CR, Burnside DM, Cianciotto NP. 2011. The surfactant of Legionella pneumophila is secreted in a TolC-dependent manner and is antagonistic toward other Legionella species. J Bacteriol 193:5971–5984. doi: 10.1128/JB.05405-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chatfield CH, Mulhern BJ, Burnside DM, Cianciotto NP. 2011. Legionella pneumophila LbtU acts as a novel, TonB-independent receptor for the legiobactin siderophore. J Bacteriol 193:1563–1575. doi: 10.1128/JB.01111-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Legionella strains of CRISPR-Cas system in this study.
Information on 173 strains of Legionella in China.
The bacterial strains and plasmids used in gene editing studies.
Primers used in this study.