Abstract
Immune responses to Plasmodium falciparum rhoptry-associated protein 1 (RAP-1), RAP-2, and RAP-3 appear to contribute to protection against infection by this human malarial parasite. This conclusion is suggested by results of monkey immunization trials and of cell culture studies showing antibody-dependent inhibition of erythrocyte invasion. In the present study, splenectomized owl monkeys were infected with P. falciparum in order to monitor anti-RAP-1 antibody production as antiparasite immunity developed. The monkeys responded to a primary infection with the production of antibodies to a fragment of RAP-1 containing amino acids 1 to 294 (RAP-11–294). After drug cure and reinfection, the monkeys had a prolonged prepatent period, indicating they had already developed partial immunity to the parasite. Sera from these animals showed major increases in anti-RAP-11–294 antibodies. In contrast, only low levels of antibodies to inhibitory B-cell epitope 1 (iB-1), an inhibitory epitope in RAP-11–294 with the sequence N200TLTPLEELYPT211, was observed after the initial parasite infection, and the anti-iB-1 antibodies were not readily boosted upon reinfection. These results suggest that iB-1 is an immunogenic but not immunodominant epitope and that anti-iB-1 antibodies do not substantially contribute to early stages of naturally acquired immunity in the owl monkey model. To identify additional epitopes bound by inhibitory antibodies, mouse monoclonal antibodies were produced with a recombinant fusion protein containing RAP-11–294. Monoclonal antibody 1D6 inhibited parasite invasion of erythrocytes in vitro. 1D6 did not bind peptide iB-1 but rather bound a second inhibitory epitope called iB-2. iB-2, like iB-1, is found near the amino terminus of p67, a RAP-1 processing product thought to be involved in merozoite invasion of erythrocytes. Since anti-iB-1 antibodies were not readily produced during parasite infection, it may be desirable to direct antibody responses to particular epitopes in RAP-1, such as iB-1 and iB-2.
The parasite Plasmodium falciparum causes the majority of malaria deaths, taking its greatest toll on young children. Over time, individuals living in areas endemic for malaria acquire a nonsterile immunity to P. falciparum. Antibodies, in conjunction with cellular immune responses, appear to play an important role in the development of this immunity. In now classical experiments, Cohen and colleagues demonstrated that the passive transfer of purified antibodies from pooled human immune serum was beneficial to children with life-threatening falciparum malaria infections (8). Using adult Thai recipients, Druilhe and coworkers confirmed Cohen’s results (2). They also showed that some pooled immunoglobulins from human immune sera, as well as immunoglobulins from mice or rabbit immune sera, inhibit parasite growth in vitro when tested in combination with normal human monocytes. This inhibition occurred even in cases where the separate use of immunoglobulins or monocytes was ineffective (2). Thus, antibodies are important elements in the development of a protective immune response to malaria parasites, and the identification and characterization of epitopes bound by inhibitory antibodies are one desirable focus in vaccine development efforts.
Proteins localized to the rhoptries, specialized organelles of the invasive P. falciparum merozoite stage, have been a focus of intense interest as potential vaccine candidates (16). One of these proteins, rhoptry-associated protein 1 (RAP-1), which is processed into molecules of 86, 82, 70, and 67 kDa, forms heterooligomeric protein complexes with the rhoptry proteins RAP-2 and RAP-3 (4, 5, 7, 13, 28). Monoclonal antibodies (MAb) that bind epitopes in RAP-1 or RAP-2 and inhibit invasion in vitro have been identified (9, 22, 28). Several of the inhibitory anti-RAP-1 MAb map to the linear sequence N200TLTPLEELYPT211 located in the amino (N-)-terminal one-third of RAP-1 (9). This epitope, inhibitory B-cell epitope 1, is here termed iB-1 (Fig. 1).
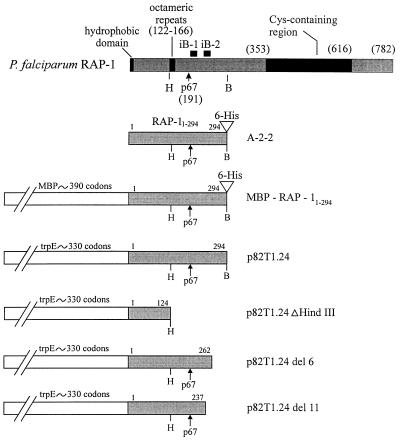
FIG. 1.
Schematic diagrams of P. falciparum RAP-1, the recombinant protein A-2-2, and recombinant fusion proteins MBP-RAP-11–294, p82T1.24, p82T1.24ΔHindIII, p82T1.24del6, and p82T1.24del11. The locations of structural features of RAP-1 are indicated (including those of the octameric repeats, iB-1, iB-2, and the Cys-containing region). Within the recombinant constructs, the termini of the RAP-1 fragments, the approximate lengths of the fusion partners MBP and trpE, and the locations of the 6-His residues are provided. The locations of BamHI (B) and HindIII (H) restriction sites are shown; a second HindIII site, located in the vector DNA of the p82T1.24 construct and used to construct p82T1.24ΔHindIII, is not shown. The RAP-11–294 fragment is found in A-2-2, MBP-RAP-11–294, and p82T1.24.
The potential role of iB-1 and other RAP-1 epitopes in the development of a protective, anti-P. falciparum immune response has not been explored. Owl and squirrel monkeys can be productively infected with P. falciparum, and antibodies from immune monkeys can inhibit P. falciparum proliferation in vitro in the absence of monocytes or other effector immune cells (6, 24). Furthermore, monkeys, in contrast to humans, rapidly develop immunity to subsequent P. falciparum challenge. Thus, these nonhuman primates provide models for studying protective immune responses after immunization or after P. falciparum infection. In the squirrel monkey model, monkeys immunized with parasite-derived RAP-1, RAP-2, and RAP-3 and challenged with a heterologous strain of P. falciparum developed a delayed and relatively low parasitemia compared to that of control animals (26). However, while the prechallenge immune sera contained anti-RAP-1 antibodies, there was no correlation between antibody concentrations and peak parasitemias in the protected animals (26). Antibodies binding particular epitopes in RAP-1, such as iB-1, may have played a role in protecting these animals, but the RAP-1 epitopes recognized by the prechallenge antibodies were not mapped. The present study was initiated to further characterize anti-RAP-1 and anti-iB-1 antibody responses in animal models and to identify additional inhibitory anti-RAP-1 MAb.
Monkey immune sera screened for anti-RAP-11–294 and anti-iB-1 antibodies.
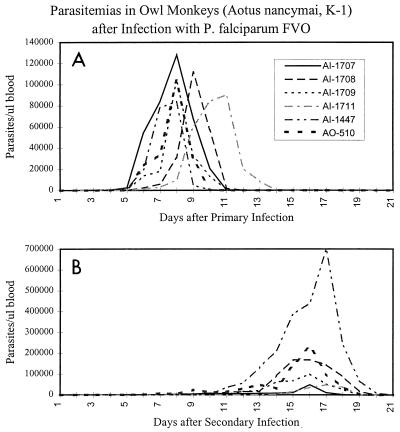
Owl monkeys were experimentally infected with P. falciparum to examine the relationship between immunity to P. falciparum and the anti-RAP-1 and anti-iB-1 antibody responses. Six naïve male splenectomized owl monkeys (Aotus nancymai, karyotype 1) were infected with blood-stage P. falciparum FVO (Vietnam Oak Knoll) isolate parasites by William Collins (Centers for Disease Control and Prevention). Splenectomized monkeys were used to promote their susceptibility to subsequent reinfection. The animals were drug treated (20 mg of quinine and 50 mg of mefloquine) 7 to 10 days after the first infection when the parasitemia exceeded 75,000 parasites/μl (∼2% parasitemia), and blood was drawn for serum 4 weeks after infection (Fig. 2A). The monkeys were reinfected with FVO-parasitized erythrocytes (RBC) 102 days after the initial infection (Fig. 2B). The monkeys were drug treated 16 days after secondary infection, and blood was drawn for serum 15 days later. Even with splenectomy, the monkeys exhibited an enhanced ability to control a secondary infection, as indicated primarily by a prolonged prepatent period (Fig. 2B).
FIG. 2.
Parasitemias of six owl monkeys following two experimental infections with P. falciparum. Parasitemias were determined from daily blood smears of individual monkeys. (A) After the first infection, monkeys were drug cured on day 7 (AI1707 and AI447), day 8 (AI1709 and AO510), day 9 (AI1708), and day 10 (AI1711). (B) All monkeys were drug cured 16 days after a second P. falciparum infection.
The primary anti-RAP-1 response in humans following infection by P. falciparum is to the first 294 amino acids of RAP-1 (RAP-11–294) (11). To measure antibody binding, RAP-11–294 was expressed in the form of recombinant protein A-2-2 (Fig. 1). The protein had a carboxy (C)-terminal 6-His tag protein to promote purification with chelated Ni+ and was expressed with the bacterial pQE70 expression system (Qiagen). The RAP-11–294 fragment was derived from the P. falciparum Honduras I/CDC RAP-1 gene (10) by amplification in a PCR with the sense primer 5′-GCGCTGCAGGCATGCGTTTCTATTTGGGTAGCTTAG-3′ (SphI site with initiation codon is underlined) and antisense primer 5′-CTTTCTTAAAAGGATTTAATTTACTG-3′. The PCR product was inserted between the SphI and BamHI sites of pQE70 (Qiagen) to produce the plasmid pA-2-2. DNA sequence analysis verified junctional sequences in the clone. A-2-2 was expressed as an ∼31-kDa protein that was purified by 250 mM imidazole elutions from Ni+-chelate agarose (Qiagen). While our purification procedure was similar to that of Stowers et al. (30), the Ni+-purified fraction contained predominantly one band (not shown) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
The purified A-2-2 protein was used in enzyme-linked immunosorbent assays (ELISA) to analyze anti-RAP-11–294 antibodies in the infected and normal owl monkey sera. ELISA were performed in triplicate with 50 μl of recombinant protein A-2-2 (0.5 μg of antigen/ml) per well. Monkey sera were used in ELISA after dilution in phosphate-buffered saline (PBS)–0.1% Tween 20–0.1% bovine serum albumin (BSA), followed by horseradish peroxidase (HRP)-conjugated protein A (10,000-fold dilution; Zymed). Bound HRP was detected with 2,2′- azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS; Zymed) and analyzed at 405 nm. These analyses show that monkeys infected with P. falciparum develop anti-RAP-11–294 antibodies (Fig. 3A). The anti-A-2-2 reactivities of all six infected monkey sera also increased greatly following reinfection of the monkeys (Fig. 3A). In comparison, normal monkey sera failed to bind A-2-2 (Fig. 3A). These results indicate that owl monkeys readily mount an anti-RAP-1 antibody response as a result of P. falciparum infection and that this response is boosted upon reinfection.
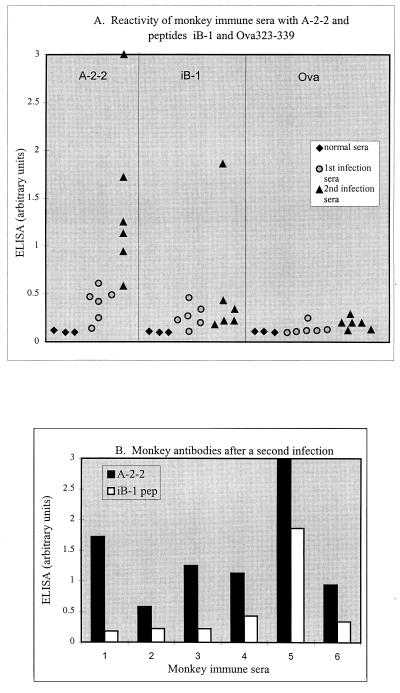
FIG. 3.
The binding of owl monkey antibodies to the recombinant RAP-1 fragment A-2-2, the peptide iB-1, and the unrelated peptide Ova323–339. (A) Scatter plots of antibody reactivities to the three antigens. The means of triplicate ELISA determinations of normal monkey serum (⧫), serum after the first infection with P. falciparum ( ), and monkey serum after a second infection (▴) (diluted 1:1,000) on the recombinant and peptide antigens are shown. The peptide FL-160#3 (not shown) produced results nearly identical to those of Ova323–339. (B) Comparison of anti-A-2-2 and anti-iB-1 antibody responses in individual monkeys. Shown are reactivities of individual monkey sera, obtained after a second P. falciparum infection, to recombinant protein A-2-2 (black) and peptide iB-1 (white). Serum samples 1 to 6 are from monkeys AI1707, AI1708, AI1709, AI1711, AI1447, and AO510, respectively. pep, peptide.
Antibodies to iB-1 (residues 200 to 211 of RAP-1) were also examined in these monkeys to determine whether a positive relationship exists between anti-iB-1 antibody responses and the development of immunity and protection from infection. Peptide iB-1 (Ac-CPKNTLTPL EELYPT-NH2) was synthesized by standard 9-fluorenylmethoxycarbonyl chemistry at Molecumetics (Bellevue, Wash.). The peptides Ova323–339 (ISQAVHAAHAEINEAGR) and FL160# (ATLTSDEQTVDPEERKDT) were used as irrelevant control peptides (gifts from A. LaFlamme, University of Washington). The peptides (50-μl peptide used at 1 μg/ml) were used to coat 96-well plates (Nunc Maxisorp), and ELISA were performed as described above. Some of the monkey sera obtained following an initial parasite infection had antibodies that appeared to specifically recognize peptide iB-1 (Fig. 3A). However, following a second infection, only two of the six monkeys (AI1711 and AI1447) exhibited an associated increase in antipeptide iB-1 reactivity (Fig. 3A and B). The precise basis for the pronounced anti-iB-1 antibody response in monkey AI1447 is uncertain, but may be related to a prolonged, elevated parasitemia in the monkey (6 days at >105 parasites/μl), illustrated in Fig. 2B. The results with iB-1 contrast with the superior boosting of antibody responses to other epitopes in RAP-11–294 observed in these monkeys (Fig. 3A and B). The relationship between anti-RAP-11–294 and anti-iB-1 antibody responses from each monkey was examined by Spearman rank correlation analysis and found to be not significant (rs = 0.086), supporting a conclusion that in these monkeys antibody responses to the iB-1 sequence was independent of antibody responses to other epitopes in a larger region (RAP-11–294) encompassing iB-1. These results argue against the hypothesis that iB-1 is an immunodominant epitope in the in vivo anti-RAP-1 response (9) during experimental infection. Recent experiments with human immune sera from Brazilian donors also fail to support the hypothesis that iB-1 is immunodominant (16a).
Mouse antibodies to RAP-11–294 within a recombinant RAP-1 fusion protein.
A majority of the anti-RAP-1 inhibitory MAb that have been identified map to the iB-1 peptide sequence (9, 28). With the goal of identifying additional inhibitory antibodies that bind within the region RAP-11–294, mice were immunized with the fusion protein maltose binding protein (MBP)–RAP-11–294. This protein consists of coding regions of the Escherichia coli MBP and RAP-11–294 with a 6-His tag (Fig. 1) and was expressed in greater relative yield than was A-2-2 (results not shown). The plasmid DNA expressing MBP–RAP-11–294 was constructed by blunting the SphI site of pA-2-2 with T4 DNA polymerase, excising the RAP-11–294 insert with BamHI, and ligating it into the XmnI and BamHI sites of the bacterial expression vector pMAL (New England Biolabs [NEB]). A 6-His tag sequence was added at the XbaI/PstI sites of pMAL, located 3′ of the RAP-11–294 sequence, using a synthetic adapter prepared from the oligonucleotides 5′-CTAGA TCTCATCACCATCACCATCACTAACTGCA-3′ (upper strand) and 5′-GTTAGTGATGGTGATGGTGATGAGAT- 3′ (lower strand). A clone expressing MBP–RAP-11–294 was identified after induction with 2 mM isopropyl-β-d-thiogalactopyranoside. The fusion protein was shown to be sequentially purified on amylose beads (NEB) and Ni+ chelating resin (Qiagen), indicating that the entire coding region was translated, including the 3′ His codons. Large-scale purification was accomplished by binding in batch to amylose beads after releasing protein from bacteria disrupted by freeze-thawing and sonication in column buffer (10 mM Tris-Cl, 200 mM NaCl, 1 mM EDTA). Protein was eluted from the amylose with 10 mM maltose in column buffer. SDS-PAGE analysis suggested that the partially purified MBP–RAP-11–294 preparation contained full-length as well as partial-length products (not shown). These products are probably produced from a combination of premature termination of translation and proteolysis of the translation products. To isolate full-length MBP–RAP-11–294 from this mixture, protein was dialyzed and solubilized in 8 M urea, bound to Ni+-chelate beads, and eluted in batch with 250 mM imidazole in 8 M urea–100 mM NaH2PO4–10 mM Tris-HCl (pH 6.3) buffer. The purified MBP–RAP-11–294 was essentially homogeneous for an ∼80-kDa band by SDS-PAGE (not shown). Purified, dialyzed protein was concentrated with Aquacide (Calbiochem) and redialyzed against PBS. Protein concentration was determined by a Coomassie protein assay (Pierce). The cleavage site for Factor Xa provided in the pMAL vector was not accessible in this fusion protein.
To produce anti-RAP-11–294 antibodies, Rb(8.12)5Bnr mice (BALB/cByJ-Rb5Bnr/J; Jackson Labs) were immunized with 55 μg of MBP–RAP-11–294 either intraperitoneally (i.p.) as a 1:1 emulsion in Freund’s complete adjuvant (Sigma) or subcutaneously as a 2:1 emulsion in Ribi adjuvant (Ribi International). The Freund’s mouse was given an i.p. booster injection with 80 μg of antigen in incomplete Freund’s adjuvant after 42 days and with antigen in PBS at 102 days. The Ribi mouse was given booster injections after 21 days with 35 μg of antigen in Ribi adjuvant and, after a further 21 days, with 35 μg of antigen in PBS. Three days after the final booster injection, blood was collected from both mice for serum samples. Sera from the immunized Freund’s mouse (not shown) and the Ribi mouse (Fig. 4, lane d) were shown to bind the RAP-1 fusion protein p82T1.24 on Western blots. p82T1.24 (Fig. 1) contained RAP-11–294 fused to the bacterial protein trpE instead of the MBP found in the immunizing protein MBP–RAP-11–294; the construction and expression of p82T1.24 as well as of p82T1.24ΔHindIII (Fig. 1; see below) have been described elsewhere (11). Equal numbers of washed splenocytes recovered from the Ribi-immunized mouse and FOX-NY cells were fused with polyethylene glycol and grown in microtiter plates containing a feeder layer of thymocytes pooled from several BALB/cByJ mice (Jackson) as described elsewhere (21). Selective medium containing AAT (31) was added after 3 days. Parental, unfused FOX-NY cells were dead after 7 days in medium containing AAT. Hybrids were initially screened by ELISA. Culture supernatants of ELISA-positive wells were then analyzed by immunoblot analysis with recombinant protein (see below) or immunofluorescence assay on fixed blood-stage parasites (15). Colonies producing antibodies reactive with parasite antigen were further expanded in growth medium containing AAT and cloned by limiting dilution. MAb 1D6 and 2D9, two MAb described in greater detail below, were of the immunoglobulin G1 (IgG1) isotype (Boehringer Mannheim Biochemicals [BMB] isotyping kit). Ascites containing MAb were produced in pristane-treated mice.
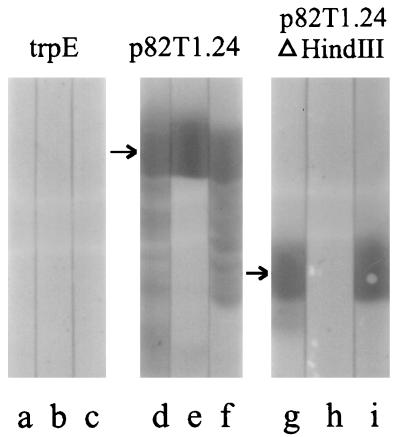
FIG. 4.
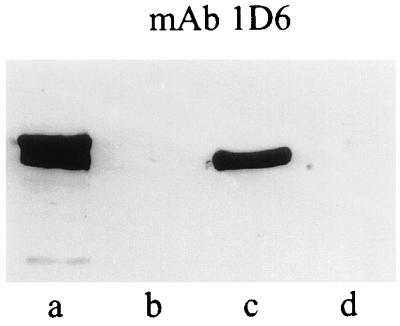
Binding of MAb 1D6 to recombinant RAP-1 on immunoblots. Nitrocellulose blots of E. coli pATH lysate containing trpE (lanes a to c) and the recombinant trpE-RAP-1 fusion proteins p82T1.24 (lanes d to f) and p82T1.24ΔHindIII (lanes g to i) were probed with polyclonal anti-MBP–RAP-11–294 mouse serum (lanes a, d, and g), 1D6 (lanes b, e, and h), and MAb 2D9 (lanes c, f, and i). Positive binding reactions are shown in lanes d to g and i. The polyclonal antiserum and MAb 2D9 also bind smaller molecules (lanes d, f, and g); these represent truncated forms of RAP-1 present in the bacterial lysate, probably resulting from incomplete translation and/or from proteolysis in the bacteria. The different binding specificities of MAb 1D6 and 2D9 (compare lanes e with f and lanes h with i) indicate that MAb 1D6 recognizes an epitope 3′ of the internal HindIII site in RAP-1 and that MAb 2D9 recognizes an epitope 5′ of the HindIII site.
The RAP-1 binding specificity of MAb from the resulting hybridomas was examined on immunoblots with two different RAP-1 recombinant proteins, p82T1.24 (containing RAP1–294) and p82T1.24ΔHindIII (containing the RAP-11–294 region of RAP-1). Nitrocellulose membranes containing the proteins were screened with a mouse primary antibody followed by HRP-goat anti-mouse antibody (Bio-Rad) and aminoethyl carbazole (Zymed) as chromogenic substrate. MAb 1D6 and 2D9 were representative of two of the binding specificities observed. Both MAb bound epitopes in the region containing residues 1 to 294 of p82T1.24 (Fig. 4, lanes e and f). However, 1D6 failed to bind p82T1.24ΔHindIII antigen (Fig. 4, lane h), suggesting that its epitope was missing from RAP-11–124. Polyclonal antibodies (in the sera of mice immunized with MBP-RAP-11–294) and MAb 2D9 also bound truncated molecules produced by bacteria expressing p82T1.24 (Fig. 4, lanes d and f), while 1D6 did not bind these smaller N-terminal fragments of p82T1.24 (lane e). These results indicate that the epitope for 1D6 is located between amino acids 124 and 294 (Fig. 4, lanes e and h) and that the epitope for 2D9 is between residues 1 and 124 (Fig. 4, lanes f and i). MAb 1D6 and 2D9 also bound parasite-derived RAP-1 products in immunoprecipitation assays, indicating that they recognize both native and denatured forms of the protein (10a).
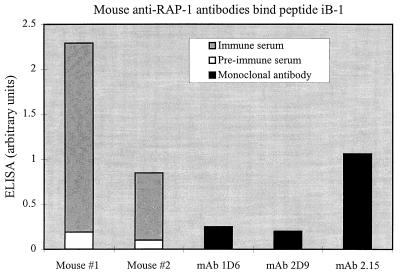
Our polyclonal and monoclonal anti-RAP-11–294 mouse antibodies were also screened on peptide iB-1 by ELISA to determine whether they contained antibodies that bind this epitope. The mouse antibodies were diluted in PBS–0.1% Tween 20–0.1% BSA and used to bind peptides, as described above for ELISA with monkey antibodies. Bound antibodies were detected with HRP-conjugated goat anti-mouse IgG heavy plus light chain (2,000-fold dilution; Bio-Rad) followed by ABTS. As a control, MAb 2.15, which binds the iB-1 epitope (9), was used in parallel. Figure 5 shows that these anti-RAP-11–294 immune sera contained antibodies that specifically recognized iB-1 and that these antibodies were absent from the preimmune sera (Fig. 5). Neither MAb 1D6 nor 2D9 bound the iB-1 peptide, even though 1D6 binds in the region of RAP-1 (residues 124 to 294) that contains the iB-1 epitope (Fig. 1), whereas 2.15 did bind (Fig. 5). Thus, antibodies are elicited to the iB-1 sequence in mice with a denatured recombinant fragment of RAP-11–294 as immunogen, and these responses are more pronounced than those in either immune sera from experimentally infected owl monkeys (present results) or sera from humans naturally infected with P. falciparum (16a).
FIG. 5.
Binding of mouse antibodies to the iB-1 peptide. ELISA were performed with plate-bound peptide iB-1. Shown are the means of triplicate determinations of preimmune (open bars) and immune (gray bars) sera diluted 1:2,000 from two mice and determinations with MAb 1D6, 2D9, and 2.15 diluted 1:1,000 (black bars). Mouse no. 1 was immunized with MBP–RAP-11–294 in Freund’s adjuvant, and mouse no. 2 received MBP–RAP-11–294 in Ribi adjuvant.
Inhibition of parasite invasion in vitro by the anti-RAP-1 MAb 1D6.
MAb 1D6 and 2D9 were tested by an in vitro RBC invasion assay to determine whether they inhibit RBC invasion. The knob-expressing Honduras I/CDC isolate of P. falciparum was used for these inhibition studies. Since MBP–RAP-11–294 contains Honduras-derived RAP-1 sequence, this provides a homologous system for testing these anti-RAP-1 antibodies. The parasites were cultured at 37°C in mixed gas with RTNGGS, an RPMI-based growth medium with 10% A+ human serum, and A+ or O+ human RBC as described elsewhere (14). Synchronous parasite cultures were produced and maintained with regular treatments of sorbitol and/or sedimentation in modified gelatin (18, 23, 29). Assays were performed in duplicate or triplicate wells of a microtiter plate with schizont-stage parasitized RBC at 0.5 to 3% starting parasitemia and 5% hematocrit in RTNGGS in a final volume of 100 μl per well. Cultures were supplemented with either no antibody or purified IgG. IgG was purified from ascites on protein A-Sepharose, concentrated with Aquacide, extensively dialyzed against PBS followed by serum-free parasite culture medium, and then filter sterilized. Control mouse IgG was purchased (Sigma). The protein concentration of the IgG solutions were determined by a Coomassie protein assay prior to use. The effects of the purified IgG preparations on parasite reinvasion were assessed by counting rings in thin blood smears after 24 h in culture. MAb 1D6 (125 μg/ml) inhibited parasite invasion by 61% relative to the control culture in the in vitro assay shown in Table 1 (experiment 1). This inhibition was statistically significant compared to medium alone, as judged by a two-tailed Student’s t test. The inhibition was not a nonspecific effect of IgG1 MAb, since MAb 2D9 appeared to increase invasion relative to medium alone. This increase might occur if antibody promotes binding of merozoites to RBC or increases adventitious protein-protein interactions. The invasion inhibition by 125 μg of 1D6 IgG/ml is consistent with inhibitory concentrations of other anti-RAP-1 antibodies (9, 22, 28). At 10 μg/ml, MAb 1D6 no longer inhibited invasion, relative to control IgG, while some enhancement of invasion was still observed with 2D9 (Table 1 [experiment 2]). Thus, MAb 1D6 inhibits parasite invasion of RBC and also fails to bind iB-1, suggesting that this mAb identifies iB-2, a second inhibitory epitope in RAP-1.
TABLE 1.
MAb 1D6 inhibits parasite growth in an in vitro assay
| Expt and treatment (μg/ml) | No
monocytes
|
With monocytes
|
||
|---|---|---|---|---|
| % Rings (± SE)a | % Inhibitionc | % Rings (± SE)a | % Inhibitionc | |
| 1 | ||||
| Medium | 6.50 (± 0.33)d | |||
| MAb 1D6 (125) | 3.55 (± 0.37)d | 61 | ||
| MAb 2D9 (125) | 7.30 (± 0.41) | −17 | ||
| 2 | ||||
| Medium | 7.8 (± 2.4) | 6.2 (± 0.49) | ||
| MAb 1D6 (10) | 7.5b | 5 | 7.1b | −23 |
| MAb 2D9 (10) | 9.9b | −35 | 9.9b | −95 |
| Normal IgG (10) | 7.1b | 13 | 7.1b | −23 |
Schizont-stage parasites were plated at initial parasitemia values of 1.7% (experiment 1) and 2.3% (experiment 2). After 24 h in culture, the percentage of parasites in the ring stage was determined for each sample.
Values are the means of two determinations.
Percent inhibition of invasion was calculated as follows: 100 − [100 × (final percent rings with antibody minus initial percent schizonts)/(final percent rings with medium alone minus initial percent schizonts)].
P < 0.05 by the two-tailed Student t test.
Human monocytes can act cooperatively in vitro with mouse, rabbit, or human antibodies to exert an antibody-dependent cellular inhibition of parasite growth (ADCI) (2, 17, 20). Moreover, monocytes have been shown to inhibit parasite growth in vitro in concert with some antibodies that alone do not inhibit invasion or parasite growth (2, 17, 20). This cooperative effect with monocytes depends on cytophilic antibodies and may explain the observation that cytophilic subclasses (IgG1 and mainly IgG3) predominate in protected individuals while noncytophilic types (IgG2 and IgM) are more abundant in various nonprotected subjects (3). Therefore, the postulate that monocytes could act synergistically with the anti-iB-2 MAb 1D6 or with MAb 2D9 to inhibit parasite invasion of RBC was tested. Some cultures containing IgG at 10 μg/ml were supplemented with fresh human monocytes (2 × 105 monocytes per well; 1 monocyte/200 RBC [20]) prepared from buffy coats by gradient centrifugation steps on Ficoll-Hypaque and Percoll. After 24 and 48 h, smears were taken from the cultures, and parasitemias were determined on Giemsa-stained smears by counting at least 2,000 RBC. We observed no inhibition of invasion or inhibition of parasite growth by the monocytes after either 24 h (Table 1) or 48 h (data not shown) in the presence of a noninhibitory concentration of either MAb (10 μg/ml), and monocytes did not modulate the 2D9-dependent enhancement of invasion. Tests with monocytes at higher MAb concentrations were not performed. These results suggest that the antibody-dependent inhibition of invasion or parasite growth by MAb 1D6 is not facilitated by monocytes. This lack of cooperative inhibition may occur because the RAP-1-bound 1D6 antibody is not accessible to monocyte receptors due to steric hindrance. Alternatively, heterogeneity in the affinity of the human FcγII receptor for mouse IgG1 (70% of humans have the responder phenotype, corresponding to a higher-affinity type of receptor, and 30% are nonresponders with a lower-affinity receptor [32]) may explain our observations. Until we have a complete understanding of the ADCI reaction, it will be difficult to distinguish among these possibilities.
Binding of MAb 1D6 to the iB-2 epitope in RAP-1.
The epitope iB-2, as defined by MAb 1D6, was shown to be located between residues 124 and 294 of RAP-1 and to be distinct from iB-1. To further localize iB-2, nested deletions from the C terminus of RAP-11–294 were produced, and the resulting recombinant proteins were probed with the MAb. These expression clones were prepared from p82T1.24 (Fig. 1) by linearizing the plasmid at the 3′ end of the RAP-1 coding region with BamHI and digesting DNA with BAL 31 nuclease (BMB) for 1, 2, and 3 min. Pooled DNAs from the three time points were agarose gel purified, blunted with T4 DNA polymerase (BMB) and deoxynucleoside triphosphates, and self-ligated. Nitrocellulose blots containing truncated RAP-1 proteins expressed from the resulting clones (11) were analyzed as described above. Mouse polyclonal anti-RAP-11–294 antibodies were used to confirm protein expression (not shown), and separate blots were incubated with MAb 1D6. Clones p82T1.24del6 (del6) and p82T1.24del11 (del11) were found to delineate the MAb 1D6 epitope. 1D6 bound a protein in clone del6 (Fig. 6, lane c) as well as in the parental clone p82T1.24 (lane a), but did not bind other deletion products (Fig. 6, lane d; data not shown). Clone del11 had the longest RAP-1 insert (restriction mapping data not shown) that failed to bind 1D6 (Fig. 6, lane d). DNA sequences of the RAP-1 inserts of the del11 and del6 plasmid DNAs were obtained after subcloning SspI-SspI restriction fragments generated from the plasmids into pBluescript. These analyses showed that the RAP-1 open reading frame of del11 terminated at Asp237 while that of del6 terminated at Leu262 (Fig. 1). These data map iB-2 between amino acids 238 and 262 in RAP-1, a site C terminal to the iB-1 sequence at amino acids 200 to 211 (Fig. 7). These results confirm our iB-1 peptide ELISA data, which suggested that iB-1 and iB-2 are distinct epitopes.
FIG. 6.
Deletion mapping of the epitope for MAb 1D6. Deletion constructs were prepared by BAL 31 nuclease digestion at the BamHI site of p82T1.24. Bacterial lysates with fusion proteins expressed from full-length p82T1.24 (lane a) and parental pATH vector (lane b) and from deletion clones del6 (lane c) and del11 (lane d) were screened by immunoblotting with MAb 1D6.
FIG. 7.
Amino acid sequence in the region of the N terminus of p67 including the locations of iB-1 and iB-2. An alignment of deduced amino acid sequences of the RAP-1 gene from P. falciparum laboratory isolates (Sierra Leone, Tanzania I/CDC, K1, and Honduras I/CDC) and the wild-type isolate India D (GenBank accession numbers L10322, L10323, M32853, M80807, and U41074, respectively) is given (11, 12, 27). The locations of the iB-1 and iB-2 epitopes are underlined, and the cleavage site responsible for the formation of p67 from the p82 precursor is marked (25). Sites of amino acid differences in this region are highlighted in boldface. Both Ala and Val were observed at X (position 184) in different genetic clones derived from the Sierra Leone isolate.
The data presented above identify iB-2, a second epitope for an inhibitory anti-RAP-1 MAb. Both iB-2 and iB-1 are located within the first amino-terminal 75 residues of the RAP-1-processing product p67 (Fig. 7). p67 is relatively abundant in purified free merozoites (10a) but is not observed in ring-stage parasites (15), indicating that p67 is secreted or degraded prior to ring formation. These observations together with the MAb inhibition data suggest that p67 performs a crucial function during merozoite invasion. What the function(s) of p67 might be is presently unknown. Another unsolved issue is how MAb that bind an organellar protein are capable of inhibiting RBC invasion. One possible explanation is that antibodies are accessible to p67 or its RAP-1 precursors within the rhoptries. Another explanation is that p67 may be transiently exposed to the medium at the apical surface of the invading merozoite during secretion of the contents of the rhoptries (4). Through either mechanism, an inhibitory anti-RAP-1 MAb may prevent invasion-related function(s) of p67 (or other RAP-1 polypeptides). One such function might be binding to a ligand or substrate of the parasite or host (19). A stimulatory MAb such as 2D9 in this case might bind and accelerate these interactions. An alternative target for the inhibitory antibodies might be prevention of p67 formation by sterically blocking cleavage of RAP-1. To do this, the MAb would gain entry to the merozoite just prior to or sometime after RBC lysis (9). Supporting this hypothesis is the observation that certain MAb which inhibit the processing of merozoite surface protein 1 (MSP1) also inhibit parasite invasion (1). Additional studies will be needed to clarify the mechanism(s) by which anti-RAP-1 antibodies inhibit invasion.
In summary, antibodies to RAP-11–294 are produced by mice after immunization with MBP–RAP-11–294 and in monkeys as a result of P. falciparum infection. Immunized mice made antibodies to iB-1 and a new inhibitory epitope, iB-2, which was identified with the anti-RAP-1 MAb 1D6. Both iB-1 and iB-2 are located near the N terminus of p67 and appear to be linear determinants (Fig. 7). There was no indication that the anti-iB-2 MAb 1D6 and monocytes cooperate to inhibit parasite growth in vitro. The iB-1 peptide does not appear to be immunodominant in owl monkeys as a result of P. falciparum infection. Consequently, since iB-1 and iB-2 appear to be suitable targets for antibody responses, it may be prudent to direct the antibody responses to these epitopes by reducing the lengths of RAP-1-derived immunogens. These two epitopes are also completely conserved among P. falciparum isolates examined to date (Fig. 7). Because any malarial vaccine will probably require periodic boosting of the immune response by natural infection with heterologous strains of the parasite, conserved sequences, such as iB-1 and iB-2, would be a distinct advantage over polymorphic antigens and would be expected to result in the successful boosting of the immune response by subsequent infections in the field.
Acknowledgments
We thank Michael Kahn for synthesis of the iB-1 peptide and Elizabeth Wayner for advice on hybridoma production. We gratefully acknowledge William Collins and his coworkers at the Centers for Disease Control for infecting monkeys and providing serum from infected owl monkeys, W. E. Collins for critical evaluation of the manuscript, and Susan G. Langreth for normal owl monkey serum. We also thank Cheryl Schmidt for assistance with expression cloning, protein purification, and parasite culture, Anne LaFlamme (University of Washington) for the control peptides Ova323–339 and FL-160#3, Jana McBride (University of Edinburgh) for MAb 2.15, and Marty Gibson for help with the graphics.
This work was supported by Public Health Service grant AI-32620 from the National Institute of Allergy and Infectious Disease and by the Royalty Research Fund from the University of Washington (to R.F.H.). Support for the monkey studies was provided by US-AID PASA no. STB-0453.23-P-HZ-00165-03 (CDC).
REFERENCES
- 1.Blackman M J, Scott-Finnigan T J, Shai S, Holder A A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:289–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparumblood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparummalaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bushell G R, Ingram L T, Fardoulys C A, Cooper J A. An antigenic complex in the rhoptries of Plasmodium falciparum. Mol Biochem Parasitol. 1988;28:105–112. doi: 10.1016/0166-6851(88)90057-6. [DOI] [PubMed] [Google Scholar]
- 5.Campbell G H, Miller L H, Hudson D, Franco E L, Andrysiak P M. Monoclonal antibody characterization of Plasmodium falciparumantigens. Am J Trop Med Hyg. 1984;33:1051–1054. doi: 10.4269/ajtmh.1984.33.1051. [DOI] [PubMed] [Google Scholar]
- 6.Chulay J D, Haynes J D, Diggs C L. Inhibition of in vitro growth of Plasmodium falciparumby immune serum from monkeys. J Infect Dis. 1981;144:270–278. doi: 10.1093/infdis/144.3.270. [DOI] [PubMed] [Google Scholar]
- 7.Clark J T, Anand R, Akoglu T, McBride J S. Identification and characterization of proteins associated with the rhoptry organelles of Plasmodium falciparummerozoites. Parasitol Res. 1987;73:425–434. doi: 10.1007/BF00538200. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, McGregor I A, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 9.Harnyuttanakorn P, McBride J S, Donachie S, Heidrich H-G, Ridley R G. Inhibitory monoclonal antibodies recognise epitopes adjacent to a proteolytic cleavage site on the RAP-1 protein of Plasmodium falciparum. Mol Biochem Parasitol. 1992;55:177–186. doi: 10.1016/0166-6851(92)90138-a. [DOI] [PubMed] [Google Scholar]
- 10.Howard R F. The sequence of the p82 rhoptry protein is highly conserved between two Plasmodium falciparumisolates. Mol Biochem Parasitol. 1992;51:327–330. doi: 10.1016/0166-6851(92)90083-v. [DOI] [PubMed] [Google Scholar]
- 10a.Howard, R. F., et al. Unpublished data.
- 11.Howard R F, Jensen J B, Franklin H L. Reactivity profile of human anti-82-kilodalton rhoptry protein antibodies generated during natural infection with Plasmodium falciparum. Infect Immun. 1993;61:2960–2965. doi: 10.1128/iai.61.7.2960-2965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard R F, Peterson C. Limited RAP-1 sequence diversity in field isolates of Plasmodium falciparum. Mol Biochem Parasitol. 1996;77:95–98. doi: 10.1016/0166-6851(96)02576-5. [DOI] [PubMed] [Google Scholar]
- 13.Howard R F, Reese R T. Plasmodium falciparum: Heterooligomeric complexes of rhoptry polypeptides. Exp Parasitol. 1990;71:330–342. doi: 10.1016/0014-4894(90)90038-e. [DOI] [PubMed] [Google Scholar]
- 14.Howard R F, Schmidt C M. The secretory pathway of Plasmodium falciparumregulates transport of p82/RAP-1 to the rhoptries. Mol Biochem Parasitol. 1995;74:43–54. doi: 10.1016/0166-6851(95)02481-6. [DOI] [PubMed] [Google Scholar]
- 15.Howard R F, Stanley H A, Campbell G H, Reese R T. Proteins responsible for a punctate fluorescence pattern in Plasmodium falciparummerozoites. Am J Trop Med Hyg. 1984;33:1055–1059. doi: 10.4269/ajtmh.1984.33.1055. [DOI] [PubMed] [Google Scholar]
- 16.Howard R J, Pasloske B L. Target antigens for asexual malaria vaccine development. Parasitol Today. 1993;9:369–372. doi: 10.1016/0169-4758(93)90085-t. [DOI] [PubMed] [Google Scholar]
- 16a.Jacobson, K. C., J. Thurman, C. M. Schmidt, E. Rickel, J. Oliviera de Ferreira, M. De Fátima Ferreira-Da-Cruz, C. T. Daniel-Ribeiro, and R. F. Howard. A study of antibody and T cell recognition of RAP-1 and RAP-2 recombinant proteins and peptides of Plasmodium falciparum in migrants and residents of the state of Rondonia, Brazil. Am. J. Trop. Med. Hyg., in press. [DOI] [PubMed]
- 17.Khusmith S, Druilhe P. Enhanced Plasmodium falciparummerozoite phagocytosis by monocytes from immune individuals. Infect Immun. 1982;35:874–879. doi: 10.1128/iai.35.3.874-879.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambros C, Vanderberg J P. Synchronization of Plasmodium falciparumerythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 19.Locher C P, Vandekerckhove F, Tam L Q. Three matrix metalloproteinases form a non-covalent association with the rhoptry-associated protein-1 of Plasmodium falciparum. Biochim Biophys Acta Prot Struct Mol Enzymol. 1992;1160:275–280. doi: 10.1016/0167-4838(92)90088-u. [DOI] [PubMed] [Google Scholar]
- 20.Oeuvray C, Bouharoun-Tayoun H, Gras-Masse H, Bottius E, Kaidoh T, Aikawa M, Filgueira M-C, Tartar A, Druilhe P. Merozoite surface protein-3: A malaria protein inducing antibodies that promote Plasmodium falciparumkilling by cooperation with blood monocytes. Blood. 1994;84:1594–1602. [PubMed] [Google Scholar]
- 21.Oi V T, Herzenberg L A. Immunoglobulin-producing hybrid cell lines. In: Mishell B B, Shiigi S M, editors. Selected methods in cellular immunology. W. H. New York, N.Y: Freeman and Co.; 1980. pp. 351–372. [Google Scholar]
- 22.Perrin L H, Ramirez E, Lambert P H, Miescher P A. Inhibition of P. falciparumgrowth in human erythrocytes by monoclonal antibodies. Nature. 1981;289:301–303. doi: 10.1038/289301a0. [DOI] [PubMed] [Google Scholar]
- 23.Reese R T, Langreth S G, Trager W. Isolation of stages of the human parasite Plasmodium falciparumfrom culture and from animal blood. Bull WHO. 1979;57:53–61. [PMC free article] [PubMed] [Google Scholar]
- 24.Reese R T, Motyl M R. Inhibition of the in vitro growth of Plasmodium falciparumI. The effects of immune serum and purified immunoglobulin from owl monkeys. J Immunol. 1979;123:1894–1899. [PubMed] [Google Scholar]
- 25.Ridley R G, Lahm H-W, Takács B, Scaife J G. Genetic and structural relationships between components of a protective rhoptry antigen complex from Plasmodium falciparum. Mol Biochem Parasitol. 1991;47:245–246. doi: 10.1016/0166-6851(91)90184-8. [DOI] [PubMed] [Google Scholar]
- 26.Ridley R G, Takacs B, Etlinger H, Scaife J G. A rhoptry antigen of Plasmodium falciparum is protective in Saimirimonkeys. Parasitology. 1990;101:187–192. doi: 10.1017/s0031182000063228. [DOI] [PubMed] [Google Scholar]
- 27.Ridley R G, Takacs B, Lahm H-W, Delves C J, Goman M, Certa U, Matile H, Woollett G R, Scaife J G. Characterisation and sequence of a protective rhoptry antigen from Plasmodium falciparum. Mol Biochem Parasitol. 1990;41:125–134. doi: 10.1016/0166-6851(90)90103-s. [DOI] [PubMed] [Google Scholar]
- 28.Schofield L, Bushell G R, Cooper J A, Saul A J, Upcroft J A, Kidson C. A rhoptry antigen of Plasmodium falciparumcontains conserved and variable epitopes recognized by inhibitory monoclonal antibodies. Mol Biochem Parasitol. 1986;18:183–195. doi: 10.1016/0166-6851(86)90037-x. [DOI] [PubMed] [Google Scholar]
- 29.Stoll H R, Nitschmann H. Succinylated gelatin as a plasma substitute. Bibl Haematol. 1969;33:81–95. doi: 10.1159/000384831. [DOI] [PubMed] [Google Scholar]
- 30.Stowers A, Prescott N, Cooper J, Takacs B, Stueber D, Kennedy P, Saul A. Immunogenicity of recombinant Plasmodium falciparumrhoptry associated proteins 1 and 2. Parasite Immunol. 1995;17:631–642. doi: 10.1111/j.1365-3024.1995.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 31.Taggart R T, Samloff I M. Stable antibody-producing murine hybridomas. Science. 1983;219:1228–1280. doi: 10.1126/science.6402815. [DOI] [PubMed] [Google Scholar]
- 32.Tax W J, Hermes F F, Willems R W, Capel P J, Koene R A. Fc receptors for mouse IgG1 on human monocytes: polymorphism and role in antibody-induced T cell proliferation. J Immunol. 1984;133:1185–1189. [PubMed] [Google Scholar]