ABSTRACT
Background: PTSD is a significant mental health problem worldwide. Current evidence-based interventions suffer various limitations. Ketamine is a novel agent that is hoped to be incrementally better than extant interventions.
Objective: Several randomized control trials (RCTs) of ketamine interventions for PTSD have now been published. We sought to systematically review and meta-analyse results from these trials to evaluate preliminary evidence for ketamine’s incremental benefit above-and-beyond control interventions in PTSD treatment.
Results: Omnibus findings from 52 effect sizes extracted across six studies (n = 221) yielded a small advantage for ketamine over control conditions at reducing PTSD symptoms (g = 0.27, 95% CI = 0.03, 0.51). However, bias-correction estimates attenuated this effect (adjusted g = 0.20, 95%, CI = −0.08, 0.48). Bias estimates indicated smaller studies reported larger effect sizes favouring ketamine. The only consistent timepoint assessed across RCTs was 24-hours post-initial infusion. Effects at 24-hours post-initial infusion suggest ketamine has a small relative advantage over controls (g = 0.35, 95% CI = 0.06, 0.64). Post-hoc analyses at 24-hours post-initial infusion indicated that ketamine was significantly better than passive controls (g = 0.44, 95% CI = 0.03, 0.85), but not active controls (g = 0.24, 95% CI = −0.30, 0.78). Comparisons one-week into intervention suggested no meaningful group differences (g = 0.24, 95% CI = 0.00, 0.48). No significant differences were evident for RCTs that examined effects two-weeks post initial infusion (g = 0.17, 95% CI = −0.10, 0.44).
Conclusions: Altogether, ketamine-for-PTSD RCTs reveal a nominal initial therapeutic advantage relative to controls. However, bias and heterogeneity appear problematic. While rapid acting effects were observed, all control agents (including saline) also evidenced rapid acting effects. We argue blind penetration to be a serious concern, and that placebo is the likely mechanism behind reported therapeutic effects.
KEYWORDS: Ketamine, PTSD, randomized control trials, meta-analysis, bias
HIGHLIGHTS
We systematically reviewed and meta-analysed all randomized control trials of ketamine intervention for PTSD.
While ketamine was associated with a reduction in symptoms, the effect was generally not stronger than control conditions.
By two-weeks post-initial infusion, no meaningful differences are evident between ketamine and controls.
Abstract
Antecedentes: El TEPT es un importante problema de salud mental en todo el mundo. Las intervenciones actuales basadas en la evidencia adolecen de varias limitaciones. La ketamina es un agente novedoso que se espera sea cada vez mejor que las intervenciones existentes.
Objetivo: Varios estudios controlados aleatorizados (ECAs) de intervenciones con ketamina para TEPT han sido actualmente publicados. Intentamos hacer una revisión y metanálisis sistemático de los los resultados de estos estudios para evaluar la evidencia preliminar del beneficio incremental de la ketamina por sobre y más allá de las intervenciones de control en el tratamiento del TEPT.
Resultados: Los resultados generales de 52 tamaños del efecto extraídos entre seis estudios (n = 221) arrojaron una pequeña ventaja para la ketamina sobre las condiciones de control en la reducción de síntomas de TEPT (g = 0.27, 95% IC = 0.03, 0.51). Sin embardo, las estimaciones de corrección de sesgo atenuaron este efecto (g ajustado = 0.20, 95% IC = 0.08, 0.48). Las estimaciones de heterogeneidad indicaron que estudios más pequeños informaron tamaños de efecto más grande favoreciendo a la ketamina. El único momento consistente evaluado en los ECAs fue a las 24 hrs post infusión inicial. Los efectos a las 24 horas post infusión inicial sugieren que la ketamina tiene una pequeña ventaja relativa sobre los controles (g = 0.35, 95% IC = 0.06, 0.64). Los análisis post-hoc a las 24 horas post infusión inicial indicaron que la ketamina fue significativamente mejor que los controles pasivos (g = 0.44, 95% IC = 0.03, 0.85), pero no con los controles activos (g = 0.24, 95% IC = 0.30, 0.78). Las comparaciones una semana después de la intervención sugirieron que no había diferencias significativas entre los grupos (g = 0.24, 95% IC = 0.00, 0.48). No hubo diferencias significativas evidentes para los ECAs que examinaron los efectos dos semanas después de la infusión inicial (g = 0.17, 95% IC = 0.10, 0.44).
Conclusiones: En conjunto, los ECAs de ketamina para el TEPT revelan una ventaja terapéutica inicial nominal en relación con los controles. Sin embargo, los sesgos y la heterogeneidad parecen ser problemáticos. Si bien se observaron efectos de acción rápida, todos los agentes de control (incluyendo solución salina) también evidenciaron efectos de acción rápida. Argumentamos que la penetración ciega es una preocupación seria y el placebo es el mecanismo probable detrás de los efectos terapéuticos reportados.
PALABRAS CLAVE: Ketamina, TEPT, ensayos de control aleatorizados, metanalisis, sesgo
Post-Traumatic Stress Disorder (PTSD) is a prolonged adverse psychological reaction to a traumatic event. The lifetime prevalence of PTSD ranges from 3.4–26.9% in civilians, and 7.7–17% among military personnel in the United States (Schein et al., 2021). While evidence-based interventions for PTSD are continually evolving, psychotherapy remains a popular option with robust scientific support (Benish et al., 2008; Steenkamp et al., 2015). However, psychological treatments typically require considerable time and financial investments. Moreover, exposure with response prevention, which is often considered the best treatment for PTSD (Rauch & Ruzek, 2012), is often associated with short-term increases in symptomology that may contribute to attrition (Schnurr et al., 2022).
Given psychotherapy’s limitations, many providers recommend adjunctive psychopharmacotherapy. Currently, paroxetine and sertraline (both selective serotonin reuptake inhibitors; SSRIs) are approved for PTSD intervention by the United States Food and Drug Administration (FDA). While being relatively affordable and accessible, they are associated with a range of negative side effects (e.g. sexual dysfunction, suicidal thoughts) that contribute to early discontinuation of treatment (Marazziti et al., 2019; Wang et al., 2018). Moreover, the SSRI programme of research has been associated with a number of criticisms, including poor action mechanism validity (Moncrieff et al., 2022) and suspicion of placebo-driven therapeutic effects (Cuijpers & Cristea, 2015).
Many other off-label pharmaceuticals (e.g. prazosin for nightmares, and risperidone for mood regulation) are regularly employed to treat PTSD (Krystal, Davis, et al., 2017). These agents show limited therapeutic benefit for PTSD symptoms in modern placebo-controlled randomized control trials (RCTs; e.g. Krystal et al., 2011; Raskind et al., 2018). The ongoing difficulties in finding valid therapeutic psychoactive agents for PTSD has led some researchers to question the entire therapeutic premise (i.e. the ‘PTSD pharmacotherapy crisis’), inspiring requests for new agents that act on PTSD-specific mechanisms (Abdallah et al., 2019; Krystal, Davis, et al., 2017).
Consequently, there has been a shift within psychiatry to identify novel agents that can withstand scientific scrutiny (Begola & Schillerstrom, 2019; Stein & Simon, 2021). This shift has been associated with a re-examination of psychedelic agents for PTSD, including 3,4-methylenedioxymethamphetamine (MDMA/‘ecstasy’), psilocybin (‘magic mushrooms’), and ketamine (‘Special-K’; Varker et al., 2021). Ketamine in particular has been explored in a number of cohort trials, case studies, and RCTs (Artin et al., 2022; Dames et al., 2022; D’Andrea & Andrew Sewell, 2013).
Ketamine is a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist and is generally classified as a dissociative agent. The therapeutic action mechanisms of ketamine on PTSD are generally theoretical (Stein & Simon, 2021), though it is thought that ketamine might disrupt the fear-response associated with traumatic memories (Asim et al., 2021; Feder et al., 2022) and/or aid in restoring synaptic connectivity that is potentially disrupted in PTSD-afflicted individuals (Krystal, Abdallah, et al., 2017). While the precise mechanisms are unknown, initial arguments for ketamine therapy for PTSD come from the depression interventional literature (Katalinic et al., 2013; Murrough et al., 2013). That is, the basic therapeutic model is that if ketamine relieves depression, it might also relieve PTSD. Though recent findings challenge this premise (Chen et al., 2023; de Laportalière et al., 2023).
Despite increased empirical attention of ketamine as a PTSD intervention, there are only a few published meta-analyses that synthesize outcomes from PTSD intervention studies (Du et al., 2022; Whittaker et al., 2021; Yousefifard et al., 2020). Du et al. (2022) examined RCTs (k = 5), case–control (k = 3), and cohort (k = 2) trials and found that ketamine treatment was not associated with PTSD incidence nor meaningful change in PTSD indicators, though their study had several limitations. Their data presentation was challenging to interpret and their findings conflate different study designs in their analyses. Additionally, more recent data, including the largest RCT examining the therapeutic potential of ketamine for PTSD (Abdallah et al., 2022), was not included in their meta-analysis. Similarly, Yousefifard et al. (2020) examined ketamine intervention, but primarily studies in which ketamine was used to treat pain in patients suffering from physical trauma (e.g. physical burns), not PTSD. Finally, Whittaker et al. (2021) meta-analysed six RCTs that utilized ketamine as an intervention for anxiety-related psychological problems. However, only three of these RCTs examined PTSD outcomes. Their findings suggest ketamine is marginally better than controls. Notably, the confidence interval around their omnibus odds ratio is sizable (OR = 2.03; 95% CI = 0.67–6.15; p = .21) suggesting low power (Whittaker et al., 2021).
Here, we sought to clarify the state of the ketamine-for-PTSD research via systematic review and meta-analysis. To do this, we strictly examined RCTs involving ketamine and control conditions to determine the incremental efficiency of this intervention. Therein, we examined how the incremental benefit might change over time, as ketamine is often purported to have rapid acting effects (Abdallah et al., 2015; Riggs & Gould, 2021). While case-series and single-condition cohort trials are useful in preliminary contexts, we were solely interested in RCTs due to their rigour. A meta-analytic approach is beneficial as effect sizes can be aggregated across observations; thus, getting a better estimate of the true therapeutic effect. Indeed, all ketamine-for-PTSD RCTs to date have involved relatively small samples. Given small samples may bias effects, meta-analysis may be useful for better determining efficacy (while estimating and adjusting for bias) in preparation for larger RCTs. Moreover, despite the emerging status of ketamine as a PTSD intervention, we considered the literature to be large enough for review. Indeed, past summaries of the Cochrane Library have reported that the median number of studies per meta-analysis across the wider field of medicine to be as low as three (Davey et al., 2011).
Taken together, we believe intervention science can be greatly aided by meta-analysis of the extant ketamine for PTSD RCTs, with accompanied systematic review of study features to help clarify the efficacy of ketamine for PTSD intervention.
1. Methods
1.1. Search strategy
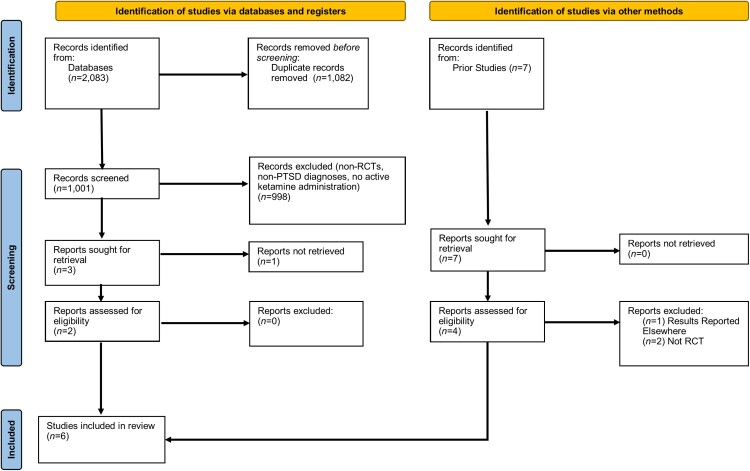
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021) guidelines were followed for this study (see Figure 1 for study selection flowchart). The databases used to gather the articles were Medline, PsycINFO, ClinicalTrials.gov, Alt HealthWatch, CINAHL Complete, PsycARTICLES, JSTOR, PTSDpubs, PubMed, Web of Science, SpringerLINK, Gale Health and Wellness, and Scopus. Terms ‘PTSD’ or ‘Post-Traumatic Stress Disorder’, were paired with ‘Ketamine’, and ‘Randomized Controlled Trial’ or ‘RCT’. Search terms were identical per search engine. Searches were conducted iteratively per term. For instance, ‘PTSD’ with ‘Ketamine’ with ‘Randomized Control Trial’ was followed by ‘PTSD’ with ‘Ketamine’ with ‘RCT’. The search interval spanned all years up until September 2023. The formal search was complemented by an informal search of Google Scholar and past systematic review citations (e.g. Du et al., 2022). The Google Scholar searches involved utilizing the same search combinations and review of up to 100 studies per term. However, we did not include the Google Scholar estimates in flowchart as each search term was associated with an unusually large number of selections (e.g. the search ‘ketamine PTSD RCT’ returns 17,400 titles) most of which appeared irrelevant to inclusion criteria beyond the first initial 50 titles.
Figure 1.
PRISMA flow chart.
1.2. Data extraction and study selection criteria
After completing the initial literature search, titles and abstracts were assessed. If the abstract suggested the study would meet inclusion criteria, the study was read by team members. Studies were then evaluated against inclusion criteria. Specifically, studies had to include adult human participants (18+), a psychometrically validated measure of PTSD, ketamine intervention, RCT design, and be published in a peer-review outlet. Exclusion criteria were human participants under 18 years old, absence of validated PTSD measures, no ketamine administration, non-RCT design, and non-peer review outlet. Studies that met inclusion criteria were then coded for quality and had means, standard deviations, n’s, and basic demographic information extracted for analyses. All studies had three quality coding raters (TO, DJ, JV). Any coding discrepancies were resolved via group discussion (none were evident). The first author did not have input on quality coding/extraction besides determining initial criteria. Non-English studies were not excluded but all studies that met inclusion criteria were English language.
1.3. Quality coding
Study quality was assessed via the National Institute of Health Study Quality Assessment of Controlled Intervention Studies (NHLBI, 2021). All studies were considered to have ‘good’ quality (for details, see Supplementary File 1).
1.4. Data analysis
Data were analysed using Comprehensive Meta-Analysis (v4) software (Borenstein et al., 2013). All analyses were modelled under random effects. Hedges’ g was selected as the index of effect size in between group analyses to adjust for small sample sizes (Zakzanis, 2001). Values were interpreted as |0.2| = small, |0.5| = medium, and |0.8| = large, where larger values represent a greater mean difference of within-group change between conditions in standard deviation units, with positive values representing a greater therapeutic effect in the ketamine conditions relative to controls (i.e., great PTSD reduction relative to controls). Cohen’s davg was used to help contextualize findings for studies with many timepoint measurements. Cohen’s davg was used in place of the traditional Cohen’s dwithin as all authors failed to report bivariate correlations between pre- and post-measures across timepoints. As such, we used Cohen’s davg in which coefficients are standardized by taking the average standard deviation of the repeated measures (Lakens, 2013).
Between-study heterogeneity in effects was assessed with Cochran’s Q test and the I2 statistic, where a non-significant Q and low I2 (i.e. < 30%) suggest variability in effects may be due to sampling error and not between-study differences (Higgins et al., 2019). Heterogeneity was further investigated when there was a significant Q test (p < .10) and I2 > 30%, but interpreted with caution given the relatively small empirical body (Von Hippel, 2015). Moreover, we only pursued heterogeneity statistics when k ≥ 3 studies were available for sub-analyses. Omnibus publication bias was assessed using Egger’s regression test, which regresses the effect sizes on the inverse of the standard error (Higgins et al., 2019). Duval and Tweedie’s (2000) Trim-and-Fill method was used to identify evidence of missing studies due to publication bias.
1.5. Data availability
Extracted data sheets are available on the Open Science Framework [https://osf.io/mtfdx/].
2. Results
2.1. Study sample characteristics
The initial search identified 131 records, of which k = 6 studies with n = 221 participants, and k = 52 effect sizes (keffects) met inclusion criteria. One study had a subgroup of low dosage ketamine (Abdallah et al., 2022), which was treated as its own condition for omnibus analyses (k = 7 condition comparisons). PTSD measures included the PTSD Checklist for DSM-5 (PCL-5; Blevins et al., 2015), the Impact of Events Scale-Revised (IES-R; Weiss, 2004), and the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5; Weathers et al., 2018). Three of the studies used active controls (keterolac and midazolam), while the rest used a saline solution (i.e. passive control). The ketamine dose for each study was 0.5 mg/kg, although the study conducted by Abdallah et al. (2022) also had a condition that received 0.2 mg/kg. Only two (33%) were double blinded, three (50%) did not have similar between-group baselines, and two (33%) had attrition rates over 20%. The two studies conducted by Feder et al. (2014, 2021) reported conflicts of interest (involving multiple funding opportunities/ketamine patents). All studies were pre-registered and conducted in the United States. One study utilized a cross-over RCT design (Feder et al., 2014). As such, we calculated effects prior to the cross-over. Study characteristic information can be found in Table 1.
Table 1.
Study characteristics.
| Reference | n | Age (Mean) | Percent Male | Percent White | Treatment Length | Funding? | Sample | Financial COI | Psychotherapy Confound | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ketamine | Control | |||||||||
| Abdallah et al. (2022) | 51 | 54 | 42.6 | 57.25 | NS | 8 weeks | Yes | Veterans | No | NS* |
| Dadabayev et al. (2020)§ | 11 | 10 | 42.7 | 62 | 95 | 1 week | No | Individuals w/Chronic Pain | No | NS** |
| Feder et al. (2021) | 15 | 15 | 38.9 | 23.3 | 53.33 | 2 weeks | Yes | Community | Yes | n = 9 in K, n = 8 C |
| Feder et al. (2014) | 19 | 16 | 36.05 | 54.65 | 31.6 | 2 weeks | Yes | Community | Yes | NS |
| Pradhan et al. (2018) | 10 | 10 | 40.7 | 40 | NS | Until Relapse | Yes | Community | No | All Enrolled |
| Pradhan et al. (2017) | 5 | 5 | 43 | 30 | NS | Until Relapse | Yes | Community | No | All Enrolled |
Note: NS = Not Specified, COI = Conflict of Interest, K = ketamine condition, C = control condition. § Dadabayev et al. (2020) also included conditions involving patients with chonic pain without PTSD. The participants were not included in meta-analyses. *were unmedicated or were stable on an antidepressant for at least 4 weeks or PTSD-focused psychotherapy for at least 6 weeks (p. 1575) **Participants engaged in psychotherapy for PTSD symptoms were required to have sessions that were stable in frequency and duration for at least 6 weeks prior to beginning of the study (p. 2).
2.2. Main analyses
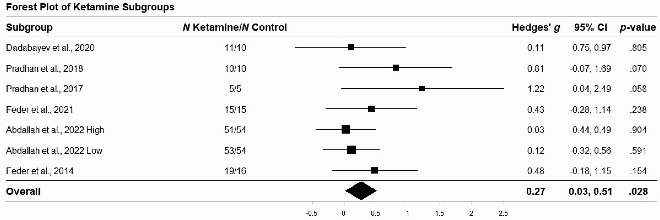
An omnibus meta-analysis (i.e. across all the studies, PTSD measures, and timepoints) yielded a small-magnitude positive effect of ketamine on PTSD symptoms relative to control interventions (g = 0.27, 95% CI = 0.03, 0.51, keffects = 52). See Table 2 for forest plot of omnibus/across-study effect size estimates. While not a single mixed effect was significant for any individual study, when all data were aggregated, a significant effect emerged. We believe this is likely a function of increased statistical power.
Table 2.
Forest plot of ketamine subgroups.
 |
2.3. Publication bias analyses
Omnibus publication bias across subgroups revealed a significant association between Hedges’ g and standard error (Egger’s t(5) = 3.41, p = .019), such that smaller studies tended to yield larger effects that favored ketamine. Trim and Fill procedure further estimated two missing studies, and imputation of these attenuated the omnibus effect to a non-significant level (adjusted g = 0.20 95% CI = −0.08, 0.48; see Supplementary file 2 for funnel plot of adjusted effects).
2.4. 24-hours post initial infusion
Effect sizes for each subgroup at 24-hours post-initial infusion can be found in Table 3.
Table 3.
Effects of ketamine 24-hours post initial infusion.
| Reference | Scale | Control | Ketamine Dose (mg/kg) | n | Number of Doses | Hedges’ g | Standard Error | |
|---|---|---|---|---|---|---|---|---|
| Ketamine | Control | |||||||
| Abdallah et al. (2022) | PCL-5 | Saline | 0.2 | 53 | 54 | 1 | 0.15 | 0.2 |
| 0.5 | 51 | 0.33 | 0.2 | |||||
| Dadabayev et al. (2020) | IES-R | Keterolac | 0.5 | 11 | 10 | 1 | −0.25 | 0.44 |
| Feder et al. (2021) | IES-R | Midazolam | 0.5 | 15 | 15 | 1 | −0.12 | 0.36 |
| Feder et al. (2014) | IES-R | Midazolam | 0.5 | 19 | 16 | 1 | 0.70* | 0.32 |
| Pradhan et al. (2018) | PCL-5 | Saline | 0.5 | 10 | 10 | 1 | 0.74 | 0.44 |
| CAPS-5 | 0.72 | 0.44 | ||||||
| Pradhan et al. (2017) | PCL-5 | Saline | 0.5 | 5 | 5 | 1 | 1.73* | 0.69 |
| CAPS-5 | 1.72* | 0.69 | ||||||
| Total Random Effect | 0.35* | 0.15 | ||||||
| Total Random Effect Vs Active Control | 0.24 | 0.28 | ||||||
| Total Random Effect Vs Passive Control | 0.44* | 0.21 | ||||||
*p < .05.
Meta-analysis of these effects resulted in a small positive effect for ketamine on PTSD symptoms at 24-hours post-infusion compared to controls (g = 0.35, 95% CI = 0.06, 0.64, keffects = 9). Overall, the effects ranged from extremely small (and negative) to extremely large. In this vein, the aggregated effect was somewhat heterogenous (Q(6) = 9.18, p = .164, I2 = 34.66% [95%CI: 0, 64.5]), but should be interpreted with caution due to the small k size, and large CI (this note applies to all following heterogeneity statistics). The only three significant effects were for the PCL-5 (g = 1.73, p = .012) and CAPS-5 (g = 1.72, p = .012) from Pradhan et al. (2017), and IES-R (g = 0.70; p = .027) from Feder et al., 2014. These effects were moderate-to-large, indicating that ketamine had a substantial effect at reducing PTSD symptoms compared to control groups (saline in Pradhan et al., 2017; and midazolam in Feder et al., 2014). However, two studies revealed small, non-significant, inverse effects (i.e. the control did better than ketamine) at 24-hour follow-up. They were from Dadabayev et al. (2020) (g = −0.03, p = .955) and Feder et al. (2021) (g = −0.12, p = .729) which both used the IES-R as the symptom outcome measure.
Based off these initial observations, we conducted follow-up post-hoc meta-analyses split by active and passive (saline) controls. When compared against a passive control, ketamine treatment was significantly better at reducing PTSD symptoms at 24-hours (g = 0.44, 95% CI = 0.03, 0.85). However, we failed to observe a significant difference when ketamine was tested against an active pharmacological control (g = 0.24, 95% CI = −0.30, 0.78). Additionally, Feder et al. (2014) reported subscales of the IES-R, finding the hyperarousal subscale had a significant difference compared to control condition (Mean Difference = 2.6, 95% CI:[0.2, 4.9]), but no other subscale relative to controls (Intrusion Mean Difference = 2.6 95% CI:[−0.8, 6.0]; Avoidance Mean Difference = 3.3 95% CI:[−0.7, 6.8]).
2.5. One-week post initial infusion
Effect sizes for all studies with a seven-day timepoint post-initial infusion are included in Table 4. Omnibus results indicated a small random effect that approached significance (g = 0.24, 95% CI:[0.00, 0.48], keffects = 6). The heterogeneity statistics suggested a wide potential for heterogeneity (Q(4) = 3.43, p = .489, I2 = 0.00% [95% CI: 0, 81]). When examining groups who received three administrations,1 a small non-significant effect size was observed (g = 0.25, 95% CI:[−0.10, 0.59]). We also examined groups that received only one administration. A small non-significant effect size was observed (g = 0.35, 95% CI:[−0.20, 0.89]).
Table 4.
Effects of ketamine one week post initial infusion.
| Reference | Scale | Control drug | Ketamine dose (mg/kg) | n | Number of doses | Hedges’ g | Standard error | |
|---|---|---|---|---|---|---|---|---|
| Ketamine | Control | |||||||
| Abdallah et al. (2022) | PCL-5 | Saline | 0.2 | 47 | 48 | 3 | 0.18 | 0.20 |
| 0.5 | 43 | 0.06 | 0.21 | |||||
| Dadabayev et al. (2020) | IES-R | Keterolac | 0.5 | 11 | 10 | 1 | 0.19 | 0.44 |
| Feder et al. (2021) | CAPS-5 | Midazolam | 0.5 | 15 | 15 | 3 | 0.80* | 0.37 |
| Feder et al. (2014) | IES-R | Midazolam | 0.5 | 19 | 16 | 1 | 0.15 | 0.34 |
| CAPS-5 | 0.70* | 0.34 | ||||||
| Total Random Effect | 0.24* | 0.12 | ||||||
*p < .05.
2.6. Two-weeks post initial infusion
Effect sizes after 14 days post-initial infusion are presented in Table 5. Omnibus results indicated a small non-significant aggregated effect size (g = 0.17, 95% CI:[−0.10, 0.44], keffects = 4). Of all observations, there was one significant effect from Feder et al. (2021) on the CAPS-5 (g = 0.92, p = .014). This effect follows three total ketamine infusions over the course of 14 days and indicates a relatively large improvement in severity of PTSD symptoms compared to the control group. Conversely, Abdallah et al. (2022) observed a small and non-significant effect at 14-days after five infusions for their low (0.2 mg/kg) ketamine condition compared to controls (g = 0.12, p = .576) and their high (0.5 mg/kg) ketamine condition compared to controls (g = 0.10, p = .652). While not formally two weeks, Feder et al. (2014) took final measurements between 10 and 13 days post single infusion. These were not included in our 14-day-post-initial-infusion statistics. However, we did calculate them for comprehensive reporting and found a small, non-significant, inverse effect for the IES-R (g = −0.06, p = .860) and a large positive effect for the CAPS-5 (g = 0.71, p = .038). This suggests that ketamine may outperform an active control condition (midazolam) in clinician-rated PTSD criteria, but is non-significant when considering patient self-report.
Table 5.
Effects of ketamine two weeks post initial infusion.
| Reference | Scale | Control drug | Ketamine dose (mg/kg) | n | Number of doses | Hedges’ g | Standard error | |
|---|---|---|---|---|---|---|---|---|
| Ketamine | Control | |||||||
| Abdallah et al. (2022) | PCL-5 | Saline | 0.2 | 48 | 44 | 5 | 0.12 | 0.21 |
| 0.5 | 43 | 0.10 | 0.21 | |||||
| Feder et al. (2021) | CAPS-5 IES-R |
Midazolam | 0.5 | 15 | 15 | 3 | 0.92* 0.12 |
0.38 0.36 |
| Total Random Effect | 0.17 | 0.14 | ||||||
*p < .05.
2.7. Time until relapse after initial infusion
Pradhan et al. (2017) and Pradhan et al. (2018) continued to evaluate participants following initial ketamine infusion until individuals relapsed. Results from these studies are included in Supplementary File 3. Pradhan et al. (2017, 2018) defined relapse as total scores >50 for the CAPS-5 and >51 for the PCL-5. In Pradhan et al. (2017), the average time to relapse was 33 days (SD = 22.98) for the ketamine group and 25 days (SD = 16.8) for the control group (non-active saline for both studies). For Pradhan et al. (2018), the average time to relapse was 34.44 days (SD = 19.12) for the ketamine group and 16.5 days (SD = 11.39) for the control group.
Omnibus results indicated a large random effect (g = 1.01, 95% CI: [0.30, 1.73], keffects = 4). This suggests that when participants relapsed, they had lower (but above threshold) PTSD scores in the ketamine condition compared to those in the passive control (saline) condition. Effect sizes at relapse varied widely from small to large (g’s ranging from 0.38 to 1.03); however only one effect, from Pradhan et al. (2018), was significant for the PCL-5 (g = 1.03, p = .025).
2.8. Within group changes for additional timepoints
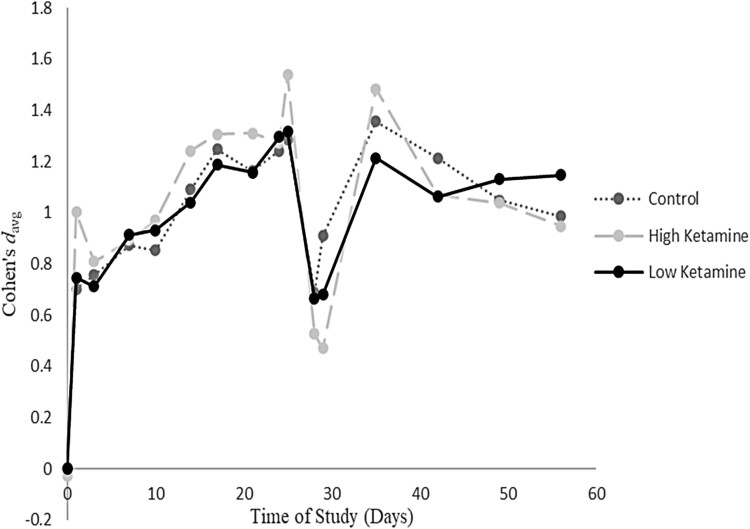
To further breakdown the effects, we analysed Abdallah et al. (2022) results with eight administrations across 24-days and with measurements until day 56 post-initial infusion. In Figure 2, we plot the change in Cohen’s davg across time for all three groups, where the effect was compared to the baseline for each group (see Supplementary File 4 for specific coefficients). We chose to plot their reported findings, as they were the only study to include many observations for a sustained period of time. As observed in Figure 2, trajectories across groups appeared similar regardless of intervention. The other study with many timepoint measurements was Feder et al. (2021). However, due to reporting, we were only able to analyse their participants baseline to post-treatment changes (i.e. two-weeks, after six infusions) on CAPS-5 scores (Treatment davg = 1.89; Active Control davg = .78).
Figure 2.
Within group effects for Abdallah et al. (2022).
Notes: The dip that occurs around day 28 for each of the groups may be due to attrition. Notably, each group had significant attrition at that period (∼78% of sample missing for ketamine group on day 29). This dip corresponds with the follow-up periods after their final infusions.
2.9. Additional considerations
When incorporating values for our meta-analyses, we observed a potential typographical error that may influence results. Specifically, Table 3 from Pradhan et al. (2018) summarizes their results for the PCL-5 and CAPS-5 at pre-study, 24 h, and relapse timepoints. The values for participants 12 through 20 were identical for both the PCL-5 at relapse and the CAPS-5 at relapse. We attempted to clarify this problem with the authors but did not receive a response. It seems unlikely that participants exactly replicated their previous scores, and this may indicate an error. Importantly, when Pradhan et al. (2018) is not included in the meta-analysis, omnibus effects become non-significant (g = 0.23, 95% CI:[−0.02, 0.48]). Similarly, the post-hoc effect at 24 h becomes non-significant (g = 0.32, 95% CI:[−0.001, 0.63]) and the effect at relapse remains non-significant (g = 0.68, 95% CI:[−0.48, 1.85]).
3. Discussion
Our results reveal several important findings. First, the omnibus (i.e. across timepoints and PTSD measures) effect of ketamine compared to all controls was weak. This effect was further attenuated when bias-correction was implemented, rendering ketamine’s incremental advantage over placebo non-significant. Several important qualifications to these findings warrant discussion. Importantly, effect sizes were not consistent across studies, likely reflecting methodological differences among the studies meta-analysed. Smaller studies generally reported larger effect sizes that favoured ketamine intervention. However, the strength of the ketamine advantage tended to dissolve as sample size increased. In other words, a decline effect was visible within the ketamine-for-PTSD literature. This observation is concerning, given it is consistent with past PTSD psychopharmaceutical trends such as when initial findings favoured risperidone as an intervention for PTSD (e.g. Padala et al., 2006), until larger RCTs were conducted (e.g. Krystal et al., 2011).
The only consistent timepoint measured across RCTs was 24-hours post initial infusion. Of which, the effect (that of all observations at 24-hours post initial infusion) also suggested a significant but weak effect favouring ketamine. Again, smaller studies favoured ketamine with large effect sizes (Pradhan et al., 2017, 2018). This positive effect shrank once power/n increased (Abdallah et al., 2022). Unlike the omnibus effects, data from two studies indicated an inverse effect at 24-hours (Dadabayev et al., 2020; Feder et al., 2021). These effects were non-significant, but their trend does not support ketamine being an incrementally beneficial intervention compared to controls at 24-hours post-infusion.
The popularity of a 24-hour outcome measurement is consistent with suggestions that ketamine is a rapid acting intervention (e.g. Asim et al., 2021). Our findings suggest such statements can be misleading as several studies reported rapid acting effects without consideration for non-significant comparisons to controls. Indeed, while ketamine does appear to have a rapid acting effect at reducing gross PTSD symptoms, the same can also be said of midazolam, ketorolac (which was descriptively better than ketamine at 24-hours), and saline (an inert placebo). In other words, the rapid acting effects might not be due to anything specific to ketamine. Rather, the reported symptom reductions are possibly due to expectancy effects and demand characteristics. This may also explain the discrepancy between active versus passive control conditions. Our sub-analyses revealed a non-significant effect when examining comparisons against active controls, and a modest effect against passive controls. This is evidence of potential blind penetration. That is, essentially a symptom reduction via placebo effect occurs for all interventions across the studies, but the effect is enhanced in participants who have interventions that actively alter perception (e.g. dissociative side effects with ketamine). These side effects may confirm receipt of an active agent (blind penetration), thereby enhancing the therapeutic placebo effect via expectancy effect. Thus, it is possible that the true therapeutic mechanism of ketamine is entirely psychological rather than biological. Future studies could evaluate this hypothesis using multiple distinct sham control conditions.
Fewer researchers monitored outcomes past 24-hours post-initial infusion in consistent intervals. This is problematic as PTSD is often long-standing. In other words, while ketamine may be efficacious as an immediate tranquilizer, additional timepoints that are distant from the initial treatment infusion are necessary to establish whether any therapeutic effect is purely acute. Future researchers are encouraged to examine outcomes several weeks post-infusion in efforts to determine incremental efficacy.
Within the extant literature, four research teams examined the effect of ketamine one-week post-initial infusion. In two of these studies (Abdallah et al., 2022; Feder et al., 2021), multiple ketamine administrations were employed, with one study examining low (0.2 mg/kg) and high (0.5 mg/kg) dosages (Abdallah et al., 2022). Concerningly, only small trials evidenced large effect sizes favouring ketamine (Feder et al., 2014, 2021−it should also be noted that these researchers reported conflicts-of-interest posing a bias risk favouring ketamine). In this context, the largest study we reviewed failed to find a significant effect one-week post-initial infusion (Abdallah et al., 2022). Neither dosage nor administration count appeared to change these trends. Of note, the clinician rating (via CAPS-5) in Feder et al. (2014) supported a large effect size at one-week post infusion (one dose), whereas the patient self-report from that same study suggested a non-significant effect. A similar discrepancy between clinician and patient ratings for PTSD symptoms was also recently observed in a separate meta-analysis looking at psychotherapy outcomes (Aita et al., 2023). While the psychometrics of this discrepancy is beyond the aims of the current discussion, it does suggest that clinicians may tend to report greater therapeutic changes than patients themselves (all effects favouring ketamine at one-week post-infusion(s) came from CAPS-5 measurements).
Only two research teams systematically reported effects at two weeks post-initial infusion (Abdallah et al., 2022; Feder et al., 2021). Consistent with previous observations, the smaller study (Feder et al., 2021) evidenced an effect size that supported ketamine’s incremental benefit, whereas the larger study (Abdallah et al., 2022) evidenced null findings. Interestingly, neither dosage nor administration count affected the outcome compared to controls. Abdallah et al. (2022) continued to monitor patients for approximately eight weeks post-initial infusion (with seven subsequent infusions). Their findings demonstrated that the within group effects across ketamine dosage levels (0.2 and 0.5 mg/kg) and controls (saline) varied across time and followed an almost identical course. On average ketamine intervention reduced self-reported PTSD symptoms within 24 h. However, the saline control group followed the same pattern. Indeed, approximately two months after initial infusion, participants in the saline control condition actually reported greater (though non-meaningful) PTSD symptom reduction than high dose-ketamine-referents (0.5 mg/kg). In short, the pattern was less about the type of intervention, but rather the presence of an intervention.
The results of the current meta-analysis call into question the incremental therapeutic efficacy of ketamine as a PTSD treatment. Even studies reporting statistical significance yielded small omnibus effects. There are many reasons for these modest findings. First, as readily admitted by all researchers of the reviewed RCTs, ketamine’s action mechanisms are poorly understood. Consistent with a Research Domain Criteria (RDoC) perspective, participants in future RCTs should be assigned based on identified markers as opposed to latent PTSD classification. Such procedures will help clarify the therapeutic efficacy of theorized action mechanisms while not risking the loss of a potentially beneficial intervention. This is of particular concern given PTSD is extremely heterogenous (Borgogna et al. 2024; Galatzer-Levy & Bryant, 2013), especially when considering the differentiation between PTSD and complex-PTSD. Distinct biopsychosocial mechanisms likely underly various PTSD presentations, meaning nuanced interventions towards such mechanisms are necessary. Ketamine may hold some incremental therapeutic potential, but RCTs need to be designed to test whether its mechanistic action is the process associated with PTSD.
In observing the analysed studies, many design considerations are needed to further enhance our understanding of ketamine as a PTSD intervention. We found it interesting that while paroxetine and sertraline are the only FDA approved agents to treat PTSD, neither agent was used as a control (in fairness Abdallah et al., 2022 indicate their participants had histories of failed FDA-approved medication intervention). Notwithstanding criticisms of extant interventions (Abdallah et al., 2019), midazolam and ketorolac are not typical PTSD interventions and are limited in their therapeutic utility. Surprisingly, across several observations, midazolam and ketorolac (and even saline) showed promise in reducing PTSD symptoms (likely due to placebo effects). Future studies utilizing more commonly employed control interventions would be helpful. An additional concern, many of the authors reported important information, such as group means and standard deviations, in supplementary contexts. Whereas the reported information within the presented manuscripts appeared biased towards showcasing ketamine as preferable/efficacious (e.g. p-values, trend figures). When the entire body of observations is evaluated, the value of ketamine for PTSD appears limited. We encourage researchers to report means, standard deviations, and effects sizes within their published materials to enhance transparency and aid future meta-analytic efforts.
The confounding role of psychotherapy adds an additional layer of complexity. Half of the studies did not clearly specify if/who were receiving adjunctive psychotherapy. Moreover, in both Pradhan trials, all participants received psychotherapy. Approximately half the participants in Feder et al. (2021) also received psychotherapy. Factors such as therapeutic alliance have been shown to have robust therapeutic effects regardless of psychotherapy approach and standardization adherence (Wampold, 2015). Stated differently, even if psychotherapy assignment was controlled, differences in therapeutic alliance could radically alter results.
Additionally, a recent systematic review of adverse events in ketamine trials for depression (de Laportalière et al., 2023) found that researchers have been underreporting the potential dangers of ketamine intervention. We did not systematically examine adverse events in our review but given the way coefficients needed for effect sizes were reported, it would not be surprising if adverse events were underreported. Specifically, de Laportalière et al. (2023) reported more than 90% of ketamine trials for depression have ‘low’ quality with regard to safety. Additionally, 45.5% of serious adverse events and 39% of non-serious adverse events were not reported in published articles and had to be located in open-access materials. We suggest transparent reporting of adverse event in ketamine-for-PTSD trials.
Our results can only confidently be generalized to PTSD cases. However, given PTSD (e.g. avoidance) features are often accompanied by traditional depression features (e.g. loneliness), our findings cast suspicion on the therapeutic efficacy of ketamine for depression. Many of the researchers who have conducted RCTs examining ketamine as a treatment for depression continue to acknowledge the specific biological mechanisms behind ketamine’s antidepressant effect remain largely unknown (e.g. Murrough et al., 2013; Phillips et al., 2019). Because placebo effect appears to be a potential explanation for most of the therapeutic efficacy of ketamine administered for PTSD, the same limitation may be evident in depression treatment. We advise meta-analyses to address this issue.
3.1. Limitations
Our study is the first systematic review and meta-analysis to comprehensively examine the effect of ketamine on PTSD exclusively compared to control interventions. In accordance with this advantage, the quantitative synthesis was limited to six studies, totalling 221 participants (n = 110 controls). Because of limited power, we were unable to examine important moderating factors. For instance, there are almost certainly additional sources of heterogeneity/bias (e.g. researcher conflict of interest). Meta-analytic considerations for the effect of ketamine across important demographic characteristics (e.g. diagnostic comorbidity, race, sex, and veteran status) are not available, limiting contextualization. All the reviewed studies came from the United States and were published in Western journals. It is possible that studies published in non-Western countries were not observed in our searches, thus limiting our generalizability. As observed, smaller samples tend to overestimate effect sizes. Hedges’ g is associated with a small upwards inflation bias of about 4%. That is, our analyses may be slightly overestimating the strength of the ketamine effect. Additionally, there appeared to be heterogeny across RCTs regarding how adjunctive treatments were involved. Some studies allowed for adjunctive psychotherapy (Abdallah et al., 2022) while others did not specify (Feder et al., 2014). It is possible that psychotherapy could be a confounding variable. We recommend future ketamine clinical trial researchers report adjunctive-therapy demographic information by participant so that such information can be appropriately reviewed and analysed. Accordingly, while our omnibus effect suggested a small significant effect favouring ketamine, this effect needs to be considered with extreme caution.
4. Conclusions
We conclude ketamine may have a small immediate/short-term incremental benefit at reducing PTSD symptoms compared to controls interventions (midazolam, ketorolac, and saline), which is indiscernible from placebo or control interventions in the extant RCTs. This advantage should be interpreted with caution given its size and susceptibility to bias. Based on our findings, we suggest placebo as the primary therapeutic mechanism driving ketamine therapeutic effects for PTSD. Patients and providers should pursue ketamine-based PTSD interventions with caution. Moreover, we suggest funders support ketamine-for-PTSD studies circumscribed to RDoC-based designs (e.g. participants assigned to treatment conditions on the basis of identified biological mechanisms that ketamine is theorized to alter vs. latent PTSD classifications). We further recommend that appropriate agents serve as controls to clarify ketamine’s incremental benefit. Finally, should future ketamine-for-PTSD RCTs be conducted, we suggest standardized long-term outcome markers such as three, six, and 12-month post-baseline measurements.
Supplementary Material
Acknowledgements
The authors would like to thank Gillian Smith for her help with this project.
Funding Statement
This work was supported by Texas Tech University.
Note
We attempted to contact the authors to clarify this issue, but did not receive a response. Based on observations of charts, three doses appears to be the accurate number of administrations and is what we modelled.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdallah, C. G., Averill, L. A., Akiki, T. J., Raza, M., Averill, C. L., Gomaa, H., Adikey, A., & Krystal, J. H. (2019). The neurobiology and pharmacotherapy of posttraumatic stress disorder. Annual Review of Pharmacology and Toxicology, 59(1), 171–189. 10.1146/ANNUREV-PHARMTOX-010818-021701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah, C. G., Averill, L. A., & Krystal, J. H. (2015). Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Annals of the New York Academy of Sciences, 1344(1), 66–77. 10.1111/nyas.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah, C. G., Roache, J. D., Gueorguieva, R., Averill, L. A., Young-McCaughan, S., Shiroma, P. R., Purohit, P., Brundige, A., Murff, W., Ahn, K. H., Sherif, M. A., Baltutis, E. J., Ranganathan, M., D’Souza, D., Martini, B., Southwick, S. M., Petrakis, I. L., Burson, R. R., Guthmiller, K. B., … Krystal, J. H. (2022). Dose-related effects of ketamine for antidepressant-resistant symptoms of posttraumatic stress disorder in veterans and active duty military: A double-blind, randomized, placebo-controlled multi-center clinical trial. Neuropsychopharmacology, 47(8), 1574–1581. 10.1038/s41386-022-01266-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aita, S. L., Kondrath, S. R., Owen, T., Borgogna, N. C., & Usset, T. (2023). The status of evidence-based treatments for moral injury syndrome: Review and meta-analysis of randomized controlled trials. Current Treatment Options in Psychiatry, 10(3), 217–233. [Google Scholar]
- Artin, H., Bentley, S., Mehaffey, E., Liu, F. X., Sojourner, K., Bismark, A. W., Printz, D., Lee, E. E., Martis, B., De Peralta, S., Baker, D. G., Mishra, J., & Ramanathan, D. (2022). Effects of intranasal (S)-ketamine on veterans with co-morbid treatment-resistant depression and PTSD: A retrospective case series. EClinicalMedicine, 48, Article 101439. 10.1016/J.ECLINM.2022.101439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asim, M., Wang, B., Hao, B., & Wang, X. (2021). Ketamine for post-traumatic stress disorders and it’s possible therapeutic mechanism. Neurochemistry International, 146, Article 105044. 10.1016/J.NEUINT.2021.105044 [DOI] [PubMed] [Google Scholar]
- Begola, M. J., & Schillerstrom, J. E. (2019). Hallucinogens and their therapeutic use: A literature review. Journal of Psychiatric Practice, 25(5), 334–346. 10.1097/PRA.0000000000000409 [DOI] [PubMed] [Google Scholar]
- Benish, S. G., Imel, Z. E., & Wampold, B. E. (2008). The relative efficacy of bona fide psychotherapies for treating post-traumatic stress disorder: A meta-analysis of direct comparisons. Clinical Psychology Review, 28(5), 746–758. 10.1016/J.CPR.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Blevins, C. A., Weathers, F. W., Davis, M. T., Witte, T. K., & Domino, J. L. (2015). The posttraumatic stress disorder checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28(6), 489–498. 10.1002/JTS.22059 [DOI] [PubMed] [Google Scholar]
- Borenstein, M., Hedges, L., Higgins, J., & Rothstein, H. (2013). Comprehesnive meta-analysis. Bisostat. [Google Scholar]
- Borgogna, N. C., Owen, T., Aita, S.L. (2024). The absurdity of latent disease model in mental health: 10,130,814 ways to have a DSM-5-TR psychological disorder. Journal of Mental Health, 10.1080/09638237.2023.2278107 [DOI] [PubMed] [Google Scholar]
- Chen, X., Hou, X., Bai, D., Lane, R., Zhang, C., Canuso, C., Wang, G., & Fu, D. J. (2023). Efficacy and safety of flexibly dosed esketamine nasal spray plus a newly initiated oral antidepressant in adult patients with treatment-resistant depression: A randomized, double-blind, multicenter, active-controlled study conducted in China and USA. Neuropsychiatric Disease and Treatment, 19, 693–707. 10.2147/NDT.S391096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers, P., & Cristea, I. A. (2015). What if a placebo effect explained all the activity of depression treatments? World Psychiatry, 14(3), 311. 10.1002/WPS.20249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadabayev, A. R., Joshi, S. A., Reda, M. H., Lake, T., Hausman, M. S., Domino, E., & Liberzon, I. (2020). Low dose ketamine infusion for comorbid posttraumatic stress disorder and chronic pain: A randomized double-blind clinical trial. Chronic Stress, 4, 1–9. 10.1177/2470547020981670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dames, S., Kryskow, P., & Watler, C. (2022). A cohort-based case report: The impact of ketamine-assisted therapy embedded in a community of practice framework for healthcare providers with PTSD and depression. Frontiers in Psychiatry, 12, 2391. 10.3389/FPSYT.2021.803279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea, D., & Andrew Sewell, R. (2013). Transient resolution of treatment-resistant posttraumatic stress disorder following ketamine infusion. Biological Psychiatry, 74(9), e13–e14. 10.1016/j.biopsych.2013.04.019 [DOI] [PubMed] [Google Scholar]
- Davey, J., Turner, R. M., Clarke, M. J., & Higgins, J. P. (2011). Characteristics of meta-analyses and their component studies in the cochrane database of systematic reviews: A cross-sectional, descriptive analysis. BMC Medical Research Methodology, 11(1), 1–11. 10.1186/1471-2288-11-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laportalière, T. T., Jullien, A., Yrondi, A., Cestac, P., & Montastruc, F. (2023). Reporting of harms in clinical trials of esketamine in depression: A systematic review. Psychological Medicine, 53(10), 4305–4315. 10.1017/S0033291723001058 [DOI] [PubMed] [Google Scholar]
- Du, R., Han, R., Niu, K., Xu, J., Zhao, Z., Lu, G., & Shang, Y. (2022). The multivariate effect of ketamine on PTSD: Systematic review and meta-analysis. Frontiers in Psychiatry, 13, 1–9. 10.3389/fpsyt.2022.813103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval, S., & Tweedie, R. (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463. 10.1111/J.0006-341X.2000.00455.X [DOI] [PubMed] [Google Scholar]
- Feder, A., Costi, S., Rutter, S. B., Collins, A. B., Govindarajulu, U., Jha, M. K., Horn, S. R., Kautz, M., Corniquel, M., Collins, K. A., Bevilacqua, L., Glasgow, A. M., Brallier, J., Pietrzak, R. H., Murrough, J. W., & Charney, D. S. (2021). A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. American Journal of Psychiatry, 178(2), 193–202. 10.1176/appi.ajp.2020.20050596 [DOI] [PubMed] [Google Scholar]
- Feder, A., Norbury, A., Rutter, S., Costi, S., Jha, M., Charney, D., & Murrough, J. (2022). Neural circuitry mechanisms of response to ketamine in PTSD: Preliminary findings. Biological Psychiatry, 91(9), S34–S35. 10.1016/j.biopsych.2022.02.104 [DOI] [Google Scholar]
- Feder, A., Parides, M. K., Murrough, J. W., Perez, A. M., Morgan, J. E., Saxena, S., Kirkwood, K., Rot, A. H., Lapidus, M., Wan, K. A. B., Ben, L., Iosifescu, D., & Charney, D. S. (2014). Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: A randomized clinical trial. JAMA Psychiatry, 71(6), 681–688. 10.1001/jamapsychiatry.2014.62 [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy, I. R., & Bryant, R. A. (2013). 636,120 ways to have posttraumatic stress disorder. Perspectives on Psychological Science, 8(6), 651–662. 10.1177/1745691613504115 [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., & Welch, V. A. (2019). Cochrane handbook for systematic reviews of interventions (2nd ed.). Wiley-Blackwell. [Google Scholar]
- Katalinic, N., Lai, R., Somogyi, A., Mitchell, P. B., Glue, P., & Loo, C. K. (2013). Ketamine as a new treatment for depression: A review of its efficacy and adverse effects. Australian and New Zealand Journal of Psychiatry, 47(8), 710–727. 10.1177/0004867413486842 [DOI] [PubMed] [Google Scholar]
- Krystal, J. H., Abdallah, C. G., Averill, L. A., Kelmendi, B., Harpaz-Rotem, I., Sanacora, G., Southwick, S. M., & Duman, R. S. (2017). Synaptic loss and the pathophysiology of PTSD: Implications for ketamine as a prototype novel therapeutic. Current Psychiatry Reports, 19(10), 1–11. 10.1007/s11920-017-0829-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal, J. H., Davis, L. L., Neylan, T. C., Raskind, A., Schnurr, M., Stein, P. P., Vessicchio, M. B., Shiner, J. B., Gleason, T. D., & Huang, D. G. (2017). It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: A consensus statement of the PTSD psychopharmacology working group. Biological Psychiatry, 82(7), e51–e59. 10.1016/J.BIOPSYCH.2017.03.007 [DOI] [PubMed] [Google Scholar]
- Krystal, J. H., Rosenheck, R. A., Cramer, J. A., Vessicchio, J. C., Jones, K. M., Vertrees, J. E., Horney, R. A., Huang, G. D., & Stock, C. (2011). Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related PTSD: A randomized trial. JAMA, 306(5), 493–502. 10.1001/JAMA.2011.1080 [DOI] [PubMed] [Google Scholar]
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 1–12. 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti, D., Mucci, F., Tripodi, B., Carbone, M. G., Muscarella, A., Falaschi, V., & Baroni, S. (2019). Emotional blunting, cognitive impairment, bone fractures, and bleeding as possible side effects of long-term Use of SSRIs. Clinical Neuropsychiatry, 16(2), 75–85. [PMC free article] [PubMed] [Google Scholar]
- Moncrieff, J., Cooper, R. E., Stockmann, T., Amendola, S., Hengartner, M. P., & Horowitz, M. A. (2022). The serotonin theory of depression: A systematic umbrella review of the evidence. Molecular Psychiatry, 28(8), 3243–3256. 10.1038/s41380-022-01661-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough, J. W., Iosifescu, D. V., Chang, L. C., Al Jurdi, R. K., Green, C. E., Perez, A. M., Iqbal, S., Pillemer, S., Foulkes, A., Shah, A., Charney, D. S., & Mathew, S. J. (2013). Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. American Journal of Psychiatry, 170(10), 1134–1142. 10.1176/appi.ajp.2013.13030392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHLBI . (2021). Study quality assessment tools.
- Padala, P. R., Madison, J., Monnahan, M., Marcil, W., Price, P., Ramaswamy, S., Din, A. U., Wilson, D. R., & Petty, F. (2006). Risperidone monotherapy for post-traumatic stress disorder related to sexual assault and domestic abuse in women. International Clinical Psychopharmacology, 21(5), 275–280. 10.1097/00004850-200609000-00005 [DOI] [PubMed] [Google Scholar]
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Medicine, 18(3), e1003583. 10.1371/JOURNAL.PMED.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, J. L., Norris, S., Talbot, J., Birmingham, M., Hatchard, T., Ortiz, A., Owoeye, O., Batten, L. A., & Blier, P. (2019). Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: A randomized controlled trial. American Journal of Psychiatry, 176(5), 401–409. 10.1176/appi.ajp.2018.18070834 [DOI] [PubMed] [Google Scholar]
- Pradhan, B. K., Mitrev, L., Moaddell, R., & Wainer, I. W. (2018). d-Serine is a potential biomarker for clinical response in treatment of post-traumatic stress disorder using (R,S)-ketamine infusion and TIMBER psychotherapy: A pilot study. Biochimica et Biophysica Acta (BBA) – Proteins and Proteomics, 1866(7), 831–839. 10.1016/J.BBAPAP.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan, B. K., Wainer, I. W., Moaddel, R., Torjman, M. C., Goldberg, M., Sabia, M., Parikh, T., & Pumariega, A. J. (2017). Trauma interventions using mindfulness based extinction and reconsolidation (TIMBER) psychotherapy prolong the therapeutic effects of single ketamine infusion on post-traumatic stress disorder and comorbid depression: A pilot randomized, placebo-controlled, crossover clinical trial. Asia Pacific Journal of Clinical Trials: Nervous System Diseases, 2(3), 80. 10.4103/2542-3932.211589 [DOI] [Google Scholar]
- Raskind, M. A., Peskind, E. R., Chow, B., Harris, C., Davis-Karim, A., Holmes, H. A., Hart, K. L., McFall, M., Mellman, T. A., Reist, C., Romesser, J., Rosenheck, R., Shih, M.-C., Stein, M. B., Swift, R., Gleason, T., Lu, Y., & Huang, G. D. (2018). Trial of prazosin for post-traumatic stress disorder in military veterans. New England Journal of Medicine, 378(6), 507–517. 10.1056/NEJMOA1507598 [DOI] [PubMed] [Google Scholar]
- Rauch, S. A. M., & Ruzek, J. (2012). Review of exposure therapy: A gold standard for PTSD treatment victimology view project mindfulness and self-compassion for trauma and PTSD view project. The Journal of Rehabilitation Research and Development, 49(5), 679–688. 10.1682/JRRD.2011.08.0152 [DOI] [PubMed] [Google Scholar]
- Riggs, L. M., & Gould, T. D. (2021). Ketamine and the future of rapid-acting antidepressants. Annual Review of Clinical Psychology, 17(1), 207–231. 10.1146/ANNUREV-CLINPSY-072120-014126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein, J., Houle, C., Urganus, A., Cloutier, M., Patterson-Lomba, O., Wang, Y., King, S., Levinson, W., Guérin, A., Lefebvre, P., & Davis, L. L. (2021). Prevalence of post-traumatic stress disorder in the United States: A systematic literature review. Current Medical Research and Opinion, 37(12), 2151–2161. 10.1080/03007995.2021.1978417 [DOI] [PubMed] [Google Scholar]
- Schnurr, P. P., Chard, K. M., Ruzek, J. I., Chow, B. K., Resick, P. A., Foa, E. B., Marx, B. P., Friedman, M. J., Bovin, M. J., Caudle, K. L., Castillo, D., Curry, K. T., Hollifield, M., Huang, G. D., Chee, C. L., Astin, M. C., Dickstein, B., Renner, K., Clancy, C. P., … Shih, M. C. (2022). Comparison of prolonged exposure vs cognitive processing therapy for treatment of posttraumatic stress disorder among US veterans: A randomized clinical trial. JAMA Network Open, 5(1), e2136921. 10.1001/JAMANETWORKOPEN.2021.36921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp, M. M., Litz, B. T., Hoge, C. W., & Marmar, C. R. (2015). Psychotherapy for military-related PTSD: A review of randomized clinical trials. JAMA, 314(5), 489–500. 10.1001/JAMA.2015.8370 [DOI] [PubMed] [Google Scholar]
- Stein, M. B., & Simon, N. M. (2021). Ketamine for PTSD: Well, isn’t that special. The American Journal of Psychiatry, 178(2), 116–118. 10.1176/APPI.AJP.2020.20121677 [DOI] [PubMed] [Google Scholar]
- Varker, T., Watson, L., Gibson, K., Forbes, D., & O’Donnell, M. L. (2021). Efficacy of psychoactive drugs for the treatment of posttraumatic stress disorder: A systematic review of MDMA, ketamine, LSD and psilocybin. Journal of Psychoactive Drugs, 53(1), 85–95. 10.1080/02791072.2020.1817639 [DOI] [PubMed] [Google Scholar]
- Von Hippel, P. T. (2015). The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Medical Research Methodology, 15(1), 35. 10.1186/S12874-015-0024-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampold, B. E. (2015). How important are the common factors in psychotherapy? An update. World Psychiatry, 14(3), 270–277. 10.1002/WPS.20238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S.-M., Han, C., Bahk, W.-M., Lee, S.-J., Patkar, A. A., Masand, P. S., & Pae, C.-U. (2018). Addressing the side effects of contemporary antidepressant drugs: A comprehensive review. Chonnam Medical Journal, 54(2), 101–112. 10.4068/cmj.2018.54.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers, F. W., Bovin, M. J., Lee, D. J., Sloan, D. M., Schnurr, P. P., Kaloupek, D. G., Keane, T. M., & Marx, B. P. (2018). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological Assessment, 30(3), 383–395. 10.1037/pas0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, D. S. (2004). The impact of events scale-revised. In Wilson J. P. & Terence M. K. (Eds.), Assessing psychological trauma and PTSD (2nd ed., pp. 168–189). Guilford Press. [Google Scholar]
- Whittaker, E., Dadabayev, A. R., Joshi, S. A., & Glue, P. (2021). Systematic review and meta-analysis of randomized controlled trials of ketamine in the treatment of refractory anxiety spectrum disorders. Therapeutic Advances in Psychopharmacology, 11, 204512532110567. 10.1177/20451253211056743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefifard, M., Askarian-Amiri, S., Rafiei Alavi, S. N., Sadeghi, M., Saberian, P., Baratloo, A., & Talebian, M. T. (2020). The efficacy of ketamine administration in prehospital pain management of trauma patients; a systematic review and meta-analysis. Archives of Academic Emergency Medicine, 8, e1. 10.22037/aaem.v8i1.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis, K. K. (2001). Statistics to tell the truth, the whole truth, and nothing but the truth: Formulae, illustrative numerical examples, and heuristic interpretation of effect size analyses for neuropsychological researchers. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 16(7), 653–667. 10.1093/arclin/16.7.653 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Extracted data sheets are available on the Open Science Framework [https://osf.io/mtfdx/].