Abstract
Introduction:
About half of adults aged ≥ 80 years suffer from frailty. Exercise is considered effective in preventing frailty but may be inapplicable to adults aged ≥ 80 years due to physical limitations. As an alternative, we aimed to explore the association of leisure activities with frailty and identify potential interaction with established polygenic risk score (PRS) among adults aged ≥ 80 years.
Methods:
Analyses were performed in a prospective cohort study of 7471 community-living older adults aged ≥ 80 years who were recruited between 2002 and 2014 from 23 provinces in China. Leisure activity was assessed using a seven-question leisure activity index and frailty was defined as a frailty index ≥0.25 using a validated 39-item health-related scale. The PRS was constructed using 59 single-nucleotide polymorphisms associated with frailty in a subsample of 2541 older adults. Cox proportional hazards models were used to explore the associations of leisure activities, PRS with frailty.
Results:
The mean age of participants was 89.4 ± 6.6 years (range: 80–116). In total, 2930 cases of frailty were identified during 42,216 person-years of follow-up. Each 1 unit increase in the leisure activity index was associated with 12% lower risk of frailty (hazard ratio [HR]: 0.88 [95%CI, 0.85-0.91]). Participants with high genetic risk (PRS>2.47×10−4) suffered from 26% higher risk of frailty. Interaction between leisure activity and genetic risk was not observed.
Conclusion:
Evidence is presented for the independent association of leisure activities and genetic risk with frailty. Engagement in leisure activities suggested to be associated with lower risk of frailty across all levels of genetic risk among adults aged ≥ 80 years.
Keywords: Leisure activity, Epidemiology, Frailty, Genetic risk, Healthy ageing
Introduction
Frailty is a common syndrome among older adults, characterized by reduced physiological reserve and function across multiple organ systems and increased vulnerability to adverse health outcomes [1-5]. The worldwide prevalence of frailty increases with age, being less than 10% among adults aged 60 years but increasing to 50% among adults aged ≥ 80 years, resulting in increased health-care utilization and cost [6]. The increasing global numbers of the oldest-old result in increased aggravation of this burden with estimates of 145.5 million oldest-old individuals in 2020 and 426.4 million by 2050.[7] We have previously found that nearly half of adults aged ≥80 years were underweight [8], reflecting unhealthy skeletal muscle mass and nutritional status and being associated with higher risk of frailty [9,10]. However, frailty incidence rates and potential preventive factors remain unclear since most cohort studies have had a limited sample size of adults aged ≥80 years [11]. Therefore, more long-term cohort studies are required to reveal feasible preventive or interventional measures to address the problem of frailty and foster healthy ageing [1].
Exercise is a recognized frailty prevention factor[1,12-14] but high prevalence of daily living disability limits the access of adults aged ≥80 years to regular exercise [15,16]. Leisure activities also have the potential to impact frailty in this population and greater understanding of this association would be an advantage. Previous studies among adults aged ≤79 years have suggested an association of leisure activities, such as hobbies, cultural engagements and other organized activities (e.g., dancing, Tai Chi, visiting museums/theatre/cinema, golf, clubs, social groups and religious activities), with lower risks of frailty [13,17-19]. However, the existence of any such association among adults aged ≥80 years remains unknown.
Candidate frailty genes are thought to be related to the immune response, cholesterol transport, apoptotic signaling, homocysteine metabolism, folate metabolism, phosphate/calcium homeostasis, stem cell maintenance, cell adhesion, growth, migration and differentiation [20-24]. However, the polygenic risk score (PRS) associated with frailty and the joint effect of PRS with leisure activities on frailty remains less studied, especially among adults aged ≥80 years.
The current study aims to explore the associations of leisure activities, PRS, and their joint effect with frailty among 7471 community-living older adults aged ≥80 years from the Chinese Longitudinal Healthy Longevity Survey (CLHLS).
Materials and Methods
Study Population
The CLHLS study was a prospective cohort study conducted in 23 of 31 provinces in China to investigate determinants of longevity [15,25,26] and 35,474 older adults were enrolled in 2002, 2005, 2008, 2011 and 2014 baseline waves. Exclusion criteria were as follows: participants aged ≤ 79 (n=9316), without follow-up measurements of frailty (n=15,905), with frailty at baseline (n=1986), with missing values of leisure activities (n=3) or with disability in activities of daily living (ADL) or functional limitations (unable to raise an arm straight upwards, put the hand behind neck or lower back, stand up from a chair or pick up a book from the floor; n=793), leaving 7471 adults aged ≥ 80 years (mean age: 89.4±6.6 years) in the final analysis (online suppl. Fig. 1). A proportion of the total, 2541 adults aged ≥80 years, had qualified single-nucleotide polymorphism (SNP) data and were included in the genetic analysis. Ethical approval was granted by the biomedical ethics committee of Peking University (IRB00001052-13074) and the ethics committee of the National Institute of Environmental Health, Chinese Center for Disease Control and Prevention (No. 201922). Written informed consent was obtained from all participants or their proxy respondents.
Assessment of Leisure Activities
A leisure activity index was generated by face-to-face interviews including seven questions related to gardening, outdoor activities excluding exercise (e.g., Tai Chi, dancing, visiting neighbors or friends), keeping poultry or pets, reading, playing cards or Mahjong, watching TV or listening to the radio and attending organized social activities [27]. One point was assigned for each answer of “Almost every day” or “Not every day, but at least once per week”. Total scores ranged from 0 to 7. The index was treated in three ways: as a continuous variable; using a cut-off considered ‘enough leisure activities’ [participants with leisure activity index ≥ 2 (median value)] [28,29] and as a quartiles variable (defined according to the quartiles of leisure activity index with score ≤ 1, score = 2, score = 3 and score ≥ 4). A binary variable (0 vs ≥1) was also constructed for each kind of leisure activity.
Assessment of Frailty
Frailty was assessed by a previously validated 39-item health-related scale for assessment of cognitive function, ADL, instrumental activities of daily living, functional limitations, prevalence of diseases, depression, hearing loss and visual function [30]. The frailty index (FI) was calculated according to a previous study (range: 0-1), computed by summing all deficits and then dividing by the total number of possible deficits (online suppl. Table 1) [30]. A higher FI indicated poorer health status and frailty was defined as FI ≥ 0.25 [6,31]. Follow-up time was calculated for each participant using the time from the baseline investigation to the incidence of frailty, loss of follow-up or the end time of the survey (September 1, 2018).
Construction of Polygenic Risk Score
Saliva and venous blood samples were collected during the CLHLS study for DNA extraction and SNP determination. 27,000 SNPs were genotyped in 2014 by the Beijing Genomics Institute using Illumina HumanOmniZhongHua-8 BeadChips [32]. CLHLS genetic data was evaluated for quality and completeness, achieving a full quality item score of 12 [33] and the suggested thresholds and procedures were used during the current study to assess the quality of samples and genetic variants [34]. Sporadic missing genotypes were imputed based on the 1000 Genomes Project Phase 3 data using IMPUTE (version 2.3.2) software to increase the power of the phenotype-genotype association. A genome-wide association study (GWAS) was performed to select frailty-related genetic variants and a linear regression method integrated into PLINK used to assess the associations of genetic variants and frailty. Overall, 59 SNPs were selected as candidate variables associated with the FI (p-values <.001) (online suppl. Table 2). These genetic variants were used to construct the PRS which was defined for an individual, , as:
where is the index of the genetic variants; are the beta coefficient associations from a linear regression analysis on the CLHLS data; is the number of FI-effect alleles in the i-th individual and is the number of available SNPs in the i-th individual.
Covariates
Information on participant characteristics and lifestyles were acquired using face-to-face questionnaires. The covariates included age, sex, race, residence, living arrangement, education, marital status, income levels, smoking, drinking, exercise and dietary diversity evaluated by nine major food types [35]. Body mass index (BMI) was calculated using height and weight.
Statistical Analysis
Baseline continuous variables are presented as mean ± standard deviation (SD) and compared by one-way ANOVA. Categorical variables are presented as numbers of participants (percentage) and compared using tests. The initial missing rates for all variables were less than 0.6%. Missing values were imputed using the mean and mode of variables from five multiple imputed datasets generated by the random forest method [36,37]. A two-step analysis was performed. In the first step (Analysis I; n=7471), data from adults aged ≥ 80 years with eligible information on leisure activities and frailty was used to explore the association of different measurements of leisure activities with frailty. In the second step (Analysis II; n=2541), the FI-associated PRS was constructed, the association of PRS with frailty assessed and potential interactions between levels of PRS (classified into high or low levels by median of 2.47×10−4) and different measurements of leisure activities (using continuous, binary or quartiles definitions of leisure activity index) with frailty explored.
Cox proportional hazards models were established to explore the association of leisure activities with frailty during analyses I and II. Models were tested to satisfy the proportional hazards assumption with scaled Schoenfeld residuals (online suppl. Fig. 2, 3) [38]. Hazard ratio (HR) and 95% confidence interval (CI) were estimated in 4 different models: a raw model that was not adjusted for any covariate; model 1 adjusted for age, sex, race and residence; model 2 adjusted for education, living arrangements, marital status and income level; model 3 adjusted for drinking, smoking, dietary diversity and BMI.
During Analysis I, we also: (1) conducted restricted cubic spline with 3 knots to investigate the non-linear associations between leisure activity index and frailty in the fully adjusted model; (2) performed subgroup analyses by age, sex, race, residence, living arrangement, education level, marital status, smoking, drinking, exercise, dietary diversity, income level and BMI.
Genetic data extraction and quality control were performed using PLINK 1.9 [39]. All analyses were completed by R 4.0.1 for Windows (R Foundation for Statistical Computing, Vienna, Austria) using packages of “table1”, “survival”, “survminer”, “forestplot”, “randomForest”, “mice” and “ggplot2”. Significance was set at a two-tailed value of p <.05.
Results
Baseline Characteristics
A total of 35,474 adults enrolled in the CLHLS 2002, 2005, 2008, 2011 and 2014 baseline waves were recruited as potential candidates and 7471 adults aged ≥ 80 years (mean age: 89.4±6.6; range: 80–116) satisfied inclusion criteria (Table 1; online suppl. Fig. 1). 3310 (44.3%) were male. The median follow-up time was 5.5 (interquartile range [IQR]: 3.3–6.3) years. 2930 cases of frailty were recorded during 42,216 person-years of follow-up from 2002 to 2018 with an incidence density of 69.4/1000 person-years. Baseline characteristics are presented by frailty status with participants who were older, female, Han race, living with family, illiterate, married, low income, never-drinkers and never-smokers being more likely to be frail (Table 1).
Table 1.
Baseline participant characteristics by frailty
| Characteristics | Participants, No. (%) | P value | ||
|---|---|---|---|---|
| All | Non-frail (FI score<0.25) |
Frail (FI score≥0.25) |
||
| Sample size | 7571 (100) | 4541 (63.4) | 2930 (36.6) | |
| Age, mean (SD), year | 89.4 (6.6) | 88.6 (6.4) | 90.8 (6.8) | <.001 |
| Sex | ||||
| Male | 3310 (44.3) | 2225 (49.0) | 1085 (37.0) | <.001 |
| Female | 4161 (55.7) | 2316 (51.0) | 1845 (63.0) | |
| Race | ||||
| Han race | 6930 (92.8) | 4169 (91.8) | 2761 (94.2) | <.001 |
| Minority race | 541 (7.2) | 372 (8.2) | 169 (5.8) | |
| Residence | ||||
| Urban | 2869 (38.4) | 1744 (38.4) | 1125 (38.4) | 1.000 |
| Rural | 4602 (61.6) | 2797 (61.6) | 1805 (61.6) | |
| Living arrangement | ||||
| With family | 5798 (77.6) | 3456 (76.1) | 2342 (79.9) | <.001 |
| Alone or in nursing home | 1673 (22.4) | 1085 (23.9) | 588 (20.1) | |
| Education | ||||
| Illiteracy | 4971 (66.5) | 2878 (63.4) | 2093 (71.4) | <.001 |
| Literacy | 2500 (33.5) | 1663 (36.6) | 837 (28.6) | |
| Marital status | ||||
| In marriage | 5626 (75.3) | 3357 (73.9) | 2269 (77.4) | <.001 |
| unmarried, divorced or widowed | 1845 (24.7) | 1184 (26.1) | 661 (22.6) | |
| Income level | ||||
| High | 1277 (17.1) | 760 (16.7) | 517 (17.6) | .034 |
| Medium | 4986 (66.7) | 3080 (67.8) | 1906 (65.1) | |
| Low | 1208 (16.2) | 701 (15.4) | 507 (17.3) | |
| Drinking | ||||
| Never-drinkers | 5113 (68.4) | 3030 (66.7) | 2083 (71.1) | <.001 |
| Current-drinkers | 1603 (21.5) | 1037 (22.8) | 566 (19.3) | |
| Former-drinkers | 755 (10.1) | 474 (10.4) | 281 (9.6) | |
| Smoking | ||||
| Never-smokers | 5129 (68.7) | 3019 (66.5) | 2110 (72.0) | <.001 |
| Current-smokers | 1302 (17.4) | 844 (18.6) | 458 (15.6) | |
| Former-smokers | 1040 (13.9) | 678 (14.9) | 362 (12.4) | |
| Exercise | ||||
| Never-exercisers | 4465 (59.8) | 2689 (59.2) | 1776 (60.6) | .199 |
| Current-exercisers | 2494 (33.4) | 1550 (34.1) | 944 (32.2) | |
| Former-exercisers | 512 (6.9) | 302 (6.7) | 210 (7.2) | |
| Dietary diversity | ||||
| Poor | 3105 (41.6) | 1887 (41.6) | 1218 (41.6) | 1.000 |
| Good | 4366 (58.4) | 2654 (58.4) | 1712 (58.4) | |
| BMI | ||||
| Underweight | 3192 (42.7) | 1946 (42.9) | 1246 (42.5) | .881 |
| Normal | 3575 (47.9) | 2173 (47.9) | 1402 (47.8) | |
| Overweight or obesity | 704 (9.4) | 422 (9.3) | 282 (9.6) | |
Abbreviations: BMI, body mass index; FI, frailty index; SD, standard deviation.
Association between Leisure Activities and Frailty
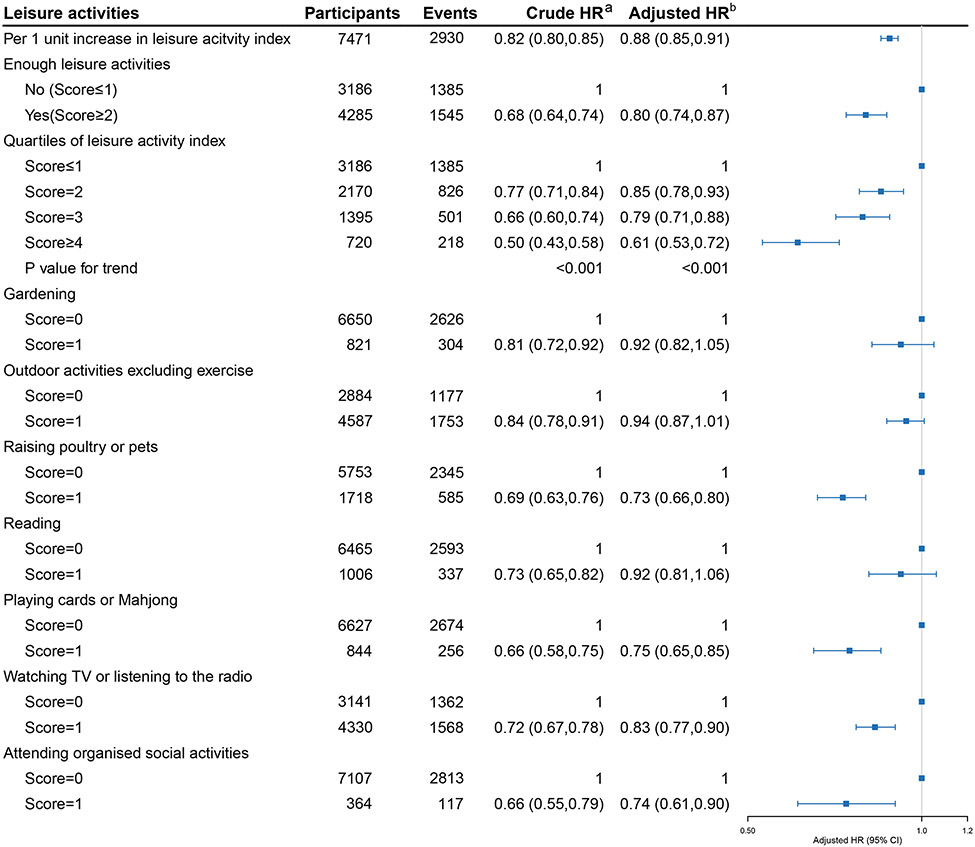
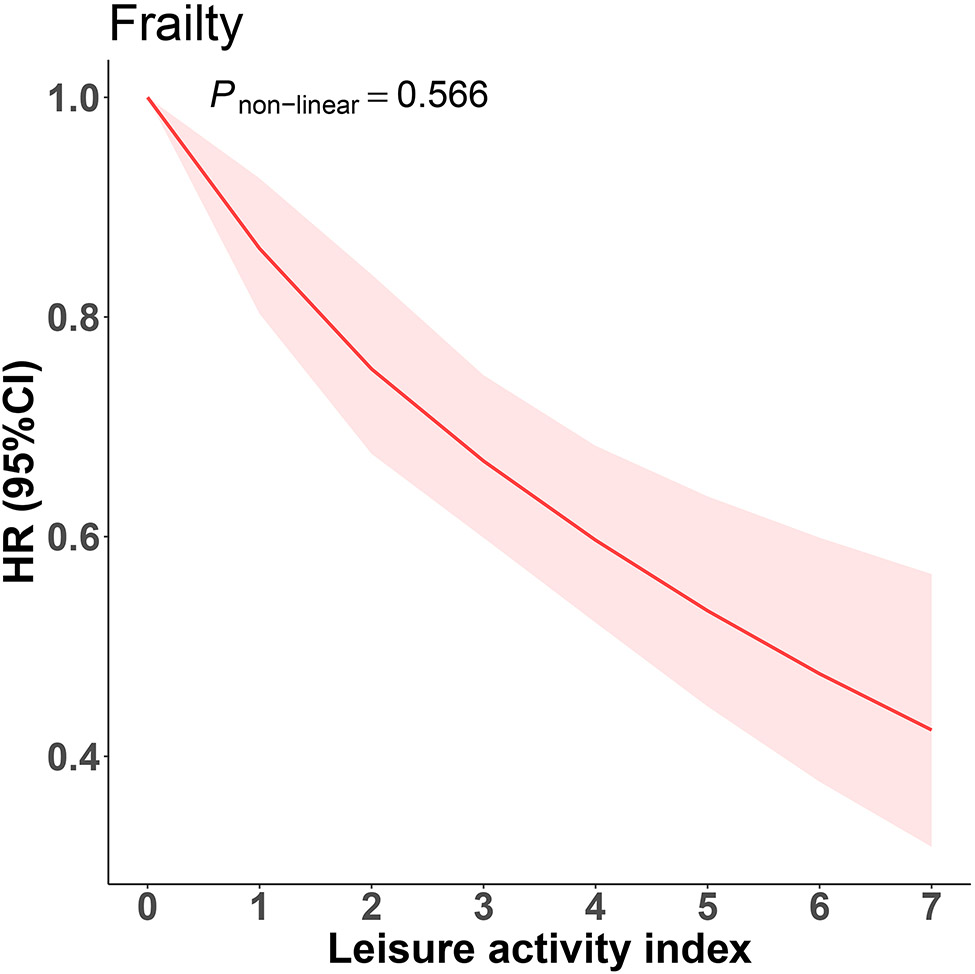
Analysis I extensively explored the association between leisure activities and frailty. We revealed a linear relationship in which every 1-point increase in the leisure activity index was associated with 12% lower risk of frailty in the fully adjusted model (HR 0.88, 95% CI 0.85-0.91) (Fig.1), the non-linear relationship between leisure activity index and frailty was not observed (p-value for nonlinearity =.566) (Fig. 2). The findings were similar for binary and quartile forms of leisure activity index. Participants with ‘enough leisure activities’ (score ≥ 2) had a 20% lower risk of frailty in the binary analysis compared with those with a leisure activity index score ≤1. Similarly, participants with score = 2, 3 and ≥ 4 had 15%, 21% and 39% lower risks of frailty in quartile analyses, respectively (Fig. 1). These results remained consistent among models adjusted for different covariates (online suppl. Table 3).
Fig. 1. Association between multiple measurements of leisure activities and frailty.
Abbreviations: HR, hazard ratio.
aWithout adjustment for any covariate.
bAdjusted for age, sex, race, residence, education, living arrangement, marital status, income level, drinking, smoking, exercise, dietary diversity and body mass index.
Fig. 2. The non-linear association between leisure activity index and frailty.
Abbreviations: HR, hazard ratio; CI, confidence interval.
The curve was plotted using Cox proportional hazards models with restricted cubic splines with 3 knots, adjusted for age, sex, race, residence, education, living arrangement, marital status, income level, drinking, smoking, exercise, dietary diversity and body mass index. Shading area indicates 95% CIs.
Fully adjusted models for each of the seven leisure activities and frailty showed that the risk reduction was greatest for keeping poultry or pets (HR 0.73, 95% CI 0.66-0.80) and least for watching TV or listening to the radio (HR 0.83, 95% CI 0.77-0.90). However, associations of gardening, outdoor activities excluding exercise and reading with frailty were no longer statistically significant after adjustment for all covariates (Fig. 1). These results remained stable in models adjusted for different covariates (online suppl. Table 3).
Interactions were not observed between leisure activity index and different subgroups (all p for interactions > .05) (online suppl. Fig. 4).
Association between PRS and Frailty
Analysis II of 2541 adults aged ≥ 80 years validated the association between PRS and FI in a linear model and found that a 0.0001 unit increase in PRS corresponded to a 0.0040 increase in FI (95% CI: 0.0037-0.0042; p<.001) when adjusted for age and sex. Assessment of the PRS and frailty association in the fully adjusted Cox model showed that a 0.0001 unit increase in PRS was associated with 3% higher risk of frailty (HR 0.97, 95% CI 1.02-1.05). Participants with high level of genetic risk (PRS greater than median) suffered from 26% higher risk of frailty compared with those with a low level of genetic risk (HR 0.74, 95% CI 1.11-1.44) (Table 2).
Table 2.
Hazard ratio (95% confidence interval) for the association between polygenic risk score and frailty
| Polygenic risk score | Raw modela | Model 1b | Model 2c | Model 3d |
|---|---|---|---|---|
| Per 0.0001 unit | 1.04 (1.02,1.05) | 1.03 (1.02,1.05) | 1.03 (1.02,1.05) | 1.03 (1.02,1.05) |
| Binary group | ||||
| Low | Reference | Reference | Reference | Reference |
| High | 1.31 (1.15,1.48) | 1.28 (1.12,1.45) | 1.27 (1.12,1.45) | 1.26 (1.11,1.44) |
Without adjustment for any covariate.
Adjusted for age, sex, race and residence.
Adjusted for age, sex, race and residence, education, living arrangement, marital status and income level.
Adjusted for age, sex, race and residence, education, living arrangement, marital status and income level, drinking, smoking, diet diversity and body mass index.
Joint Association of Leisure Activities and PRS with Frailty
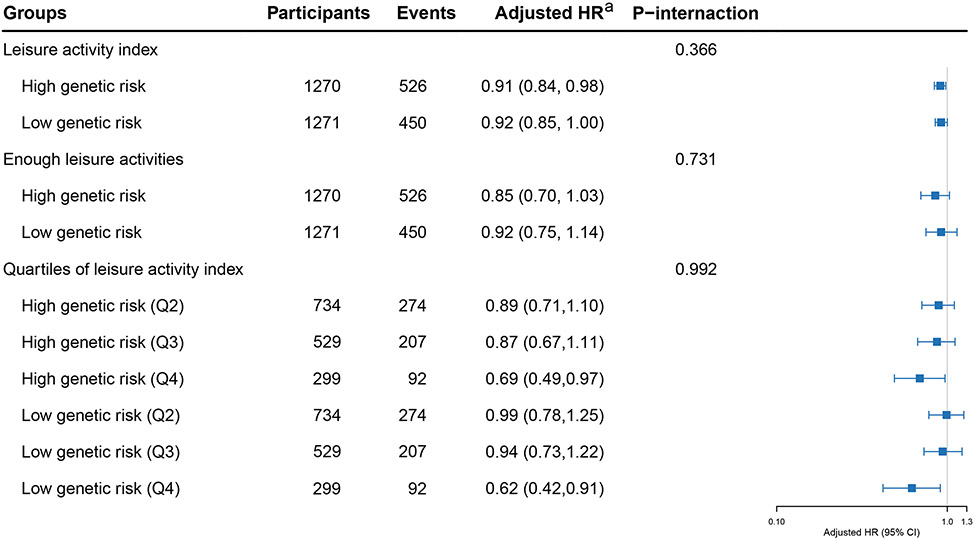
Analysis II revealed no significant interactions between leisure activity index and PRS in the fully adjusted models and this finding remained unchanged in analyses using binary or quartile measurements of leisure activities (all p for interactions>.05) (Fig. 3).
Fig. 3. The potential interactions between leisure activities, polygenic risk score and frailty.
Abbreviations: HR, hazard ratio; CI, confidence interval.
aadjusted for age, sex, race, residence, education, living arrangement, marital status, income level, drinking, smoking, exercise, dietary diversity and body mass index. The shading indicates 95% CIs.
Discussion
The current large prospective cohort study of 7471 adults aged ≥ 80 years with a median follow-up time of 5.5 (IQR:3.3-6.3) years found that both leisure activities and PRS were independently associated with lower risk of frailty. However, interdependence of leisure activities and genetic risk was not observed. The findings suggest that the preventive effect of leisure activities on frailty might be constant for older adults with different genetic backgrounds.
Association between Leisure Activities and Frailty
The incidence of frailty among adults aged ≥ 80 years was 69.4/1000 person-years, 1.6 times higher than that found among adults aged 60 years or older [11]. The association between leisure activities and frailty has been understudied. Evidence from previous cross-sectional or cohort studies with limited follow-up times indicated that leisure activities like dancing, Tai Chi, visiting museums, theatre or cinema, playing golf, joining clubs, engaging in social groups and religious activities were associated with lower risk of frailty among adults aged 79 years or younger [13,17-19]. The current study systematically explored the association between different measurements of leisure activity and frailty and the impact of genetic risk in this association. The current findings are consistent with those of previous studies suggesting daily leisure activities may be beneficial for elderly people. For example, Tai Chi was reported to improve physical performance and hemodynamic outcomes in a 48-week randomized clinical trial among adults aged ≥ 70 years and may reduce the risk of future frailty [40]. Prolonged TV watching was found to be a risk factor for frailty and functional limitations in European older adults [41] but might prove beneficial to adults aged ≥ 80 years by reducing the risk of cognitive impairment [16,42]. Keeping pets [43,44] and playing cards or Mahjong were also protective factors against frailty [45]. In addition, leisure activities can facilitate the interaction of older adults with family or friends, and therefore prevent frailty by improving social connection, sense of belonging, and life satisfaction [46,47]. The preventive effects of these leisure activities have also been reported in our previous findings on ADL, cognitive impairment and mortality among adults aged ≥ 80 years [16,42,48].
Association between PRS and Frailty
Previous studies in participants aged ≥ 50 years have revealed frailty related genes associated with the immune response, cholesterol transport, apoptotic signaling, homocysteine metabolism, folate metabolism, phosphate and calcium homeostasis, stem cell maintenance, cell adhesion, growth, migration and differentiation [20-24]. The current PRS analysis found that the genetic variants associated with frailty in the ≥ 80s were involved in cancer, mental disorders and immune and inflammatory responses.
Several frailty-related SNPs were associated with diverse kinds of cancers. CPO-rs10179420 was reported to be associated with lung adenocarcinoma [49], ZNRD1ASP-rs6928966 with breast cancer [50] and PRKAA1-rs1002424 with gastric cancer [51]. Furthermore, some SNPs were related to many kinds of cancer. For example, OSBPL10-rs13317583 was associated with diffuse large B-cell lymphoma and bladder cancer [52,53], PTPRT-rs73271475 with colorectal cancer [54,55] and gastric cancer [56], ABCC4-rs4148497 with pancreatic cancer [57], breast cancer [58] and epithelial ovarian cancer [59].
SNPs affecting the mental health of older adults also contributed to the development of frailty. PTPRT-rs73271475 was associated with depression [60,61], rs55767117 (close to HPHP3-AS1 ∣ TMEM108) with neuropsychiatric disorders [62] and ITIH3-rs2535629 with many mental disorders, including depression, schizophrenia and autism spectrum disorder [63-66]. These SNPs were selected for the construction of frailty-associated PRS.
Nonetheless, a majority of frailty-related SNPs were associated with immune and inflammatory responses. IRF1-AS1-rs11745587, ATF6B-rs8283, ABCC4-rs4148497 and SLC22A5-rs2073643 were associated with asthma [67-71] and ATF6B-rs8283 and MUCL3-rs9501035 with risk of systemic lupus erythematosus (SLE) [72,73]. Rs9271640 (close to HLA-DRB1 ∣ LOC124901301), LOC643339-rs7302522 and RPL37-rs6876367 were associated with multiple sclerosis [74-77], SLAMF8-rs2501341, CDC25B-rs3761218 and rs17207986 (close to ATF6B ∣ TNXB) with inflammatory bowel disease [78-80] and IRF1-AS1-rs11745587 and ADORA2A-rs2236624 with rheumatoid arthritis [81,82]. These SNPs could potentially play roles in immune dysfunction and inflammation, mediating the incidence of frailty.
Joint Association of Leisure Activities and PRS with Frailty
The joint association of leisure activities and genetic risk with frailty has not been previously investigated. No potential interactions between leisure activities and FI-associated PRS in adults aged ≥ 80 years were observed during the current analysis, suggesting that leisure activities are an intervention measure suitable for older adults with different genetic backgrounds to reduce risk of frailty. More research is required to investigate potential genes and gene-lifestyle interactions associated with frailty.
Strengths and Limitations
The strengths of this study include the national survey database with a large sample size of adults aged ≥ 80 years, the prospective cohort design with follow-up time up to 17 years, abundant covariates on personal characteristics and lifestyles and the inclusion of PRS construction. These advantages enabled us to evaluate the association of leisure activities and PRS with frailty in an extensive manner. However, there remain several limitations. First, although diverse measurements of leisure activities were considered to prove the robustness of our findings, the duration of these activities was not recorded which might cause bias in the exposure measurement. Second, the specific group of adults aged ≥ 80 years may limit the generalizability of our findings to younger populations. Third, the current frailty PRS requires validation in other racial groups to allow generalization.
Conclusion
Leisure activities and genetic risk were each independently associated with risk of frailty among Chinese adults aged ≥ 80 years in a long-term cohort study. Engagement in leisure activities provides a more feasible modifiable factor as an alternative to exercise to prevent frailty among adults aged ≥ 80 years, many of whom suffer limitations in daily activities and are less likely to finish regular exercise. The preventive effect of leisure activities on frailty did not differ by the level of genetic risks, suggesting that leisure activities constitute an appropriate intervention measure for all older adults, even for those with high genetic risk of frailty. Evidence is presented to support the primary prevention of frailty among the adults aged ≥ 80 years to promote healthy ageing.
Supplementary Material
Acknowledgements
The authors would like to thank all the CLHLS staff and participants for their time and valuable contribution.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding Sources
This work was supported by the National Natural Science Foundation of China (grant numbers 82025030, 82222063, 81941023, and 81872707) and Claude D. Pepper Older Americans Independence Centers grant (grant number 5P30 AG028716 from NIA).
Footnotes
Statement of Ethics
Ethical approval was granted by the biomedical ethics committee of Peking University (IRB00001052-13074) and the ethics committee of the National Institute of Environmental Health, Chinese Center for Disease Control and Prevention (No. 201922). Written informed consents were obtained for all participants or their proxy respondents.
Conflict of Interest Statement
The authors declared that they have no competing interests.
Data Availability Statement
The survey data are available from the Peking University Open Research Data Platform (Website: https://opendata.pku.edu.cn/dataverse/CHADS) and the data manager (chads@nsd.pku.edu.cn) for researchers who meet the criteria for data access. Further enquiries can be directed to the corresponding authors.
References
- 1.WHO Clinical Consortium on Healthy Ageing. Report of consortium meeting 1–2 December 2016 in Geneva, Switzerland. Geneva: World Health Organization; 2017; [cited 2022 May 13]. Available from: https://www.who.int/publications-detail-redirect/WHO-FWC-ALC-17.2. [Google Scholar]
- 2.Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–86. [DOI] [PubMed] [Google Scholar]
- 3.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–92. [DOI] [PubMed] [Google Scholar]
- 5.Cesari M, Calvani R, Marzetti E. Frailty in older persons. Clin Geriatr Med. 2017;33(3):293–303. [DOI] [PubMed] [Google Scholar]
- 6.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–75. [DOI] [PubMed] [Google Scholar]
- 7.United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2019. New York: United Nations; 2020; [cited 2022 May 23]. Available from: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Report.pdf. [Google Scholar]
- 8.Lv Y, Mao C, Gao X, Ji JS, Kraus VB, Yin Z, et al. The obesity paradox is mostly driven by decreased noncardiovascular disease mortality in the oldest old in China: a 20-year prospective cohort study. Nat Aging. 2022;2(5):389–96. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe D, Yoshida T, Watanabe Y, Yamada Y, Kimura M, Kyoto-Kameoka Study Group. A U-shaped relationship between the prevalence of frailty and body mass index in community-dwelling Japanese older adults: the Kyoto-Kameoka Study. J Clin Med. 2020;9(5):E1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan L, Chang M, Wang J. Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: a systematic review and meta-analysis. Age Ageing. 2021;50(4):1118–28. [DOI] [PubMed] [Google Scholar]
- 11.Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watts P, Webb E, Netuveli G. The role of sports clubs in helping older people to stay active and prevent frailty: a longitudinal mediation analysis. Int J Behav Nutr Phys Act. 2017;14(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fushiki Y, Ohnishi H, Sakauchi F, Oura A, Mori M. Relationship of hobby activities with mortality and frailty among community-dwelling elderly adults: results of a follow-up study in Japan. J Epidemiol. 2012;22(4):340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morley JE, Vellas B, Abellan van Kan G, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel JW. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet. 2017;389(10079):1619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Chen Q, Byers Kraus V, Shen D, Zhang X, Zhang P, et al. Leisure activities and disability in activities of daily living among the oldest-old Chinese population: evidence from the Chinese Longitudinal Healthy Longevity Study. Aging. 2020;12(11):10687–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers NT, Fancourt D. Cultural engagement is a risk-reducing factor for frailty incidence and progression. J Gerontol B Psychol Sci Soc Sci. 2020;75(3):571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim A, Yi E, Kim J, Kim M. A study on the influence of social leisure activities on the progression to the stage of frailty in Korean seniors. Int J Environ Res Public Health. 2020;17(23):8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasim NF, Veldhuijzen van Zanten J, Aldred S. Tai Chi is an effective form of exercise to reduce markers of frailty in older age. Exp Gerontol. 2020;135:110925. [DOI] [PubMed] [Google Scholar]
- 20.Sathyan S, Verghese J. Genetics of frailty: a longevity perspective. Transl Res. 2020;221:83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mekli K, Marshall A, Nazroo J, Vanhoutte B, Pendleton N. Genetic variant of Interleukin-18 gene is associated with the frailty index in the English Longitudinal Study of Ageing. Age Ageing. 2015;44(6):938–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho Y-Y, Matteini AM, Beamer B, Fried L, Xue Q, Arking DE, et al. Exploring biologically relevant pathways in frailty. J Gerontol A Biol Sci Med Sci. 2011;66(9):975–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida OP, Norman PE, van Bockxmeer FM, Hankey GJ, Flicker L. CRP 1846G>A polymorphism increases risk of frailty. Maturitas. 2012;71(3):261–6. [DOI] [PubMed] [Google Scholar]
- 24.Sathyan S, Barzilai N, Atzmon G, Milman S, Ayers E, Verghese J. Genetic insights into frailty: association of 9p21-23 locus with frailty. Front Med (Lausanne). 2018;5:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Y Toward deeper research and better policy for healthy aging – using the unique data of Chinese Longitudinal Healthy Longevity Survey. China Econ J. 2012;5(2–3):131–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu D, Feng Q, Zeng Y. Chinese Longitudinal Healthy Longevity Study. In: Pachana NA, editor. Encyclopedia of Geropsychology. Singapore: Springer Singapore; 2016; pp 1–14. [Google Scholar]
- 27.Zhu A, Wu C, Yan LL, Wu C-D, Bai C, Shi X, et al. Association between residential greenness and cognitive function: analysis of the Chinese Longitudinal Healthy Longevity Survey. BMJ Nutr Prev Health. 2019;2(2):72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Fu S, Ding D, Lutz MW, Zeng Y, Yao Y. Leisure activities, APOE ε4, and cognitive decline: a longitudinal cohort study. Front Aging Neurosci. 2021;13:736201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin Z, Brasher M, Kraus V, Lv Y, Shi X, Zeng Y. Dietary diversity was positively associated with psychological resilience among elders: a population-based study. Nutrients. 2019;11(3):650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu D, Dupre ME, Sautter J, Zhu H, Liu Y, Yi Z. Frailty and mortality among Chinese at advanced ages. J Gerontol B Psychol Sci Soc Sci. 2009;64(2):279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–43. [DOI] [PubMed] [Google Scholar]
- 32.Jin X, He W, Zhang Y, Gong E, Niu Z, Ji J, et al. Association of APOE ε4 genotype and lifestyle with cognitive function among Chinese adults aged 80 years and older: a cross-sectional study. PLoS Med. 2021;18(6):e1003597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng Y, Nie C, Min J, Chen H, Liu X, Ye R, et al. Sex differences in genetic associations with longevity. JAMA Netw Open. 2018;1(4):e181670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marees AT, de Kluiver H, Stringer S, Vorspan F, Curis E, Marie-Claire C, et al. A tutorial on conducting genome-wide association studies: quality control and statistical analysis. Int J Methods Psychiatr Res. 2018;27(2):e1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv Y, Kraus VB, Gao X, Yin Z, Zhou J, Mao C, et al. Higher dietary diversity scores and protein-rich food consumption were associated with lower risk of all-cause mortality in the oldest old. Clin Nutr. 2020;39(7):2246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waljee AK, Mukherjee A, Singal AG, Zhang Y, Warren J, Balis U, et al. Comparison of imputation methods for missing laboratory data in medicine. BMJ Open. 2013;3(8):e002847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abeysekera W, Sooriyarachchi M. Use of Schoenfeld’s global test to test the proportional hazards assumption in the Cox proportional hazards model: an application to a clinical study. J Natn Sci Foundation Sri Lanka. 2009;37(1):41–51. [Google Scholar]
- 39.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf SL, O’Grady M, Easley KA, Guo Y, Kressig RW, Kutner M. The influence of intense Tai Chi training on physical performance and hemodynamic outcomes in transitionally frail, older adults. J Gerontol A Biol Sci Med Sci. 2006;61(2):184–9. [DOI] [PubMed] [Google Scholar]
- 41.García-Esquinas E, Andrade E, Martínez-Gómez D, Caballero FF, López-García E, Rodríguez-Artalejo F. Television viewing time as a risk factor for frailty and functional limitations in older adults: results from 2 European prospective cohorts. Int J Behav Nutr Phys Act. 2017;14(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao C, Li Z, Lv Y, Gao X, Kraus VB, Zhou J, et al. Specific leisure activities and cognitive functions among the oldest-old: the Chinese Longitudinal Healthy Longevity Survey. J Gerontol A Biol Sci Med Sci. 2020;75(4):739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taniguchi Y, Seino S, Nishi M, Tomine Y, Tanaka I, Yokoyama Y, et al. Association of dog and cat ownership with incident frailty among community-dwelling elderly Japanese. Sci Rep. 2019;9(1):18604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kojima G, Aoyama R, Taniguchi Y. Associations between pet ownership and frailty: a systematic review. Geriatrics. 2020;5(4):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao M, Gao J, Li M, Wang K. Relationship between loneliness and frailty among older adults in nursing homes: the mediating role of activity engagement. J Am Med Dir Assoc. 2019;20(6):759–64. [DOI] [PubMed] [Google Scholar]
- 46.Sun J, Kong X, Li H, Chen J, Yao Q, Li H, et al. Does social participation decrease the risk of frailty? Impacts of diversity in frequency and types of social participation on frailty in middle-aged and older populations. BMC Geriatr. 2022;22(1):553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hikichi H, Aida J, Matsuyama Y, Tsuboya T, Kondo K, Kawachi I. Community-level social capital and cognitive decline after a natural disaster: a natural experiment from the 2011 Great East Japan Earthquake and Tsunami. Soc Sci Med. 2020;257:111981. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Zhang X, Lv Y, Shen D, Li F, Zhong W, et al. Leisure activities and all-cause mortality among the Chinese oldest-old population: a prospective community-based cohort study. J Am Med Dir Assoc. 2020;21(6):713–719.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhan J, Sun S, Chen Y, Xu C, Chen Q, Li M, et al. MiR-3130-5p is an intermediate modulator of 2q33 and influences the invasiveness of lung adenocarcinoma by targeting NDUFS1. Cancer Med-US. 2021;10(11):3700–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dashti S, Taherian-Esfahani Z, Kholghi-Oskooei V, Noroozi R, Arsang-Jang S, Ghafouri-Fard S, et al. In silico identification of MAPK14-related lncRNAs and assessment of their expression in breast cancer samples. Sci Rep. 2020;10(1):8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heinrichs SKM, Hess T, Becker J, Hamann L, Vashist YK, Butterbach K, et al. Evidence for PTGER4, PSCA, and MBOAT7 as risk genes for gastric cancer on the genome and transcriptome level. Cancer Med. 2018;7(10):5057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dobashi A, Togashi Y, Tanaka N, Yokoyama M, Tsuyama N, Baba S, et al. TP53 and OSBPL10 alterations in diffuse large B-cell lymphoma: prognostic markers identified via exome analysis of cases with extreme prognosis. Oncotarget. 2018;9(28):19555–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang J, Bi Y, Liu X-P, Yu D, Yan X, Yao J, et al. To construct a ceRNA regulatory network as prognostic biomarkers for bladder cancer. J Cell Mol Med. 2020;24(9):5375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y, Scott A, Zhang P, Hao Y, Feng X, Somasundaram S, et al. Regulation of paxillin-p130-PI3K-AKT signaling axis by Src and PTPRT impacts colon tumorigenesis. Oncotarget. 2017;8(30):48782–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laczmanska I, Sasiadek MM. Tyrosine phosphatases as a superfamily of tumor suppressors in colorectal cancer. Acta Biochim Pol. 2011;58(4):467–70. [PubMed] [Google Scholar]
- 56.Chen C, Shi C, Huang X, Zheng J, Zhu Z, Li Q, et al. Molecular profiles and metastasis markers in Chinese patients with gastric carcinoma. Sci Rep. 2019;9(1):13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Z, Wang J, Shen B, Peng C, Zheng M. The ABCC4 gene is a promising target for pancreatic cancer therapy. Gene. 2012;491(2):194–9. [DOI] [PubMed] [Google Scholar]
- 58.Low FG, Shabir K, Brown JE, Bill RM, Rothnie AJ. Roles of ABCC1 and ABCC4 in proliferation and migration of breast cancer cell lines. Int J Mol Sci. 2020;21(20):E7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bagnoli M, Beretta GL, Gatti L, Pilotti S, Alberti P, Tarantino E, et al. Clinicopathological impact of ABCC1/MRP1 and ABCC4/MRP4 in epithelial ovarian carcinoma. Biomed Res Int. 2013;2013:143202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim S-H, Shin S, Kim M-H, Kim EC, Lee DY, Moon J, et al. Depression-like behaviors induced by defective PTPRT activity through dysregulated synaptic functions and neurogenesis. J Cell Sci. 2020;133(20):jcs243972. [DOI] [PubMed] [Google Scholar]
- 61.Hyde CL, Nagle MW, Tian C, Chen X, Paciga SA, Wendland JR, et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet. 2016;48(9):1031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O He, H E, H Mj, T Cc, R Mj, M Je, et al. Expression quantitative trait loci in the developing human brain and their enrichment in neuropsychiatric disorders. Genome biology. 2018;19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium, Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18(4):497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andreassen OA, Thompson WK, Schork AJ, Ripke S, Mattingsdal M, Kelsoe JR, et al. Improved detection of common variants associated with schizophrenia and bipolar disorder using pleiotropy-informed conditional false discovery rate. PLoS Genet. 2013;9(4):e1003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brandl EJ, Lett TA, Chowdhury NI, Tiwari AK, Bakanidze G, Meltzer HY, et al. The role of the ITIH3 rs2535629 variant in antipsychotic response. Schizophr Res. 2016;176(2–3):131–5. [DOI] [PubMed] [Google Scholar]
- 66.Xie X, Meng H, Wu H, Hou F, Chen Y, Zhou Y, et al. Integrative analyses indicate an association between ITIH3 polymorphisms with autism spectrum disorder. Sci Rep. 2020;10(1):5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burren OS, Reales G, Wong L, Bowes J, Lee JC, Barton A, et al. Genetic feature engineering enables characterisation of shared risk factors in immune-mediated diseases. Genome Med. 2020;12(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park T-J, Kim J-H, Pasaje CF, Park B-L, Bae JS, Uh S-T, et al. Polymorphisms of ATF6B are potentially associated with FEV1 decline by aspirin provocation in asthmatics. Allergy Asthma Immunol Res. 2014;6(2):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palikhe S, Uuganbayar U, Trinh HKT, Ban G-Y, Yang E-M, Park H-S, et al. A role of the ABCC4 gene polymorphism in airway inflammation of asthmatics. Mediators Inflamm. 2017;2017:3549375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramasamy A, Kuokkanen M, Vedantam S, Gajdos ZK, Couto Alves A, Lyon HN, et al. Genome-wide association studies of asthma in population-based cohorts confirm known and suggested loci and identify an additional association near HLA. PLoS ONE. 2012;7(9):e44008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.James G, Reisberg S, Lepik K, Galwey N, Avillach P, Kolberg L, et al. An exploratory phenome wide association study linking asthma and liver disease genetic variants to electronic health records from the Estonian Biobank. PLoS ONE. 2019;14(4):e0215026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Systemic Lupus Erythematosus Genetics Consortium, Morris DL, Fernando MMA, Taylor KE, Chung SA, Nititham J, et al. MHC associations with clinical and autoantibody manifestations in European SLE. Genes Immun. 2014;15(4):210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barcellos LF, May SL, Ramsay PP, Quach HL, Lane JA, Nititham J, et al. High-density SNP screening of the major histocompatibility complex in systemic lupus erythematosus demonstrates strong evidence for independent susceptibility regions. PLoS Genet. 2009;5(10):e1000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Emmer A, Brütting C, Kornhuber M, Staege M. Genetic determinants of antibody levels in cerebrospinal fluid in multiple sclerosis: possible links to endogenous retroviruses. Int J Mol Sci. 2018;19(3):786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goris A, Pauwels I, Gustavsen MW, van Son B, Hilven K, Bos SD, et al. Genetic variants are major determinants of CSF antibody levels in multiple sclerosis. Brain. 2015;138(3):632–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mehta D, Wani S, Wallace L, Henders AK, Wray NR, McCombe PA. Cumulative influence of parity-related genomic changes in multiple sclerosis. J Neuroimmunol. 2019;328:38–49. [DOI] [PubMed] [Google Scholar]
- 77.Matesanz F, González-Pérez A, Lucas M, Sanna S, Gayán J, Urcelay E, et al. Genome-wide association study of multiple sclerosis confirms a novel locus at 5p13.1. PLoS ONE. 2012;7(5):e36140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. 2017;49(2):256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peters JE, Lyons PA, Lee JC, Richard AC, Fortune MD, Newcombe PJ, et al. Insight into genotype-phenotype associations through eQTL mapping in multiple cell types in health and immune-mediated disease. PLoS Genet. 2016;12(3):e1005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haritunians T, Taylor KD, Targan SR, Dubinsky M, Ippoliti A, Kwon S, et al. Genetic predictors of medically refractory ulcerative colitis: Inflamm Bowel Dis. 2010;16(11):1830–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo S, Jin Y, Zhou J, Zhu Q, Jiang T, Bian Y, et al. MicroRNA variants and HLA-miRNA interactions are novel rheumatoid arthritis susceptibility factors. Front Genet. 2021;12:747274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hider SL, Thomson W, Mack LF, Armstrong DJ, Shadforth M, Bruce IN. Polymorphisms within the adenosine receptor 2a gene are associated with adverse events in RA patients treated with MTX. Rheumatology. 2008;47(8):1156–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The survey data are available from the Peking University Open Research Data Platform (Website: https://opendata.pku.edu.cn/dataverse/CHADS) and the data manager (chads@nsd.pku.edu.cn) for researchers who meet the criteria for data access. Further enquiries can be directed to the corresponding authors.