Abstract
The purpose of this study was to evaluate if phytocannabinoids, synthetic cannabidiol (CBD), and tetrahydrocannabivarin (THCV), and their combination, could protect mice from Paclitaxel-induced peripheral neuropathy (PIPN). Six groups of C57BL/6J mice (n = 6) were used in this study. The mice were given paclitaxel (PTX) (8 mg/kg/day, i.p.) on days 1, 3, 5, and 7 to induce neuropathy. Mice were evaluated for behavioral parameters, and dorsal root ganglions (DRG) were collected from the animals and subjected to RNA sequencing and westernblot analysis at the end of the study. On cultured DRGs derived from adult male rats, immunocytochemistry and mitochondrial functional assays were also performed. When compared to individual treatments, the combination of CBD and THCV improved thermal and mechanical neurobehavioral symptoms in mice by twofold. Targets for CBD and THCV therapy were identified by KEGG (RNA sequencing). PTX reduced the expression of p-AMPK, SIRT1, NRF2, HO1, SOD2, and catalase while increasing the expression of PI3K, p-AKT, p-P38 MAP kinase, BAX, TGF-β, NLRP3 inflammasome, and caspase 3 in DRG homogenates of mice. Combination therapy outperformed monotherapy in reversing these protein expressions. The addition of CBD and THCV to DRG primary cultures reduced mitochondrial superoxides while increasing mitochondrial membrane potentials. WAY100135 and rimonabant altered the neuroprotective effects of CBD and THCV respectively by blocking 5-HT1A and CB1 receptors in mice and DRG primary cultures. The entourage effect of CBD and THCV against PIPN appears to protect neurons in mice via 5HT1A and CB1 receptors respectively.
Keywords: Peripheral Neuropathy, Cannabidiol, Tetrahydrocannabivarin, AMPK, CB1 receptors, 5-HT1A receptors
1. Introduction
Peripheral neuropathies are the most frequent type of neurodegeneration affecting millions of people worldwide. Chemotherapeutic drugs such as taxanes, vinca alkaloids, and proteasome inhibitors like bortezomib, aromatase inhibitors (letrozole, anastrozole, exemestane) induce peripheral neuropathy (CIPN) [1]. CIPN is the leading cause of dose reductions, skipping, and limitation during cancer treatment. Treatment of CIPN is a substantial unmet medical need in the United States and significantly impacts clinical outcomes and survival. Paclitaxel (PTX) has been used to treat aggressive and metastatic breast, pancreatic, ovarian, non-small-cell lung cancers and other solid organ malignancies [2]. However, with incidence rates in patients ranging from 11 to 90%, PTX-induced neuropathy (PIPN) is one of the most common clinical issues [3]. It can cause sensory dominant neuropathy, which mostly affects small diameter sensory fibers and manifests clinically as paraesthesias, dysesthesias, numbness, impaired proprioception and loss of dexterity in toes and fingers. These symptoms are dose-dependent and they usually go away after the medication is stopped. Symptoms in some people can linger for up to 3 years after the therapy is stopped, and in other cases, they can last a lifetime [4]. However, existing treatments for PIPN are only partially effective, and the processes underlying the development of PIPN remain unknown. As a result, there is an urgent need to find a therapeutic that maintains anti-tumor efficacy and effectively manages PIPN.
PTX has an uncertain mechanism of action; however, it affects cellular cytoskeleton, DNA synthesis, endoplasmic reticulum and mitochondrial function [5]. According to recent reports, PTX alters the activity of voltage-gated sodium [6], potassium, and transient receptor potential vanilloid (TRPV) channels, affecting neuronal firing and synchronization [7]. Infiltration of activated microglia by accumulated PTX in DRGs resulted in the release of pro-inflammatory cytokines, which hampered peripheral nerve system synchronization with CNS [8]. PTX also interferes with noradrenaline and 5-HT signaling, exacerbating the consequences of central sensitization [8].
Several studies have demonstrated that cannabinoids effectively alleviate neuropathic, inflammatory and cancer-related pain [9]. CBD and THCV are major phytocannabinoids that have received significant attention as therapeutic agents in various applications (e.g., anxiety, aging, obesity, cancer, pain, and other CNS diseases) [10]. Unlike THC, which is psychoactive and acts as an agonist at both the CB1 and CB2 receptors, THCV is a non-psychoactive, neutral CB1 antagonist/reverse agonist that can behave as a partial agonist or antagonist at the CB2 receptors depending on the dose. The mechanism by which THCV antagonizes the impact of THC is unknown and is thus hypothesized to prevent the psychological effects of THC. In contrast to THC, THCV causes hypophagia in both fasted and non-fasted mice [11]. However, THCV showed potential beneficial effects on metabolic disturbances associated with obesity, such as hyperglycemia, dyslipidemia, and fatty liver, in dietary-induced obese and genetically obese (ob/ob) mice, suggesting pharmacology distinct from that of CB1 inverse agonists/antagonists [12]. In another study, THCV was observed to alleviate insulin resistance and obesity in diet-induced obese mouse models by interacting with TRPV1 ion channels and modifying metabolic processes [13]. On the contrary, CBD’s therapeutic effect in inflammatory and neuropathic pain models is demonstrated by reduced levels of various mediators such as prostaglandin E2 (PGE2), lipid peroxidation and nitric oxide (NO), constitutive endothelial NO synthase (eNOS), and tumor necrosis factor-alpha (TNF-α) [14]. CBD has been reported to interfere with a range of receptors, including CB1, CB2, 5HT1A receptors, and TRPV1 ion channels, in reducing pain produced by a variety of ailments in several animal models [15].
Despite their outstanding clinical benefits, CBD and THCV have been shown to be safe when used in appropriate doses. CBD doses of up to 200 mg per day and THCV doses of up to 10 mg per day have been used for up to 8–13 weeks with no side effects [11,16]. However, the safety of this treatment is still unknown, but most studies have found no negative side effects when administered acutely and mild to moderate side effects when administered chronically. Further, more preclinical and clinical research is needed to determine their long-term side effects.
Currently, no reports have conclusively demonstrated the role of any specific agents against CIPN, which is a potential problem: CBD and THCV work directly or indirectly on transient receptor potential cation channel subfamily V member 1 (TRPV1), cannabinoid (CB 1 & 2), 5-HT1A, and 3-Glycine receptors to reduce thermal, mechanical, and drug-induced pain hypersensitivities. In this study, we have evaluated for the first time, the neuroprotective effects of THCV and its combination with synthetic CBD against PIPN. We extracted DRGs from the treated mice and used RNA sequencing to evaluate the transcriptome changes related to CBD and THCV against PTX-induced neuropathy. Furthermore, to assess the effect of CBD and THCV on these receptors in altering PTX-induced nociception in mice, we utilized CB1, TRPV1, and 5HT1A blockers. The overall hypothesis here is that combination of CBD and THCV will have significant effect against CIPN in vivo. Additionally, blocking Cannabinoid and non cannabinoid receptors in DRG primary cultures, allowed us to investigate the specific mechanism of action of these drugs on neurite outgrowths and mitochondrial function. Further, in this study we used rats for DRG culture experiments instead of mice because we have established the conditions for growing DRGs in rats only. However, we are currently in the process of growing DRGs from mice and future experiments will be conducted with them.
2. Materials and methods
2.1. Materials
Paclitaxel (Sigma Aldrich, MO, USA, Cat# T7191), synthetic Cannabidiol (PurisysTM, Athens, GA, Cat# NQS1951), Tetrahydrocannabivarin (Open Book Extracts, Roxboro, NC, USA, Cat# THCV-003–201106), WAY100135 (Sigma Aldrich, MO, USA, Cat# W1895), Rimonabant (Sigma Aldrich, MO, USA, Cat# SML0800), AM630 (Sigma Aldrich, MO, USA, Cat# SML0327), DMEM/HAMS F12 media (Genesee scientific, CA, USA, Cat# 25–503), Neurobasal Media (Thermo Fischer scientific, Waltham, MA, USA, Cat# 21103049), N2 supplements (Thermo Fischer scientific, Waltham, MA, USA, Cat# 17502048), NGF (Sigma Aldrich, MO, USA, Cat# N8133), MITOSOX Stain (Abcam, Boston, MA, USA, Cat# ab219943), Neurite outgrowth assay kit (Thermo Fischer scientific, Waltham, MA, USA, Cat# A15001), TPER (Thermo Fischer scientific, Waltham, MA, USA, Cat# 78510), Collagenase (Sigma Aldrich, MO, USA, Cat# C6885), fetal bovine serum (Atlanta biological, MN, USA, Cat# S11150). Unless specified all the chemicals (GLP/GMP grade) were purchased from Sigma Aldrich, USA.
2.2. Invivo
2.2.1. Animals
C57BL/6J mice (4–5 weeks old) and male Sprague Dawley rats (8–9 weeks old) were provided by Envigo (Indianapolis, IN) for the current study on PIPN. FAMU has AAALAC-accredited animal facilities, and following NIH recommendations (Guide for the care and use of laboratory animals), the current protocol was evaluated and approved by Florida Agricultural and Mechanical University’s Institutional Animal Use and Care Committee (protocol numbers: 020–06 & 021–04). The animals were sacrificed using the carbon dioxide (CO2) asphyxiation procedure.
2.2.2. Study design
To establish peripheral neuropathy, C57BL/6J female mice (4–5 weeks old) were given PTX (8 mg/kg, i.p.) every other day for four injections. In first set of experiments, animals were divided into three groups after they had developed neuropathy: a. vehicle treated age-matched normal mice, b. PTX (8 mg/kg, i.p.) every other day for four injections, c. cannabidiol (CBD) group: animals were given 10 mg/kg CBD (i.p.) twice a week for six weeks following the previous PTX injection. d. Tetrahydrocannabivarin (THCV) group: animals were given 15 mg/kg of THCV (i.p.) twice a week for a total of six weeks after the last dose of PTX injection, e. Cannabidiol (CBD) + Tetrahydrocannabivarin (THCV) group: animals were given 10 mg/kg of CBD and 15 mg/kg of THCV (i.p.) and in the second set of experiments, the effects of CB1, CB2, and 5HT1A blockers on CBD and THCV-induced neurobehavioral changes in PTX-induced neuropathic mice were studied by dividing the animals into six groups; a &b: (PTX + CB1B + CBD or THCV): CB1 blocker (CB1B) 3 mg/kg/day, i.p., was given to mice for four weeks and three hours before administering CBD or THCV. c&d: (PTX + 5HT1AB + CBD or THCV): 5HT1A blocker (5HT1AB), 10 mg/kg/day, i.p., was given to mice for four weeks and three hours before administering CBD or THCV e&f: (PTX + CB2B + CBD or THCV): CB2 blocker (CB2B) 1 mg/kg/day, i.p., was given to mice for four weeks and three hours before administering CBD or THCV. Further, PTX, CBD, THCV, AM630, Rimonabant and WAY10065 solutions and dosages were prepared and determined using existing literature reports [17–25]. After the animals were euthanized using CO2 asphyxiation, dorsal root ganglions (L1-L5) were extracted and biochemical and molecular parameters were examined.
2.2.3. Behavioral studies
2.2.3.1. Thermal hyperalgesia
2.2.3.1.1. Hargreaves plantar test.
This test was carried out with some modifications [26,27]. Briefly, the animals were housed in 12 plexiglass enclosures kept at a constant temperature (30 °C) above a horizontal glass surface. With a cut-off time of the 20 s, the time it took a mouse to lift its right or left paw after being exposed to a radiant heat source of infrared irradiation (40 IR units) was measured. The results are reported in seconds as paw withdrawal latency, with a 10-minute time interval between each consecutive reading.
2.2.3.1.2. Hot and cold plate method.
Thermal hyperalgesia was measured using the hot and cold plate method, as previously reported [28]. Immediately after acclimatization, the mice were placed on a hot plate (55 °C) and a cold plate (10 °C) where the time latency for the animal to lick its right/left foot was measured with a cut-off time of 20 s and an interval of 10 min at each reading, and the results were reported as paw withdrawal latency in seconds.
2.2.3.2. Mechanical hyperalgesia.
The Vonfrey and Randall Selitto tests were used to assess mechanical hypersensitivity in mice. The mice were poked with standard vonfrey fibres of varying weights (g), and the weight at which they lifted their paws was monitored using a digital electronic readout unit and reported as paw withdrawal threshold (g). The paw withdrawal pressure of mice was measured using Randall sellito pincture pressure on both paws. Each animal was tested five times, with a 10–15 min delay between each reading [29]
2.2.4. RNA sequencing
RNA sequencing on DRG homogenates from control, PTX, CBD, and THCV-treated mice was performed by Novogene Corporation Inc (Sacramento, CA). Briefly, messenger RNA was isolated from total RNA using poly-T-oligo magnetic beads. This was followed by second strand cDNA synthesis using either dUTP or dTTP depending on the library type. Except in directed library preparation, user enzyme digestion was included after size selection. The library was quantified with Qubit, and the size distribution was detected with a bioanalyzer. Quantified libraries were pooled and sequenced on Illumina platforms. Clustering and sequencing were done following manufacturer’s instructions. After generating clusters and paired-end reads, the library preparations were sequenced on Illumina. Initially, quality control of raw data in fastq format was handled using perl programs. Raw reads were cleaned by eliminating adaptor, poly-N, and low quality reads. The cleaned data Q20, Q30, and GC content were calculated and used for downstream analysis. For better mapping results, clean reads were mapped to the reference genomes using Hisat2 v2.0.5, which developed a database of splice junctions based on the gene model annotation file. Feature Counts v 1.5.0-p3 was used to count the reads mapped to each gene. In order to assess gene expression levels, FPKM (fragments per kilobase of exon per million mapped fragments) was calculated. DESeq2 R software was used to analyze differential gene expression (1.20.0). In order to reduce the false discovery rate, Benjamini and Hochberg’s procedure was used. Genes with a P < 0.05 difference across groups were considered differentially expressed. The online Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/) was used to examine the statistical enrichment of differential expression genes in KEGG pathways. The effective genes and critical pathways in regulating neuronal function were predicted using Reactome, disease ontology, and DisGeNET databases.
2.2.5. Western blotting
For this study, separated DRGs were homogenized in TPER (1:100), centrifuged at 20,000 rpm for 20 min, and the supernatant was collected and evaluated for total protein content using a bicinchonic acid test kit. To denature the proteins, the sample was heated to 95 °C for 10 min with 4X laemmli buffer containing 5% mercapto-ethanol. SDS PAGE gel electrophoresis resolved 40 μg protein. Semi-dry transfer of resolved gel containing segregated proteins to PVDF/nitrocellulose membranes (Transbloto, Biorad, USA). Incubation with primary antibodies (P-38 MAPkinase (Cell signalling technology, USA, Cat# 4631), p-AMPK (Cell signalling technology, USA, Cat# 2535S), PI3K (Cell signalling technology, USA, Cat# 4249), p-AKT (Cell signalling technology, USA, Cat# 4060), TFAM (Santacruz biotechnologies, CA, USA, Cat# 166965), HO-1 (Cell signalling technology, USA, Cat# 43966S), Catalase (Santacruz biotechnologies, CA, USA, Cat# 271803), Nrf2 (Cell signalling technology, USA, Cat# 12721S), Bax (Cell signalling technology, USA, Cat# 14796), and TGF-beta (Cell signalling technology, USA, Cat# 3711) of rabbit or mouse origin) in 1:1000 dilution in 3% BSA solution at 4 °C overnight followed by three washes with PBST. We next probed the membranes for 2 h at room temperature with HRP conjugated secondary anti-rabbit (Cell signalling technology, USA, Cat# 7074) and anti-mouse (Cell signalling technology, USA, Cat# 56970) antibodies in 1:20000 dilution in 3% BSA solution. The ChemiDocTMXRS + imaging system (BIO-RAD) was used to collect the luminescence signal which was quantified by using image J software (version 1.48, NIH, USA) [30].
2.3. In-vitro DRG primary cultures
Primary DRG neuronal cells were generated in vitro using adult rat dorsal root ganglions from the (L1-l5) lumbar region of the spinal cord with slight modifications as earlier reported [31]. Briefly, 9–10 week old rats were sacrificed and DRGs were separated aseptically into HAMS F12 medium with 10% FBS and 5% antibiotic/antimycotic solution. This was followed by centrifugation at 1200 rpm for 2 min before adding Trypsin (Thermo Fischer scientific, Waltham, MA, USA, Cat# 15050065) (0.25 percent) for 30 min and triturating with a glass pipette to dissociate into cells. This cell and tissue suspension was filtered through 70 μM nylon gauge. The monosuspended DRG cells were centrifuged for 3 min at 1200 rpm and resuspended in neurobasal media containing 10% FBS and 0.5 percent antibiotic and antimycotic solution. A 1:50 vol ratio of matrigel matrix (Corning, USA, Cat# 356234) to neurobasal media was used to grow the cells. The neurite extensions appeared two–three days after culture and were treated with various treatments at optimal concentrations.
2.3.1. Neurite outgrowth assay
Three days after DRG primary cultures in 12 well plates were treated with 3 μM of PTX followed by post treatment with CBD and THCV at (12 μM each) and 5HT1A blocker (WAY100135) and CB1 blocker (rimonabant) at 1 μM each for 48 h. At specified intervals (48 h), the cells were washed with fresh PBS and neurite outgrowth assay was performed using the manufacturer’s kit-based protocol. Five fields were chosen at random and examined using a phase contrast microscope (Nikon ECLIPSE, Ti-U, Japan). The length of neurite outgrowths in 30 cells from each field was measured using Image J software (NIH, USA). The number of neurite outgrowths/axon-like extensions that are twice or more than the diameter of the cell body were counted [32].
2.3.2. Immunocytochemistry
DRG neurons were cultured on glass Coverslips in a 6-well plate at 5000 cells/well seeding density and fixed with 4% paraformaldehyde solution and permeabilized with 0.5 percent Triton-X 100 for 15 min at room temperature, as described elsewhere. These cells were blocked for 2 h at room temperature with a 3 percent BSA solution in PBS. After blocking, the cells were incubated overnight at 4 °C with primary antibodies (p-AMPK, Complex I, and TFAM) at 1:200 dilutions in 3% BSA solution. The following day, cells were washed with PBST and incubated with secondary Goat anti-Mouse IgG (H + L) Secondary Antibody, DyLight 633 (Thermo Fischer scientific, Waltham, MA, USA, Cat# 35512) and Zenon™ Alexa Fluor™ 488 Rabbit IgG Labeling Kit (Thermo Fischer scientific, Waltham, MA, USA, Cat# Z25302) at room temperature for 2 h in 3% BSA solution at 1:100 dilution. Finally, the coverslips were adhered to the glass slide with DAPI mounting medium (Sigma Fluoroshield™). A confocal microscope was used to capture the images (Leica TCS SP8 Laser Scanning Spectral Confocal microscope, Germany) [33].
2.3.3. Assay for JC1
DRG primary cultures were stained with JC1 as previously described [34,35]. Cultured DRG primary cells were incubated for 15 min with 5 μM JC-1 in PBS. The cells were washed with PBS to remove unbound red stain before being examined with a fluorescence microscope (Nikon ECLIPSE, Ti-U, Japan) with red and green filters. The mean red and green fluorescence intensity ratio was calculated using Image J software (NIH, USA).
2.3.4. Mitosox test
The Mitosox red assay was carried out in DRG primary cultures according to published protocols [36]. Briefly, the cultures were treated with 3 μM of PTX followed by post treatment with CBD and THCV at (12 μM each) and 5HT1A blocker (WAY100135) and CB1 blocker (rimonabant) at 1 μM each for 48 h. The cultures were washed with PBS and incubated for 15 min at 37 °C with 5 μM Mitosox reagent. The cultures were then washed to remove any unbound Mitosox red reagent before being examined with a fluorescence microscope (Nikon ECLIPSE, Ti-U, Japan) with a green filter. Image J software was used to calculate the mean red fluorescence intensity (NIH, USA).
2.4. Statistical analysis
Excel was used to calculate mean, SD, and SEM for each parameter. The results were analyzed with the newest version of graph pad prism program, with one way ANOVA to compare the groups (multiple comparison tests). When one way ANOVA demonstrated statistical significance, Bonferroni’s multiple comparisons test was used for post hoc analysis. Statistical significance was defined as P < 0.05 or less.
3. Results
3.1. CBD, THCV, and their combination on PTX-induced neurobehavioral changes
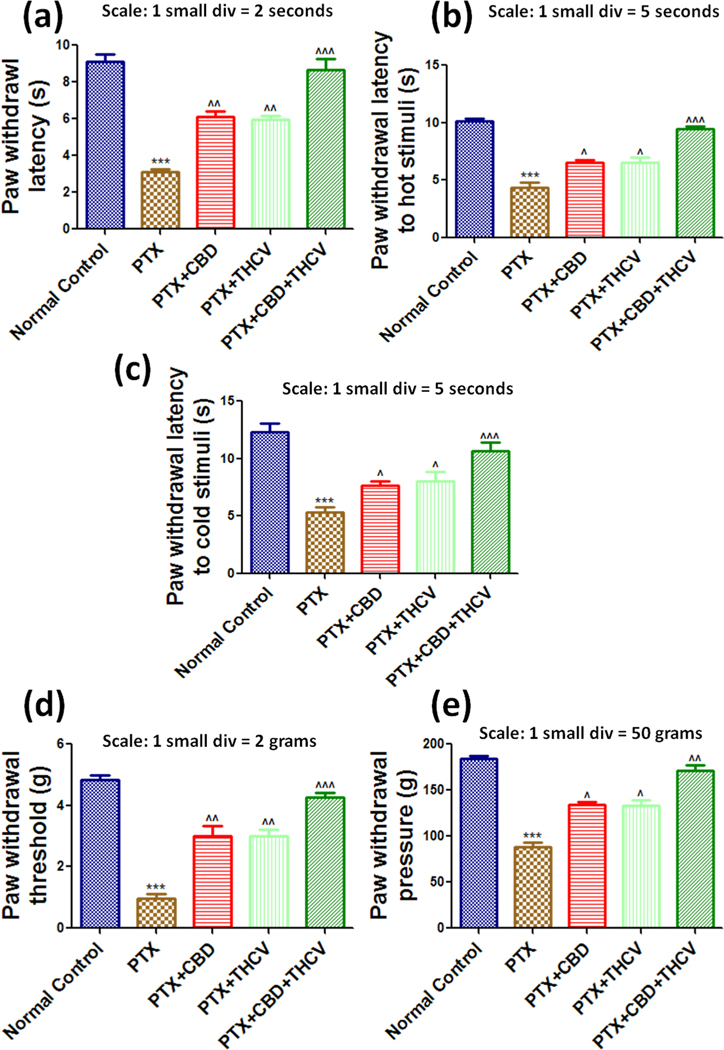
PTX (8 mg/kg) treatments to the animals significantly (p < 0.001) produced mechanical and thermal hyperalgesia in mice, and the neuropathy in animals lasted until the last day of the study (Fig. 1 and suppl Figs. 3 & 4). The neuropathic mice were given CBD (10 mg/kg) and THCV (15 mg/kg) twice a week for six weeks which reduced PTX-induced thermal hypersensitivity (p < 0.01), as determined Hot and cold plate method (Fig. 1 & suppl Fig. 3). Their combination alleviated neuropathic pain by reducing PTX-induced thermal hyperalgesia. We also evaluated the role of CBD, THCV, and their combination on PTX-induced mechanical hyperalgesia. The paw withdrawal thresholds and pressures of CBD and THCV treated mice were significantly different from PTX treated animals in Electronic-Vonfrey and Randall selitto studies (Fig. 1 & suppl Fig. 4). Besides that, administration of 3 mg/kg rimonabant (CB1 blocker, i.p.) and 1 mg/kg AM630 (CB2 blocker) blocked the effect of CBD on mechanical and thermal hypersensitivity in mice. Further, administration of 1 mg/kg AM630 and 10 mg/kg WAY10065 (5HT1A blocker) had no effect on the neurobehavioral changes associated with THCV. However, in PTX-induced neuropathic mice, 10 mg/kg WAY10065 and 3 mg/kg rimonabant significantly (p < 0.05 to 0.01) reduced the neurobehavioral effects of CBD and THCV, respectively (Table 1). Further, to investigate the gene regulation of CBD and THCV in DRG homogenates from PTX-treated mice, we used RNA sequencing on isolated DRG neurons from different groups of mice.
Fig. 1. Effects of CBD, THCV, and their combination on the neurobehavioral parameters in PTX-induced neuropathic mice.
(a) The Hargreaves plantar test, (b) the hot plate method, (c) the cold plate method, (d) the Vonfrey test, and (e) the Randall selitto test are all represented by bar graphs. The data is presented as a mean ± standard error of the mean (n = 5). ***p < 0.001 Vs normal control, ^p < 0.05, ^^p < 0.01 and ^^^p < 0.001 Vs PTX (8 mg/kg).
Table 1. Effect of CB1 blocker (Rimonabant-3 mg/kg, i.p), CB2 blocker (AM630, 1 mg/kg/day, i.p), and 5HT1A blocker (WAY100135, 10 mg/kg, i.p) on CBD and THCV induced neurobehavioral changes:
The values are expressed as mean standard error of the mean (n = 6), normal control: untreated age matched mice, PTX: mice received (8 mg/kg/day) on alternate days for four injections cumulatively, PTX + CB1B + CBD/THCV: Mice were given a CB1 blocker (CB1B) 3 mg/kg/day, i.p. for four weeks three hours before receiving CBD or THCV, PTX + 5HT1AB + CBD/THCV: Mice were given a 5HT1A blocker (5HT1AB) 10 mg/kg/day, i.p. for four weeks three hours before receiving CBD or THCV, PTX + CB2B + CBD/THCV: Mice were given a CB2 blocker (CB2B) 1 mg/kg/day, i.p. for four weeks three hours before receiving CBD or THCV.
| Parameter | Normal Control | PTX | PTX + CB1B + CBD | PTX + 5HT1B + CBD | PTX + CB2B + CBD | PTX + CB1 + THCV | PTX + 5HT1AB + THCV | PTX + CB2B + THCV |
|---|---|---|---|---|---|---|---|---|
| Paw withdrawal latency to hot stimuli (s) | 10.2 ± 0.4 | 4.9 ± 0.2*** | 6.9 ± 0.3^ | 5.7 ± 0.4 | 7.4 ± 0.4^^ | 5.2 ± 0.8 | 7.8 ± 0.8^^ | 6.8 ± 0.3^ |
| Paw withdrawal latency to cold stimuli (s) | 12.5 ± 0.6 | 5.3 ± 0.4*** | 7.2 ± 0.5^^ | 5.1 ± 0.2 | 7.3 ± 0.8^^ | 5.3 ± 0.4± | 7.9 ± 0.5^^ | 7.1 ± 0.4^^ |
| Paw withdrawal latency (s) | 9.4 ± 0.2 | 3.2 ± 0.7*** | 5.2 ± 0.5^ | 3.4 ± 0.1 | 5.0 ± 0.4^ | 3.7 ± 0.5 | 5.6 ± 0.1^ | 6.3 ± 0.3^^ |
| Paw withdrawal threshold (g) | 3.9 ± 0.1 | 0.9 ± 0.6*** | 2.9 ± 0.6^^ | 1.3 ± 0.1 | 2.9 ± 0.7^^ | 1.6 ± 0.8 | 2.7 ± 0.3^^ | 3.2 ± 0.1^^^ |
| Average Body weight (g) | 21.98 ± 1.09 | 20.87 ± 1.8 | 23.15 ± 0.99 | 21.97 ± 1.32 | 22.13 ± 0.89 | 23.06 ± 1.56 | 20.19 ± 0.53 | 19.78 ± 1.87 |
p < 0.001 Vs Normal control &
p < 0.05
p < 0.01
p < 0.001 Vs PTX.
3.2. RNA seq analysis in DRG homogenates of CBD and THCV treated neuropathic mice
Primary sequencing data produced by RNA-Seq were subjected to quality control and after cleaning, the total reads and mapping ratio reads were calculated as shown in Table 2 which denotes the quality of RNA seq data.
Table 2.
Total reads and mapping ratio in DRG homogenates of normal control, paclitaxel, CBD and THCV treated mice (n = 3) by RNA Sequencing.
| Sample | Raw reads | Raw Bases | Clean reads | Clean bases | Error rate | Q20 | Q30 | Mapping ratio (%) |
|---|---|---|---|---|---|---|---|---|
| Normal control 1 | 52,558,934 | 7.88G | 51,976,984 | 7.8G | 0.02 | 97.98 | 94.2 | 88.9 |
| Normal control 2 | 47,350,494 | 7.1G | 46,825,174 | 7.02G | 0.03 | 97.44 | 93.19 | 89.05 |
| Normal control 3 | 43,345,782 | 6.5G | 42,842,116 | 6.43G | 0.03 | 97.95 | 94.18 | 89.97 |
| PTX 1 | 40,755,866 | 6.11G | 40,256,088 | 6.04G | 0.03 | 97.86 | 93.95 | 90.55 |
| PTX 2 | 48,838,682 | 7.33G | 48,264,496 | 7.24G | 0.03 | 97.83 | 93.89 | 91.5 |
| PTX 3 | 45,696,120 | 6.85G | 45,249,740 | 6.79G | 0.03 | 97.61 | 93.53 | 91.16 |
| PTX + CBD1 | 49,807,284 | 7.47G | 48,461,610 | 7.27G | 0.03 | 97.48 | 93.56 | 83.97 |
| PTX + CBD2 | 61,609,912 | 9.24G | 60,338,254 | 9.05G | 0.02 | 98.17 | 95.01 | 86.79 |
| PTX + CBD3 | 52,513,590 | 7.88G | 51,467,086 | 7.72G | 0.02 | 98.21 | 95.12 | 86.31 |
| PTX + THCV1 | 46,498,850 | 6.97G | 46,002,094 | 6.9G | 0.02 | 97.99 | 94.29 | 91.52 |
| PTX + THCV2 | 42,721,422 | 6.41G | 41,875,364 | 6.28G | 0.03 | 97.62 | 93.74 | 89.7 |
| PTX + THCV3 | 40,949,016 | 6.14G | 39,903,266 | 5.99G | 0.03 | 97.64 | 93.79 | 89.93 |
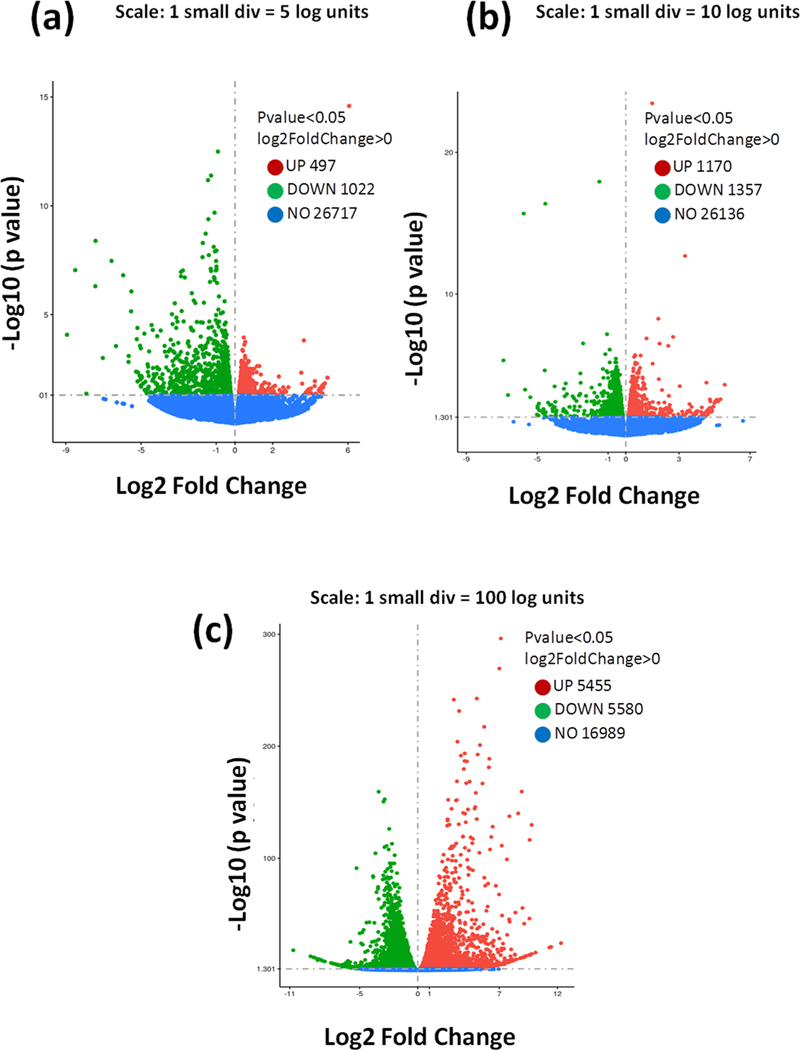
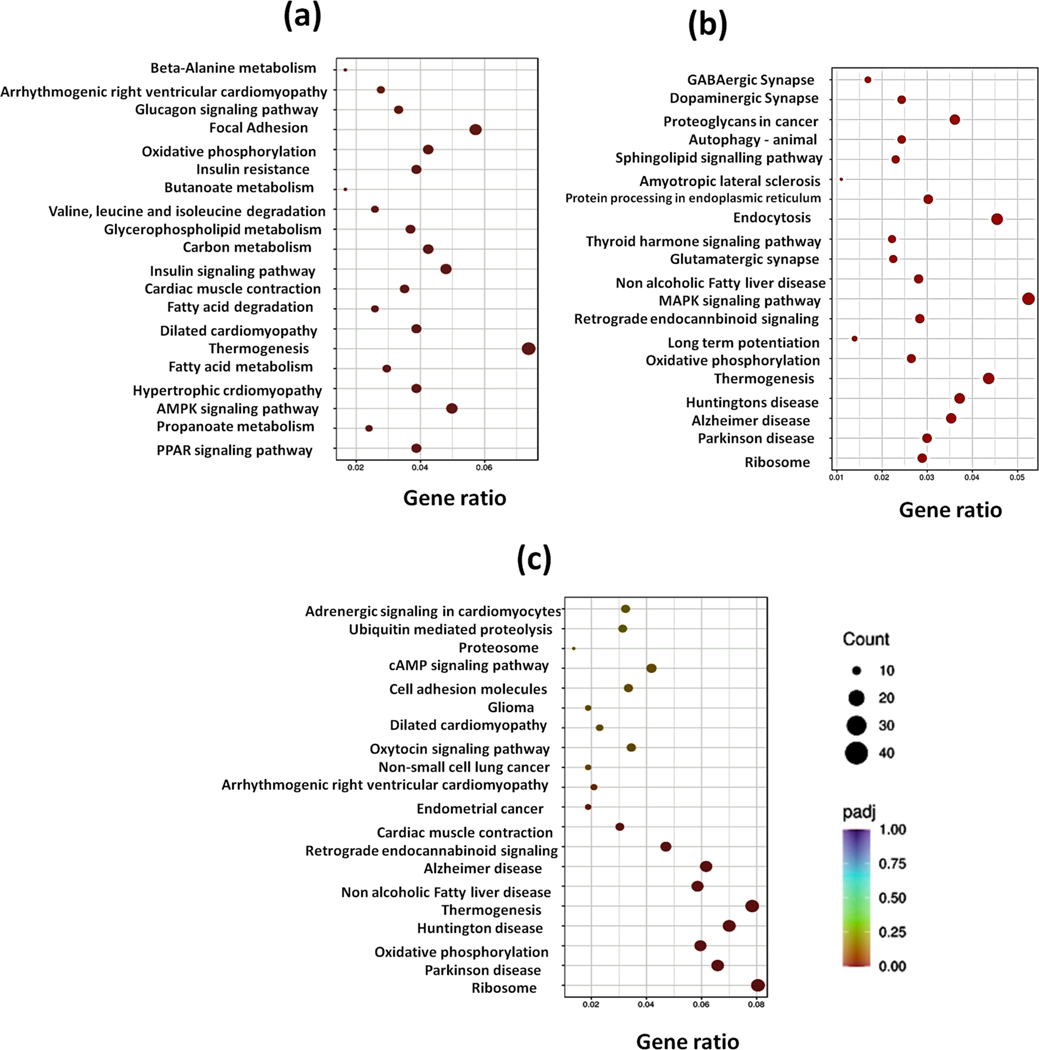
Further, we performed cluster analysis and heat map visualizations of gene expression patterns using the heatmap software. A total of 1519 genes were differentially expressed in control and PTX group, 11,035 genes in CBD treatment and PTX group and 2527 genes in THCV treatment and PTX group. The number of upregulated and downregulated genes was plotted in volcano graphs as shown in Fig. 2, suppl Fig. 1 & suppl Fig. 2. Further, KEGG analysis unraveled several signaling pathways enriched in DRG homogenates of control and treated mice. Among these pathways, cannabinoid signaling, serotonin (5HT1A receptors), AMPK-PGC1 alpha, MAPK signalling, PI3K-AKT and NLRP3 inflammasome pathways especially attracted our attention since they have been implicated in mediating chronic pain (Fig. 3). These pathways are important for regulating neuron-glial activation, cytokine production, neuroinflammation, oxidative stress, mitochondrial function, apoptosis and autophagy. Based on RNA seq analysis we further validated this pathways by performing western blotting analysis in DRG homogenates.
Fig. 2. RNA-Seq analysis showing expression alterations of differentially expressed mRNAs (DEmRNAs) in dorsal root ganglia collected form paclitaxel-induced neuropathic mice after respective treatments.
a, DEmRNAs in DRGs of PTX group mice vs. control group mice in volcano plots. b, DEmRNAs in DRGs of PTX + CBD group mice vs. PTX group mice in volcano plots. c, DEmRNAs in DRGs of PTX + THCV group mice vs. PTX group mice in volcano plots. Upregulated DEmRNAs are indicated by red points, downregulated DEmRNAs are indicated by blue points, and non– DEmRNAs are indicated by grey patches. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3. DEmRNA KEGG pathway analysis.
a. Bubble plots comparing the top 20 significant DEmRNA pathways in DRGs of PTX and control group mice. b. Bubble plots of the top 20 significant DEmRNA pathways in DRGs of PTX + CBD animals vs. PTX group mice. c. In volcano plots, bubble plots depicting the top 20 important DEmRNA pathways in DRGs of PTX + THCV group mice vs. PTX group mice. More genes are represented by larger bubbles and the significance and count are shown by the color and size of each bubble.
3.3. Effect of CBD, THCV and their combination on PI3K-AKT, P38 MAPkinase and AMPK pathway
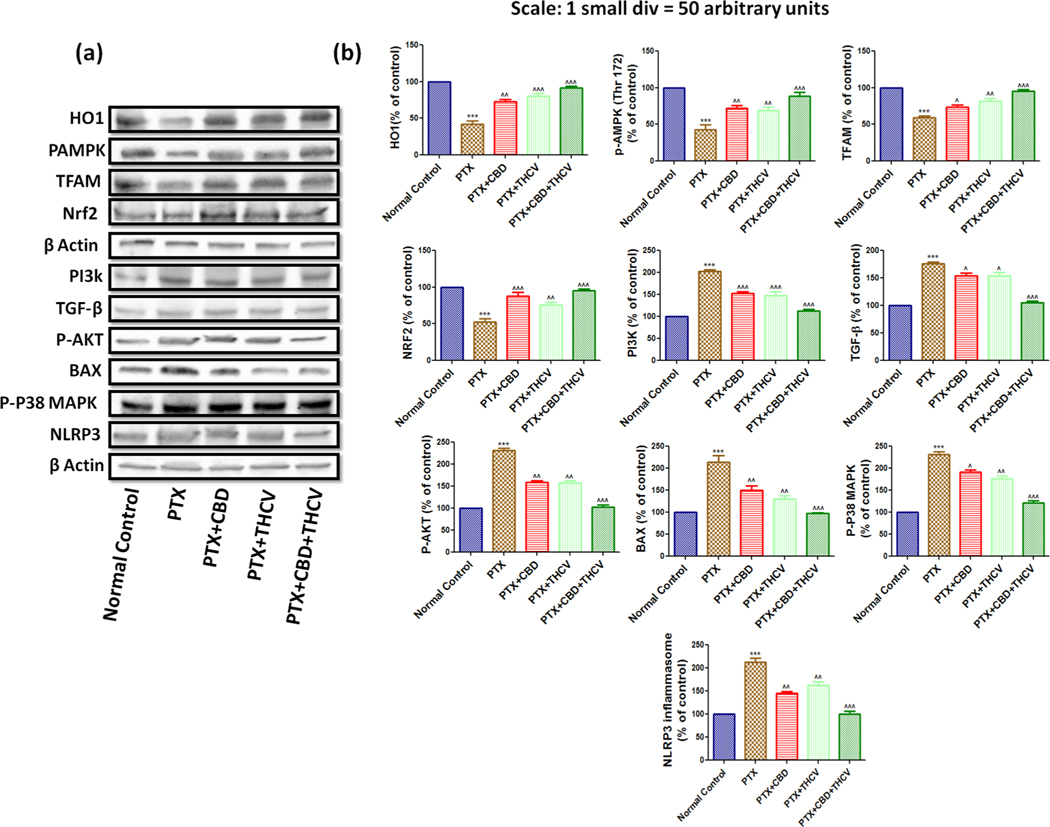
Western blotting analysis in DRG homogenates of PTX treated mice showed increased expression of PI3K (p < 0.001), p-AKT (p < 0.001), p-P38 MAP kinase (p < 0.001), BAX (p < 0.001), TGF-β (p < 0.001), NLRP3 inflammasome (p < 0.001) and caspase 3 (p < 0.001) and decreased expression of p-AMPK (p < 0.001), SIRT1 (p < 0.001), NRF2 (p < 0.001), HO1 (p < 0.001), SOD2 (p < 0.001) and catalase (p < 0.001) when compared to normal control group as shown in Fig. 4. CBD and THCV treatment significantly reversed the expressions of these proteins in DRG homogenates (from PTX treated mice) (Fig. 4). However the combination of CBD and THCV treatment to PTX induced mice significantly reversed the expression of these proteins when compared to CBD and THCV alone treatment groups as indicated in Fig. 4. These results suggest that the combination of these drugs would be a superior therapeutic strategy in improving neuropathy against PTX induced pain in mice. Further to understand the role of cannabinoid and non cannabinoid receptors in regulating these pathways after CBD and THCV treatment, we isolated DRGs (L1-L5 region) from rats and cultured them in our laboratory and evaluated the neuroprotective and mitoprotective effects of these drugs in presence of CB1 and 5HT1A receptor blockers. Rationality behind the usage of these blockers was based on the previous literature and on our in vivo results.
Fig. 4. The effects of CBD, THCV, and its combination on the p38 MAPKinase, AMPK, and PI3-AKT pathways.
(a) Western blots of DRG homogenates from PTX-treated mice demonstrate CBD, THCV, and their combination therapy for six weeks after the final PTX dose. (b) Western blot quantification is represented by bar graphs. Values are expressed as mean ± SEM (n = 3). ***p < 0.001 Vs Normal control, ^p < 0.05, ^^p < 0.01 and ^^^p < 0.001 Vs PTX (8 mg/kg).
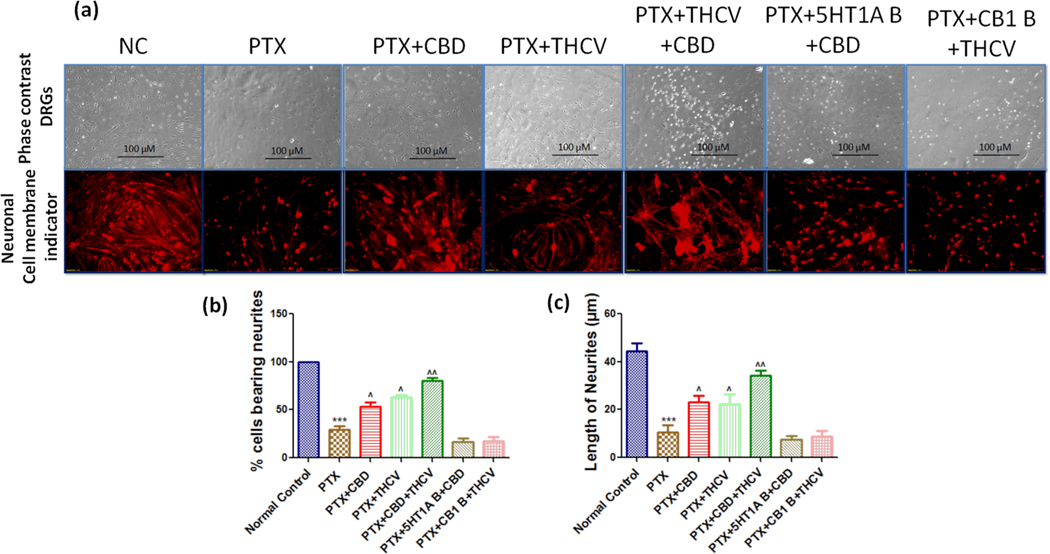
3.4. Effect of CBD, THCV and their combination on neurite outgrowths of PTX insulted DRG primary cultures
We measured the number of neurite outgrowths/ axon like extensions which were double/more than the diameter of cell body. PTX treated DRG cells significantly (p < 0.001) reduced the neurite outgrowths and percentage of neurite bearing cells when compared to normal DRG cells (Fig. 5).
Fig. 5. Effects of CBD, THCV, and their combination on neuritogenesis in cultured DRG cells derived from rats’ L1 to L5 spinal regions.
(a) Representative photomicrographs taken with a phase contrast microscope (20X) and a florescence microscope (20X) showing neurite extensions emerging from each DRG cell, (b) bar graphs representing the percent of cells bearing neurites, and (c) bar graphs representing image j analysis of neurite length in the untreated and treated groups. ***p < 0.001 Vs normal control, ^p < 0.05, ^^p < 0.01 and ^^^p < 0.001 Vs PTX.
CBD and THCV at 12 μM concentration significantly (p < 0.01) improved the neurite outgrowth and percentage of neurite bearing cells when compared to PTX treated primary DRG cells. Interestingly, CBD and THCV combination improved the neurite outgrowths in PTX treated primary DRG cells two folds better than the CBD and THCV alone treatment (Fig. 5). However, two hours before CBD and THCV treatment, CB1 receptors and 5HT1A receptors were blocked with WAY100135 (1 μM) (5HT1A antagonist), Rimonabant (1 μM) (CB1 antagonist). CBD and THCV failed to improve neurite outgrowths after blocking 5 HT1A receptors and CB1 receptors respectively as shown in Fig. 5. These results suggest that the neuroprotective effects of CBD and THCV depend upon 5 HT1A receptors and CB1 receptors respectively.
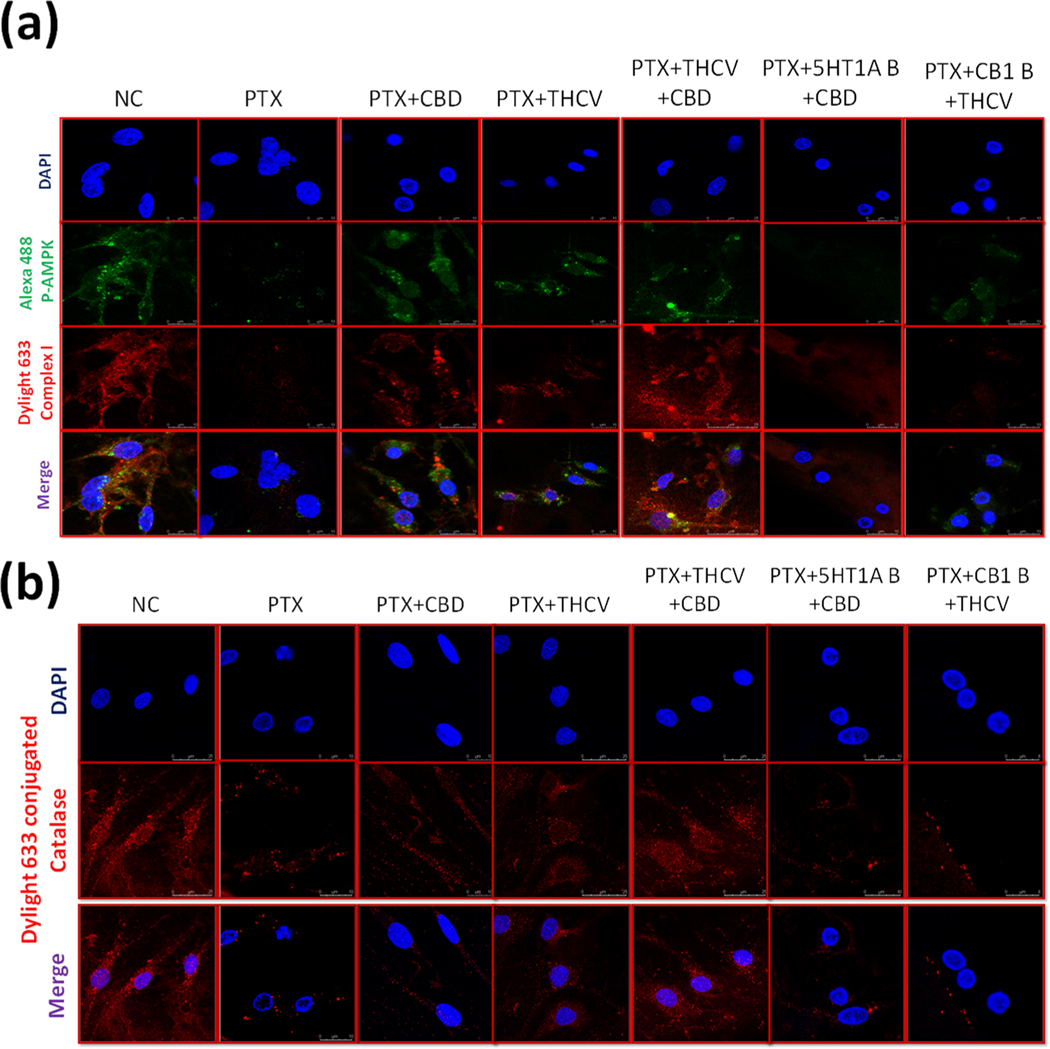
3.5. Immunoexpression of p-AMPK, complex I and catalase in treated DRG primary cultures
After confirming neuroprotective effects of CBD and THCV in presence of 5HT1A and CB1 blockers, we sought to study the molecular effects of these drugs by blocking the same receptors. In line with the invivo study, PTX induced toxicity in DRG neuronal cells, reduced the immunoexpression of p-AMPK, complex I and Catalase as shown in Fig. 6. Nevertheless, CBD and THCV treatment significantly increased the expression of these proteins and their combination significantly reversed these protein expression in PTX treated DRG neuronal cells as shown in Fig. 6. However, CBD and THCV failed to increase the expression of these proteins after blocking with 5HT1A and CB1 receptors respectively. These results signify the importance of CBD and THCV combination in attenuating neuropathic pain and also suggest the mechanism of CBD and THCV in mitigating neuropathic pain against PTX induced toxicity in DRG neurons. Further, we sought to study the mitoprotective effects of these compounds in presence of same blockers.
Fig. 6. Immunoexpression of p-AMPK, complex I, and catalase in primary DRG cells cultured in vitro.
(a) Representative confocal microscope images showing i) the first panel enumerates nuclear (DAPI) staining ii) the second panel shows immuno expression of p-AMPK tagged with Alexa488 fluorochrome, iii) the third panel is for immuno expression of mitochondrial complex I labeled with dylight 633 conjugated secondary antibody, and the fourth panel is the merge of all the above three channels (b) Representative confocal microscope images showing the upper panel nuclear (DAPI) staining, middle panel: immuno expression of Catalase tagged with dylight 633 conjugated secondary antibody and lower panel showing the merge of above two channels.
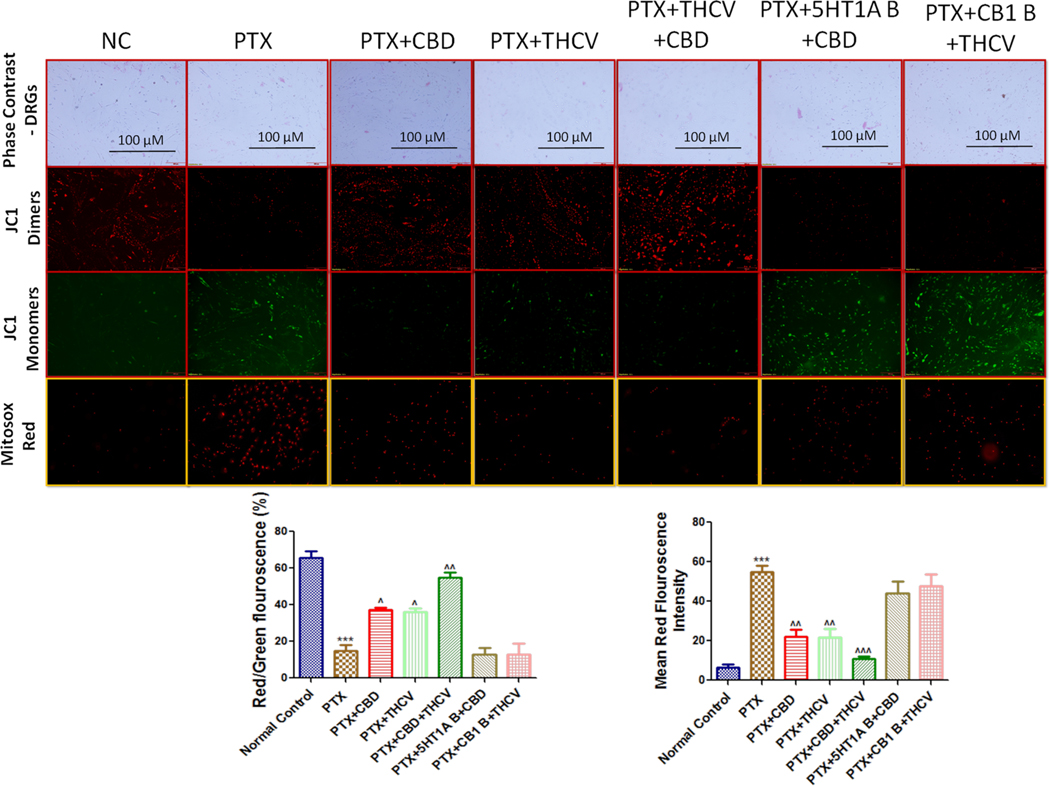
3.6. Effect of CBD, THCV and their combination on mitochondrial membrane potentials and mitochondrial superoxide production in PTX exposed DRG cells
JC1 assay explains about the mitochondrial transmembrane potentials (ΔΨm) which directly correlates the integrity and health of the mitochondria. Mitosox staining assay quantifies the mitochondrial superoxide production in cultured cells. Fluorescence imaging assay carried out in cultured DRG primary cells isolated from Rat (L1-L5 region) using mitoprobe JC1 assay kit and Mitosox staining, demonstrated mitochondrial membrane repolarization effects and reduced mitochondrial superoxides with CBD and THCV treatment respectively as shown by concentration dependent formation of red:green fluorescent J1 aggregates and decreased Mitosox red fluorescence in PTX treated DRG cells (Fig. 7). However, CBD and THCV combination therapy have significantly (p < 0.001) improved mitochondrial membrane potentials and reduced mitochondrial superoxides which is demonstrated by increased JC1 dimers formation (red fluorescent J1 aggregates) and decreased Mitosox red fluorescence respectively against PTX induced toxicity in DRG primary neuronal cells as shown in Fig. 7. Interestingly, mitochondrial membrane potentials and mitochondrial superoxides were unaltered in CBD and THCV treated DRG primary cells after blocking 5HT1A and CB1 receptors respectively. These results would suggest that mitoprotective effects are prominent with CBD and THCV combination and these effects of CBD and THCV depends upon the activation of 5HT1A and CB1 receptors respectively.
Fig. 7. Effect of CBD, THCV and their combination on mitochondrial membrane potential (ΔΨm) and mitochondrial superoxides.
(a) Representative fluorescence microscopic images showing phase contrast images (10X) (first lane), JC-1 aggregates emitting red fluorescence (second lane), JC1 monomers emitting green fluorescence (third lane) and mitochondrial superoxide production by Mitosox staining florescence (fourth lane lane). (b) Respective quantification of red: green fluorescence ratio indicating depolarized mitochondria and (c) respective mitosox staining red florescence quantification indicating the mitochondrial superoxides production in different groups (n = 3). ***p < 0.001 Vs normal control, ^p < 0.05, ^^p < 0.01 and ^^^p < 0.001 Vs PTX. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Chemotherapy-induced peripheral neuropathy (CIPN) is a critical challenge for most cancer patients. For many solid organ cancers, PTX is a mainstay chemotherapeutic drug and it triggers irreversible neurotoxicity, rendering it inappropriate for long-term usage. Currently no specific treatment is available in clinical settings to reduce the severity of this disease [2]. In this study, we investigated for the first time, the neuroprotective effects of synthetic CBD, THCV, and their combination in PTX-induced neuropathic mice. Although there have been reports of CBD’s impact on PTX-induced neuropathic pain, the molecular mechanism behind their neuroprotective benefits still remains to be investigated
Nonpsychoactive phytocannabinoids constitute a viable therapeutic for treatment of neuropathic pain [37]. CBD-containing gelatin given ad libitum orally for three weeks after surgery dramatically reduced (p < 0.01) allodynia in a sciatic nerve damage mouse model [38]. Also, CBD and its modified derivatives reduced (p < 0.001) thermal and mechanical hyperalgesia in mice and rats after intraplantar injection of 10 μl complete Freund’s adjuvant and ligation of the L5 spinal nerve respectively [39]. Further, CBD also inhibited mechanical and thermal allodynia in mice and prevented mechanical sensitivity in investigating PTX-induced neuropathic pain [25]. Despite CBD’s importance in the treatment of neuropathic pain, THCV has also been shown to have similar effects. For example, in mice, THCV reduced the thermal and mechanical hyperalgesia generated by formalin and carrageenan [40]. In line with earlier studies, current study also demonstrated that synthetic CBD and THCV treatment reduced thermal and mechanical hyperalgesia against PTX induced neuropathic mice. Interestingly, combining these two drugs proved to be more effective in providing neuroprotection against PTX-induced neuropathy in mice (P < 0.01).
PTX has been shown to damage peripheral sensory neurons, including dorsal root ganglions, and dorsal root ganglions axons, as shown by a number of studies in PTX-induced peripheral neuropathy [41,42]. In the current study, PTX treatment induced damage in isolated DRGs from mice, as demonstrated by decreased neurite outgrowths and the quantity of neurites carrying cells, as well as a change in neuron morphology. On the other hand, CBD and THCV therapy restored all of the PTX-induced alterations in the neurons with higher efficacy when used together. We used primary DRG cultures isolated from rats instead of mice because we had previously standardized and established primary DRG cultures isolated from rats.
RNA sequencing (KEGG analysis) in DRG homogenates of neuropathic mice treated with CBD and THCV revealed differences in gene expression levels, indicating the involvement of cannabinoid signaling, serotonin signaling, p38 MAPkinase, AMPK, PI3-AKT, autophagy, oxidative phosphorylation, retrograde endocannabinoid signaling, GABAmergic, glutamergic and dopamergic synapse, axon guidance and inflammatory pathways. These pathways are linked to mitochondrial biogenesis and function, oxidative stress, autophagy, apoptosis, ER stress, and neuroinflammation [43,44]. Although similar transcriptome findings with PTX injury in DRG neurons have previously been published [45], the RNA seq data with CBD and THCV therapy in neuropathic mice is unique and is being reported for the first time.
The modulation of nociceptive information induced by PTX exposure in DRGs is well established to involve MAPK activation pathways [46]. MAPKs are a family of serine/threonine protein kinases that have been implicated in different aspects of cell communication and gene expression in the peripheral nervous system [47,48]. Inflammation-induced pain hyperalgesia is considered to be modulated by MAPKs in DRGs and the spinal cord. P38 MAPK is activated, and expression levels in the spinal dorsal horns are increased following peripheral nerve damage [49]. P38 MAPK has been shown to stimulate several inflammatory pathways, including the generation of inflammasomes, and initiate apoptosis in the cellular milieu by activating Bax and caspases [50]. In the current study, we observed an increase in p-p38 MAPKinase expression, NLRP3 inflammasome formation, TGF beta expression, and BAX expression following PTX assault, which is consistent with earlier results. Surprisingly, CBD and THCV therapy lowered the expressions of these proteins, but the reduction in protein expressions with their combination was two times greater than with each treatment alone. In another study, CBD delivery intranasally and intravenously reduced type 1 diabetic neuropathic pain and suppressed microglial density and phosphorylation of p38 mitogen-activated protein kinases [51], thus corroborating our findings. Moreover, THCV has been shown to diminish inflammation caused by formalin and carrageenan in rodents, and a number of studies have observed that p-p38 MAPKinase is important in regulating inflammation caused by formalin and carrageenan in rats [52]. These studies and our findings suggest that phytocannabinoids’ suppression of p-p38 MAPkinase is important in reducing PTX-induced neuropathic pain in mice.
On the other hand, we observed an increase in PI3K and AKT protein expression in PTX-treated DRGs isolated from rodents, which has been previously documented [53]. These protein expressions were reduced in CBD and THCV-treated mice, and the decrease was even greater (p < 0.001) in their combined treatment. According to a growing body of evidence, blocking the PI3K/Akt signaling pathway has been shown to be analgesic in neuropathic pain models [54]. According to the findings, activation of PI3K and PI3K/Akt appears to be implicated in the progression of PTX-induced neuropathic pain. Previous research has also documented that the PI3K and PI3K/Akt signaling pathways are critical in modulating the actions of inflammatory markers, including NLRP3 inflammasome, IL-1, and TNF-α, which are significant in neuropathic pain [55]. Similarly, the current study observed increased NLRP3 inflammasome production in DRG homogenates of PTX-treated mice, which was reduced by CBD, THCV, and their combination treatment; however the combination showed higher efficacy. With these findings, we can deduce that PTX-induced activation of the P-38 MAPKinase, PI3K, and PI3K/Akt signaling pathways may cause changes in inflammatory markers and contribute to the development of neuropathic pain, while CBD, THCV, and their combination treatments reduce neuropathic pain by regulating these signaling pathways (Fig. 8).
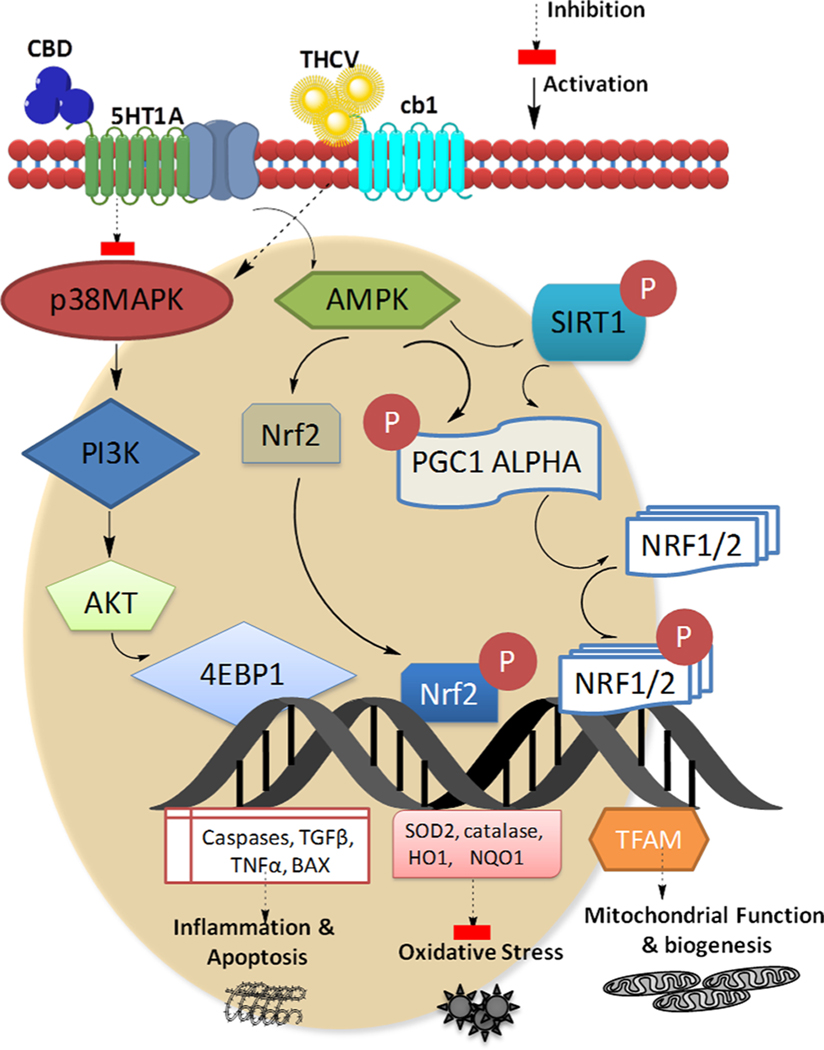
Fig. 8. Mechanisms by which CBD and THCV may alleviate paclitaxel-induced peripheral neuropathy.
Cannabidiol and Tetrahydrocannabivarin regulate apoptosis, inflammation, oxidative stress, and mitochondrial homeostasis in dorsal root ganglions via interfering with 5HT1A and CB1 receptors, respectively. Apoptosis and inflammation are regulated by the P-38 MAPkinase and the PI3K-AKT pathways, while mitochondrial homeostasis and oxidative stress are regulated by the AMPK-TFAM and AMPK-Nrf2 pathways respectively. AMPK: Adenosine monophosphate kinase, AKT: protein kinase B, BAX: bcl-2-like protein 4, CBD: Cannabidiol, 4EBP1: Eukaryotic translation initiation factor 4E-binding protein 1, NRF1/2: Nuclear respiratory factor 1/2, Nrf2: nuclear erythoid related factor-2, NQO1: NAD(P)H dehydrogenase 1, PGC1 Alpha: Peroxisome proliferator-activated receptor-γ coactivator 1 alpha, PI3K: Phosphoinositide 3-kinases, SIRT1: Sirtuin 1, SOD2: superoxide dismutase 2, TNFα: Tumor necrosis factor alpha, TGFβ: Transforming growth factor beta.
AMP-activated protein kinase (AMPK) is an energy-sensing kinase that can block mitogen-activated protein kinase (MAPK) signaling, which has been associated to pain enhancement following injury and the development of hyperalgesic priming [56]. Of note, Inyang et al., observed that PTX administration induced mechanical hypersensitivity in both male and female mice, which was counteracted by administering AMPK activators [56]. In agreement with previous studies, current study revealed that AMPK was significantly downregulated in PTX-treated DRGs when compared to the normal control group. Despite this, phytocannabinoids can boost AMPK activation through a variety of signaling pathways, which can help with appetite and heart function [57]. Similarly, we observed that CBD and THCV treatment elevated p-AMPK expression, and that their combination was superior in this effect, suggesting that combination therapy may provide additive to synergistic neuroprotection against PTX-induced neuropathy in mice which however has to be further investigated. Nrf2 is known to be activated by p-AMPK and plays a key role in regulating endogenous antioxidant defense by regulating the transcription of downstream target genes such as heme oxygenase-1 (HO-1), superoxide dismutase, glutathione reductase, and NAD(P)H: quinone oxidoreductase 1 [58]. As a result, Nrf2 activators reduced PTX-induced neuropathic pain in experimental animals [59]. Moreover, CBD’s antioxidant potential has been established via its effect on the activity of the Nrf2 transcription factor, which is involved in the development of cytoprotective proteins such as antioxidant enzymes [60]. In this work, we also discovered that neuropathic mice with CBD and THCV treatment had elevated expressions of Nrf2, HO1, and catalase in DRGs, and that these protein expressions were considerably higher (p < 0.001) in the combination treatment. This data implies that when these phytocannabinoids are combined, their antioxidant capacity increases in reducing the neuropathic pain (Fig. 8).
Further, by activating TFAM, p-AMPK reduced mitochondrial functional deficiencies in various animal and cell culture models of diseases by increasing mitochondriogenesis and respiratory capacity [61,62]. TFAM is a nuclear protein that binds to the mitochondrial genome and controls the transcription of subunits of mitochondrial complexes [63]. Overexpression of TFAM has also been shown to have protective effects in neuropathological disorders such as age related hearing loss, amyotrophic lateral sclerosis, alzheimer disease, and memory loss in animal systems. CBD, THCV, and their combination treatment improved TFAM levels (p < 0.01 to 0.001) in DRG homogenates of PTX-induced neuropathic mice in the current investigation [64]. Immunocytochemistry investigations in DRG primary cultures demonstrated a rise in mitochondrial complex I subunits and JC1 labeling suggesting mitochondrial repolarisation or restoration of mitochondrial membrane potentials. These findings imply that CBD and THCV increase mitochondrial function via activating the AMPK-Nrf2-TFAM axis, and that their combination is more effective than either medication alone.
THCV is a non-psychoactive, neutral CB1 antagonist/reverse agonist that can act as an agonist or antagonist at CB2 receptors depending on the dose [19]. When THCV was given to genetically obese mice at doses of 0.1–12.5 mg/kg once daily for 45 days, the total fat content was reduced by 31.1 percent when compared to the obese control group [12]. It was observed that THCV had a high affinity for CB1 receptors and high brain penetration, resulting in metabolically favorable effects that are typical of CB1 receptor inverse agonists. Further, THCV has been demonstrated to improve insulin sensitivity and reduce obesity in diet-induced obese mice models via altering metabolic processes through interacting with TRPV1 ion channels [65]. However, in the current investigation, we discovered that administering a selective CB1 blocker (rimonabant) to PTX-induced neuropathic mice prevented the antinociceptive effects of THCV. Furthermore, we observed that inhibiting CB1 receptors with Rimonabant in DRG primary cultures also prevented THCV-induced increases in AMPK, complex I, and catalase expression. These findings suggest that THCV modulates mitochondrial bioenergetics via the CB1/AMPK/nrf2/TFAM axis and the exact process by which THCV interacts with CB1 receptors still needs more investigations.
Our research also demonstrated that CBD treatment, when combined with a 5HT1A receptor blocker, it had no effect on nociception caused by PTX injection in mice. CBD provided protection against STZ-induced diabetes pain by specifically activating 5HT1A receptors. Pascual et al. observed that the CB1/CB2 dual agonist WIN55,212–2 decreased the thermal hyperalgesia and tactile allodynia elicited by PTX in rats, and that this action was prevented by the CB1 antagonist SR141716, implying that the CB1 receptor is involved [66,67]. Another study suggested that injecting CBD into the infralimbic cortex of the rat brain reduced fear by activating CB1 receptors, as determined by lower levels of freezing during an extinction test [68]. However, neither CB1 nor CB2 receptors are involved in CBD’s ability to reduce neuropathic pain, according to Segrado et al. and Sara et al [69,70]. Furthermore, despite its indirect activation of CB1/CB2 receptors via increasing endocannabinoid levels, very few studies have shown that CBD has a lower binding affinity for CB1 or CB2 receptors. In this study, we also observed that CBD’s anti-nociceptive effects are not dependent on CB1 activation, but rather on 5HT1A receptor activation, which is consistent with earlier results. Additionally, 5HT1A receptors have been shown to be important in the phosphorylation of CaMKII, an upstream regulator of the AMPK pathway [71]. As a result of the activation of 5HT1A receptors, CBD may have the capacity to regulate mitochondrial bioenergetics, redox, and inflammatory homeostasis in neuronal cells by regulating AMPK pathway. It is important to point out that in our animal studies, there was no group of PTX and 5HT1A/CB2 blocker because the focus of our studies is to understand the mechanism of action of CBD and THCV after the use of these blockers in PTX treated mice. Because CBD and THCV work in distinct ways, our study demonstrates that combining them results in entourage neuroprotective advantages.
5. Conclusion
Synthetic CBD and THCV showed neuroprotective benefits in PTX-induced neuropathic mice, as measured by a variety of neurobehavioral characteristics. MAPkinase, PI3K-AKT, AMPK, and inflammasome pathways, along with mitochondrial function-related genes, were observed to be involved in the pathogenesis of PTX-induced neuropathy by transcriptome analysis of DRG homogenates from diverse treatment groups of mice. The neuroprotective effects of CBD and THCV in mouse/rat DRGs are dependent on the modulation of 5HT1A and CB1 receptors, respectively, in reducing PTX-induced neuropathic pain. The combination of CBD and THCV has been shown to provide better neuro and mitoprotection against PTX-induced insult than either treatment alone. Future knock-down/knock-in experiments are needed to fully comprehend the pharmacology of CBD and THCV in the treatment of neuropathic pain.
Supplementary Material
Acknowledgements
The Consortium for Medical Marijuana Clinical Outcomes Research, Grant/Award number: SUB00002097, National Institute on Minority Health and Health Disparities, Grant/Award Number: U54 MD007582, and NSF-CREST Center for Complex Materials Design for Multidimensional Additive Processing (CoManD), Grant/Award Number: 1735968 provided funding for this study.
Footnotes
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2022.108693.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Ibrahim EY, Ehrlich BE, Prevention of chemotherapy-induced peripheral neuropathy: a review of recent findings, Critical Rev. Oncol./Hematol. 145 (2020), 102831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Klein I, Lehmann HC, Pathomechanisms of Paclitaxel-Induced Peripheral Neuropathy, Toxics 9 (10) (2021) 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J, Mechanisms of chemotherapy-induced peripheral neuropathy, Int. J. Mol. Sci. 20 (6) (2019) 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kober KM, Lee M-C, Olshen A, Conley YP, Sirota M, Keiser M, Hammer MJ, Abrams G, Schumacher M, Levine JD, Differential methylation and expression of genes in the hypoxia-inducible factor 1 signaling pathway are associated with paclitaxel-induced peripheral neuropathy in breast cancer survivors and with preclinical models of chemotherapy-induced neuropathic pain, Mol. Pain 16 (2020), 1744806920936502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang M, Xu N.a., Xu W, Ling G, Zhang P, Potential Therapies and Diagnosis Based on Golgi-targeted Nano Drug Delivery Systems, Pharmacol. Res. 175 (2022) 105861, 10.1016/j.phrs.2021.105861. [DOI] [PubMed] [Google Scholar]
- [6].K.F.A.E.S. Al, Modulatory effect of mesenchymal stem cells on pregabalin and lacosamide in paclitaxel-induced neuropathic pain in rats, CU Theses (2019). [Google Scholar]
- [7].Sałat K, Chemotherapy-induced peripheral neuropathy: Part 1—current state of knowledge and perspectives for pharmacotherapy, Pharmacol. Rep. 72 (2020) 486–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vermeer CJC, Hiensch AE, Cleenewerk L, May AM, Eijkelkamp N, Neuro-immune interactions in paclitaxel-induced peripheral neuropathy, Acta Oncol. 60 (10) (2021) 1369–1382. [DOI] [PubMed] [Google Scholar]
- [9].Aviram J, Lewitus GM, Pud D, Procaccia S, Berman P, Yellin B, Vysotski Y, Hazan O, Eisenberg E, Meiri D, Specific phytocannabinoid compositions are associated with analgesic response and adverse effects in chronic pain patients treated with medical cannabis, Pharmacol. Res. 169 (2021), 105651. [DOI] [PubMed] [Google Scholar]
- [10].Filipiuc LE, Ababei DC, Alexa-Stratulat T, Pricope CV, Bild V, Stefanescu R, Stanciu GD, Tamba B-I, Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 13 (11) (2021) 1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, O’Sullivan SE, Tan GD, Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study, Diabetes Care 39 (10) (2016) 1777–1786. [DOI] [PubMed] [Google Scholar]
- [12].Wargent ET, Zaibi MS, Silvestri C, Hislop DC, Stocker CJ, Stott CG, Guy GW, Duncan M, Di Marzo V, Cawthorne MA, The cannabinoid Δ 9-tetrahydrocannabivarin (THCV) ameliorates insulin sensitivity in two mouse models of obesity, Nutr. Diabetes 3 (5) (2013) e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Riedel G, Fadda P, McKillop-Smith S, Pertwee RG, Platt B, Robinson L, Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice, Br. J. Pharmacol. 156 (7) (2009) 1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M, The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain, Eur. J. Pharmacol. 556 (1–3) (2007) 75–83. [DOI] [PubMed] [Google Scholar]
- [15].Mlost J, Bryk M, Starowicz K, Cannabidiol for pain treatment: focus on pharmacology and mechanism of action, Int. J. Mol. Sci. 21 (22) (2020) 8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Larsen C, Shahinas J, Dosage, efficacy and safety of cannabidiol administration in adults: a systematic review of human trials, J. Clin. Med. Res. 12 (3) (2020) 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kaplan JS, Wagner JK, Reid K, McGuinness F, Arvila S, Brooks M, Stevenson H, Jones J, Risch B, McGillis T, Budinich R, Gambell E, Predovich B, Cannabidiol Exposure During the Mouse Adolescent Period Is Without Harmful Behavioral Effects on Locomotor Activity, Anxiety, and Spatial Memory, Front. Behav. Neurosci. 15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chahal SK, Sodhi RK, Madan J, Duloxetine hydrochloride loaded film forming dermal gel enriched with methylcobalamin and geranium oil attenuates paclitaxel-induced peripheral neuropathy in rats, IBRO Rep. 9 (2020) 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Abioye A, Ayodele O, Marinkovic A, Patidar R, Akinwekomi A, Sanyaolu A, Δ9-Tetrahydrocannabivarin (THCV): a commentary on potential therapeutic benefit for the management of obesity and diabetes, J. Cannabis Res. 2 (1) (2020) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Delis F, Polissidis A, Poulia N, Justinova Z, Nomikos GG, Goldberg SR, Antoniou K, Attenuation of cocaine-induced conditioned place preference and motor activity via cannabinoid CB2 receptor agonism and CB1 receptor antagonism in rats, Int. J. Neuropsychopharmacol. 20 (3) (2017) 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, Fujiwara M, Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor–dependent mechanism, Stroke 36 (5) (2005) 1071–1076. [DOI] [PubMed] [Google Scholar]
- [22].Mori MA, Meyer E, Silva FF, Milani H, Guimarães FS, Oliveira RMW, Differential contribution of CB1, CB2, 5-HT1A, and PPAR-γ receptors to cannabidiol effects on ischemia-induced emotional and cognitive impairments, Eur. J. Neurosci. 53 (6) (2021) 1738–1751. [DOI] [PubMed] [Google Scholar]
- [23].Kyte SL, Toma W, Bagdas D, Meade JA, Schurman LD, Lichtman AH, Chen Z-J, Del Fabbro E, Fang X, Bigbee JW, Damaj MI, Gewirtz DA, Nicotine prevents and reverses paclitaxel-induced mechanical allodynia in a mouse model of CIPN, J. Pharmacol. Exp. Ther. 364 (1) (2018) 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S, Pathogenesis of paclitaxel-induced peripheral neuropathy: A current review of in vitro and in vivo findings using rodent and human model systems, Exp. Neurol. 324 (2020), 113121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA, Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT1A receptors without diminishing nervous system function or chemotherapy efficacy, Br. J. Pharmacol. 171 (3) (2014) 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kalvala AK, Yerra VG, Sherkhane B, Gundu C, Arruri V, Kumar R, Kumar A, Chronic hyperglycemia impairs mitochondrial unfolded protein response and precipitates proteotoxicity in experimental diabetic neuropathy: focus on LonP1 mediated mitochondrial regulation, Pharmacol. Rep. 72 (6) (2020) 1627–1644. [DOI] [PubMed] [Google Scholar]
- [27].Cheah M, Fawcett J, Andrews M, Assessment of thermal pain sensation in rats and mice using the Hargreaves test, Bio-protocol 7 (16) (2017), 10.21769/BioProtoc.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yalcin I, Charlet A, Freund-Mercier M-J, Barrot M, Poisbeau P, Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents, J. Pain 10 (7) (2009) 767–773. [DOI] [PubMed] [Google Scholar]
- [29].Kalvala AK, Kumar R, Sherkhane B, Gundu C, Arruri VK, Kumar A, Bardoxolone methyl ameliorates hyperglycemia induced mitochondrial dysfunction by activating the keap1-Nrf2-ARE pathway in experimental diabetic neuropathy, Mol. Neurobiol. 57 (8) (2020) 3616–3631. [DOI] [PubMed] [Google Scholar]
- [30].Patel N, Kommineni N, Surapaneni SK, Kalvala A, Yaun X, Gebeyehu A, Arthur P, Duke LC, York SB, Bagde A, Cannabidiol loaded extracellular vesicles sensitize triple-negative breast cancer to doxorubicin in both in-vitro and in vivo models, Int. J. Pharm. 607 (2021), 120943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saleh A, Smith DR, Balakrishnan S, Dunn L, Martens C, Tweed CW, Fernyhough P, Tumor necrosis factor-α elevates neurite outgrowth through an NF-κB-dependent pathway in cultured adult sensory neurons: diminished expression in diabetes may contribute to sensory neuropathy, Brain Res. 1423 (2011) 87–95. [DOI] [PubMed] [Google Scholar]
- [32].Areti A, Komirishetty P, Kalvala AK, Nellaiappan K, Kumar A, Rosmarinic acid mitigates mitochondrial dysfunction and spinal glial activation in oxaliplatin-induced peripheral neuropathy, Mol. Neurobiol. 55 (9) (2018) 7463–7475. [DOI] [PubMed] [Google Scholar]
- [33].Kalvala AK, Yerra VG, Kumar A, LONP1 induction by SRT1720 attenuates mitochondrial dysfunction against high glucose induced neurotoxicity in PC12 cells, Toxicol. In Vitro 62 (2020), 104695. [DOI] [PubMed] [Google Scholar]
- [34].Chow RT, David MA, Armati PJ, 830 nm laser irradiation induces varicosity formation, reduces mitochondrial membrane potential and blocks fast axonal flow in small and medium diameter rat dorsal root ganglion neurons: implications for the analgesic effects of 830 nm laser, J. Peripheral Nervous Syst. 12 (1) (2007) 28–39. [DOI] [PubMed] [Google Scholar]
- [35].Yerra VG, Kumar A, Adenosine monophosphate-activated protein kinase abates hyperglycaemia-induced neuronal injury in experimental models of diabetic neuropathy: effects on mitochondrial biogenesis, autophagy and neuroinflammation, Mol. Neurobiol. 54 (3) (2017) 2301–2312. [DOI] [PubMed] [Google Scholar]
- [36].Kirkland RA, Saavedra GM, Franklin JL, Rapid activation of antioxidant defenses by nerve growth factor suppresses reactive oxygen species during neuronal apoptosis: evidence for a role in cytochrome c redistribution, J. Neurosci. 27 (42) (2007) 11315–11326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Casey SL, Atwal N, Vaughan CW, Cannabis constituent synergy in a mouse neuropathic pain model, Pain 158 (12) (2017) 2452–2460. [DOI] [PubMed] [Google Scholar]
- [38].Abraham AD, Leung EJY, Wong BA, Rivera ZMG, Kruse LC, Clark JJ, Land BB, Orally consumed cannabinoids provide long-lasting relief of allodynia in a mouse model of chronic neuropathic pain, Neuropsychopharmacology 45 (7) (2020) 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xiong W, Cui T, Cheng K, Yang F, Chen S-R, Willenbring D, Guan Y, Pan H-L, Ren K.e., Xu Y, Zhang L.i., Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors, J. Exp. Med. 209 (6) (2012) 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bolognini D, Costa B, Maione S, Comelli F, Marini P, Di Marzo V, Parolaro D, Ross RA, Gauson LA, Cascio MG, Pertwee RG, The plant cannabinoid Δ9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice, Br. J. Pharmacol. 160 (3) (2010) 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhong S, Zhou Z, Lin X, Liu F, Liu C, Liu Z, Deng W, Zhang X, Chang H, Zhao C, Ketogenic diet prevents paclitaxel-induced neuropathic nociception through activation of PPARγ signalling pathway and inhibition of neuroinflammation in rat dorsal root ganglion, Eur. J. Neurosci. 54 (4) (2021) 5341–5356. [DOI] [PubMed] [Google Scholar]
- [42].Xiong C, Chua KC, Stage TB, Priotti J, Kim J, Altman-Merino A, Chan D, Saraf K, Ferracini A. Canato, Fattahi F, Kroetz DL, Human induced pluripotent stem cell derived sensory neurons are sensitive to the neurotoxic effects of paclitaxel, Clin. Transl. Sci. 14 (2) (2021) 568–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang B-F, Jiang H, Chen J, Guo X, Li Y, Hu Q.i., Yang S, Nobiletin ameliorates myocardial ischemia and reperfusion injury by attenuating endoplasmic reticulum stress-associated apoptosis through regulation of the PI3K/AKT signal pathway, Int. Immunopharmacol. 73 (2019) 98–107. [DOI] [PubMed] [Google Scholar]
- [44].Xu Y, Zhang Y, Xu Y, Zang G, Li B, Xia H, Yuan W, Activation of CD137 signaling promotes macrophage apoptosis dependent on p38 MAPK pathway-mediated mitochondrial fission, Int. J. Biochem. Cell Biol. 136 (2021), 106003. [DOI] [PubMed] [Google Scholar]
- [45].Li Y, Yin C, Liu B, Nie H, Wang J, Zeng D, Chen R, He X, Fang J, Du J, Liang Y. i., Jiang Y, Fang J, Liu B, Transcriptome profiling of long noncoding RNAs and mRNAs in spinal cord of a rat model of paclitaxel-induced peripheral neuropathy identifies potential mechanisms mediating neuroinflammation and pain, J. Neuroinflammation 18 (1) (2021), 10.1186/s12974-021-02098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lan Y-Y, Chen Y-H, Liu C, Tung K-L, Wu Y-T, Lin S-C, Wu C-H, Chang H-Y, Chen Y-C, Huang B-M, Role of JNK activation in paclitaxel-induced apoptosis in human head and neck squamous cell carcinoma, Oncol. Lett. 22 (4) (2021) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Reshkin SJ, Bellizzi A, Cardone RA, Tommasino M, Casavola V, Paradiso A, Paclitaxel induces apoptosis via protein kinase A-and p38 mitogen-activated protein-dependent inhibition of the Na+/H+ exchanger (NHE) NHE isoform 1 in human breast cancer cells, Clin. Cancer Res. 9 (6) (2003) 2366–2373. [PubMed] [Google Scholar]
- [48].Edelman AM, Blumenthal DK, Krebs EG, Protein serine/threonine kinases, Annu. Rev. Biochem. 56 (1) (1987) 567–613. [DOI] [PubMed] [Google Scholar]
- [49].Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K, Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury, Glia 45 (1) (2004) 89–95. [DOI] [PubMed] [Google Scholar]
- [50].Li D, Ren W, Jiang Z, Zhu L, Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury, Mol. Med. Rep. 18 (5) (2018) 4399–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Toth CC, Jedrzejewski NM, Ellis CL, Frey WH, Cannabinoid-Mediated Modulation of Neuropathic Pain and Microglial Accumulation in a Model of Murine Type I Diabetic Peripheral Neuropathic Pain, Mol Pain 6 (2010), 1744–8069-6–16, 10.1186/1744-8069-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M, Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia, Eur. J. Neurosci. 22 (10) (2005) 2431–2440. [DOI] [PubMed] [Google Scholar]
- [53].Huang J, Chen D, Yan F, Wu S, Kang S, Xing W, Zeng W, Xie J, JTC-801 alleviates mechanical allodynia in paclitaxel-induced neuropathic pain through the PI3K/Akt pathway, Eur. J. Pharmacol. 883 (2020), 173306. [DOI] [PubMed] [Google Scholar]
- [54].Chen S-P, Zhou Y-Q, Liu D-Q, Zhang W, Manyande A, Guan X-H, Tian Y-K, Ye D-W, Mohamed Omar D, PI3K/Akt pathway: a potential therapeutic target for chronic pain, Curr. Pharm. Des. 23 (12) (2017) 1860–1868. [DOI] [PubMed] [Google Scholar]
- [55].Islam MT, Bardaweel SK, Mubarak MS, Koch W, Gaweł-Beben K, Antosiewicz B, Sharifi-Rad J, Immunomodulatory effects of diterpenes and their derivatives through NLRP3 inflammasome pathway: A review, Front. Immunol. 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Inyang KE, Burton MD, Szabo-Pardi T, Wentworth E, McDougal TA, Ramirez ED, Pradhan G, Dussor G, Price TJ, Indirect AMP-activated protein kinase activators prevent incision-induced hyperalgesia and block hyperalgesic priming, whereas positive allosteric modulators block only priming in mice, J. Pharmacol. Exp. Ther. 371 (1) (2019) 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M, Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase, J. Biol. Chem. 280 (26) (2005) 25196–25201. [DOI] [PubMed] [Google Scholar]
- [58].Sun W, Yan C, Frost B, Wang X, Hou C, Zeng M, Gao H, Kang Y, Liu J, Pomegranate extract decreases oxidative stress and alleviates mitochondrial impairment by activating AMPK-Nrf2 in hypothalamic paraventricular nucleus of spontaneously hypertensive rats, Sci. Rep. 6 (1) (2016) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sun H, Guo X.i., Wang Z, Wang P, Zhang Z, Dong J, Zhuang R, Zhou Y, Ma G, Cai W, Alphalipoic acid prevents oxidative stress and peripheral neuropathy in nab-paclitaxel-treated rats through the Nrf2 signalling pathway, Oxid. Med. Cell. Longevity 2019 (2019) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jastrząb A, Gęgotek A, Skrzydlewska E, Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation, Cells 8 (8) (2019) 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nanjaiah H, Vallikannan B, Lutein upregulates the PGC-1α, NRF1, and TFAM expression by AMPK activation and downregulates ROS to maintain mtDNA integrity and mitochondrial biogenesis in hyperglycemic ARPE-19 cells and rat retina, Biotechnol. Appl. Biochem. 66 (6) (2019) 999–1009. [DOI] [PubMed] [Google Scholar]
- [62].Li PA, Hou X, Hao S, Mitochondrial biogenesis in neurodegeneration, J. Neurosci. Res. 95 (10) (2017) 2025–2029. [DOI] [PubMed] [Google Scholar]
- [63].Kang I, Chu CT, Kaufman BA, The mitochondrial transcription factor TFAM in neurodegeneration: emerging evidence and mechanisms, FEBS Lett. 592 (5) (2018) 793–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kober KM, Olshen A, Conley YP, Schumacher M, Topp K, Smoot B, Mazor M, Chesney M, Hammer M, Paul SM, Expression of mitochondrial dysfunction-related genes and pathways in paclitaxel-induced peripheral neuropathy in breast cancer survivors, Molecular pain 14 (2018), 1744806918816462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, Stott CG, Di Marzo V, Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes, Br. J. Pharmacol. 163 (7) (2011) 1479–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rahn E, Makriyannis A, Hohmann A, Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats, Br. J. Pharmacol. 152 (5) (2007) 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pascual D, Goicoechea C, Suardíaz M, Martín MI, A cannabinoid agonist, WIN 55,212–2, reduces neuropathic nociception induced by paclitaxel in rats, Pain 118 (1–2) (2005) 23–34. [DOI] [PubMed] [Google Scholar]
- [68].Kuhnert S, Meyer C, Koch M, Involvement of cannabinoid receptors in the amygdala and prefrontal cortex of rats in fear learning, consolidation, retrieval and extinction, Behav. Brain Res. 250 (2013) 274–284. [DOI] [PubMed] [Google Scholar]
- [69].Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA, Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy, Br J Pharmacol 171 (3) (2014) 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sagredo O, Pazos MR, Satta V, Ramos JA, Pertwee RG, Fernández-Ruiz J, Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington’s disease, J. Neurosci. Res. 89 (9) (2011) 1509–1518. [DOI] [PubMed] [Google Scholar]
- [71].Albert PR, Vahid-Ansari F, The 5-HT1A receptor: Signaling to behavior, Biochimie 161 (2019) 34–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.