Abstract
Background/Aims
Asymptomatic esophageal eosinophilia (aEE) is considered to be a potential precursor of eosinophilic esophagitis (EoE). However, there are few clinical parameters that can be used to evaluate the disease. Therefore, we aimed to clarify the factors involved in the symptoms of EoE by examining the clinicopathological differences between aEE and EoE.
Methods
We reviewed 41 patients with esophageal eosinophilia who underwent endoscopic ultrasonography and high-resolution manometry. They were divided into the aEE group (n=16) and the EoE group (n=25) using the Frequency Scale for the Symptoms of Gastroesophageal Reflux Disease score. The patients’ clinicopathological findings were collected and examined.
Results
The median Frequency Scale for the Symptoms of Gastroesophageal Reflux Disease score was 3.0 in the aEE group and 10.0 in the EoE group. There was no significant difference in patient characteristics, endoscopic findings and pathological findings. The cutoff value for wall thickening was 3.13 mm for the total esophageal wall thickness and 2.30 mm for the thickness from the surface to the muscular layer (total esophageal wall thickness 84.0% sensitivity, 75.0% specificity; thickness from the surface to the muscular layer 84.0% sensitivity, 68.7% specificity). The high-resolution manometry study was abnormal in seven patients (43.8%) in the aEE group and in 12 (48.0%) in the EoE group. The contractile front velocity was slower in the EoE group (p=0.026).
Conclusions
The esophageal wall thickening in the lower portion of the esophagus is an important clinical factors related to the symptoms in patients with EoE.
Keywords: Eosinophilic esophagitis, Endosonography, High resolution manometry
INTRODUCTION
Eosinophilic esophagitis (EoE) has been understood to be a clinicopathological entity in which symptoms and histology must always be considered together.1,2 Previous studies have shown that the symptoms of EoE worsen as the condition progresses3 and that the chronic eosinophilic inflammation that occurs in EoE causes thickening of the esophageal wall.4 Esophageal wall thickness can be assessed by computed tomography or endoscopic ultrasonography (EUS), and it has been suggested that the esophageal wall thickening is involved in the esophageal motility disorders.5,6 Furthermore, several studies have found that EoE causes esophageal motility disorder. High-resolution manometry (HRM) is useful for evaluation of EoE, and 27% to 76% of patients with EoE evaluated by HRM have been found to have abnormal esophageal motility.7,8
The concept of asymptomatic esophageal eosinophilia (aEE), which has the same etiological and clinicopathological features as EoE but no symptoms, is becoming established.9-11 aEE is considered to be a potential precursor of EoE. However, given that aEE is often detected incidentally and is asymptomatic, there are few clinical parameters that can be used to evaluate the disease. In the early 2010s, only symptomatic cases of EoE were studied and reported. However, since that time, we have believed that asymptomatic cases with esophageal eosinophilic infiltration may have the same pathogenesis and lie on the same spectrum of disease pathology. Therefore, for the purpose of pathophysiology interpretation and exploratory research, we have been evaluating patients with aEE without any esophageal symptom as well as those with EoE by EUS and esophageal HRM when consent was obtained.
Evaluation by EUS and HRM suggests that the esophageal wall may be thicker and the esophageal body pressure higher in EoE than in aEE.12 In the previous report, cases with abnormal esophageal motility in HRM were excluded, and only cases with normal esophageal motility were included. However, as mentioned above, a certain percentage of EoE cases also show abnormal esophageal motility. In addition, EUS was evaluated locally at only 2 points using an EUS probe, and the phenotype in endoscopic findings was not taken into account. Thus, the degree of wall thickening and its area has not been examined, and there is limited information available concerning the relationship between esophageal wall thickness or esophageal motility and other endoscopic or histological findings, including the lamina propria fibrosis (LPF), which is considered to be important for esophageal stenosis and pathogenesis of EoE.
Therefore, in the present study, we aimed to clarify the factors involved in the symptoms of EoE by examining the clinicopathological differences between aEE and EoE, including evaluation by EUS and HRM.
MATERIALS AND METHODS
1. Study population and design
This is a retrospective observational study. This study included consecutive patients with esophageal eosinophilia (EE) who underwent EUS and HRM at Toranomon Hospital, Tokyo, Japan, between January 1, 2010, and April 1, 2022. Patients with EE were defined as those who underwent upper gastrointestinal endoscopy and were histopathologically confirmed to have esophageal infiltration of at least 15 eosinophils per high-power field, as stated in the guidelines for EE.1,2 We excluded patients who had a history of esophageal surgery, chemoradiation therapy that included the esophagus in the radiation field, and secondary causes of EE, such as eosinophilic gastroenteritis, esophageal candidiasis, achalasia, graft-versus-host disease, and eosinophilic granulomatosis with polyangiitis. The 41 patients who met the criteria were then divided into an aEE group (n=16) and an EoE group that met the diagnostic criteria for EoE (n=25). Clinical, endoscopic, and histopathological data were collected and compared between the two groups.
2. Evaluation of symptoms
Symptoms were recorded using questionnaires and assessed using the Frequency Scale for the Symptoms of Gastroesophageal Reflux Disease (F-scale) score,13 Eckardt score (cutoff ≥3),14,15 and the self-reported GerdQ score (cutoff ≥8).16,17 Symptoms related to the chief complaint were evaluated, namely, those indicating esophageal dysfunction, such as dysphagia, heartburn, chest pain, food impaction, and other digestive symptoms. Patients with an F-scale score ≥7 were defined as symptomatic. We defined “asymptomatic” as the absence of any of the aforementioned symptoms related to esophageal dysfunction within the previous 6 months and an F-scale score of ≤6. The F-scale cutoff score was set at 7 in view of an earlier report indicating that patients with aEE consistently had an F-scale score of ≤6.11
3. Histopathological assessment of biopsy specimens
All biopsy specimens were fixed in 10% formalin, stained with hematoxylin and eosin, and assessed pathologically using the EoE histology scoring system (EoEHSS).18 The EoEHSS evaluates eosinophilic inflammation and other features, including epithelial basal zone hyperplasia, eosinophilic abscesses, eosinophil surface layering, dilated intercellular spaces, changes in surface epithelium, dyskeratotic epithelial cells, and LPF. The grade and stage of abnormalities are scored using a 4-point scale (0, normal; 3, maximum change).
4. Endoscopic findings
Endoscopic findings were evaluated based on the EoE endoscopic reference score.19 Scores were calculated for inflammation and fibrostenosis.20 The location of each finding considered to be typical of EoE, such as esophageal rings, white exudate, longitudinal furrows/ridges, edema, and stricture, was determined from images obtained by white-light endoscopy and narrow-band imaging endoscopy. In a previous study, the location of endoscopic findings was divided into a diffuse EE phenotype and a localized EE phenotype.21 The localized phenotype was defined as a small area of EE localized within 1 to 2 cm and the diffuse phenotype as a widespread area of EE involving one or more of the upper, middle, and lower locations in the esophagus. Diagnoses were made retrospectively by two board-certified fellows of the Japan Gastroenterological Endoscopy Society. In this study, examiners were in agreement in most cases. In cases where the examiners’ diagnoses differed, both examiners discussed with each other before making a decision.
5. Endoscopic ultrasonography
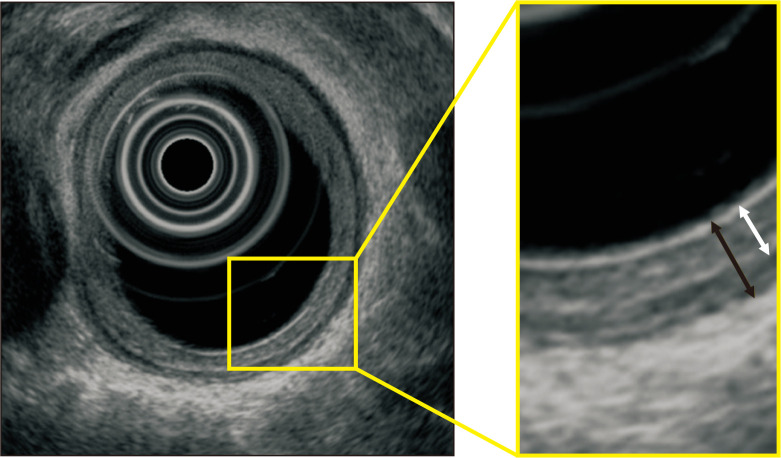
The thickness from the surface to the muscular layer (TSM) and the total esophageal wall thickness (TWT) were determined using a GF-UM2000 mechanical radial scope (Olympus Medical Systems Corp., Tokyo, Japan). The difference between TWT minus TSM was defined as the thickness of the muscle layer. The proximal area of the aortic arch was defined as the upper portion of esophagus, the area from the esophagogastric junction (EGJ) to 5 cm proximal as the lower portion of esophagus, and the area between these two as the middle portion of esophagus. Measurements were obtained at 1- to 2-cm intervals from the EGJ and recorded where the thickness was most noticeable in the upper, middle, and lower portions of esophagus. The frequencies used for EUS were 20 and 12 MHz. A typical measurement obtained by EUS is shown in Fig. 1.
Fig. 1.
Typical measurements obtained by endoscopic ultrasonography. The total esophageal wall thickness (black double-headed arrow) and the thickness from the surface to the muscular layer (white double-headed arrow) were measured at 20 MHz and 12 MHz using the GF-UM2000.
6. High-resolution manometry
HRM was performed using a StarletⓇ system (Star Medical, Tokyo, Japan). This system has a catheter with a 36-channel solid-state sensor spaced at 1 cm intervals (Unisensor AG, Attikon, Switzerland). Esophageal motility was described according to the Chicago classification, version 3.0.22
7. Study endpoints
The primary study endpoint was set as identification of the relationship between esophageal wall thickness and symptoms. The secondary endpoint was confirmation of the relationship between esophageal motility and symptoms, clinicopathological characteristics, and other endoscopic findings.
8. Statistical analyses
The data are presented as the median and interquartile range. The chi-square test and Fisher exact test were used as appropriate to compare qualitative variables between groups, and the Mann-Whitney U test was used to compare quantitative variables. The point that was the minimum distance from the upper left corner of the receiver operating characteristic curve was defined as the cutoff point. All statistical analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA). A p-value <0.05 was considered statistically significant.
9. Ethical approval
This study was approved by the Ethics Committee of Toranomon Hospital (approval number: 1783) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients provided written informed consent to undergo the proposed procedure. Written informed consent for inclusion in the study was not mandatory due to the retrospective observational nature of the research. However, patients were given the opportunity to opt out via the hospital’s website.
RESULTS
1. Patient demographics and clinicopathological characteristics
Table 1 shows the patients’ demographics and clinicopathological characteristics. There was no significant difference in median age between the aEE group and the EoE group or in gender, current smoking status, daily alcohol consumption, body mass index, history of concurrent allergic disease, or serum biochemistry, including the mean peripheral blood eosinophil count and mean nonspecific immunoglobulin E level.
Table 1.
Patients’ Clinicopathological Characteristics
| Characteristic | aEE (n=16) | EoE (n=25) | p-value |
|---|---|---|---|
| Age, yr | 57 (44-64) | 49 (45–54) | 0.230 |
| Sex | 0.215 | ||
| Male | 15 (93.8) | 19 (76.0) | |
| Female | 1 (6.2) | 6 (24.0) | |
| Symptoms | |||
| Chief complaint (duplicates counted) | |||
| Dysphagia | 14 (56.0) | ||
| Heartburn | 9 (36.0) | ||
| Chest pain | 4 (16.0) | ||
| Food impaction | 13 (52.0) | ||
| Others | 1 (4.0) | ||
| F-scale score | 3 (2–4) | 10 (8–14) | <0.001* |
| Eckardt score | 0.5 (0.0–1.3) | 1.0 (1.0–2.0) | 0.007* |
| GerdQ score | 6 (6–6) | 7 (6–7) | 0.120 |
| Body mass index, kg/m2 | 23.8 (22.6–24.5) | 24.1 (22.4–27.1) | 0.220 |
| Current smoking | 1 (6.3) | 5 (20.0) | 0.376 |
| Brinkman index | 0 (0–0) | 0 (0–26) | 0.904 |
| Alcohol consumption, g/day | 20 (0–39) | 10 (0–35) | 0.203 |
| Concurrent allergic disease (duplicates counted) | 7 (43.8) | 18 (72.0) | 0.104 |
| Allergic rhinitis | 3 (18.8) | 10 (40.0) | 0.187 |
| Bronchial asthma | 4 (25.0) | 5 (20.0) | 0.717 |
| Atopic dermatitis | 3 (18.8) | 4 (16.0) | >0.999 |
| Food or other allergies | 0 | 4 (16.0) | 0.143 |
| Peripheral blood eosinophil,/μL | 274 (129–379) | 252 (189–391) | 0.308 |
| Nonspecific IgE, IU/mL | 93 (47–148) | 247 (70–757) | 0.267 |
| Helicobacter pylori infection | 0.497 | ||
| Current infection | 0 | 2 (8.0) | |
| Past infection | 3 (18.8) | 5 (20.0) | |
| Negative infection | 13 (81.3) | 18 (72.0) | |
| Histopathological findings | |||
| Peak eosinophil count, eos/hpf | 67 (46–119) | 57 (40–67) | 0.132 |
| EoEHSS grade score | |||
| Eosinophilic inflammation | 2 (2–3) | 2 (2–3) | 0.451 |
| Basal cell hyperplasia | 2 (2–3) | 2 (2–3) | 0.271 |
| Dilated intercellular spaces | 3 (3–3) | 3 (3–3) | 0.697 |
| Lamina propria fibrosis | 1 (0–2) | 2 (2–3) | 0.064† |
| Eosinophilic abscess | 0 (0–0) | 0 (0–0) | >0.999 |
| Eosinophil surface layering | 0 (0–0) | 0 (0–0) | 0.328 |
| Surface epithelial alteration | 1 (0–1) | 1 (1–2) | 0.388 |
| Dyskeratotic epithelial cells | 0 (0–0) | 0 (0–0) | 0.237 |
| Total score/full score | 0.44 (0.32–0.47) | 0.46 (0.41–0.51) | 0.090† |
Data are presented as median (interquartile range) or number (%).
aEE, asymptomatic esophageal eosinophilia; EoE, eosinophilic esophagitis; F-scale, Frequency Scale for the Symptoms of Gastroesophageal Reflux Disease; IgE, immunoglobulin E; eos/hpf, eosinophils per high-power field; EoEHSS, eosinophilic esophagitis histologic scoring system.
*p<0.05; †p<0.1.
The symptoms noted in the EoE group were dysphagia in 14 patients (56.0%), food impaction in 13 (52.0%), heartburn in nine (36.0%), chest pain in four (16.0%), and others (including vomiting and abdominal discomfort) in one (4.0%). The median F-scale score was 3.0 in the aEE group and 10.0 in the EoE group. The Eckardt and GerdQ scores were below the cutoff in both groups; however, the Eckardt score was significantly higher in the EoE group (p<0.01).
A proton pump inhibitor (PPI) or a potassium-competitive acid blocker (PCAB) was administered in two patients in the aEE group (rabeprazole 10 mg and vonoprazan 10 mg) and in one patient in the EoE group (esomeprazole 20 mg). The patients in the aEE group had a history of taking the PPI or PCAB on an as-needed basis for a feeling of heavy stomach. However, in these cases, the medication was discontinued for at least 2 months prior to the examination, and no obvious symptoms were observed during that time.
Histologically, there was no significant difference between the two groups in the peak eosinophils per high-power field value or in the scores for any of the items evaluated in the EoEHSS. However, LPF tended to be higher in the EoE group (p=0.064).
2. Endoscopic findings and HRM
Tables 2 and 3 show the endoscopic and HRM findings in the two groups. The prevalence of typical endoscopic findings of EoE did not significantly differ between the aEE and EoE groups. There was no significant difference in total inflammatory and fibrostenotic score of EoE endoscopic reference score between the two groups. Furthermore, neither the location of these endoscopic findings nor in the phenotype was significantly different between the groups. There was also no significant different in endoscopic findings for atrophic gastritis, reflux esophagitis, and hiatal hernia.
Table 2.
Endoscopic Findings
| Variable | aEE (n=16) | EoE (n=25) | p-value |
|---|---|---|---|
| Endoscopic findings, EREFS | |||
| Longitudinal furrows/ridges, No. (%) | 13 (81.3) | 23 (92.0) | |
| Median score (IQR) | 1 (1–1) | 1 (1–1) | 0.359 |
| Esophageal rings, No. (%) | 7 (43.8) | 14 (56.0) | |
| Median score (IQR) | 0 (0–1) | 1 (0–1) | 0.270 |
| White exudates, No. (%) | 12 (75.0) | 17 (68.0) | |
| Median score (IQR) | 1.0 (0.8-1.0) | 1.0 (0.0-1.0) | 0.660 |
| Stricture, No. (%) | 0 | 2 (8.0) | |
| Median score (IQR) | 0 (0–0) | 0 (0–0) | 0.083* |
| Edema, No. (%) | 16 (100) | 25 (100) | >0.999 |
| Median score (IQR) | 1 (1–1) | 1 (1–1) | |
| Inflammatory score, median (IQR) | 3 (2–3) | 3 (2–3) | 0.872 |
| Fibrostenotic score, median (IQR) | 0 (0–1) | 1 (0–1) | 0.132 |
| Total score, median (IQR) | 3.0 (2.8-4.0) | 3.0 (3.0-4.0) | 0.261 |
| Phenotype, No. (%) | >0.999 | ||
| Localized type | 2 (12.5) | 3 (12.0) | |
| Diffuse type | 14 (87.5) | 22 (88.0) | |
| Location, No. (%) | 0.148 | ||
| Ut-Lt | 4 (25.0) | 14 (56.0) | |
| Mt-Lt | 9 (56.3) | 8 (32.0) | |
| Lt | 3 (18.8) | 3 (12.0) | |
| Other endoscopic findings, No. (%) | |||
| Atrophic gastritis | 2 (12.5) | 4 (16.0) | >0.999 |
| Reflux esophagitis (LA classification; N-M/A/B) | 15/1/0 | 22/1/2 | 0.492 |
| Hiatus hernia | 8 (50.0) | 16 (64.0) | 0.518 |
aEE, asymptomatic esophageal eosinophilia; EoE, eosinophilic esophagitis; EREFS, EoE endoscopic reference score; IQR, interquartile range; Ut, upper part of the thoracic esophagus; Lt, lower part of the thoracic esophagus; Mt, middle part of the thoracic esophagus; LA, Los Angeles.
*p<0.1.
Table 3.
High-Resolution Manometry
| Variable | aEE (n=16) | EoE (n=25) | p-value |
|---|---|---|---|
| IRP, mm Hg | 18.8 (9.4–24.6) | 16.6 (12.6–22.7) | 0.653 |
| LES pressure, mm Hg | 29.5 (21.7–35.1) | 32.6 (27.4–41.1) | 0.492 |
| LES residual pressure, mm Hg | 20.1 (11.9–26.7) | 16.0 (12.4–22.5) | 0.489 |
| UES basal pressure, mm Hg | 33.3 (21.5–75.0) | 76.6 (59.3–104.3) | 0.250 |
| DCI mean, mm Hg·s·cm | 2,344 (1,557–3,319) | 1,828 (1,071–2,648) | 0.219 |
| DCI max, mm Hg·s·cm | 3,379 (2,140–4,270) | 2,987 (1,741–3,737) | 0.635 |
| Contractile front velocity, cm/s | 3.7 (3.8–4.0) | 4.2 (3.4–5.3) | 0.026* |
| Distal latency, s | 7.3 (7.0–8.4) | 7.2 (6.9–7.9) | 0.910 |
| Abnormal study | 7 (43.8) | 12 (48.0) | 0.755 |
| Ineffective esophageal motility | 3 (18.8) | 7 (58.3) | |
| EGJ outflow obstruction | 4 (25.0) | 4 (33.3) | |
| Fragmented peristalsis | 0 | 1 (8.3) |
Data are presented as median (interquartile range) or number (%).
aEE, asymptomatic esophageal eosinophilia; EoE, eosinophilic esophagitis; IRP, integrated relaxation pressure; LES, lower esophageal sphincter; UES, upper esophageal sphincter; DCI, distal contractile integral; EGJ, esophagogastric junction.
*p<0.05.
The HRM study was abnormal in seven patients (43.8%) in the aEE group (ineffective esophageal motility [IEM], n=3; EGJ outflow obstruction [EGJOO], n=4) and in 12 (48.0%) in the EoE group (IEM, n=7; EGJOO, n=4; fragmented peristalsis, n=1). Although the median values of the metrics related to manometric diagnosis based on the Chicago classification version 3.0 were within normal limits, the contractile front velocity was significantly slower in the EoE group (p=0.026).22
3. Endoscopic ultrasonography
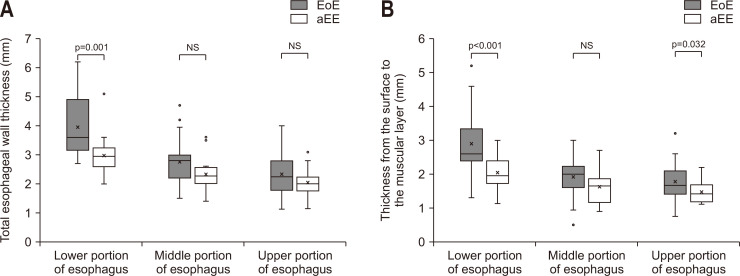
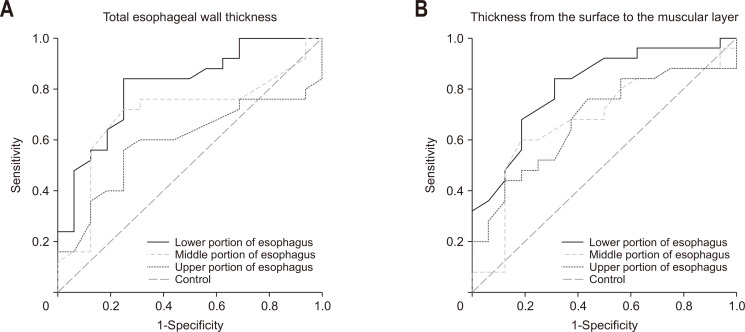
The esophageal wall thickness measured by EUS is shown in Table 4. Mean TWT and mean TSM in the lower portion of esophagus were significantly larger in the EoE group than in the aEE group (TWT, 3.0 mm vs 3.6 mm; TSM, 2.0 mm vs 2.6 mm). However, the thickness of the muscular layer was not significantly different between the two groups. The ratio of TSM to TWT in the lower esophagus tended to be higher in the EoE group than in the aEE group (p=0.059). The cutoff values in the lower portion of esophagus gave high sensitivity and specificity (TWT, 3.13 mm; 84.0% of sensitivity, 75.0% of specificity; TSM, 2.30 mm; 84.0% of sensitivity, 68.7% of specificity) (Fig. 2). The positive predictive value was at least 80% for both TWT and TSM (84.0% and 80.8%, respectively). The box plots and the receiver operating characteristic curves are shown in Fig. 3.
Table 4.
Endoscopic Ultrasonography Measurement of Esophageal Wall Thickness
| Variable | aEE (n=16) | EoE (n=25) | p-value | AUC | 95% CI | |
|---|---|---|---|---|---|---|
| Ut | TSM | 1.4 (1.2–1.7) | 1.7 (1.5–2.1) | 0.038† | 0.677 | 0.512–0.843 |
| TWT | 2.0 (1.8–2.1) | 2.2 (1.8–2.8) | 0.193 | 0.590 | 0.414–0.766 | |
| Muscular layer | 0.6 (0.5–0.8) | 0.6 (0.4–0.8) | 0.355 | 0.409 | 0.229–0.589 | |
| TSM/TWT | 0.71 (0.65–0.75) | 0.71 (0.70–0.81) | 0.091* | 0.630 | 0.457–0.803 | |
| Mt | TSM | 1.7 (1.2–1.8) | 2.0 (1.6–2.2) | 0.101 | 0.676 | 0.503–0.849 |
| TWT | 2.3 (2.0–2.5) | 2.8 (2.4–3.0) | 0.051* | 0.701 | 0.532–0.871 | |
| Muscular layer | 0.7 (0.5–0.8) | 0.7 (0.5–1.1) | 0.279 | 0.496 | 0.319–0.673 | |
| TSM/TWT | 0.74 (0.64–0.75) | 0.75 (0.67–0.81) | 0.795 | 0.601 | 0.428–0.774 | |
| Lt | TSM | 2.0 (1.7–2.4) | 2.6 (2.4–3.3) | <0.001‡ | 0.809 | 0.673–0.945 |
| TWT | 3.0 (2.6–3.2) | 3.6 (3.2–4.4) | 0.001‡ | 0.811 | 0.676–0.947 | |
| Muscular layer | 0.9 (0.7–1.0) | 1.0 (0.7–1.4) | 0.396 | 0.438 | 0.394–0.751 | |
| TSM/TWT | 0.67 (0.63–0.76) | 0.75 (0.69–0.82) | 0.059* | 0.665 | 0.492–0.838 | |
Data are presented as median (interquartile range).
aEE, asymptomatic esophageal eosinophilia; EoE, eosinophilic esophagitis; AUC, area under the curve; CI, confidence interval; Ut, upper part of the thoracic esophagus; Mt, middle part of the thoracic esophagus; Lt, lower part of the thoracic esophagus; TSM, thickness from surface to submucosal layer; TWT, total esophageal wall thickness.
*p<0.1, †p<0.05, ‡p<0.01.
Fig. 2.
Box plots for total esophageal wall thickness (A) and thickness from the surface to the muscular layer (B). Median total esophageal wall thickness and median thickness from the surface to the muscular layer in the lower portion of esophagus were significantly larger in the EoE group than in the aEE group. EoE, eosinophilic esophagitis; aEE, asymptomatic esophageal eosinophilia; NS, not significant.
Fig. 3.
Receiver operating characteristic curves for total esophageal wall thickness (A) and thickness from the surface to the muscular layer (B). The area under curve was high for total esophageal wall thickness and thickness from the surface to the muscular layer in the lower portion of the esophagus.
DISCUSSION
In this study, we sought to identify clinical factors that contribute to symptoms of EoE by comparing the clinicopathological findings of EoE with those of aEE. The strength of this study is that TWT and TSM were evaluated in detail at 1- to 2-cm intervals using mechanical radial EUS scope and were compared with respect to other clinicopathological findings. Histological findings were assessed based on the EoEHSS by specialist pathologists, and LPF tended to be higher in the EoE group. Thus, this study is more in-depth into the pathogenesis of EoE symptoms. Furthermore, the ratio of TSM to TWT was also evaluated to provide objectivity. We demonstrated that thickening of the lower portion of the wall of the esophagus, especially from the surface to the muscular layer, is one of the important clinical factors related to EoE symptoms. This suggests that the submucosal layer may be the primary site of inflammation in EoE. We also showed that the cutoff value for wall thickening was 3.13 mm for TWT and 2.30 mm for TSM.
The concept of aEE has not yet been established in guidelines,1,2 and there is no set diagnostic or treatment algorithm for the aEE. While a part of aEE patients is believed to become symptomatic,9,11 there is insufficient evidence that aEE is a precursor of EoE. Therefore, future research on aEE is expected. Clinical, endoscopic and histological follow-up is recommended at least when aEE is suspected clinically.23
We objectively evaluated the study participants according to the presence or absence of symptoms and classified them into two groups using the F-scale score. Although there is no clear definition of “asymptomatic,” we used an F-scale score of 7 as the cutoff to be consistent with previous research11 and found it to be a useful indicator of symptoms in patients with EoE. Furthermore, there was a significant difference between the groups in scores when using the Eckardt questionnaire, which is designed for achalasia. In the future, it would be useful to define “asymptomatic” more comprehensively when using these questionnaires.
We found no significant differences between the two groups in patient background characteristics or in endoscopic findings, including the affected area and phenotype, which suggests that endoscopic findings probably appear well before symptoms and may not be a major contributor to symptom onset. Previous studies have also found that the severity of symptoms does not necessarily correlate with endoscopic findings or extent of eosinophilic inflammation.9,11,24
It is generally assumed that thickening of the esophageal wall is associated with esophageal dysmotility. A recent study demonstrated that the degree of muscle thickening in the lower esophageal sphincter is associated with the severity of the disease in terms of achalasia, distal esophageal spasm, and hypercontractile esophagus.6 As with other esophageal diseases, symptoms of EoE may be associated with esophageal wall thickness and increased esophageal body pressure.12 In our study, there was a significant difference between the aEE group and the EoE group in esophageal wall thickness, especially in the lower portion of esophagus. Therefore, the degree of wall thickening in the lower portion of esophagus may be an important factor contributing to the onset of EoE symptoms that can be evaluated clinically.
It has also been reported that dense eosinophilic infiltration in the esophageal muscular layer is associated with dysmotility in the esophagus and disease severity not only in patients with EoE but also in those with jackhammer esophagus or nutcracker esophagus.25 In this study, TSM was also significantly greater in the EoE group. In addition, there was no significant difference in the thickness of the muscular layer and the ratio of TSM to TWT in the lower esophagus tended to be higher in the EoE group. It is also reported that EoE symptoms may associated with the esophageal distensibility due to abnormalities in esophageal wall biomechanics related to tissue remodeling.26 Therefore, it can be inferred that chronic inflammation associated with eosinophil infiltration progresses primarily in the mucosal and submucosal layers and that fibrosis and wall thickening in the lamina propria are contributors to the onset of symptoms in EoE. In support of this, the EoE group also tended to have a high LPF score on the EoEHSS notion. Although histological diagnosis by biopsy can only evaluate the superficial layer of the esophagus, evaluation of LPF could also help to predict onset of symptoms of EoE in the future.
Several studies have evaluated esophageal motor function in patients with EoE by HRM and shown that motor abnormalities in the esophagus are not uncommon in these patients, although the evidence currently available indicates that esophageal motility is not disease-specific.27-29 There is a report suggesting that the severity of EoE is associated with esophageal dysmotility.30 Esophageal remodeling, interleukins associated with eosinophilic inflammation, eosinophilic neurotoxic mediators, mast cell degranulation, primary motor dysfunction, and myoactive eosinophilic mediators are thought to be involved in the background.25,29,31 Therefore, even if there are no endoscopic findings, symptoms may still occur as a result of the motor disorder in the esophagus caused by inflammation beneath the surface. In this study, 43.8% of aEE patients was diagnosed with abnormal esophageal motility based on the Chicago classification version 3.0, and all patients with abnormal results were either EGJOO or IEM. In many cases, the clinical significance of IEM is uncertain. EGJOO is defined only by the integrated relaxation pressure value that evaluates EGJ, not the esophageal body, and EGJOO tends to be overdiagnosed in Chicago classification version 3.0. Therefore, there may be insufficient evidence to consider that esophageal motility disorders were frequently caused by aEE. On the other hand, from this perspective, 11 of 12 patients with abnormal results in the EoE group were also diagnosed with either EGJOO or IEM. It has been reported that 27% to 76% of patients with EoE have abnormal esophageal motility.7,8 In this study, 12 of 25 (48.0%) patients with EoE were diagnosed with abnormal esophageal motility, the frequency of which was similar to that reported previously. These findings suggest that esophageal motility disorders may be present in the background even in asymptomatic cases diagnosed with EGJOO or IEM.
EUS and HRM are very advanced methods and are difficult to do in every facility. In addition, the endoscope used for EUS is more invasive than a conventional endoscope, making it more difficult to perform EUS in all patients, especially in asymptomatic patients. Therefore, HRM and EUS for EoE are currently not beyond the modality for research purposes. If a minimally invasive and simple modality is constructed in the future, measurement of esophageal wall thickening is expected to be important for understanding the progression of diseases in individual patients and predicting the onset of symptoms.
The esophageal inflammatory infiltrate can influence esophageal contractility directly and indirectly, potentially inducing both hypocontractility and hypercontractility.31,32 In our study, a strong tendency was observed in contractile front velocity in the EoE group. It is possible that thickening of the esophageal wall centering on the lamina propria as a result of eosinophilic inflammation affects the contractile front velocity. This esophageal wall thickening may cause esophageal dysmotility and be directly or indirectly involved in the onset of gastrointestinal symptoms.
This study has some limitations. First, it had a retrospective single-center design and a limited sample size. Therefore, a larger-scale prospective study is needed to verify our findings. Second, we did not consider the long-term outcomes or the subsequent course of treatment, such as administration of a PPI or topical steroid therapy. In this study, EUS and HRM were basically performed at the time of initial diagnosis, and the thickness of the esophageal wall after treatment was not compared to that before treatment. Future evaluation of esophageal wall thickness after treatment is needed to make this study more meaningful. In addition, although most of the patients examined were not receiving treatment, three were on a PPI or PCAB at the time of the examination. Two patients in the aEE group stopped taking medication at least 2 months before the examination, which may have affected the evaluation of esophageal wall thickness and esophageal motility. Third, expansion of the lumen during filling with water, individual differences, and peristalsis of the esophagus may affect the wall thickness measured using EUS and reduce objectivity. In order to reduce these effects as much as possible, butylscopolamine was used for the examination and the filling water for EUS measurement was kept to the minimum necessary. In addition, the ratio of TSM to TWT, which is considered to be more objective than the length itself, tended to be higher in the EoE group than in the aEE group, although the difference was not significant. This finding is considered to support the conclusions of this study.
In conclusion, the results of this study suggest that wall thickening in the lower portion of esophagus is one of the important clinical factors related to symptoms in patients with EoE. At present, esophageal wall thickness is not included as an evaluation item for EoE, but could be an aid in elucidating the pathogenesis of EoE. It would be desirable to make the measurement of esophageal wall thickness a minimally invasive and simple examination in the future.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: Y.S. Data acquisition: all authors. Data analysis and interpretation: Y.S. Drafting of the manuscript: Y.S. Critical revision of the manuscript for important intellectual content: Y.S. Statistical analysis: Y.S. Administrative, technical, or material support; study supervision: S.H. Approval of final manuscript: all authors.
REFERENCES
- 1.Lucendo AJ, Molina-Infante J, Arias Á, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017;5:335–358. doi: 10.1177/2050640616689525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 2018;155:1022–1033. doi: 10.1053/j.gastro.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato H, Honma T, Nozawa Y, et al. Eosinophilic esophagitis in Japanese patients: a mild and slow-progressing disorder. PLoS One. 2018;13:e0206621. doi: 10.1371/journal.pone.0206621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straumann A, Conus S, Degen L, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400–409. doi: 10.1016/j.cgh.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Tanomkiat W, Chongchitnan P. Transabdominal sonography of gastroesophageal junctions. J Clin Ultrasound. 1999;27:505–512. doi: 10.1002/(SICI)1097-0096(199911/12)27:9<505::AID-JCU4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Santander C, Perea E, Caldas M, Clave P. Catheter-based high-frequency intraluminal ultrasound imaging is a powerful tool to study esophageal dysmotility patients. Ann N Y Acad Sci. 2017;1395:60–66. doi: 10.1111/nyas.13313. [DOI] [PubMed] [Google Scholar]
- 7.Roman S, Hirano I, Kwiatek MA, et al. Manometric features of eosinophilic esophagitis in esophageal pressure topography. Neurogastroenterol Motil. 2011;23:208–e111. doi: 10.1111/j.1365-2982.2010.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martín Martín L, Santander C, Lopez Martín MC, et al. Esophageal motor abnormalities in eosinophilic esophagitis identified by high-resolution manometry. J Gastroenterol Hepatol. 2011;26:1447–1450. doi: 10.1111/j.1440-1746.2011.06770.x. [DOI] [PubMed] [Google Scholar]
- 9.Ishimura N, Sumi S, Okada M, et al. Is asymptomatic esophageal eosinophilia the same disease entity as eosinophilic esophagitis? Clin Gastroenterol Hepatol. 2019;17:1405–1407. doi: 10.1016/j.cgh.2018.08.048. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura H, Tanaka F, Nadatani Y, et al. Eosinophilic esophagitis and asymptomatic esophageal eosinophilia display similar immunohistological profiles. J Clin Biochem Nutr. 2021;68:246–252. doi: 10.3164/jcbn.20-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki Y, Iizuka T, Hosoi A, et al. Clinicopathological differences between eosinophilic esophagitis and asymptomatic esophageal eosinophilia. Intern Med. 2022;61:1319–1327. doi: 10.2169/internalmedicine.8241-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muroi K, Kakushima N, Furukawa K, et al. Subjective symptoms in patients with eosinophilic esophagitis are related to esophageal wall thickness and esophageal body pressure. Dig Dis Sci. 2021;66:2291–2300. doi: 10.1007/s10620-020-06527-5. [DOI] [PubMed] [Google Scholar]
- 13.Kusano M, Shimoyama Y, Sugimoto S, et al. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39:888–891. doi: 10.1007/s00535-004-1417-7. [DOI] [PubMed] [Google Scholar]
- 14.Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732–1738. doi: 10.1016/0016-5085(92)91428-7. [DOI] [PubMed] [Google Scholar]
- 15.Taft TH, Carlson DA, Triggs J, et al. Evaluating the reliability and construct validity of the Eckardt symptom score as a measure of achalasia severity. Neurogastroenterol Motil. 2018;30:e13287. doi: 10.1111/nmo.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030–1038. doi: 10.1111/j.1365-2036.2009.04142.x. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki H, Matsuzaki J, Okada S, Hirata K, Fukuhara S, Hibi T. Validation of the GerdQ questionnaire for the management of gastro-oesophageal reflux disease in Japan. United European Gastroenterol J. 2013;1:175–183. doi: 10.1177/2050640613485238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins MH, Martin LJ, Alexander ES, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus. 2017;30:1–8. doi: 10.1111/dote.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62:489–495. doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 20.Dellon ES, Cotton CC, Gebhart JH, et al. Accuracy of the eosinophilic esophagitis endoscopic reference score in diagnosis and determining response to treatment. Clin Gastroenterol Hepatol. 2016;14:31–39. doi: 10.1016/j.cgh.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kon T, Abe Y, Sasaki Y, et al. Clinical features of esophageal eosinophilia according to endoscopic phenotypes. Intern Med. 2020;59:2971–2979. doi: 10.2169/internalmedicine.4447-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreiner P, Biedermann L, Greuter T, Wright BL, Straumann A. How to approach adult patients with asymptomatic esophageal eosinophilia. Dis Esophagus. 2021;34:doaa105. doi: 10.1093/dote/doaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pentiuk S, Putnam PE, Collins MH, Rothenberg ME. Dissociation between symptoms and histological severity in pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2009;48:152–160. doi: 10.1097/MPG.0b013e31817f0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakajima N, Sato H, Takahashi K, et al. Muscle layer histopathology and manometry pattern of primary esophageal motility disorders including achalasia. Neurogastroenterol Motil. 2017;29:e12968. doi: 10.1111/nmo.12968. [DOI] [PubMed] [Google Scholar]
- 26.Nicodème F, Hirano I, Chen J, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11:1101–1107. doi: 10.1016/j.cgh.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nennstiel S, Bajbouj M, Becker V, et al. High-resolution manometry in patients with eosinophilic esophagitis under topical steroid therapy: a prospective observational study (HIMEOS-study) Neurogastroenterol Motil. 2016;28:599–607. doi: 10.1111/nmo.12753. [DOI] [PubMed] [Google Scholar]
- 28.von Arnim U, Kandulski A, Weigt J, Malfertheiner P. Correlation of high-resolution manometric findings with symptoms of dysphagia and endoscopic features in adults with eosinophilic esophagitis. Dig Dis. 2017;35:472–477. doi: 10.1159/000458407. [DOI] [PubMed] [Google Scholar]
- 29.Visaggi P, Ghisa M, Barberio B, Marabotto E, de Bortoli N, Savarino E. Systematic review: esophageal motility patterns in patients with eosinophilic esophagitis. Dig Liver Dis. 2022;54:1143–1152. doi: 10.1016/j.dld.2022.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Carlson DA, Shehata C, Gonsalves N, et al. Esophageal dysmotility is associated with disease severity in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2022;20:1719–1728. doi: 10.1016/j.cgh.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santander C, Chavarría-Herbozo CM, Becerro-González I, Burgos-Santamaría D. Impaired esophageal motor function in eosinophilic esophagitis. Rev Esp Enferm Dig. 2015;107:622–629. doi: 10.17235/reed.2015.3801/2015. [DOI] [PubMed] [Google Scholar]
- 32.Spechler SJ, Konda V, Souza R. Can eosinophilic esophagitis cause achalasia and other esophageal motility disorders? Am J Gastroenterol. 2018;113:1594–1599. doi: 10.1038/s41395-018-0240-3. [DOI] [PubMed] [Google Scholar]