Abstract
An ompD mutation caused by a Tn10 insertion was transduced into Salmonella typhimurium SL1344 and UK-1. The adherence and invasion capabilities of the resultant ompD mutants were examined by tissue culture analysis. The virulence of the S. typhimurium ompD mutants was ascertained by a 50% lethal dose (LD50) study and by determining colonization ability with BALB/c mice. We found no statistically significant difference in adherence and invasion capacities between the S. typhimurium wild type strains and their corresponding ompD mutants. Furthermore, the LD50 and colonization studies revealed that there is no statistically significant difference in virulence between the S. typhimurium wild type strains and their corresponding ompD mutants. These results differ from those reported previously (C. J. Dorman, S. Chatfield, C. F. Higgins, C. Hayward, and G. Dougan, Infect. Immun. 57:2136–2140, 1989).
Salmonella enterica serovar Typhimurium, a gram-negative bacterial species, is a facultative intracellular pathogen which infects its hosts through the oral route (25). Human diseases caused by Salmonella serotypes include gastroenteritis, bacteremia, and typhoid fever (11). Most infections occur as a result of ingestion of undercooked eggs or contaminated food (meats and dairy products) or water (1, 11, 22). Each year in the United States two to four million cases of gastroenteritis are caused by Salmonella bacteria (23), along with a few hundred cases of typhoid fever (3). According to the World Health Organization, Salmonella is probably the most common cause of diarrhea globally (11), and at least 12 million cases of typhoid fever are reported each year, with a mortality rate of 10 to 12% (7).
Like other gram-negative bacteria, S. typhimurium has an outer membrane surrounding the periplasmic space. The outer membrane contains numerous proteins, referred to as OMPs. A subset of these, called porins, form water-filled channels across the outer membrane to facilitate the transport of small hydrophilic molecules (16). S. typhimurium expresses three porins when grown under normal conditions (Lennox broth at 37°C): OmpD (34 kDa), OmpF (35 kDa), and OmpC (36 kDa) (12, 15, 21). OmpD is found in S. typhimurium but is absent from other gram-negative bacteria, including Escherichia coli. OmpD is homologous with the NmpC and Lc porins in E. coli K-12 (21), both of which (NmpC and Lc) can only be expressed in E. coli K-12 mutants which lack normal outer membrane proteins (18). Little is known about the OmpD porin, apart from the genomic location of the ompD gene and the immunochemical and topological structure of the porin itself (20, 21).
Dorman and colleagues (6) showed that mutations in some porin-associated genes affect the virulence of S. typhimurium in BALB/c mice. Specifically, a mutation in the ompR gene, which encodes a positive regulator of porin gene expression, has a dramatic effect on virulence, increasing the 50% lethal dose (LD50) by more than three log units compared to that of a wild-type strain. In the same study, Dorman et al. characterized the effect of mutations in the ompC, ompF, and ompD genes. They report that strains containing ompF or ompC mutations were as virulent as their wild-type parent, while a strain containing an ompD mutation showed a slight reduction in virulence (23-fold increase in LD50 between the wild type and the ompD mutant). Interestingly, OmpR regulates the expression of the genes coding for porins OmpC and OmpF; but it does not seem to regulate expression of ompD (6).
In a subsequent study, Chatfield et al. (2) showed that a mutant lacking both the OmpF and OmpC porins is attenuated, displaying an oral LD50 that is three log units greater than that of the wild-type parent. This result explains, in part, the attenuation of ompR mutants. However, because ompR mutants display higher oral and intravenous LD50s than the ompF ompC double mutant, it is likely that there are other genes regulated by OmpR which encode proteins involved in virulence.
Traditional programs to design live, attenuated oral vaccines against Salmonella have concentrated on using mutations in the bacterial biochemical pathways or using mutations in global regulators (19). Eliminating global regulators can render a strain avirulent and immunogenic. Inactivating some of the genes regulated by a global regulator should account for some of the avirulence and immunogenicity seen in strains containing a mutation in the global regulator. The ompD gene is regulated by adenylate cyclase and the cyclic AMP regulatory protein (CRP) (16). Strains of S. typhimurium which have cya and crp mutations are avirulent (4). Our goal was to determine if a mutation in ompD could account for some of the avirulence of the cya and crp mutants. In this study, an ompD mutation was transduced into virulent S. typhimurium SL1344 and UK-1. The resultant transductants were compared with their wild-type parents with respect to their abilities to adhere to and invade cells in culture and to colonize tissues and cause disease in BALB/c mice. In contrast to the results of a previous study (6), our results show that ompD mutants are not attenuated, eliminating the possibility that nonexpression of OmpD contributes to the avirulence of cya and crp mutants.
Bacterial strains, media, and phenotypic screens.
The bacterial strains used in this study are listed in Table 1. To generate strains specifically for this project, standard P22HTint transductions were performed. Strains were constructed by transducing the ompD::Tn10 mutation from strain BRD455 (6) into virulent S. typhimurium SL1344 strain χ3339 (9) and UK-1 strain χ3761 (5) to yield strains χ8201 and χ8202, respectively. Transductants were purified, and the presence of the ompD mutation was verified by examining outer membrane fractions from χ8201 and χ8202 by protein electrophoresis as described below.
TABLE 1.
Bacterial strains
| Strain | Genotype | Source (reference) |
|---|---|---|
| S. typhimurium SL1344 | ||
| χ3339 | rpsL hisG | This laboratory (9) |
| BRD454 | rpsL hisG ompC396::Tn10 | Dorman et al. (6) |
| BRD455 | rpsL hisG ompD159::Tn10 | Dorman et al. (6) |
| χ3643 | rpsL hisG invA::TnphoA | Galán and Curtiss (8) |
| χ8201 | rpsL hisG ompD159::Tn10 | This study |
| S. typhimurium UK-1 | ||
| χ3761 | Wild type | This laboratory (5) |
| χ8202 | ompD159::Tn10 | This study |
The standard media used for this study were Lennox broth (13), Luria-Bertani broth (14), and MacConkey agar (Difco). Lennox broth was supplemented with 1.5% agar for plates. MacConkey agar was supplemented with lactose at 1.0%. Tetracycline (Gibco BRL, Grand Island, N.Y.) was added to Lennox broth and MacConkey agar at a concentration of 15 μg/ml.
Membrane isolation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
Bacterial cells were grown in Lennox broth overnight and then sedimented by centrifugation at 5,000 rpm (Sorvall SS-34 rotor in a Sorvall RC5C centrifuge) at 4°C for 10 min and resuspended in phosphate-buffered saline at pH 7.4. Cells were lysed with a French press at 2,000 psi. After the cellular debris was removed by centrifugation at 6,000 rpm (Sorvall SS-34 rotor in a Sorvall RC5C centrifuge) at 4°C for 10 min, the outer membrane proteins were specifically selected by ultracentrifugation at 36,000 rpm (Sorvall SW41Ti rotor in a Sorvall OTD65B ultracentrifuge) at 4°C for 1 h. The pellet containing both cytoplasmic and outer membrane proteins was resuspended in phosphate-buffered saline containing 0.5% Sarkosyl (sodium lauryl sarcosinate) (Sigma, St. Louis, Mo.), followed by another round of ultracentrifugation at 36,000 rpm at 4°C for 1 h to precipitate the outer membrane fractions. Tris-glycine SDS-polyacrylamide gels (10% acrylamide and 1.5 M Tris [pH 8.8] for the slab gel; 5% acrylamide and 1.0 M Tris [pH 6.8] for the stacking gel) were used to separate the outer membrane proteins. The gels were stained with 0.25% Coomassie blue stain and destained with a solution of 10% glacial acetic acid and 30% methanol.
Virulence assays.
The abilities of S. typhimurium mutants to adhere to and invade Intestine-407 (Int-407) cells (10) were analyzed by using a protocol based on a method developed by Galán and Curtiss (8), as described previously (24).
Seven- to ten-week-old female BALB/c mice were used for all animal experiments. The mice were obtained from Harlan Sprague Dawley (Indianapolis, Ind.) and kept at least 1 week prior to inoculation. Virulence was assayed by a comparison of the LD50s of wild-type and mutant strains and by a comparison of the abilities of wild-type and mutant strains to colonize various tissues at 1, 3, and 6 days postinfection.
For the LD50 experiment, strains were grown in Luria-Bertani broth overnight and then subcultured at a 1:200 dilution and grown to an optical density at 600 nm of between 0.7 and 1.0. The cells were concentrated by centrifugation and resuspended in buffered saline with gelatin (BSG), after which dilutions were made in BSG to obtain three different doses for each strain. At each dose, four mice were given oral inoculations of 20 μl of S. typhimurium suspension per mouse. The mice were observed for a period of 4 weeks. LD50s were calculated by the method of Reed and Meunch (17).
For the colonization experiment, bacteria were grown as described above and concentrated in BSG approximately 10-fold. The wild-type and mutant suspensions were mixed to give a ratio of mutant/wild-type bacteria of approximately 1.0. Twelve mice were each infected perorally with 20 μl of the bacterial suspension after being deprived of food and water for 4 to 6 h. Food and water were returned 30 min after infection. At 1, 3, and 6 days postinoculation, four mice were euthanized and the Peyer’s patches, intestinal wall, intestinal contents, spleen, and liver were removed from each mouse. Each tissue was placed in 2 ml of cold BSG and homogenized with a Brinkmann homogenizer. The bacteria were enumerated after the dilutions were plated on MacConkey lactose agar as well as MacConkey lactose agar plus tetracycline.
Verification of the ompD mutants.
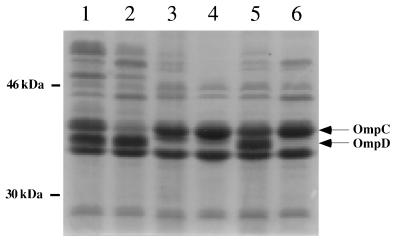
As described above, the ompD::Tn10 mutation was transduced into S. typhimurium SL1344 strain χ3339 and S. typhimurium UK-1 strain χ3761 to obtain χ8201 and χ8202, respectively. SDS-PAGE analysis of outer membrane fractions from χ8201 and χ8202 was performed to verify the absence of the protein band corresponding to OmpD. The results confirm that both χ8201 and χ8202 strains did not express OmpD (Fig. 1).
FIG. 1.
Separation of outer membrane fractions of six strains of S. typhimurium by SDS–10% PAGE. Lane 1, wild-type SL1344 strain χ3339; lane 2, ompC mutant BRD454; lane 3, ompD mutant BRD455; lane 4, ompD mutant SL1344 strain χ8201; lane 5, wild-type UK-1 strain χ3761; lane 6, ompD mutant UK-1 strain χ8202.
Adherence and invasion capabilities of the ompD S. typhimurium mutants.
Dorman and colleagues (6) reported that a mutation in ompD had only a small effect on the virulence of S. typhimurium SL1344 when BALB/c mice were inoculated perorally. Consequently, the abilities of ompD mutants to adhere to and invade intestinal epithelial cells were compared with those of the wild type. The effect of the ompD mutation was examined in both the virulent SL1344 and UK-1 strain backgrounds. As shown in Table 2, in both SL1344 and UK-1 backgrounds, the ompD mutants show no significant difference from the wild type in either adherence to or invasion of Int-407 cells. This result indicates that the OmpD porin is not involved in either the adherence or invasiveness of S. typhimurium.
TABLE 2.
Adherence to and invasion of Int-407 cells by wild-type, ompD, and invA strains of S. typhimurium
| Strain | % Adherence (P valuea) | % Invasion (P valuea) |
|---|---|---|
| χ3339 | 37.7 ± 3.4 | 15.8 ± 8.8 |
| χ8201 | 40.4 ± 14.4 (NS) | 25.4 ± 14.9 (NS) |
| χ3643 | 27.0 ± 6.9 (NS) | 0.7 ± 0.4 (≤0.025) |
| χ3761 | 41.6 ± 11.5 | 7.0 ± 3.8 |
| χ8202 | 39.8 ± 4.9 (NS) | 9.5 ± 4.9 (NS) |
P values are given only when the percent adherence or invasion for a mutant is significantly different from that of its wild-type parent (NS, not significant). The test statistic was calculated from the following equation: t = (ȳ1 − ȳ2)/s [(1/n1) + (1/n2)]1/2. ȳ1 is the mean for percent adherence or percent invasion for wild type; ȳ2 is the mean for percent adherence or percent invasion for each mutant strain; s is the standard deviation; n1 is the number of samples averaged in ȳ1; n2 is the number of samples averaged in ȳ2.
Determination of the virulence of wild-type and ompD strains of S. typhimurium with BALB/c mice.
As our results showed no effect of the ompD mutation on adherence or invasion, we decided to assay virulence in an animal model. The LD50s of the ompD mutants and their corresponding wild-type parents were determined. This was done to verify the original observation made by Dorman et al. (6) that ompD mutants were somewhat attenuated. Concurrently, we performed colonization studies to determine at which point in the infection process these mutants may be blocked.
The peroral LD50s for wild-type strains and ompD mutants are 1.2 × 105 for strain χ3339 (SL1344), 7.5 × 104 for strain χ8201 (SL1344 ompD::Tn10), 3.1 × 105 for strain χ3761 (UK-1), and 1.1 × 105 for strain χ8202 (UK-1 ompD::Tn10). In our study, the ompD::Tn10 mutation did not increase the LD50 in either background. In fact, the LD50s are slightly lower for the ompD mutants.
For colonization studies, mice were coinfected with an ompD mutant and its respective wild-type parent. Representative data from one of the colonization studies are presented in Table 3. Colonization experiments were conducted twice with S. typhimurium SL1344 and three times with S. typhimurium UK-1. Our studies indicate that there is no consistently significant difference between the ompD mutants and their respective wild-type parents in their abilities to colonize either the Peyer’s patches, intestinal wall, intestinal contents, spleen, or liver at any of the time points. An occasional increase was seen in either an ompD mutant’s or its parent’s ability to colonize a tissue; however, these differences were not consistent or repeatable.
TABLE 3.
Statistical analysis of a colonization study of S. typhimurium SL1344 and an ompD derivative by using a paired difference testa
| Time postinoculation (days) | Tissue | Mean paired differenceb | P valuec |
|---|---|---|---|
| 3 | Peyer’s patches | −1.1 ± 0.7 | NS |
| Intestinal wall | −0.6 ± 0.8 | NS | |
| Intestinal contents | 0.2 ± 0.3 | NS | |
| Spleen | 0.9 ± 2.4 | NS | |
| Liver | −0.2 ± 1.3 | NS | |
| 6 | Peyer’s patches | −1.3 ± 1.0 | NS |
| Intestinal wall | −1.1 ± 0.6 | NS | |
| Intestinal contents | −1.3 ± 1.3 | NS | |
| Spleen | −0.1 ± 0.1 | NS | |
| Liver | −0.4 ± 0.2 | NS |
Mice were inoculated with a mixed suspension of bacteria at a ratio of strain χ3339 cells/χ8201 cells of 1.5.
Mean of the paired difference (log10 CFU of strain χ3339 − log10 CFU of strain χ8201) ± the standard deviation.
P values are given only when significant (NS, not significant). The test statistic (t) was calculated as follows: t = (d̄ − μ0)/sd/n1/2 where d is the mean paired difference, μ0 is the difference of the inoculum (log10 CFU of strain χ3339 − log10 CFU of strain χ8201 [0.18]), sd is the standard deviation, and n is the sample size (the sample size was four mice, except in a few cases where four mice were tested but results were obtained only for two or three mice).
Discussion.
Dorman and colleagues (6) have reported that an ompD mutant of S. typhimurium SL1344 is less virulent than the wild type, with an oral LD50 about 23-fold higher. LD50s determined in this study show that there is no difference in virulence between ompD mutants of S. typhimurium SL1344 or UK-1 and their corresponding wild-type parents. In support of this conclusion, our colonization data show that there is no significant difference between the abilities of two different wild-type S. typhimurium strains and their respective ompD mutants to colonize or reach the Peyer’s patches, intestinal wall, intestinal contents, spleen, or liver. Furthermore, adherence and invasion assays performed with cultured intestinal epithelial cells showed no significant difference between the wild type and ompD mutants of either S. typhimurium UK-1 or SL1344 in their abilities to adhere to or invade host cells.
Why are the results from our study different from those obtained by Dorman et al. (6)? It is possible that differences exist in the BALB/c mice used in the two studies. For example, there may be mild genetic differences between the different mouse colonies which affect their susceptibilities to S. typhimurium. Alternatively, the BALB/c mice used by Dorman et al. may have had an additional infection, possibly compromising their ability to recover from a Salmonella infection. Another possibility is that subtle differences exist in the manner in which the mice were infected or cared for and that these may account for the differences observed.
The differences in experimental results are unlikely to be due to the nature of the mutations tested, as the particular ompD::Tn10 insertion used in this study was the same as that described previously (6). However, the specific ompD strain used by Dorman et al. (6) may have acquired an additional mutation during construction which could be the actual cause of the decrease in virulence. It is possible that when the OmpD porin is eliminated by mutation, another may function in its place; hence, a “backup system” may exist. The fact that an ompC mutant or an ompF mutant (either of which still synthesizes the two other porins) is still virulent (6) could support this hypothesis. If the ompD mutant used by Dorman et al. (6) had an additional mutation in this backup system, the mutation may account for the strain’s slight attenuation.
Acknowledgments
Support for this study was provided by a grant to Washington University from the Howard Hughes Medical Institute through the Undergraduate Biological Sciences Education Program and by a grant from the National Institute of Allergy and Infectious Diseases.
We thank Lisa Burns-Keliher for help in the preparation of Fig. 1 and Cheryl Nickerson for helpful discussion.
REFERENCES
- 1.Centers for Disease Control and Prevention. Surveillance for foodborne-disease outbreaks—United States, 1988–1992. Morbid Mortal Weekly Rep. 1996;45:1–66. . (CDC surveillance summary.) [PubMed] [Google Scholar]
- 2.Chatfield S N, Dorman C J, Hayward C, Dougan G. Role of ompR-dependent genes in Salmonella typhimurium virulence: mutants deficient in both OmpC and OmpF are attenuated in vivo. Infect Immun. 1991;59:449–452. doi: 10.1128/iai.59.1.449-452.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collinson S K, Doig P C, Doran J L, Clouthier S, Trust T J, Kay W W. Thin, aggregative fimbriae mediate binding of Salmonella enteriditis to fibronectin. J Bacteriol. 1993;175:12–18. doi: 10.1128/jb.175.1.12-18.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtiss R, III, Kelly S M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987;55:3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtiss R, III, Porter S B, Munson M, Tinge S A, Hassan J O, Gentry-Weeks C, Kelly S M. Nonrecombinant and recombinant avirulent Salmonella live vaccines for poultry. In: Blakenship L C, Bailey J S, Cox N A, Stern N J, Meinersmann R J, editors. Colonization control of human bacterial enteropathogens in poultry. New York, N.Y: Academic Press, Inc.; 1991. pp. 169–198. [Google Scholar]
- 6.Dorman C J, Chatfield S, Higgins C F, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989;57:2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelman R A, Levine M M. Summary of an international workshop of typhoid fever. Rev Infect Dis. 1986;8:329–349. doi: 10.1093/clinids/8.3.329. [DOI] [PubMed] [Google Scholar]
- 8.Galán J E, Curtiss R., III Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulig P A, Curtiss R., III Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987;55:2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henle G, Deinhardt F. The establishment of strains of human cells in tissue culture. J Immunol. 1957;79:54–59. [PubMed] [Google Scholar]
- 11.Hook E W. Salmonella species (including typhoid fever) In: Mandel G L, Douglas R G, editors. Principles and practices in infectious diseases. New York, N.Y: Wiley and Sons; 1985. pp. 1256–1269. [Google Scholar]
- 12.Lee D R, Schnaitman C A. Comparison of outer membrane porin proteins produced by Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980;142:1019–1022. doi: 10.1128/jb.142.3.1019-1022.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lennox E S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 14.Luria S E, Burrous J J. Hybridization between Escherichia coli and shigella. J Bacteriol. 1957;74:461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikaido H. Outer membrane. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: ASM Press; 1996. pp. 29–47. [Google Scholar]
- 17.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 18.Riley M, Krawiec S. Genome organization. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 2. Washington, D.C: American Society for Microbiology; 1987. pp. 967–981. [Google Scholar]
- 19.Roberts M, Chatfield S N, Dougan G. Salmonella as carriers of heterologous antigens. In: O’Hagen D T, editor. Novel delivery systems for oral vaccines. Ann Arbor, Mich: CRC Press; 1994. pp. 27–58. [Google Scholar]
- 20.Singh S P, Miller S, Williams Y, Rudd K E, Nikaido H. Immunochemical structure of the OmpD porin from Salmonella typhimurium. Microbiology. 1996;142:3201–3210. doi: 10.1099/13500872-142-11-3201. [DOI] [PubMed] [Google Scholar]
- 21.Singh S P, Upshaw Y, Abdullah T, Singh S, Klebba P E. Structural relatedness of enteric bacterial porins assessed with monoclonal antibodies to Salmonella typhimurium OmpD and OmpC. J Bacteriol. 1992;174:1965–1973. doi: 10.1128/jb.174.6.1965-1973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.St. Louis M, Morse D L, Potter M E, DeMelfi T M, Guzewich J J, Tauxe R V, Blake P A M. The emergence of grade A eggs as a major source of Salmonella enteritidis infections. JAMA. 1988;259:2103–2107. [PubMed] [Google Scholar]
- 23.Tauxe R V. Salmonella: a postmodern pathogen. J Food Prot. 1991;54:563–568. doi: 10.4315/0362-028X-54.7.563. [DOI] [PubMed] [Google Scholar]
- 24.Wilmes-Riesenberg M R, Bearson B, Foster J W, Curtiss R., III The role of the acid tolerance response in the virulence of Salmonella typhimurium. Infect Immun. 1996;64:1085–1092. doi: 10.1128/iai.64.4.1085-1092.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwadyk P. Enterobacteriaceae: Salmonella and Shigella, intestinal pathogens. In: Joklik W K, Willett H P, Amos D B, Wilfert C M, editors. Zinsser microbiology. 19th ed. Norwalk, Conn: Appleton and Lange; 1988. pp. 473–479. [Google Scholar]