Abstract
Background/Aims
Based on their anatomy, cholangiocarcinomas (CCAs) are classified into intrahepatic, hilar, and distal CCAs. Although the diagnosis and treatment of each type of CCA are thought to be different, real-world data studies on the current practice are limited. Therefore, this study was designed to capture the current practice of diagnosing and treating perihilar CCA in Korea.
Methods
We conducted a survey using an online platform. The questionnaire consisted of 18 questions designed to evaluate the current practice of diagnosing and treating perihilar CCA in Korea. The targets of this survey were biliary endoscopists who are members of the Korean Pancreatobiliary Association.
Results
In total, 119 biliary endoscopists completed the survey. Of the respondents, 89.9% thought that the use of the International Classification of Diseases, 11th Revision (ICD-11) system is necessary to classify CCA. Approximately half of the respondents would recommend surgery or chemotherapy until patients were 80 years of age. For the pathological diagnosis of CCA, endoscopic retrograde cholangiopancreatography with biopsy was the most preferred modality. Routine preoperative biliary drainage was performed by 44.5% of the respondents. For operable CCAs, 64.7% of the respondents preferred endoscopic biliary drainage using plastic stents. For palliative biliary drainage, 69.7% of the respondents used plastic stents. For palliative endoscopic biliary drainage using metal stents, 63% of the respondents preferred the stent-in-stent method.
Conclusions
A new coding system using the ICD-11 is needed for classifying CCAs. Guidelines for diagnosing and treating CCA based on the clinical situation in Korea are needed.
Keywords: Cholangiocarcinoma, Diagnosis, Treatment, Surveys and questionnaires
INTRODUCTION
Cholangiocarcinoma (CCA) is a malignant tumor that occurs in the epithelium of the bile duct and is classified into intrahepatic CCA (iCCA), perihilar CCA (pCCA), and distal CCA (dCCA) according to its location.1 The most common type of CCA is pCCA (50% to 60%), followed by dCCA (20% to 30%) and iCCA (10% to 20%).2 CCA accounts for approximately 3% of all gastrointestinal malignancies. However, its incidence is increasing globally.2 The incidence of CCA is much higher in Eastern countries than in Western countries.3 According to the Annual Report of Cancer Statistics 2018 announced by the Korea Central Cancer Registry in 2021, the incidence of CCAs and gallbladder cancers was 3,840 cases (crude incidence rate 15/100,000) for men and 3,339 cases (crude incidence rate 3.6/100,000) for women, ranking 9th in the overall cancer incidence.4 The 5-year relative survival rate was 28.8%, which showed a poor prognosis compared with other gastrointestinal cancers, such as gastric (77%) and colon (74.3%) cancers.4
Each CCA type has distinct characteristics. Therefore, the diagnosis, treatment, and prognosis are thought to be different.5,6 However, few studies have focused on this issue. One reason is the lack of a separate code for the most common type of CCA (i.e., pCCA) in the 10th revision of the International Classification of Diseases (ICD-10).3 The ICD-10 divides CCAs into intrahepatic (C22.1) and extrahepatic (C24.0) CCAs. For this reason, pCCAs are sometimes misclassified as iCCA. Although several guidelines on the diagnosis and treatment of CCAs have been published,7-9 few real-world data studies on the clinical practice of diagnosing and treating pCCA have been conducted. Therefore, this study was designed to capture the current practice in diagnosing and treating pCCA in Korea.
MATERIALS AND METHODS
1. Study design and contents of the questionnaire
A national online survey was organized by the Committee on Bile Duct Diseases of Korean Pancreatobiliary Association. An 18-item online questionnaire was developed and revised through three meetings after drafting by the committee members. The questionnaire was divided into five categories: (1) demographic characteristics of the respondents, (2) CCA classification, (3) treatment of CCA in elderly patients, (4) diagnosis of pCCA, and (5) biliary drainage in pCCA. E-mails with direct links to the web survey were sent four times to the members of the Korean Pancreatobiliary Association between May 2021 and September 2021.
2. ICD and Korean Standard Classification of Disease and Cause of Death (KCD)
Endorsed by the World Health Organization in 1990 and first used in 1994, the ICD-10 is currently the most widely used medical classification coding system (Table 1).10 The disease code for CCA in Korea follows the KCD-8, which is based on the ICD-10 and the 3rd International Classification of Diseases for Oncology (Table 2).11 Therefore, the disease code for CCA in Korea also divides CCAs into intrahepatic (C22) and extrahepatic (C24) CCAs. The 11th revision of the ICD (ICD-11) came into effect on January 1, 2022 and categorizes CCAs into iCCA (2C12), pCCA (2C18), and dCCA (2C14 and 2C15) (Table 3).12
Table 1.
International Classification of Diseases, 10th Revision (ICD-10)
| Code | Code description |
|---|---|
| C22 | Malignant neoplasm of liver and intrahepatic bile ducts |
| |
| C22.0 | Liver cell carcinoma
|
| C22.1 | Intrahepatic bile duct carcinoma
|
| C22.2 | Hepatoblastoma |
| C22.3 | Angiosarcoma of liver
|
| C22.4 | Other sarcomas of liver |
| C22.7 | Other specified carcinomas of liver |
| C22.9 | Liver, unspecified |
| C24 | Malignant neoplasm of other and unspecified parts of biliary tract |
| |
| C24.0 | Extrahepatic bile duct
|
| C24.1 | Ampulla of Vater |
| C24.8 | Overlapping lesion of biliary tract
|
| C24.9 | Biliary tract, unspecified |
NOS, not otherwise specified.
Data from: https://icd.who.int/browse10/2019/en#/C15-C26.10
Table 2.
Korean Standard Classification of Disease and Cause of Death, 8th Revision (KCD-8)
| Code | Code description |
|---|---|
| C22.1 | Malignant neoplasm of intrahepatic bile duct carcinoma |
| C22.10 | Malignant neoplasm of cholangiocarcinoma |
| C24.0 | Extrahepatic bile duct, malignant neoplasm |
| C24.00 | Biliary duct or passage NOS, malignant neoplasm |
| C24.01 | Common bile duct, malignant neoplasm |
| C24.02 | Cystic duct, malignant neoplasm |
| C24.03 | Hepatic duct, malignant neoplasm |
| C24.1 | Ampulla of Vater, malignant neoplasm |
| C24.8 | Overlapping lesion of biliary tract, malignant neoplasm |
| C24.80 | Malignant neoplasm involving both intrahepatic and extrahepatic bile ducts |
| C24.9 | Biliary tract, unspecified malignant neoplasm |
NOS, not otherwise specified.
Data from: http://kssc.kostat.go.kr/.11
Table 3.
International Classification of Diseases, 11th Revision (ICD-11)
| Code | Code description |
|---|---|
| 2C12 | Malignant neoplasms of liver or intrahepatic bile ducts |
| 2C12.0 | Malignant neoplasm of liver |
| 2C12.1 | Malignant neoplasm of intrahepatic bile ducts 2C12.10 Intrahepatic cholangiocarcinoma 2C12.1Y Other specified malignant neoplasms of intrahepatic bile ducts |
| 2C12.Z | Malignant neoplasms of liver or intrahepatic bile ducts, unspecified |
| 2C14 | Malignant neoplasms of proximal biliary tract, cystic duct |
| 2C14.0 | Adenocarcinoma of proximal biliary tract, cystic duct |
| 2C14.1 | Mucinous cystic neoplasm with associated invasive carcinoma of cystic duct |
| 2C14.2 | Neuroendocrine neoplasms of cystic duct |
| 2C14.Y | Other specified malignant neoplasms of biliary tract, cystic duct |
| 2C14.Z | Malignant neoplasms of proximal biliary tract, cystic duct, unspecified |
| 2C15 | Malignant neoplasms of biliary tract, distal bile duct |
| 2C15.0 | Adenocarcinoma of biliary tract, distal bile duct |
| 2C15.1 | Mucinous cystic neoplasm with associated invasive carcinoma of distal bile duct |
| 2C15.2 | Neuroendocrine neoplasms of distal bile duct |
| 2C15.Y | Other specified malignant neoplasms of biliary tract, distal bile duct |
| 2C15.Z | Malignant neoplasms of biliary tract, distal bile duct, unspecified |
| 2C17 | Malignant neoplasms of other or unspecified parts of biliary tract |
| 2C18 | Malignant neoplasms of perihilar bile duct |
| 2C18.0 | Hilar cholangiocarcinoma |
| 2C18.1 | Mucinous cystic neoplasm with associated invasive carcinoma of perihilar bile duct |
| 2C18.2 | Neuroendocrine neoplasm of perihilar bile duct |
| 2C18.Y | Other specified malignant neoplasms of perihilar bile duct |
| 2C18.Z | Malignant neoplasms of perihilar bile duct, unspecified |
Data from: https://icd.who.int/browse11/l-m/en.12
3. Statistical analysis
Categorical variables were presented as proportions. Univariate analysis was performed using the chi-square test for categorical variations. p-values of less than 0.05 were used to denote statistical significance. All statistical analyses were performed using Statistical Package for the Social Sciences, version 26 (IBM Corp., Armonk, NY, USA).
RESULTS
1. Demographic characteristics of the respondents
Of the 209 certified pancreatobiliary endoscopists, 119 completed the survey (56.9%). Among them, 113 were male doctors and six were female doctors. Fig. 1 shows the demographic characteristics of the respondents in this survey. Twenty respondents (16.8%) were in their 30s, 55 (46.2%) in their 40s, 34 (28.6%) in their 50s, and 10 (8.4%) in their 60s (Fig. 1A). The experience on endoscopic retrograde cholangiopancreatography (ERCP) was >10 years in 65 endoscopists (54.6%), 7 to 9 years in 13 endoscopists (10.9%), 3 to 6 years in 23 endoscopists (19.3%), <3 years in 18 endoscopists (15.1%) (Fig. 1B). Most endoscopists (107/119, 89.9%) worked in tertiary hospitals (Fig. 1C). The number of ERCP procedures per month in the hospitals where the respondents are working was >100 in 43 (36.2%), 50 to 99 in 43 (36.1%), and <50 in 33 (27.7%) (Fig. 1D).
Fig. 1.
Demographic characteristics of the respondents. (A) Age. (B) Personal experience in endoscopic retrograde cholangiopancreatography (ERCP). (C) Types of respondent hospitals. (D) Monthly ERCP cases at each respondent’s hospital.
2. Classification of CCA
Most respondents (98/119, 82.4%) used separate codes for iCCA and extrahepatic CCA (eCCA) at the time of diagnosis. However, 20 respondents (16.8%) did not use distinguished codes for iCCAs or eCCAs (Fig. 2A). Most respondents (107/119, 89.9%) thought that the replacement of ICD-10 by ICD-11 is needed (Fig. 2B).
Fig. 2.
Classification of cholangiocarcinoma. (A) Actual coding of cholangiocarcinoma at the time of diagnosis. (B) The need for the ICD-10 versus the ICD-11. ICD, International Classification of Diseases; CCA, cholangiocarcinoma.
3. Treatment of CCA in the elderly patients
The maximum age of the patients at which the respondents would recommend surgery was 80 years in 62 (52.1%), followed by 85 years in 29 (24.4%), 75 years in 17 (14.3%), >85 years in nine (7.6%), and 70 years in two (1.7%) (Fig. 3A). Moreover, the maximum age of the patients at which the respondents would recommend chemotherapy was 80 years in 57 (47.9%), followed by 85 years in 46 (38.7%), 75 years in 10 (8.4%), >85 years in five (4.2%), and 70 years in one (0.8%) (Fig. 3B).
Fig. 3.
Treatment of cholangiocarcinoma in elderly patients. (A) What is the maximum age at which you recommend surgery? (B) What is the maximum age at which you recommend chemotherapy?
4. Diagnosis of pCCA
For the pathological confirmation of pCCA, most respondents (114/119, 95.8%) preferred using ERCP with biopsy and cytology as the initial diagnostic method (Fig. 4A). If the first biopsy result showed atypia and curative resection is possible, most respondents (100/119, 84%) thought that a second biopsy would be unnecessary (Fig. 4B). Overall, 102 endoscopists (85.7%) chose ERCP with re-biopsy as the next diagnostic method for the pathological confirmation of unresectable pCCA. Twelve endoscopists (10.1%) used cholangioscopy, three (2.5%) used percutaneous transhepatic biliary drainage (PTBD) with bile juice cytology, and two (1.7%) used endoscopic ultrasound-guided fine-needle aspiration/biopsy (EUS-FNA/B) as the second diagnostic method (Fig. 4C).
Fig. 4.
Diagnosis of perihilar cholangiocarcinoma (pCCA). (A) What is the first diagnostic modality used for the pathological diagnosis of suspected pCCA? (B) Do you think that preoperative pathological confirmation is necessary in case of curative pCCA if the first biopsy result shows atypia? (C) What is the second diagnostic method for the pathological confirmation of inoperable pCCA? ERCP, endoscopic retrograde cholangiopancreatography; EUS-FNA/B, endoscopic ultrasound-guided fine-needle aspiration/biopsy; PTBD, percutaneous transhepatic biliary drainage.
5. Biliary drainage in pCCA
For preoperative biliary drainage, 66 respondents (55.5%) performed biliary drainage in selected patients, whereas 53 (44.5%) performed routine preoperative biliary drainage (Fig. 5A). Regarding the preoperative biliary drainage method, 77 respondents (64.7%) preferred performing endoscopic retrograde biliary drainage (ERBD) using plastic stents, followed by endoscopic nasobiliary drainage in 25 (21.0%), PTBD in 15 (12.6%), and ERBD using metal stents in two (1.7%) (Fig. 5B).
Fig. 5.
Preoperative biliary drainage in perihilar cholangiocarcinoma (pCCA). (A) Do you routinely perform preoperative biliary drainage in patients with operable pCCA? (B) What is the most preferred method for preoperative biliary drainage in pCCA? ENBD, endoscopic nasobiliary drainage; ERBD, endoscopic retrograde biliary drainage; PTBD, percutaneous transhepatic biliary drainage.
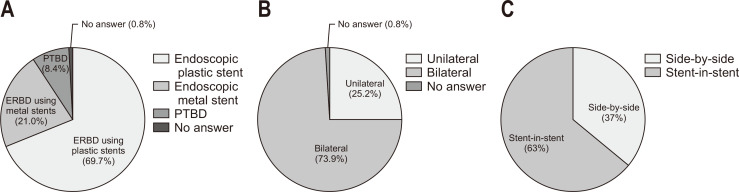
For palliative biliary drainage in inoperable pCCAs, ERBD using plastic stents was the most preferred primary method (83/119, 69.7%), followed by ERBD using metal stents (25/119, 21.0%) and PTBD (10/119, 8.4%) (Fig. 6A). When endoscopic biliary drainage was performed, most endoscopists (88/119, 73.9%) preferred bilateral drainage over unilateral drainage (Fig. 6B). When endoscopic bilateral metal stenting was performed, the stent-in-stent (SIS) method was preferred (75/119, 63%) over the side-by-side (SBS) method (Fig. 6C).
Fig. 6.
Palliative biliary drainage in inoperable perihilar cholangiocarcinoma (pCCA). (A) Which is the preferred initial method for palliative biliary drainage in pCCA? (B) When endoscopic palliative biliary drainage is required in pCCA, which method is preferred, unilateral or bilateral drainage? (C) If endoscopic bilateral metal stenting is performed for palliative biliary drainage in pCCA, which method would you prefer, side-by-side or stent-in-stent? ERBD, endoscopic retrograde biliary drainage; PTBD, percutaneous transhepatic biliary drainage.
6. Differences in the diagnosis and treatment of CCA according to age group
Table 4 shows the difference in the diagnosis and treatment of CCAs among four age groups of respondents. No significant differences in the coding of CCA, the treatment of CCA in the elderly patients, the initial method for the pathological confirmation of CCA, and the need for preoperative pathological confirmation were found among the four age groups. However, a significant difference in the second method for the pathological confirmation of pCCA (p<0.05) was observed among the four age groups. Furthermore, >80% of the respondents in the age 30 to 59-year group preferred ERCP with re-biopsy as the second diagnostic method. In contrast, only 50% of the respondents in their 60s favored ERCP with re-biopsy. EUS-FNA/B was chosen by the respondents in their 30s only. Cholangioscopy was preferred in 40% of the respondents in their 60s. A trend where preoperative biliary drainage was preferred more by respondents in their 60s than by the respondents of the 30 to 59-year group was noticed (90% vs 50.9%–55%), although this difference was statistically insignificant (p=0.146). Furthermore, the respondents of the 60 to 69-year group exclusively preferred ERCP using plastic or metal stents as the preoperative biliary drainage method (p<0.05). No significant differences in the palliative biliary drainage method were observed among the four age groups.
Table 4.
Differences in the Diagnosis and Treatment of Cholangiocarcinoma According to the Endoscopists’ Age Group
| Variable | Age group, No. (%) | p-value | |||
|---|---|---|---|---|---|
| 30–39 yr (n=20) |
40–49 yr (n=55) |
50–59 yr (n=34) |
60–69 yr (n=10) |
||
| Coding of CCA | |||||
| Separate codes for iCCA and eCCA | 17 (85) | 46 (83.6) | 27 (79.4) | 8 (80) | 0.938 |
| Need for ICD-11 | 20 (100) | 46 (83.6) | 32 (94.1) | 9 (90) | 0.465 |
| Treatment of CCA in the elderly | |||||
| Maximum age of surgery | 0.816 | ||||
| 70 yr | 0 | 2 (3.6) | 0 | 0 | |
| 75 yr | 3 (15) | 10 (18.2) | 2 (5.9) | 2 (20) | |
| 80 yr | 11 (55) | 28 (50.9) | 18 (52.9) | 5 (50) | |
| 85 yr | 4 (20) | 11 (20.0) | 12 (35.3) | 2 (20) | |
| >85 yr | 2 (10) | 4 (7.3) | 2 (5.9) | 1 (10) | |
| Maximum age of chemotherapy | 0.794 | ||||
| 70 yr | 0 | 0 | 1 (2.9) | 0 | |
| 75 yr | 2 (10) | 6 (10.9) | 2 (5.9) | 0 | |
| 80 yr | 11 (55) | 25 (45.5) | 18 (52.9) | 3 (30) | |
| 85 yr | 6 (30) | 22 (40.0) | 12 (35.3) | 6 (60) | |
| >85 yr | 1 (5) | 2 (3.6) | 1 (2.9) | 1 (10) | |
| Diagnosis of pCCA | |||||
| Initial method for pathological confirmation | 0.597 | ||||
| ERCP | 19 (95) | 54 (98.2) | 32 (94.1) | 9 (90) | |
| PTBD with bile juice cytology | 1 (5) | 1 (1.8) | 2 (5.9) | 1 (10) | |
| Preoperative pathological confirmation in curative pCCA necessary | 0.249 | ||||
| Yes | 3 (15) | 12 (21.8) | 2 (5.9) | 2 (20) | |
| No | 17 (85) | 43 (78.2) | 32 (94.1) | 8 (80) | |
| Second method for pathological confirmation | <0.05 | ||||
| ERCP with re-biopsy | 16 (80) | 49 (89.1) | 32 (94.1) | 5 (50) | |
| EUS-FNA/B | 2 (10) | 0 | 0 | 0 | |
| Cholangioscopy | 2 (10) | 4 (7.3) | 2 (5.9) | 4 (40) | |
| PTBD with bile juice cytology | 0 | 2 (3.6) | 0 | 1 (10) | |
| Treatment of pCCA | |||||
| Routine preoperative biliary drainage | 0.146 | ||||
| Yes | 9 (45) | 27 (49.1) | 16 (47.1) | 1 (10) | |
| No | 11 (55) | 28 (50.9) | 18 (52.9) | 9 (90) | |
| Preferred preoperative biliary drainage method | <0.05 | ||||
| ENBD | 4 (20) | 11 (20.0) | 10 (29.4) | 0 | |
| ERCP using plastic stents | 14 (70) | 38 (69.1) | 17 (50.0) | 8 (80) | |
| ERCP using metal stents | 0 | 0 | 0 | 2 (20) | |
| PTBD | 2 (10) | 6 (10.9) | 7 (20.6) | 0 | |
| Palliative biliary drainage method | 0.712 | ||||
| ERBD using plastic stents | 16 (80) | 37 (67.3)* | 24 (70.6) | 6 (60) | |
| ERBD using metal stents | 4 (20) | 13 (23.6) | 5 (14.7) | 3 (30) | |
| PTBD | 0 | 4 (7.3) | 5 (14.7) | 1 (10) | |
| Palliative endoscopic drainage | 0.171 | ||||
| Bilateral | 2 (10)* | 14 (25.5) | 12 (35.3) | 2 (20) | |
| Unilateral | 17 (85) | 41 (74.5) | 22 (64.7) | 8 (80) | |
| Bilateral metal stenting method | 0.630 | ||||
| Side-by-side | 9 (45) | 19 (34.5) | 11 (32.4) | 5 (50) | |
| Stent-in-stent | 11 (55) | 36 (65.5) | 23 (67.6) | 5 (50) | |
CCA, cholangiocarcinoma; iCCA, intrahepatic CCA; eCCA, extrahepatic CCA; pCCA, perihilar CCA; ICD, International Classification of Diseases; ERCP, endoscopic retrograde cholangiopancreatography; PTBD, percutaneous transhepatic biliary drainage; EUS-FNA/B, endoscopic ultrasound-guided fine-needle aspiration/biopsy; ENBD, endoscopic nasobiliary drainage.
*No response (n=1).
DISCUSSION
This study represents the first survey on the current practices of Korean pancreatobiliary endoscopists in diagnosing and treating pCCA.
In this survey, 82.4% of the respondents used separate diagnostic codes for iCCA and eCCA. However, 16.8% of the respondents did not use distinguished codes. There may be several reasons for this. First, certain KCD-8 codes are ambiguous; for example, clinicians might think that “C22.10 Malignant neoplasm of cholangiocarcinoma” refers to any type of CCA. It is the same code as “C22.1 malignant neoplasm of intrahepatic bile duct carcinoma,” which refers to iCCA. Furthermore, “C24.9 Biliary tract, unspecified malignant neoplasm” might seem to refer to any type of CCA, but it exclusively refers to eCCA. Second, some clinicians may not feel the need to use appropriate diagnostic codes. Most clinicians focus hard on the correct diagnosis and treatment. However, some may not pay as much attention to the coding itself. Improper coding of CCA may result in accumulation of inaccurate data on the prevalence, incidence, or mortality of each CCA type.
Therefore, a new Korean coding system with clearly defined codes is required. Approximately 90% of the respondents thought that using the ICD-11 instead of the ICD-10 was necessary. Hence, a new coding system must include separate codes for each CCA type. Furthermore, educating clinicians on the use of appropriate KCD-8 codes is essential. Until a new Korean coding system is developed, we suggest using C22.1 (malignant neoplasm of intrahepatic bile duct carcinoma) and C22.10 (malignant neoplasm of CCA) for iCCA and C24.0 (extrahepatic bile duct, malignant neoplasm), C24.00 (biliary duct or passage NOS, malignant neoplasm), and C24.01 (common bile duct, malignant neoplasm) for dCCA and pCCA should be used. Tailored codes for the three types of CCA might provide more accurate and meaningful epidemiological data.
According to the Annual Report of Cancer Statistics 2018, the incidence rate of CCA increases with age, peaking at >85 years.4 The life expectancy in South Korea is 80.3 years for males and 86.1 years for females, which ranks Korea 3rd in the world life expectancy.13 These data suggest that the number of elderly patients with CCA requiring surgery or chemotherapy will increase. Few reports have investigated the efficacy and safety of curative surgery for CCA in elderly patients. Sumiyoshi et al.14 reported no significant difference in the overall 5-year survival rate between elderly patients (≥75 years) with pCCA and younger patients with pCCA (<75 years) (42.4% vs 51.3%, p=0.718). Furthermore, Akashi et al.15 reported that curative surgery can be performed with low mortality and better long-term survival in octogenarians. To the best of our knowledge, no report has been published on the efficacy and safety of chemotherapy in elderly patients with CCA. However, many reports suggest that chemotherapy prolongs the survival of the elderly.16,17 In this survey, approximately half of the respondents showed that the maximum age of the patients at which the respondents would recommend surgery and chemotherapy was 80 years. The second preferred age limit was 85 years. Given that healthy life expectancy is increasing, more octogenarians may benefit from surgery or chemotherapy in the future.
Most biliary strictures are malignant (70% to 80%), and only 10% to 40% of patients with pCCA can undergo curative surgical resection.18 The incidence of benign disease after the resection of suspected pCCA is 3.1% to 46%.19 Therefore, pathological diagnosis is needed in many cases to avoid unnecessary surgery. In this survey, approximately 95% of the respondents preferred ERCP with biopsy and cytology as the first diagnostic method for the pathological confirmation of pCCA. ERCP with biopsy and cytology is considered a standard diagnostic method for the pathological confirmation of pCCA.18,20 However, the diagnostic yield of this test remains low, with its sensitivity and specificity ranging from 18% to 60%.20 In cases where the first biopsy result shows atypia and curative resection is possible, 84% of the respondents replied that they would go straight to surgical resection rather than re-biopsy. Potential reasons for this may be that further invasive diagnostic procedures would result in complications, such as cholangitis or pancreatitis, and surgical delays. This practice agrees with a recent guideline, which suggests that preoperative biopsy is not essential in resectable pCCA if the index of suspicion is high.21
Surprisingly, >85% of the respondents preferred ERCP with re-biopsy as the second diagnostic method for the pathological confirmation of unresectable pCCA. However, no data showed that repeated ERCP with biopsy significantly increases the diagnostic yield. Furthermore, other diagnostic methods have superior diagnostic yields, such as cholangioscopy and EUS-FNA/B. The reported sensitivity and specificity of cholangioscopy were 72% to 94% and 87% to 99%, respectively.18 The diagnostic accuracy of cholangioscopy was reported to be 85%–96%.22 Additionally, the sensitivity and diagnostic accuracy of EUS-FNA/B were reported to be 75%–94% and 79%–94%, respectively.18
There may be some reasons for the unpopularity of cholangioscopy (10.1%) and EUS-FNA/B (1.7%) as the second diagnostic method for the pathological confirmation of CCA. The high cost of these procedures may be one reason. Single-operator cholangioscopy (SpyGlass DS, Boston Scientific) has been covered by the Korean national health insurance only since July 2021, when the survey was being conducted. Moreover, EUS-guided procedures are currently not covered by the national health insurance. Another reason may be that certain procedures require that endoscopists should have more experience or familiarity. Interestingly, EUS-FNA/B was chosen only by the respondents in their 30s, whereas cholangioscopy was preferred in 40% of the respondents in their 60s (p<0.05). The significant difference in these results suggests that there are some age or generational differences in endoscopic procedural preference among endoscopists in Korea.
Although more respondents (55.5%) performed preoperative biliary drainage in selected cases, still many respondents (44.5%) performed routine biliary drainage. A trend where respondents in their 60s (90%) preferred selective preoperative biliary drainage more than the other age groups (50.9% to 55%) was noted, although this difference was statistically insignificant (p=0.146). Most guidelines recommend against routine preoperative biliary drainage in pCCA.7,9,21,23 Meta-analyses have concluded that routine preoperative biliary drainage is associated with increased morbidity, postoperative infection complications, prolonged hospital stay, and increased cost.24,25 The suggested indications for preoperative drainage are obstructive cholangitis, intractable pruritus, long-lasting or severe jaundice (total bilirubin >15 mg/dL), or patients who are eligible for neoadjuvant chemotherapy, portal embolization, or major hepatic resection.7,9,21,23 Given the possible side effects of biliary drainage procedures, more efforts should be made to perform preoperative biliary drainage in selected patients.
Moreover, 64.7% of the respondents preferred ERBD using plastic stents as the preoperative biliary drainage method, followed by endoscopic nasobiliary drainage (21%), PTBD (12.6%), and ERBD using metal stents (1.7%). This wide variety of clinical practice may be because there is no single superior preoperative biliary drainage method. Studies on the optimal preoperative biliary drainage method had controversial results. A multicenter European study reported no differences in postoperative mortality between percutaneous and endoscopic approaches.26 In a Japanese multicenter study, ERBD was not associated with increased adverse events, unplanned re-interventions, or a poor prognosis compared with endoscopic nasobiliary drainage.27 A meta-analysis reported that postoperative complications were fewer in the PTBD group than in the endoscopic group,28 whereas another study showed that the incidence of seeding metastasis in patients undergoing endoscopic drainage was reduced compared with that in patients undergoing percutaneous drainage.29
For palliative biliary drainage in inoperable pCCA, ERBD using plastic stents was the most preferred modality (69.7%), followed by ERBD using metal stents (21%) and PTBD (8.4%). Biliary obstruction in pCCA is often complex and requires multi-segment biliary drainage. Generally, endoscopic biliary drainage is preferred in Bismuth type I-II pCCA.30 However, no single drainage method appears superior to another in Bismuth type III-IV pCCA. In a meta-analysis and systematic review, PTBD showed higher rates of successful biliary drainage and lower rates of cholangitis than ERBD.31 However, ERBD showed lower bleeding complications than PTBD.31 Recent randomized prospective multicenter studies on endoscopic biliary drainage in advanced pCCA showed clinical success rates of 84.9% to 91.4%.32,33 Therefore, the optimal biliary drainage method may depend on the patient’s biliary obstruction status, experience and skills of the endoscopist or radiologist, and the hospital’s facilities.34
More respondents preferred plastic stents over metal stents for endoscopic biliary drainage. A possible reason for this practice may be the frequent need for re-intervention in patients with pCCA. Patients with pCCA might undergo re-intervention for re-biopsy or recurrent cholangitis, and re-intervention has been shown to be easier using plastic stents.35
When endoscopic biliary drainage is performed, most respondents (73.9%) preferred bilateral drainage over unilateral drainage. This finding was probably influenced by a Korean multicenter, prospective, randomized study.33 In this study, Lee et al.33 reported that bilateral metal stenting had a similar technical success rate, but with fewer re-interventions and more stent patency in patients with advanced pCCA. In a recent retrospective multicenter study which compared bilateral and trisegment drainage using metal stents in Bismuth type III-IV pCCA, clinical success rates with re-intervention were higher in the trisegment drainage method.36 Moreover, the drainage of >50% of the liver volume, which frequently requires bilateral stenting, was shown to have survival gain in patients with pCCA.37
Regarding bilateral metal stenting, more respondents (63%) preferred the SIS method over the SBS method. Several studies have shown that the performance of the SIS and SBS methods are comparable.32 In a Korean prospective, randomized, multicenter study, total adverse events, technical and clinical success, stent patency, and survival were similar between the SIS and SBS methods.32 In a recent meta-analysis study, the SIS method had higher technical success and lower complication rates, whereas the SBS method was associated with longer stent patency.38 Further research is needed to develop good selection criteria for the SIS and SBS methods.
This study has several limitations. The data in this study were collected through a survey, which is prone to recall bias. Furthermore, the respondents might have selected what they thought to be the “correct” answer rather than their real-life practice. Finally, selection bias could not be avoided. All respondents in this survey worked in tertiary or general hospitals. However, the diagnosis and treatment of pCCA are almost exclusively performed in tertiary and general hospitals in Korea, where a multidisciplinary approach is possible. Moreover, >99% of ERCP procedures are performed in these hospitals in Korea. Therefore, the findings of this survey could represent the current practice of diagnosing and treating pCCA in Korea.
In conclusion, a new tailored coding system for CCA is needed for more accurate and meaningful epidemiological data. Guidelines for diagnosing and treating CCA according to the clinical situation in Korea are needed.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: H.J.K. Data acquisition: E.C. Data analysis and interpretation: S.J.C., M.K.J., B.J.S., J.M.P., J.K., W.S.P., J.K.P., S.M.W. Drafting of manuscript: E.C., S.H.K. Critical revision of manuscript for important intellectual content: S.H.K., M.K.J., J.M.P. Statistical analysis: E.C. Approval of final manuscript: all authors.
REFERENCES
- 1.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma: evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banales JM, Marin JJ, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19–31. doi: 10.1111/liv.14095. [DOI] [PubMed] [Google Scholar]
- 4.Ministry of Health and Welfare, author; Korea Central Cancer Registry, author; National Cancer Center, author. Annual report of cancer statistics in Korea in 2018 [Internet] Ministry of Health and Welfare; Sejong: c2021. [cited 2023 Feb 7]. Available from: https://www.mohw.go.kr/react/gm/sgm0704vw.jsp?PAR_MENU_ID=13&MENU_ID=13040801&page=1&CONT_SEQ=368889&PAR_CONT_SEQ=355717 . [Google Scholar]
- 5.Waseem D, Tushar P. Intrahepatic, perihilar and distal cholangiocarcinoma: management and outcomes. Ann Hepatol. 2017;16:133–139. doi: 10.5604/16652681.1226927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertuccio P, Malvezzi M, Carioli G, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104–114. doi: 10.1016/j.jhep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Cholangiocarcinoma Working Group, author. Italian clinical practice guidelines on cholangiocarcinoma - part II: treatment. Dig Liver Dis. 2020;52:1430–1442. doi: 10.1016/j.dld.2020.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Cholangiocarcinoma Working Group, author. Italian clinical practice guidelines on cholangiocarcinoma - part I: classification, diagnosis and staging. Dig Liver Dis. 2020;52:1282–1293. doi: 10.1016/j.dld.2020.06.045. [DOI] [PubMed] [Google Scholar]
- 9.Nagino M, Hirano S, Yoshitomi H, et al. Clinical practice guidelines for the management of biliary tract cancers 2019: the 3rd English edition. J Hepatobiliary Pancreat Sci. 2021;28:26–54. doi: 10.1002/jhbp.870. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO), author International Classification of Diseases 10th Revision [Internet] WHO; Geneva: c2019. [cited 2023 Feb 7]. Available from: https://icd.who.int/ [Google Scholar]
- 11.Statistics Korea, author. Korean Standard Classification of Disease and Cause of Death, 8th Revision (KCD-8) [Internet] Statistic Korea; Daejeon: c2020. [cited 2023 Feb 7]. Available from: https://kssc.kostat.go.kr:8443/ksscNew_web/index.jsp . [Google Scholar]
- 12.World Health Organization (WHO), author International Classification of Diseases 11th Revision for Mortality and Morbidity Statistics [Internet] WHO; Geneva: c2023. [cited 2023 Feb 7]. Available from: https://icd.who.int/browse11/l-m/en . [Google Scholar]
- 13.World Health Organization (WHO), author Life expectancy and healthy life expectancy: data by country [Internet] WHO; Geneva: c2022. [cited 2023 Feb 7]. Available from: https://apps.who.int/gho/data/node.main.688 . [Google Scholar]
- 14.Sumiyoshi T, Uemura K, Kondo N, et al. Is surgery justified for elderly patients with extrahepatic cholangiocarcinoma? Reappraisal from a viewpoint of comorbidity and organ function. Surg Today. 2021;51:1787–1794. doi: 10.1007/s00595-021-02340-3. [DOI] [PubMed] [Google Scholar]
- 15.Akashi K, Ebata T, Mizuno T, et al. Surgery for perihilar cholangiocarcinoma from a viewpoint of age: is it beneficial to octogenarians in an aging society? Surgery. 2018;164:1023–1029. doi: 10.1016/j.surg.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 16.Garcia G, Odaimi M. Systemic combination chemotherapy in elderly pancreatic cancer: a review. J Gastrointest Cancer. 2017;48:121–128. doi: 10.1007/s12029-017-9930-0. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH. Chemotherapy for colorectal cancer in the elderly. World J Gastroenterol. 2015;21:5158–5166. doi: 10.3748/wjg.v21.i17.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pouw RE, Barret M, Biermann K, et al. Endoscopic tissue sampling - Part 1: upper gastrointestinal and hepatopancreatobiliary tracts. European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2021;53:1174–1188. doi: 10.1055/a-1611-5091. [DOI] [PubMed] [Google Scholar]
- 19.Otsuka S, Ebata T, Yokoyama Y, et al. Benign hilar bile duct strictures resected as perihilar cholangiocarcinoma. Br J Surg. 2019;106:1504–1511. doi: 10.1002/bjs.11257. [DOI] [PubMed] [Google Scholar]
- 20.Anderson MA, Appalaneni V, et al. American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee, author. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc. 2013;77:167–174. doi: 10.1016/j.gie.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Benson AB, D'Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 22.Tanisaka Y, Mizuide M, Fujita A, et al. Diagnostic process using endoscopy for biliary strictures: a narrative review. J Clin Med. 2021;10:1048. doi: 10.3390/jcm10051048.58a026b80825449c8c4cd10e29c3310d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:691–699. doi: 10.1111/hpb.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng F, Tang YY, Dai JL, Li Y, Chen ZY. The effect and safety of preoperative biliary drainage in patients with hilar cholangiocarcinoma: an updated meta-analysis. World J Surg Oncol. 2020;18:174. doi: 10.1186/s12957-020-01904-w.79eec08a4be44cdfa9c2af6524e114d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu YD, Bai JL, Xu FG, Ding YT. Effect of preoperative biliary drainage on malignant obstructive jaundice: a metaanalysis. World J Gastroenterol. 2011;17:391–396. doi: 10.3748/wjg.v17.i3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farges O, Regimbeau JM, Fuks D, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013;100:274–283. doi: 10.1002/bjs.8950. [DOI] [PubMed] [Google Scholar]
- 27.Nakai Y, Yamamoto R, Matsuyama M, et al. Multicenter study of endoscopic preoperative biliary drainage for malignant hilar biliary obstruction: E-POD hilar study. J Gastroenterol Hepatol. 2018;33:1146–1153. doi: 10.1111/jgh.14050. [DOI] [PubMed] [Google Scholar]
- 28.Liu JG, Wu J, Wang J, et al. Endoscopic biliary drainage versus percutaneous transhepatic biliary drainage in patients with resectable hilar cholangiocarcinoma: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A. 2018;28:1053–1060. doi: 10.1089/lap.2017.0744. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Lin N, Xin F, Zeng Y, Liu J. Comparison of longterm efficacy between endoscopic and percutaneous biliary drainage for resectable extrahepatic cholangiocarcinoma with biliary obstruction: a systematic review and metaanalysis. Saudi J Gastroenterol. 2019;25:81–88. doi: 10.4103/sjg.SJG_429_18.ef27974a89964c428592542845061153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 31.Moole H, Dharmapuri S, Duvvuri A, et al. Endoscopic versus percutaneous biliary drainage in palliation of advanced malignant hilar obstruction: a meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2016;2016:4726078. doi: 10.1155/2016/4726078.56b457923d0c4c8787c42adc1063bdde [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee TH, Moon JH, Choi JH, et al. Prospective comparison of endoscopic bilateral stent-in-stent versus stent-by-stent deployment for inoperable advanced malignant hilar biliary stricture. Gastrointest Endosc. 2019;90:222–230. doi: 10.1016/j.gie.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Lee TH, Kim TH, Moon JH, et al. Bilateral versus unilateral placement of metal stents for inoperable high-grade malignant hilar biliary strictures: a multicenter, prospective, randomized study (with video) Gastrointest Endosc. 2017;86:817–827. doi: 10.1016/j.gie.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 34.Takenaka M, Kudo M. endoscopic reintervention for recurrence of malignant biliary obstruction: developing the best strategy. Gut Liver. 2022;16:525–534. doi: 10.5009/gnl210228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwasaki A, Kubota K, Kurita Y, et al. The placement of multiple plastic stents still has important roles in candidates for chemotherapy for unresectable perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2020;27:700–711. doi: 10.1002/jhbp.804. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto K, Kato H, Morimoto K, et al. Comparison of bilateral and trisegment drainage in patients with high-grade hilar malignant biliary obstruction: a multicenter retrospective study. Gut Liver. 2023;17:170–178. doi: 10.5009/gnl220012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vienne A, Hobeika E, Gouya H, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010;72:728–735. doi: 10.1016/j.gie.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 38.Chen L, Gao GM, Li DL, Chen ZK. Side-by-side versus stent-in-stent bilateral stenting for malignant hilar biliary obstruction: a meta-analysis. Wideochir Inne Tech Maloinwazyjne. 2022;17:279–288. doi: 10.5114/wiitm.2021.112477.1bd18ea611f842738b099e448f6ef2f5 [DOI] [PMC free article] [PubMed] [Google Scholar]